Abstract
Arable soils significantly contribute to atmosphere pollution through N2O emissions due to the massive use of N-based fertilizers and soil managements. N2O formation in the soil occurs mainly through nitrification and denitrification processes, which are influenced by soil moisture, temperature, oxygen concentration, pH, and the amount of available organic carbon and nitrogen. This review synthetically presents the mechanisms of N2O formation and emission in arable land and some of the current strategies to improve crop nutrient use efficiency. Biological nitrification inhibitor-based agronomic strategies are also presented as future prospects for the sustainable management of crops, which is missing in most of the reviews.
1. Introduction
Agricultural activities are currently heavily affected by climate change but are also a driver of climate change itself. Agronomic practices significantly contribute to emissions of a key greenhouse gases (GHGs), mainly in the form of nitrous oxide (N2O) emitted directly from managed soils through microbial activities and due to the use of synthetic/organic nitrogen fertilizers. Strategies to mitigate soil N2O emissions are critical, as they are long-living GHGs with a radiative force that is ~300 times higher than CO2 [1]. Over the last decades, N2O emissions from the agricultural sector showed a worldwide increase, and the largest contributions were from Asia (63%), followed by the Americas (20%), Europe (13%), and Africa (3%) [2]; moreover, N2O represents the main stratospheric ozone-depleting substance and is predicted to remain so in this century [3].
There are different agricultural practices responsible for GHG emissions, in particular for N2O emissions into the atmosphere. Among these, we can include the following:
- -
- Nitrogenous mineral fertilization [4,5] in its scheduling [6], methods, and fertilizer form [7];
- -
- Organic fertilization [8,9];
- -
- Irrigation in its methods [10] and scheduling [11,12];
- -
- Pastures [6,13];
- -
- Tillage systems [10];
- -
- Cultivated plant species [14].
Current agricultural practices, such as soil management and fertilization, are often responsible for low nitrogen use efficiency. Approximately only half of the N fertilizer inputs are recovered in the harvested yield [15]. Consequently, N application is likely to cause losses in the agroecosystem. Nitrate can be leached to the soil and surface waters [16,17], ammonia emissions can easily volatilize from topsoil soon after N fertilizer application [18], and gaseous N2O is emitted because of nitrification and denitrification processes [19]. Accordingly, increasing NUE is regarded as an effective measure to protect the environment and enhance crop production [20].
To enrich our understanding of the concurrent effect of crop type (being characterized by physiological and genetic traits) and agronomic management on NUE, it is crucial to analyze the whole cropping system following the sequence of crops and fallow over time [21] and to study the N processes driven by microbiota, which is responsible for the immobilization of N and the availability of N mineral forms and tightly interacts with C dynamics [22]. Although the extent that agricultural soils are the dominating source of N2O is well established, the complex relationship underlying microbial production/consumption processes and its links to biotic (e.g., inter- and intraspecies competition, plant–microbe interaction) and abiotic (e.g., soil, climate, and agronomic practices) factors requires an interdisciplinary cooperation and knowledge transfer between the scientific communities working on agronomic practices, soil microbial processes, microbial diversity, biosphere–atmosphere exchange, and modeling.
The aim of this review is to provide a brief overview of the biological processes involved in soil N2O emissions and the key factors controlling its production, as well as the mitigation strategies. Prospectives to mitigate GHG emissions from cropping systems are also provided through new strategies that allow for ammonium to be retained longer in the soil. This issue is not addressed much in most of the reviews, and this paper aims also to fill this gap.
2. Soil Nitrous Oxide Emissions from Arable Soils: Microbial Processes and Key Controlling Factors
2.1. Nitrous Oxide Production Pathways
N2O is produced through pathways interrelated and interacting to share intermediates or products. These pathways include the following:
- (a)
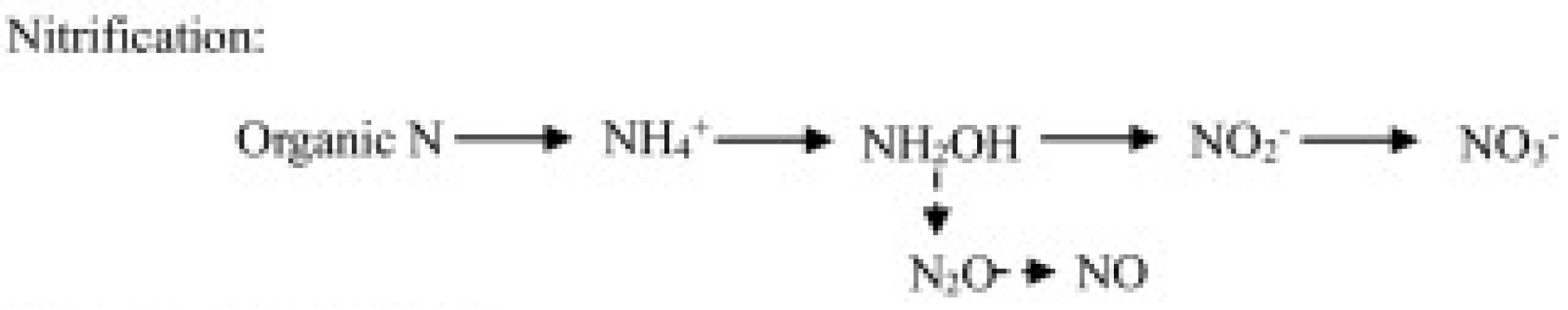
- Nitrification-related pathways, including ammonia (hydroxylamine) oxidation and nitrite oxidation—respectively operated by chemoautotrophic ammonia-oxidizing bacteria (AOB) and archaea (AOA), and nitrite-oxidizing bacteria (NOB)—and the nitrifier denitrification consisting in the reduction in nitrite by ammonia-oxidizing bacteria (Figure 1).
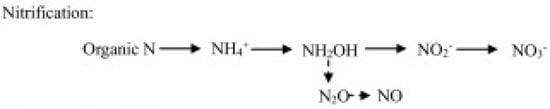 Figure 1. N2O production through the nitrification pathway operated by nitrifiers.
Figure 1. N2O production through the nitrification pathway operated by nitrifiers. - (b)
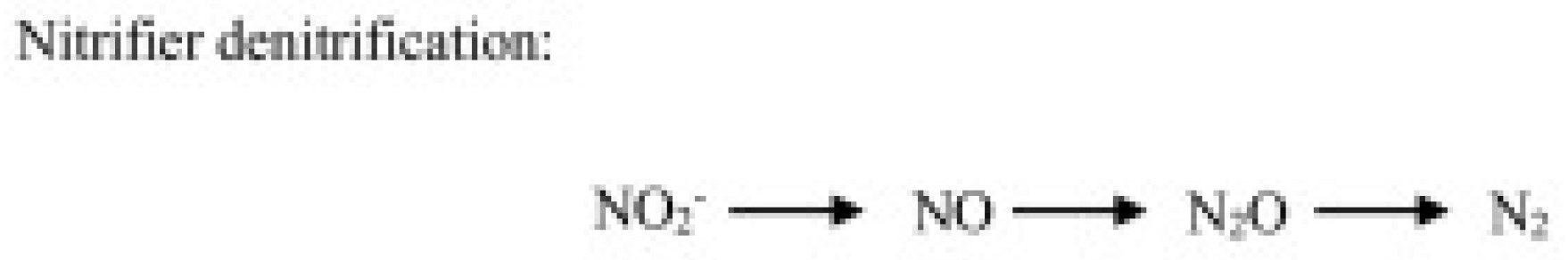
- Heterotrophic nitrification, operated by heterotrophic bacteria and fungi, consists of ammonia or organic compound oxidation (Figure 2).
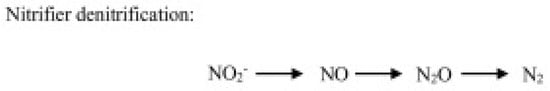 Figure 2. N2O production through the autotrophic denitrification pathway operated by nitrifiers.
Figure 2. N2O production through the autotrophic denitrification pathway operated by nitrifiers. - (c)
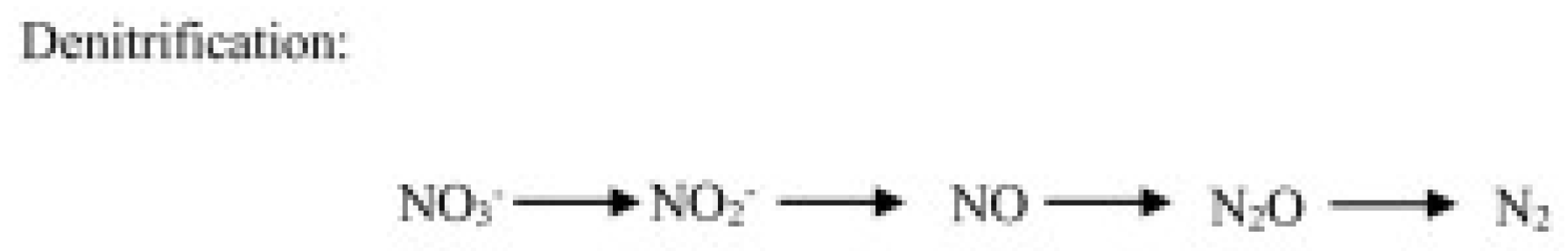
- Heterotrophic denitrification, operated by bacteria and fungi, consists of a microbial respiratory process that reduces oxidized mineral forms of N to gases, including N2O (Figure 3).
