Abstract
The demand for common hazel (Corylus avellana) fruit increases constantly. Powdery mildew (PM) on hazels in Hungary and throughout Europe was previously caused mainly by Phyllactinia guttata. However, less than a decade ago, another fungus of Asian origin, Erysiphe corylacearum, appeared on hazels in Europe, including Hungary. Our investigation aimed to develop a species-specific PCR (ssPCR) to aid the identification of P. guttata and E. corylacearum, and to assess the presence of the latter, non-native fungus in Hungary. For this study, 59 samples were collected from Hungary between 2021 and 2023. The chasmothecial morphology of the PM fungi was observed, and the internal transcribed spacer (ITS) of ribosomal DNA was sequenced in representative samples. Morphological analysis distinguished two types of chasmothecia. Parts of the chasmothecia, typical of P. guttata, were flattened and spherical with bristle-like appendages, while other chasmothecia, characteristic of E. corylacearum, were distinctly smaller, bearing appendages with branched apices. Sequence data also verified the presence of P. guttata and E. corylacearum in our samples. The developed ssPCR revealed that E. corylacearum was present in more than three-quarters of the samples, more than a quarter of the samples contained both fungi and about one-fifth carried solely P. guttata. The alien fungus E. corylacearum was found in all but one of the sampled regions and was found on C. avellana and also on C. colurna. Erysiphe corylacearum spreads rapidly and can be considered an invasive pathogen. Its practical importance lies in its ability to infect hazelnuts, potentially causing economic losses. Our ssPCR ensures accurate and quick identification of the fungus, which is essential for effective plant protection.
1. Introduction
The genus Corylus belongs to the family Betulaceae and subfamily Coryloideae [1]. One of the most well-known species, the European or common hazel (Corylus avellana), is a deciduous shrub, mostly multi-stemmed and up to five meters