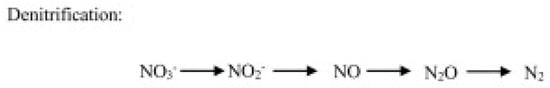 Figure 3. N2O production through the denitrification pathway operated by heterotrophic microbes.
Figure 3. N2O production through the denitrification pathway operated by heterotrophic microbes.
Nitrification is the biological conversion of ammonium in nitrite or nitrate (nitrifier nitrification, NN) and is performed by different groups of microorganisms, including ammonia-oxidizing bacteria (AOB), ammonia-oxidizing archaea (AOA), and nitrite-oxidizing bacteria (NOB) [23]. Ammonium oxidation, the first step of the NN pathway and catalyzed by the amoA gene encoding ammonia monooxygenase (AMO), is performed by AOA and AOB [24]. Nitrite oxidation, the second step of NN, is catalyzed by the nxrA or nxrB genes encoding nitrite oxidoreductase and is performed by NOB [25,26]. N2O is produced during ammonium oxidation as a byproduct of the conversion of NH2OH, an intermediate product of NN.
Heterotrophic nitrification is a further pathway producing N2O. This pathway, operated by bacteria and fungi, consists of transformations of ammonium ions or labels of organic molecules to produce NO2− or NO3−, but the heterotrophic bacteria perform ammonia transformation by genes coding for AMO-type enzymes [27]. However, information regarding this pathway is scarce compared to that on autotrophic nitrification. It is known that this pathway prevails in acidic soils, such as forest soils, but recent studies also found it in arable soils [27].
Ammonia-oxidizing bacteria can also perform NO2− reduction through the nitrifier denitrification (ND) pathway [28]. In this pathway, ammonium is oxidized to NO2−, followed by NO production that is further reduced to N2O. Ammonia oxidation is the rate-limiting step for the whole nitrification process [29], so it represents a critical step for the production of the nitrification-originated N2O.
Denitrification is performed by heterotrophic microbes and involves the reduction in nitrate and nitrite in the presence of carbon availability and leads to N2 synthesis. During these transformations, N2O is generated as a byproduct. Different reductases are involved in the bacteria denitrification pathway [30]. The nitrate reductase, encoded by narG or napA genes, catalyzes the NO3− reduction to NO2−; the nitrite reductase, encoded by nirK or nirS genes, catalyzes the NO2− reduction to NO; the nitric oxide reductase, leading to N2O formation, is mediated by the cnorB or qnorB genes; and the nitrous oxide reductase, encoded by the nosZ gene, which catalyzes the reduction in N2O to N2. Fungi produce N2O through a nitrite-reducing system consisting of a copper–nitrite-containing reductase encoded by nirK homologs and a cytochrome P45 nitric oxide reductase encoded by the P450nor gene [31].
Extensive field measurements of N2O exchange between soils and the atmosphere and laboratory incubation studies under controlled conditions provide a good understanding of the emission. Nevertheless, the dynamic and temporal trend of N2O soil emissions are characterized by ‘hot spots’ with an enormous spatio-temporal variability [32,33] caused by a multitude of interacting control factors [34]. Among them, the availability of reactive nitrogen, coming from the use of fertilizer, represents the major driver of soil N2O emissions. To better understand how soil microorganisms contribute to N2O emissions, studies that applied next-generation sequencing (NGS) and bioinformatics technologies for the metagenomic analysis of soil microbiota appear to be of pivotal importance, as well as a quantitative real-time polymerase chain reaction (qPCR) for the quantification of the abundance of key genes [35,36]. The extraction of total genomic DNA from soils is conventionally used as a template to perform metagenomic studies by High-Throughput Sequencing (HTS) or qPCR [37].
2.2. Drivers of Nitrous Oxide Production and Soil Emissions
Microbial N2O production is affected by several key factors such as soil oxygen levels, water content, pH, temperature, and the availability of nitrogen and carbon. Those factors are, in turn, influenced by climate conditions, soil texture, soil and crop management, and fertilizer type. Soil microbial biomass, responsible for nitrification and denitrification and of N2O production emissions, requires adequate environmental conditions that control the N2/N2O ratio [38].
2.2.1. O2 Levels
Soil oxygen level (O2) is the key factor simultaneously controlling nitrification and denitrification by influencing both processes and determining the partitioning of the end products between N2 and N2O [39]. Enzymes involved in N2O production are sensitive to O2. soil O2 concentration is probably the most critical variable that directly regulates N2O production and consumption at the cellular level. It is well known that high O2 levels in the soil promote N2O production by nitrifier nitrification and reduce N2O production by heterotrophic denitrification and nitrifier denitrification. Moreover, high O2 levels inhibit N2O reductase, the only enzyme catalyzing a reduction in N2O to N2, resulting in a net emission of nitrous oxide gas. On the other side, at moderately low O2 levels, N2O production is mainly promoted by nitrifier denitrification due to an increasing expression of denitrification enzymes in AOB, whereas at very low O2 levels, heterotrophic denitrification dominates on the other N2O producing processes. Song et al. [40] found that the soil N2O concentrations increased quadratically as soil O2 decreased during hot moments in dryland agricultural soil in Northern China, with the highest N2O production occurring when O2 levels dropped below 0.2%.
O2 levels in the soil are affected by soil texture. It is generally considered that fine-textured soils provide more favorable conditions for N2O emissions by denitrification than coarser-textured soils that favor nitrification [39,41]. This occurs because fine-textured soils have small pores that become more easily anaerobic after rainfall or irrigation and remain that way for longer durations than coarser-textured soils.
2.2.2. Water Content
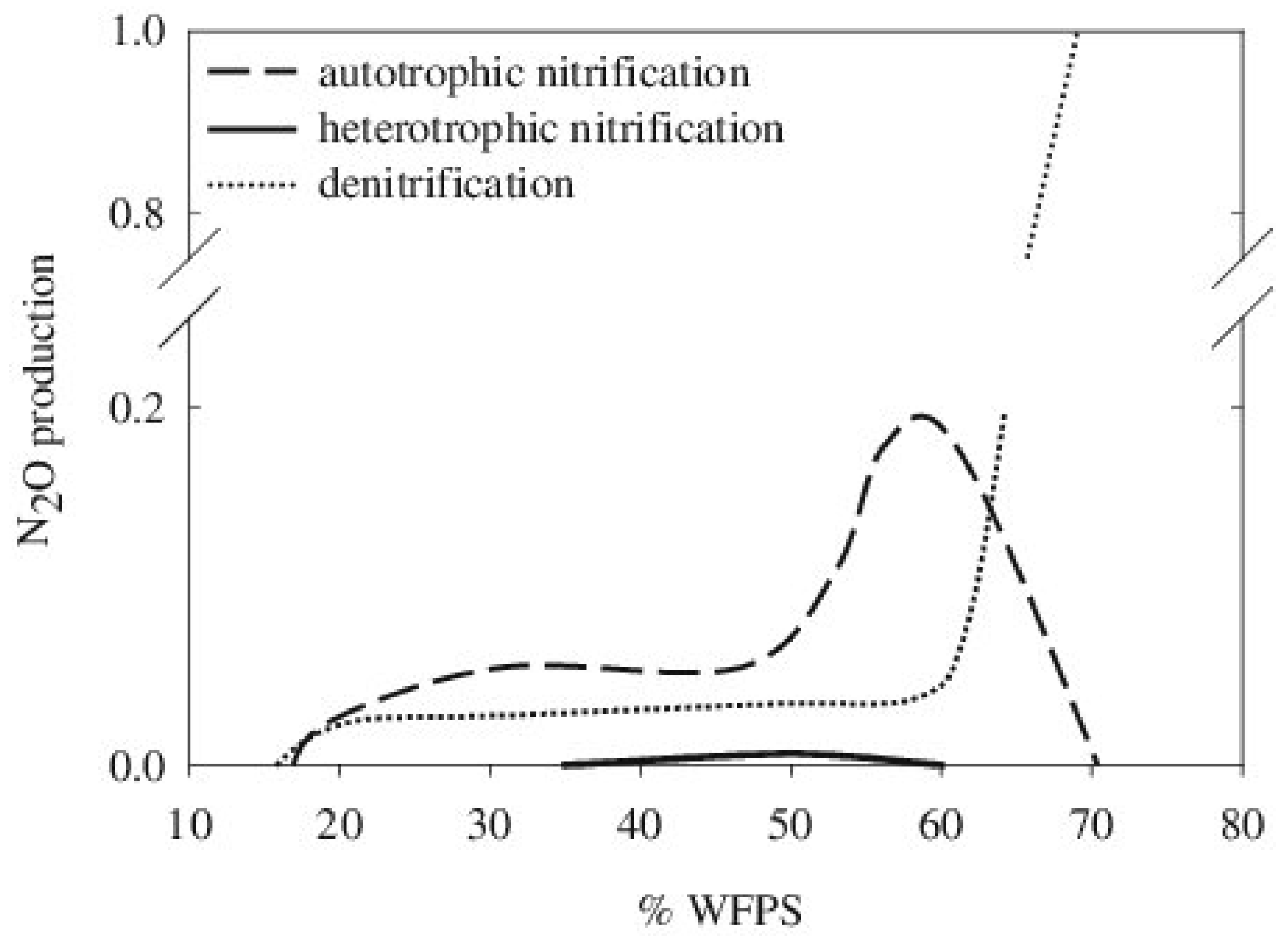
Soil humidity affects N2O emissions by influencing O2 availability for biological transformations. Irrigation and precipitation increase soil water content, thus reducing the pore space that can be occupied by oxygen and influencing the soil O2 diffusion in the water phase, which is much lower than in air. From this consideration, it follows that the diffusion rate of O2 in the soil following heavy rain or irrigation declines below the soil O2 consumption rate by microbes. The water-filled pore space (WFPS), a parameter that combines the soil water content with the soil’s physical properties, such as soil porosity and apparent density, is inversely correlated with O2 availability. The highest N2O emissions occur for a WFPS of 60–65% and are mainly attributed to denitrification, whereas nitrification is responsible for N2O emissions for a WFPS below 60% [11]. For higher WFPS levels (70–90%), N2O emissions are mainly due to denitrification, but in soils saturated by water, N2O emissions are minimal due to N2O consumption during heterotrophic denitrification (Figure 4).
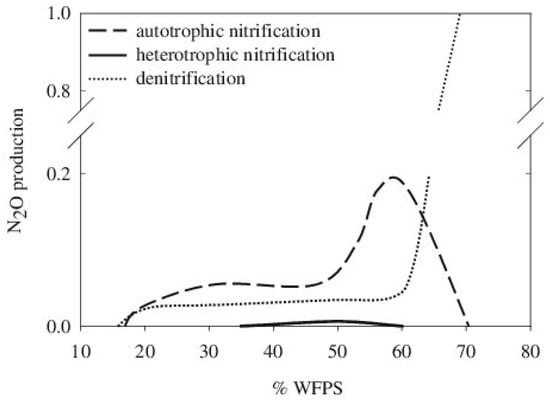
Figure 4.
Contribution of nitrification and denitrification to N2O soil emissions; from Bateman and Baggs (2005).
The irrigation dose, by influencing the soil moisture, affects N2O emissions. Carbonell-Bojollo et al. [42] report that a full irrigation dose on crop demand produces more N2O than a reduced dose, whereas Guo and colleagues [43] report that an excessive water in a vineyard increased N2O loss. These findings highlight the role of irrigation management in nitrous oxide production.
2.2.3. pH
pH affects N2O emissions by affecting the growth of microbes. Acid pH is more beneficial to nitrifiers than denitrifiers. More specifically, the growth of ammonia-oxidizing bacteria (AOB) is limited in acid soils—in which optimal growth is restricted to neutral and subalkaline soils—but not ammonia-oxidizing archaea (AOA), which have been detected in a wide range of soil pH from acid (pH < 5) to alkaline values (pH > 7) [44,45]. The causes of limited growth of AOB in acid soil might be attributed to the limited diffusivity of NH4+ across the AOB membrane upon acid pH, which instigates the strong ionization of NH3 to NH4+, limiting AOB’s ability to oxidize. Acid pH also favors heterotrophic nitrification, promoting fungi growth using organic over inorganic N [46]. On the contrary, the alkaline environment strongly affects microbial denitrification [47]. Alkaline soils promote denitrification because reductase activities are more instigated at alkaline pH [48].
Long-term or excessive application of N fertilizers can lead to soil acidification [49], which promotes N2O emissions. Different approaches have been tested to counteract soil acidification. Among these, pH change through soil liming is one of the most efficient strategies to mitigate soil N2O emissions from acid soils [50].
2.2.4. Temperature
Temperature is another key factor controlling N2O production. N2O emissions increase with increasing temperature up to 35 °C [51]. Nitrification and denitrification seem to respond similarly to temperature. Temperatures above 35 °C reduce N2O production, likely due to enhanced O2 consumption through an increased microbial respiration, creating anaerobic conditions, a circumstance that increases the reduction in N2O to N2 [51]. Soil texture is a factor affecting the temperature sensitivity of N2O emissions. Cui et al. [52] showed that the temperature sensitivity of N2O emissions was lower as soil texture became more clayey. This might occur because in a warming environment, clay soil has a greater possibility to create an anaerobic microenvironment as a result of the greater oxygen consumption resulting from C mineralization in small unconnected pores, which would accelerate N2O production by denitrifiers.
2.2.5. Nitrogen and Carbon Availability
Soil nitrogen (N) availability is the main driver of N2O production [53]. Different N forms are used by soil microbes for their growth, contributing to nitrous oxide emissions from arable soils. Therefore, N fertilization is the main cause for environmental pollution by nitrogen. The ammonium in urea and ammonium nitrate fertilizers is rapidly transformed after their application in the field into nitrate through nitrification, thus producing a substrate (NO3−) utilizable by autotrophic and heterotrophic microbes through denitrification. Under soil conditions promoting nitrification, a decrease in the amount of NH4+ and an increase in NO3− in the soil occurs during the N2O emission peak, as NO3− is being produced while NH4+ is being consumed by nitrifiers [54].
Heterotrophic denitrifiers use a carbon (C) source as an electron donor to produce nitrous oxide under anaerobic conditions [53]. However, the quantity and quality of soil carbon affect denitrification [55]. As the quantity of C available to denitrifiers increases, the rate of denitrification increases if a sufficient NO3− substrate is present and anaerobic conditions occur. C in soil derives from organic fertilization through the deposition of manures and slurries and from the mineralization of organic matter or root exudation. Increasing availability of labile C in the soil can enhance O2 consumption due to an enhanced microbial respiration, thus increasing anaerobic conditions that favor denitrification and, in turn, N2O production. However, excessive oxygen consumption due to enhanced respiration because of higher labile carbon content can limit oxygen availability to microbes and favors complete denitrification to N2.
The C/N ratio might affect soil N2O emissions, even if contrasting results have been found. For instance, negative [56], positive [57], or no relationships [58] between the soil C/N ratio and N2O emissions were observed in field studies, evidencing that the soil C/N ratio alone may be insufficient to predict N2O emissions, unlike labile forms, such as soluble organic carbon and particulate and light fraction organic carbon, which are more palatable to N2O producers.
3. Strategies for Improving Nitrogen Use Efficiency by Crops and Mitigating N2O Emissions in Agro-Ecosystems
In the last 50 years, the EU has become increasingly concerned with the efficiency of the use of fertilizers. Indeed, these substances may exert strong beneficial effects in agriculture, but they also hold the potential to negatively impact human health and environmental safety.
This is particularly true for nitrogen due to the peculiar characteristics of such a nutrient. Indeed, on one side, N has a huge effect on crop productivity and, therefore, its fertilization rate is often pushed far beyond the actual crop demand, as cheap insurance against yield losses. On the other side, however, this element is known to be rather mobile in soil and prone to high losses through leaching, especially with high rainfall levels and irrigation volumes [59]. Furthermore, several crops, especially leafy vegetables, can accumulate luxury nitrogen in their leaves, leading to health problems for humans and animals. These possible side effects of N fertilization have promoted great concern regarding the efficiency with which the crops use the N fertilizer that we provide. Obviously, the efficiency of the use of fertilizers is a general problem that concerns all nutrients, but the way we have to tackle it for N is rather peculiar due to the aforementioned mobility of this element in soil.
3.1. Nitrogen Use Efficiency (NUE)
Nitrogen use efficiency (NUE) has been the object of a remarkable research effort. In general, it has been shown that NUE is rather small for most crops; for example, years ago, it was estimated that worldwide NUE for cereals used to be as low as 33% [60]. Ref. [61] highlighted that NUE comprises three main components: (i) nitrogen uptake efficiency (NUpE), (ii) nitrogen utilization efficiency (NUtE) in producing biomass, and (iii) the nitrogen harvest index (NHI). NUtE and the NHI can be merged into one component: the utilization efficiency for harvestable products.
Of those three components, NUpE is rather important since, despite the high demands, the great majority of crops can only absorb a small fraction of mineral N in the soil. In this respect, [62] studied the amount of N absorbed by the crop (excluding fibrous roots) with and without the application of N fertilization and defined the concept of the Apparent Recovery (REC) of the N fertilizer; these authors proved that the REC declined when the fertilization rate was increased. Later studies showed that the REC is strongly variable depending on the crop species, according to their root physiology and architecture [61,63]. Furthermore, the REC was also shown to be influenced by soil conditions (e.g., it is reduced by water shortage, which decreases crop growth and N uptake), weather conditions (e.g., high rainfall levels), agronomic practices (e.g., high irrigation volumes), and fertilization methods (e.g., broadcast application over localized application) [64,65].
In order to improve NUpE, we can use different tools and strategies as follows [66]:
- -
- Breeding N-efficient genotypes. In the past, several research has been carried out in order to select physiological, biochemical, and molecular traits affecting nitrogen uptake by plants through roots. In particular, studies have addressed the spatial root arrangement in the soil evaluating the root depth, lateral root expansion, and root length densities, together with a variety of components, such as roots and root segments [63]. Studies have also been carried out in order to understand the molecular basis of the transport systems involved in the absorption of NO3− from the soil and its systemic fluxes within plants, as well as studies that have been addressed to identify the Ammonium Transporters (AMTs) and the gene family of the Amino acid Transport Family (ATF) [63]. All that knowledge has made it possible to start crop breeding programs with high nitrogen absorption through the introgression of the improved trait into the gene pool of the new genotype [63].
- -
- Adopting appropriate agronomic practices, with particular reference to, e.g., tillage, crop rotation, green manuring, intercropping, grafting, the use of biostimulants, and, obviously, organic/mineral fertilization. This latter aspect (mineral fertilization) is understandably receiving a lot of attention, for example, through the 4R Nutrient Stewardship [67,68], which provides a framework for applying the right nutrient source at the right rate, at the right time, and at the right place. Recently, the use of biostimulants to improve NUE by crops has received more and more attention, with encouraging results. Navarro-León et al. [69] demonstrated that L-α-amino-acid-based biostimulants enhance plant productivity through improved photosynthesis and increased the assimilation of essential nutrients, such as nitrogen (N). Cozzolino et al. [70] found that legume-derived protein hydrolysates and the extract of brown seaweed Ecklonia maxima applied on the leaves improved the yield and quality of tomato grown in the field. The use of lignite-derived humic substances applied to the soil has also proven effective in improving NUE through an improvement in nitrogen uptake efficiency (NUpE) and nitrogen utilization efficiency (NUtE) by tomato crops [71]. Among biostimulants, microbial biostimulants also are currently used in agriculture to improve the efficiency of the use of fertilizers and, in particular, N fertilizers. Bacteria and other microorganisms, through their metabolic processes, exude or secrete a wide range of biochemical compounds into the soil that allow plants to efficiently use nutrients [72].
With particular reference to nitrogen, increasing NUpE should be based on the following [66]:
- Determination of the N demand from the crop.
- Determination of the right N fertilizer rates to meet crop demand, considering the interactions between crop uptake, soil supply, environmental risks, and field operation logistics.
- Correct application techniques, favoring localized over broadcast applications.
- Selection of the proper nutrient source to ensure the best match between nutrient availability and crop requirements.
The problem of selecting the right nutrient source brings in the so-called Enhanced Efficiency N Fertilizers (EEFs), which may play a key role in ensuring a better synchronization between N root uptake and N release kinetics, which is one of the fundamental aspects used to reduce the spread of N into the environment. Currently, EEFs can be classified into five categories: (i) slow-release N fertilizers, (ii) controlled-release N fertilizers, (iii) nitrification inhibitor-treated N fertilizers, (iv) urease inhibitor-treated N fertilizers, and (v) fertilizers treated with both nitrification and urease inhibitors [73,74,75].
3.2. Slow- and Controlled-Release Fertilizers (SRFs and CRFs)
Slow-release fertilizers (SRFs) are characterized by low solubility in water, and they include urea-formaldehyde (UF) and isobutylenediurea (IBDU); the first compound releases N through microbial decomposing activities, which are strongly affected by several environmental factors, such as temperature and soil characteristics. In contrast, IBDU releases N through hydrolysis at low temperatures and is, therefore, preferred for winter applications [76]. Field studies demonstrated the effectiveness of slow-release fertilizers. For example, SRFs improved the yield of sugarcane [77] and winter Chinese chives [78] and, at the same time, mitigated the adverse effects of excessive fertilizer use in agricultural practices.
In controlled-release fertilizers (CRFs), N release is regulated by the presence of a water-insoluble coating, which is made of, e.g., sulfur, organic polymers, inorganic polymers, or several types of combinations. This coating material slows down the release of fertilizers, depending on the type of coating, soil temperature, and soil humidity [76,79,80]. Regarding the type of coating, organic and inorganic materials have been used. Organic polymer materials are mostly made of thermoplastic resins, such as polyolefin, polyvinylidene chloride, and copolymers, which cannot degrade easily in soil. Sometimes, natural organic polymers have been used, such as starch, chitosan, and cellulose. In general, [81] lists more than 10 types of organic coating materials, with very different characteristics and release times. Inorganic coating materials are usually made from sulfur and minerals, such as hydroxyapatite, bentonite, zeolite, and attapulgite. Among these inorganic coating materials, calcium phosphates (CaPs) have been gaining attention as CRFs due to their relatively high phosphorus content, pH-dependent solubility in water, and ability to act as carriers of micro- and macro-nutrients, including nitrogen. The beneficial effects of CRFs on grain yield and NUE largely depend on the synchronization of the N release rate with the N needs of crops. By slowing down the release of available N, the CRF reduces N availability for microbial transformation, limiting soil N2O emissions [82].
In general, SRFs and CRFs have been successfully used in field and vegetable crops, wherein they have demonstrated an ability to improve NUE, reduce nutrient losses due to leaching and the volatilization of fertilizers, and provide economic advantages due to decreased fertilization rates [83] and increased agronomic efficiency. Furthermore, SRFs and CRFs have been proven to reduce possible toxicity phenomena to plants and seedlings by reducing the local concentration of ions, which can induce osmotic stress and damage in the case of traditional fertilizers [84]. On the other hand, SRFs and CRFs have also shown some disadvantages, mainly related to the lack of reliable methods to forecast the release of nutrients to the plant; indeed, it has been shown that especially with short-cycled crops, nutrients may continue to be released after crop harvest in a prolonged manner [85]. In addition, the use of sulfur-coated urea (SCU) can increase soil acidity, while polymer-coated CRFs with synthetic materials can be difficult to degrade, producing a different form of pollution. Last, but not least, SRFs and CRFs can be more costly than conventional N fertilizers [59].
3.3. Nitrification Inhibitors (NIs)
Another way to obtain EEFs is based on the use of the so-called nitrification inhibitors (NIs); these compounds decrease the speed of conversion of ammonium to nitrate, thereby reducing the risk of nitrate leaching and N2O emissions. The most used NIs are 3,4-dimethylpyrazole phosphate (DMPP) and dicyandiamide (DCD) [86,87,88]. Several studies show the potential usefulness of such compounds in terms of NUE and environmental side effects, such as nitrate leaching and N2O emissions [89]. According to a comprehensive meta-analytic work [89], the reduction in N leaching following the application of NIs was, on average, 48%. Other authors showed that the use of NIs permitted a decrease in the number of N applications [86] and a reduction in nitrate concentration in leafy vegetables [90]. Analogously to CRFs and SRFs, the activity of NIs may depend on soil properties, such as temperature and pH. In particular, the activity of DMPP is more dependent on soil type than that of DCD, although the efficacy of both inhibitors was lower in more alkaline, low-organic matter soil [91]. The inhibitory activity of DCD and DMPP was shown to last for, respectively, 50 and 95 days, as measured in incubation experiments with cauliflower residues [92].
Apart from NIs, urease inhibitors (UIs) can also be used in EEFs. These compounds block the activity of the urease enzyme, resulting in the inhibition of the hydrolysis of urea into ammonium. Such activity is beneficial because it reduces the volatilization of ammonia—which may occur in soils with high pH or poorly buffered against pH changes where the rapid hydrolysis of the urea may result in the accumulation of ammonia rather than ammonium—and limits N2O production and, in turn, emissions from the soil to the atmosphere. Products with known UI activity are N-(n-butyl) thiophosphoric triamide (NBPT) and N-(n-propyl) thiophosphoric triamide (NPPT). These active ingredients are found in products with tradenames of Agrotain™ (NBPT) and Limus™ (NBPT and NPPT), but, to date, it is also possible to find NBPT in other commercial products since this compound is no longer patent protected. UIs have proven effective in increasing yields and NUE and protecting against ammonia losses.
Of course, NIs and NUs can also be used in combination with each other in order to ‘close’ all possible N escape routes, which is not achieved when these inhibitors are applied individually [73]. It has been shown that the use of UIs reduced the volatilization of ammonia but increased the volatilization of nitrogen oxides, which was avoided by combining the two inhibitors [93]. Ref. [94] found that the application of a double inhibitor combining NBPT and 2-(3,4-dimethylpyrazole-1-YL)-succinic acid (DMPSA) produced a 50% reduction in N volatilization.
Although both NIs and NUs have been proven useful [95], in some circumstances, their activity may not be sufficient, especially when weather and soil conditions are favorable to N losses or when excess N doses are used [96]. For these conditions, research is needed to obtain improved formulations and elucidate possible ways for improved distribution and better integration with correct agronomic practices to reduce N losses and increase NUE. Moreover, [59] observed that given the concern of consumers and retailers regarding healthy food, attention will need to be paid to ensure that there are no or minimal residues of NUs and NIs and their degradation products in edible vegetable organs.
A survey of the literature shows that all the above types of EEFs have been around for several years, and a lot of information is available in relation to their behavior in the environment. However, far less is known about their optimal usage in agriculture (e.g., timings, dosages, and methods of application), which is strongly affected by the complex interactions between the environment (soil and weather conditions), crop development, and the dynamics of the release of nutrients. Furthermore, it is not yet totally clear to what extent these EEFs can increase NUE, depending on the crop and on main environmental and agronomic factors. Last, but not least, not much information is available to predict the environmental impact of these compounds using reliable life cycle assessment methods.
3.4. Fertigation
Fertigation is a valid strategy to increase NUE [96] by crops while decreasing leached N [97] and fertilizer-induced N2O emissions from soil. Fertigation consists in the application of nutrients through irrigation systems; it presents advantages over dry fertilization, such as a more precise N management in coordination with crop demand [98]. By splitting the nitrogen fertilizer application, so-called ‘high frequency’ fertigation, the risk of nitrate leaching is minimized [99] and the N uptake by roots is improved [100]. High frequencies with a low quantity of N fertigation also have a third priority, i.e., to reduce N2O emissions. By allowing the reduction in fertilizer quantity to be applied each time, high-frequency N fertigation limits N availability for microorganisms under environmental conditions that promote N2O production during nitrification and denitrification processes. However, the benefits of fertigation in improving crop NUE and mitigating N2O production depend on the N source. It should be expected that there will be greater N2O emissions in crops fertigated with urea or ammonium fertilizer than with nitrate-based fertigation [101], resulting in greater NUE by crops for NO3−-based fertilizers under high frequencies [102].
4. Future Prospective
To limit soil N2O emissions and improve fertilizer use efficiency by crops, it is necessary to create a low-cost and convenient agronomic strategy that keeps (or improves) crops’ quantity and quality. Regulating nitrification is central to any strategy for improving nitrogen use efficiency; one of these strategies could be represented by the biological nitrification inhibition (BNI) where nitrification inhibitors exuded from plant roots suppress soil nitrifying activity, thus favoring N uptake by plant roots [103]. Different botanical species produce NIs; among these are pasture grasses, such as Panicum maximum Jacq. and Lolium perenne L., cereal crops, such as Sorghum bicolor L. and Pennisetum glaucum L., and legume crops, such as Arachis hypogaea L. To some extent, the release of NIs from roots is related to the plant N status [104] and by the N form applied. So, plants grown with NO3 as a nitrogen source do not release NIs from roots, whereas plants grown with NH4 as their nitrogen source release them.
Different molecules have been identified as nitrification inhibitors. For example, methyl 3-(4-hydroxyphenyl) propionate and sorgoleone are exuded from sorghum roots, 1,9-decanediol from rise roots, and brachialactone linolenic acid and linoleic acid from Brachiaria humidicola roots. Karanjin, a furanoflavonoid (3-methoxy furano-2′,3′,7,8-flavone), is obtained from Pongamia glabra seeds. All these BNIs block both AMO and HAO enzymatic pathways [105]. However, new molecules potentially having inhibitory properties have to be discovered or tested. For example, cannabinoids have a molecular structure, like synthetic inhibitors, and, for this reason, they have the potential ability to inhibit the enzymatic pathways involved in nitrification. By exploiting the ability of plants to produce nitrification-inhibiting substances, it could be interesting to develop new cultivation strategies involving target species in association with BNI species or introduce the BNI-controlling chromosome region in target plants, which is unable to produce inhibiting substance constitutively [106]. These new strategies will allow ammonium to be retained longer in the soil, which will be absorbed and assimilated easily by the target crops. In this way, the effectiveness of fertilizer uses increases, and fewer nitrogen fertilizers will be required by crops, thus preventing the salinization and eutrophication of water bodies.
Author Contributions
Conceptualization: L.V. and V.M. Writing—original draft preparation: A.M., M.G. (Matteo Giaccone), T.Z., A.O. and L.V. Writing—review and editing: A.M., M.G. (Matteo Giaccone), T.Z., A.O., F.T., M.F., M.G. (Mara Gabbrielli), M.A., A.P. and V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out within the Agritech National Research Center and received funding from the European Union Next-Generation EUGeneration EU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4—D.D. 1032 17 June 2022, CN00000022). This manuscript only reflects the authors’ views and opinions, and neither the European Union nor the European Commission can be considered responsible for them.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- IPCC. Climate Change: The Physical Science Basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge Univ Press: Cambridge, UK, 2007. [Google Scholar]
- Tubiello, F.N.; Salvatore, M.; Cóndor Golec, R.D.; Ferrara, A.; Rossi, S.; Biancalani, R.; Federici, S.; Jacobs, H.; Flammini, A. Agriculture, Forestry and Other Land Use Emissions by Sources and Removals by Sinks; ESS Working Paper No. 2; FAO: Rome, Italy, 2014. [Google Scholar]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, T.K.; Kongshaug, G. Energy Consumption and Greenhouse Gas Emissions in Fertiliser Production; Proceedings No. 509; The International Fertiliser Society: New York, NY, USA, 2004. [Google Scholar]
- Menegat, S.; Ledo, A.; Tirado, R. Greenhouse gas emissions from global production and use of nitrogen synthetic fertilizers in agriculture. Sci. Rep. 2022, 12, 14490. [Google Scholar] [CrossRef] [PubMed]
- Mumford, M.T.; Rowlings, D.W.; Scheer, C.; De Rosa, D.; Grace, P.R. Effect of irrigation scheduling on nitrous oxide emissions in intensively managed pastures. Agric. Ecosyst. Environ. 2019, 272, 126–134. [Google Scholar] [CrossRef]
- Tian, D.; Zhang, Y.; Mu, Y.; Liu, J.; He, K. Effect of N fertilizer types on N2O and NO emissions under drip fertigation from an agricultural field in the North China Plain. Sci. Total Environ. 2019, 715, 136903. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhu, B.; Wang, S.; Zhu, X.; Vereecken, H.; Bruggemann, N. Stimulation of N2O emission by manure application to agricultural soils may largely offset carbon benefits: A global meta-analysis. Global Chang. Biol. 2017, 23, 4068–4083. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Chang, C.; Larney, V.; Travis, G.R. Greenhouse gas emissions during cattle feedlot manure composting. J. Environ. Qual. 2002, 31, 376–386. [Google Scholar] [CrossRef]
- Wang, C.; Amon, B.; Schulz, K.; Mehdi, B. Factors that influence nitrous oxide emissions from agricultural soils as well as their representation in simulation models: A Review. Agronomy 2021, 11, 770. [Google Scholar] [CrossRef]
- Trost, B.; Prochnow, A.; Drastig, K.; Meyer-Aurich, A.; Ellmer, F.; Baumecker, M. Irrigation, soil organic carbon and N2O emissions. A review. Agron. Sustain. Dev. 2013, 33, 733–749. [Google Scholar] [CrossRef]
- Zhang, Q.; Niu, W.; Du, Y.; Sun, J.; Cui, B.; Zhang, E.; Wang, Y.; Siddique, K.H.M. Effect of aerated drip irrigation and nitrogen doses on N2O emissions, microbial activity, and yield of tomato and muskmelon under greenhouse conditions. Agric. Water Manag. 2022, 283, 108321. [Google Scholar] [CrossRef]
- Smith, H.P.J.; Reinsch, T.; Swanepoel, P.A.; Kluß, C.; Taube, F. Grazing under irrigation affects N2O-emissions substantially in south Africa. Atmosphere 2020, 11, 925. [Google Scholar] [CrossRef]
- Chataut, G.; Bhatta, B.; Joshi, D.; Subedi, K.; Kafle, K. Greenhouse gases emission from agricultural soil: A review. J. Agric. Food Res. 2023, 11, 100533. [Google Scholar] [CrossRef]
- Conant, R.T.; Berdanier, A.B.; Grace, P.R. Patterns and trends in nitrogen use and nitrogen recovery efficiency in world agriculture. Global Biogeochem. Cycles 2023, 27, 558–566. [Google Scholar] [CrossRef]
- Basile, A.P.; Bonfante, A.; De Mascelis, R.; Terribile, F.; Brenna, S.; Acutis, M. Nitrate leaching under maize cropping systems in Po Valley (Italy). Agric. Ecosyst. Environ. 2012, 147, 57–65. [Google Scholar]
- Bancheri, M.; Coppola, A.; Colombi, A.; Basile, A. The extended transfer function model for the simulation of pesticides transport along the unsaturated zone. In Proceedings of the 3rd ISMC Conference—Advances in Modeling Soil Systems, Online, 18–22 May 2021. [Google Scholar]
- Hafner, S.D.; Pacholski, A.; Bittman, S.; Burchill, W.; Bussink, W.; Chatigny, M.; Carozzi, M.; Gnemont, S.; Häni, C.; Hansen, M.; et al. The ALFAM2 database on ammonia emission from field-applied manure: Description and illustrative analysis. Agric. For. Meteorol. 2018, 258, 66–79. [Google Scholar] [CrossRef]
- Abalos, D.; Recous, S.; Butterbach-Bahl, K.; De Notaris, C.; Rittl, T.F.; Topp, C.F.E.; Petersen, S.O.; Hansen, S.; Bleken, M.A.; Rees, R.M.; et al. A review and meta-analysis of mitigation measures for nitrous oxide emissions from crop residues. Sci. Tot. Environ. 2022, 828, 154388. [Google Scholar] [CrossRef] [PubMed]
- Basso, B.; Liu, L.; Ritchie, J.T. A comprehensive review of the CERES-wheat, -maize and-rice models’ performances. Adv. Agron. 2022, 136, 27–132. [Google Scholar]
- Martinez-Feria, R.A.; Castellano, M.J.; Dietzel, R.N.; Helmers, M.J.; Liebman, M.; Huber, I.; Archontoulis, S.V. Linking crop- and soil-based approaches to evaluate system nitrogen-use efficiency and tradeoffs. Agric. Ecosyst. Environ. 2018, 256, 131–143. [Google Scholar] [CrossRef]
- Kushwaha, C.P.; Tripathi, S.K.; Singh, K.P. Variations in soil microbial biomass and N availability due to residue and tillage management in a dryland rice agro-ecosystem. Soil Till. Res. 2000, 56, 153–166. [Google Scholar] [CrossRef]
- Huang, L.; Chakrabarti, S.; Cooper, J. Ammonia-oxidizing archaea are integral to nitrogen cycling in a highly fertile agricultural soil. ISME Commun. 2021, 1, 19. [Google Scholar] [CrossRef]
- Hu, T.; Wei, J.; Du, L. The effect of biochar on nitrogen availability and bacterial community in farmland. Ann. Microbiol. 2023, 73, 4. [Google Scholar] [CrossRef]
- Poly, F.; Wertz, S.; Brothier, E.; Degrange, V. First exploration of Nitrobacter diversity in soils by a PCR cloning-sequencing approach targeting functional gene nxrA. FEMS Microbiol. Ecol. 2008, 63, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Pester, M.; Maixner, F.; Berry, D.; Rattei, T.; Koch, H.; Lücker, S.; Nowka, B.; Richter, A.; Spieck, E.; Lebedeva, E.; et al. NxrB encoding the beta subunit of nitrite oxidoreductase as functional and phylogenetic marker for nitrite-oxidizing Nitrospira. Environ. Microbiol. 2014, 16, 3055–3071. [Google Scholar] [CrossRef] [PubMed]
- Martikainen, P.J. Heterotrophic nitrification—An eternal mystery in the nitrogen cycle. Soil Biol. Biochem. 2022, 168, 108611. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; van Beusichem, M.L.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Kowalchuk, G.A.; Stephen, J.R. Ammonia-oxidizing bacteria: A model for molecular microbial ecology. Annu. Rev. Microbiol. 2001, 55, 485–529. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.J.; Watmough, N.J. Inorganic nitrogen metabolism in bacteria. Curr. Opin. Chem. Biol. 1999, 3, 207–219. [Google Scholar] [CrossRef]
- Shoun, H.; Fushinobu, S.; Jiang, L.; Kim, S.W.; Wakagi, T. Fungal denitrification and nitric oxide reductase cytochrome P450nor. Philos. Trans. R Soc. Lond B Biol. Sci. 2012, 5, 1186–1194. [Google Scholar] [CrossRef]
- Groffman, P.M. Methods for measuring denitrification: Diverse approaches to a difficult problem. Ecol. Appl. 2006, 6, 2091–2122. [Google Scholar] [CrossRef]
- Wolf, B. Grazing-induced reduction of natural nitrous oxide release from continental steppe. Nature 2010, 464, 881–884. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Dannenmann, M. Denitrification and associated soil N2O emissions due to agricultural activities in a changing climate. Curr. Opin. Environ. Sustain. 2011, 3, 389–395. [Google Scholar] [CrossRef]
- Jousset, A.; Bienhold, C.; Chatzinotas, A.; Gallien, L.; Gobe, A.; Kurm, V.; Küsel, K.; Rillig, M.C.; Rivett, D.W.; Salles, J.F.; et al. Where less may be more: How the rare biosphere pulls ecosystems strings. ISME J. 2017, 11, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Orellana, L.H.; Chee-Sanford, J.C.; Sanford, R.A.; Löffler, F.E.; Konstantinidis, K.T. Year-round shotgun metagenomes reveal stable microbial communities in agricultural soils and novel ammonia oxidizers responding to fertilization. Appl. Environ. Microbiol. 2017, 84, e01646-17. [Google Scholar] [CrossRef] [PubMed]
- Ercolini, D. High-throughput sequencing and metagenomics: Moving forward in the culture-independent analysis of food microbial ecology. Appl. Environ. Microbiol. 2013, 79, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Saggar, S.; Jha, N.; Deslippe, J.; Bolan, N.S.; Luo, J.; Giltrap, D.L.; Kim, D.-G.; Zaman, M.; Tillman, R.W. Denitrification and N2O:N2 production in temperate grasslands: Processes, measurements, modelling and mitigating negative impacts. Sci. Total. Environ. 2013, 465, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, A.; Conrad, R. Influence of O2 availability on no and N2O release by nitrification and denitrification in soils. Global Chang. Biol. 1998, 4, 387–396. [Google Scholar] [CrossRef]
- Song, X.; Wei, H.; Rees, R.M.; Ju, X. Soil oxygen depletion and corresponding nitrous oxide production at hot moments in an agricultural soil. Environ Pollut. 2022, 292, 118345. [Google Scholar] [CrossRef] [PubMed]
- Saxton, K.E.; Rawls, W.; Romberger, J.S.; Papendick, R.I. Estimating generalized soil-water characteristics from texture. Soil Sci. Soc. Am. J. 1986, 50, 1031–1036. [Google Scholar] [CrossRef]
- Carbonell-Bojollo, R.M.; Veroz-González, Ó.; GonzálezSánchez, E.J.; Ordóñez-Fernández, R.; Moreno-García, M.; RepulloRuibérriz de Torres, M.A. Soil management, irrigation and fertilisation strategies for N2O emissions mitigation in mediterranean agricultural systems. Agronomy 2022, 12, 1349. [Google Scholar] [CrossRef]
- Guo, Y.; Ji, Y.; Zhang, J.; Liu, Q.; Han, J.; Zhang, L. Effects of water and nitrogen management on N2O emissions and NH3 volatilization from a vineyard in North China. Agric. Water Manag. 2022, 266, 107601. [Google Scholar] [CrossRef]
- Lu, L.; Han, W.; Zhang, J.; Wu, Y.C.; Wang, B.; Lin, X. Nitrification of archaeal ammonia oxidizers in acid soils is supported by hydrolysis of urea. ISME J. 2012, 6, 1978–1984. [Google Scholar] [CrossRef]
- Zhang, L.M.; Hu, H.W.; Shen, J.P.; He, J.Z. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2012, 6, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Zhong, W.; Cai, Z. Organic nitrogen stimulates the heterotrophic nitrification rate in an acidic forest soil. Soil Biol. Biochem. 2015, 80, 293–295. [Google Scholar] [CrossRef]
- Brenzinger, K.; Dörsch, P.; Braker, G. pH-driven shifts in overall and transcriptionally active denitrifiers control gaseous product stoichiometry in growth experiments with extracted bacteria from soil. Front. Microbiol. 2015, 6, 961. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Mørkved, P.T.; Frostegård, A.; Bakken, L.R. Denitrification gene pools, transcription and kinetics of NO, N2O and N2 production as affected by soil pH. FEMS Microbiol. Ecol. 2010, 72, 407–417. [Google Scholar] [CrossRef]
- Shaaban, M.; Peng, Q.; Lin, S.; Wu, Y.; Zhao, J.; Hu, R. Nitrous oxide emission from two acidic soils as affected by dolomite application. Soil Res. 2014, 52, 841–848. [Google Scholar] [CrossRef]
- Shaaban, M.; Wu, Y.; Wu, L.; Hu, R.; Younas, A.; Nunez-Delgado, A.; Xu, P.; Sun, Z.; Lin, S.; Xu, X.; et al. The effects of pH change through liming on soil N2O emissions. Processes 2020, 8, 702. [Google Scholar] [CrossRef]
- Lai, T.V.; Denton, M.V. N2O and N2 emissions from denitrification respond differently to temperature and nitrogen supply. J. Soils Sed. 2018, 18, 1548–1557. [Google Scholar] [CrossRef]
- Cui, P.; Chen, Z.; Fan, F.; Yin, C.; Song, A.; Li, T.; Zhang, H.; Liang, Y. Soil texture is an easily overlooked factor affecting the temperature sensitivity of N2O emissions. Sci. Total Environ. 2023, 862, 160648. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Liu, L.; Wang, C.; Wan, Y.; Yang, R.; Mou, J.; Liu, J.; Wu, Y.; Tang, S.; Zhu, T.; et al. Carbon and nitrogen fractions control soil N2O emissions and related functional genes under land-use change in the tropics. Environ. Pollut. 2023, 335, 122370. [Google Scholar] [CrossRef]
- Akiyama, H.; Tsuruta, H.; Watanabe, T. N2O and NO Emissions from Soils after the Application of Different Chemical Fertilizers; Chemosphere: Global Change Science:: Oxford, UK, 2000; Volume 2, pp. 313–320. [Google Scholar]
- Schlüter, S.; Henjes, S.; Zawallich, J.; Bergaust, L.; Horn, M.; Ippisch, O.; Vogel, H.-J.; Dörsch, P. Denitrification in soil aggregate analogues-effect of aggregate size and oxygen Diffusion. Front. Environ. Sci. 2018, 6, 17. [Google Scholar] [CrossRef]
- Santos, C.; Fonseca, J.; Coutinho, J.; Trindade, H.; Jensen, L.S. Chemical properties of agro-waste compost affect greenhouse gas emission from soils through changed C and N mineralisation. Biol. Fertil. Soils. 2021, 57, 781–792. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, J.; Yang, W.; Cai, Z. Effects of organic material amendment and water content on NO, N2O, and N2 emissions in a nitrate-rich vegetable soil. Biol. Fertil. Soils. 2012, 49, 53–163. [Google Scholar] [CrossRef]
- Lu, C.; Bowman, D.; Rufty, T.; Shi, W. Reactive nitrogen in turfgrass systems: Relations to soil physical, chemical, and biological properties. J. Environ. Qual. 2015, 44, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.B.; Martínez-Gaitan, C.; Gallardo, M.; Giménez, C.; Fernández, M.D. Identification of irrigation and N management practices that contribute to nitrate leaching loss from an intensive vegetable production system by use of a comprehensive survey. Agri Water Manag. 2017, 89, 261–274. [Google Scholar] [CrossRef]
- Raun, W.R.; Johnson, G.V. Improving nitrogen use efficiency for cereal production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef]
- Lammerts van Bueren, E.T.; Struik, P.C. Diverse concepts of breeding for nitrogen use efficiency. A review. Agron. Sustain. Dev. 2017, 37, 50. [Google Scholar] [CrossRef]
- Greenwood, D.J.; Kubo, K.; Burn, S.I.G.; Draycott, A. Apparent recovery of fertilizer N by vegetable crops. Soil Sci. Plant Nutr. 1989, 35, 367–381. [Google Scholar] [CrossRef]
- Ferrante, A.; Nocito, F.F.; Morgutti, S.; Sacchi, G.A. Plant breeding for improving nutrient uptake and utilization efficiency. In Advances in Research on Fertilization Management of Vegetable Crops; Advances in Fertilization Management of Vegetable Crops; Tei, F., Nicola, S., Benincasa, P., Eds.; Springer: Cham, Switzerland, 2017; pp. 221–246. [Google Scholar]
- Tremblay, N.; Scharpf, H.C.; Weier, U.; Laurence, H.; Owen, J. Nitrogen Management in Field Vegetables: A Guide to efficient fertilization. Horticultural Research and Development Centre, Canada. 2001. Available online: https://publications.gc.ca/collections/Collection/A42-92-2001E.pdf (accessed on 27 April 2023).
- Benincasa, P.; Guiducci, M.; Tei, F. The nitrogen use efficiency: Meaning and sources of variation—Case studies on three vegetable crops in Central Italy. HortTechnology 2011, 21, 266–273. [Google Scholar] [CrossRef]
- Tei, F.; De Neve, S.; de Haan, J.; Kristensen, H.L. Nitrogen management of vegetable crops. Agric. Water Manag. 2020, 240, 106316. [Google Scholar] [CrossRef]
- Reetz, H.F., Jr.; Heffer, P.; Bruulsema, T.W. Chapter 4: 4R nutrient stewardship: A global framework for sustainable fertilizer management. In Managing Water and Fertilizer for Sustainable Agricultural Intensification; Drechseler, P., Heffer, P., Magen, H., Mikkelsen, R., Wichelns, D., Eds.; International Fertilizer Industry Association (IFA), International Water Management Institute (IWMI), International Plant Nutrition Institute (IPNI), and International Potash Institute (IPI): Paris, France, 2015; pp. 65–86. [Google Scholar]
- Bruulsema, T. Managing nutrients to mitigate soil pollution. Environ. Pollut. 2018, 243, 1602–1605. [Google Scholar] [CrossRef]
- Navarro-León, E.; López-Moreno, F.J.; Borda, E.; Marín, C.; Sierras, N.; Blasco, B.; Ruiz, J.M. Effect of L-amino acid-based biostimulants on nitrogen use efficiency (NUE) in lettuce plants. J. Sci. Food Agric. 2022, 102, 7098–7106. [Google Scholar] [CrossRef]
- Cozzolino, E.; Di Mola, I.; Ottaiano, L.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Foliar application of plant-based biostimulants improve yield and upgrade qualitative characteristics of processing tomato. Ital. J. Agron. 2021, 16, 1825. [Google Scholar] [CrossRef]
- Qin, K.; Dong, X.; Leskovar, D.I. Improving tomato nitrogen use efficiency with lignite-derived humic substances. Sci. Hortic. 2023, 321, 112243. [Google Scholar] [CrossRef]
- Schütz, L.; Gattinger, A.; Meier, M.; Müller, A.; Boller, T.; Mäder, P.; Mathimaran, N. Improving crop yield and nutrient use efficiency via biofertilization—A global meta-analysis. Front. Plant Sci. 2018, 8, 2204. [Google Scholar] [CrossRef] [PubMed]
- Snyder, C.S. Enhanced nitrogen fertilizer technologies support the ‘4R’ concept to optimize crop production and minimize environmental losses. Soil Res. 2017, 55, 463–472. [Google Scholar] [CrossRef]
- Chen, J.; Lü, S.; Zhang, Z.; Zhao, X.; Li, X.; Ning, P.; Liu, M. Environmentally friendly fertilizers: A review of materials used and their effects on the environment. Sci. Tot. Environ. 2018, 613–614, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Haque, K.M.S.; Khan, M.Z.H. A review on application of controlled released fertilizers influencing the sustainable agricultural production: A Cleaner production process. Environ. Technol. Innov. 2021, 23, 101697. [Google Scholar] [CrossRef]
- Morgan, K.T.; Cushman, K.E.; Sato, S. Release Mechanisms for slow- and controlled-release fertilizers and strategies for their use in vegetable production. HortTechnology 2009, 19, 10–12. [Google Scholar] [CrossRef]
- Yang, S.; Xiao, J.; Liang, T.; Tan, H. Response of bacterial compositions to the use of slow-release fertilizers with long-acting agents and synergists. Appl. Soil Ecol. 2023, 182, 104699. [Google Scholar] [CrossRef]
- Wang, C.; Lv, J.; Coulter, J.A.; Xie, J.; Yu, J.; Li, J.; Zhang, J.; Tang, C.; Niu, T.; Gan, Y. Slow-release fertilizer improves the growth, quality, and nutrient utilization of wintering chinese chives (Allium tuberosum Rottler ex Spreng.). Agronomy 2020, 10, 381. [Google Scholar] [CrossRef]
- Simonne, E.H.; Hutchinson, C.M. Controlled-release fertilizers for vegetable production in the era of best management practices: Teaching New Tricks to an Old Dog. HortTechnology 2005, 15, 36–46. [Google Scholar] [CrossRef]
- Van Eerd, L.L. Nitrogen dynamics and yields of fresh bean and sweet corn with different cover crops and planting dates. Nutr. Cycl. Agroecosyst. 2018, 111, 33–46. [Google Scholar] [CrossRef]
- Lawrencia, D.; Wong, S.K.; Low, D.Y.S.; Goh, B.H.; Goh, J.K.; Ruktanonchai, U.R.; Soottitantawat, A.; Lee, L.H.; Tang, S.Y. Controlled release fertilizers: A review on coating materials and mechanism of release. Plants 2021, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, B.; Zhou, Y.; Zhao, M.; Chen, Y.; Zhang, J.; Wang, J.; Liang, L. Effects of long-term controlled-release urea on soil greenhouse gas emissions in an open-field lettuce system. Plants 2024, 13, 1071. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ortiz, R.; Naranjo, M.Á.; Ruiz-Navarro, A.; Caballero-Molada, M.; Atares, S.; García, C.; Vicente, O. New eco-friendly polymeric-coated urea fertilizers enhanced crop yield in wheat. Agronomy 2020, 10, 438. [Google Scholar] [CrossRef]
- Sempeho, S.I.; Kim, H.T.; Mubofu, E.; Hilonga, A. Meticulous overview on the controlled release fertilizers. Adv. Chem. 2014, 2014, 363071. [Google Scholar] [CrossRef]
- Shaviv, A. Advances in controlled release of fertilizers. Adv. Agron. 2000, 71, 1–49. [Google Scholar]
- Pasda, G.; Hähndel, R.; Zerulla, W. Effect of fertilizers with the new nitrification inhibitor DMPP (3,4-dimethylpyrazole phosphate) on yield and quality of agricultural and horticultural crops. Biol. Fertil. Soils 2001, 34, 85–97. [Google Scholar] [CrossRef]
- Gilsanz, C.; Báez, D.; Misselbrook, T.H.; Dhanoa, M.S.; Cárdena, L.M. Development of emission factors and efficiency of two nitrification inhibitors, DCD and DMPP. Agric. Ecosyst. Environ. 2016, 216, 1–8. [Google Scholar] [CrossRef]
- Ruser, R.; Schulz, R. The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils—A review. J. Plant Nutr. Soil Sci. 2015, 178, 171–188. [Google Scholar] [CrossRef]
- Qiao, C.; Liu, L.; Hu, S.; Compton, J.A.; Greaver, T.; Li, Q. How inhibiting nitrification affects nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input. Global Chang. Biol. 2015, 21, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Irigoyen, I.; Lamsfus, C.; Aparicio-Tejo, P.; Muro, J. The influence of 3, 4-di- methylpyrazole phosphate and dicyandiamide on reducing nitrate accumulation in spinach under Mediterranean conditions. J. Agric. Sci. 2006, 144, 555–562. [Google Scholar] [CrossRef]
- Guardia, G.; Marsden, K.; Vallejo, A.; Jones, D.L.; Chadwick, D.R. Determining the influence of environmental and edaphic factors on the fate of the nitrification inhibitors DCD and DMPP in soil. Sci. Total Environ. 2018, 624, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Chaves, B.; Opoku, A.; De Neve, S.; Boeckx, P.; Can Cleemput, O.; Hofman, G. Influence of DCD and DMPP on soil N dynamics after incorporation of vegetable crop residues. Biol. Fertil. Soils 2006, 43, 62–68. [Google Scholar] [CrossRef]
- Drury, C.F.; Yang, X.; Reynolds, W.D.; Calder, W.; Oloya, T.O.; Woodley, A.L. Combining urease and nitrification inhibitors with incorporation reduces ammonia and nitrous oxide emissions and increases corn yields. J. Environ. Qual. 2017, 46, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Corrochano-Monsalve, M.; Bozal-Leorri, A.; Sánchez, C.; González-Murua, C.; Estavillo, J.-M. Joint application of urease and nitrification inhibitors to diminish gaseous nitrogen losses under different tillage systems. J. Clean. Prod. 2017, 289, 125701. [Google Scholar] [CrossRef]
- Cantarella, H.; Otto, R.; Soares, J.R.; Silva, A.G.D.B. Agronomic efficiency of NBPT as a urease inhibitor: A review. J. Adv. Res. 2018, 13, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Li, P.; Li, B. Effects of fertigation scheme on N uptake and N use efficiency in cotton. Plant Soil 2007, 290, 115–126. [Google Scholar] [CrossRef]
- Lv, H.; Lin, S.; Wang, Y.; Lian, X.; Zhao, Y.; Li, Y.; Du, J.; Wang, Z.; Wang, J.; Butterbach-Bahl, K. Drip fertigation significantly reduces nitrogen leaching in solar greenhouse vegetable production system. Environ. Pollut. 2019, 245, 694–701. [Google Scholar] [CrossRef]
- Lamm, F.R.; Schlegel, A.J.; Clark, G.A. Development of a best management practice for nitrogen fertigation of corn using SDI. Appl. Eng. Agric. 2004, 20, 211–220. [Google Scholar] [CrossRef]
- Bhat, R.; Sujatha, S. Soil fertility and nutrient uptake by arecanut (Areca catechu L.) as affected by level and frequency of fertigation in a laterite soil. Agric. Water Manag. 2009, 96, 445–456. [Google Scholar] [CrossRef]
- Silber, A.; Xu, G.; Levkovitch, I.; Soriano, S.; Bilu, A.; Wallach, R. High fertigation frequency: The effects on uptake of nutrients, water and plant growth. Plant Soil 2003, 253, 467–477. [Google Scholar] [CrossRef]
- Wolff, M.W.; Hopmans, J.W.; Stockert, C.M.; Burger, M.; Sanden, B.L.; Smart, D.R. Effects of drip fertigation frequency and N-source on soil N2O production in almonds. Agric. Ecosyst. Environ. 2017, 238, 67–77. [Google Scholar] [CrossRef]
- Quaggio, J.A.; Souza, T.R.; Zambrosi, F.C.B.; Boaretto, R.M.; Mattos, D., Jr. Nitrogen fertilizer forms affect the nitrogen use efficiency in fertigated citrus groves. J. Plant Nutrit. Soil Sci. 2014, 177, 404–411. [Google Scholar] [CrossRef]
- Bozal-Leorri, A.; Subbarao, G.V.; Kishii, M.; Urmeneta, L.; Kommerell, V.; Karwat, H.; Braun, H.J.; Aparicio-Tejo, P.M.; Ortiz-Monasterio, I.; González-Murua, C.; et al. Biological nitrification inhibitor-trait enhances nitrogen uptake by suppressing nitrifier activity and improves ammonium assimilation in two elite wheat varieties. Front. Plant Sci. 2022, 13, 1034219. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Ishikawa, T.; Ito, O.; Nakahara, K.; Wang, H.Y.; Berry, W.L. A bioluminescence assay to detect nitrification inhibitors released from plant roots: A case study with Brachiaria humidicola. Plant Soil 2006, 288, 101–112. [Google Scholar] [CrossRef]
- Lan, T.; Li, M.; He, X.; Deng, O.; Zhou, W.; Luo, L. Effects of synthetic nitrification inhibitor (3,4-dimethylpyrazole phosphate; DMPP) and biological nitrification inhibitor (methyl 3-(4-hydroxyphenyl) propionate; MHPP) on the gross N nitrification rate and ammonia oxidizers in two contrasting soils. Biol. Fertil. Soils 2022, 58, 333–344. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Searchinger, T.D. A “more ammonium solution” to mitigate nitrogen pollution and boost crop yields. Proc. Natl. Acad. Sci. USA 2021, 118, 2107576118. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).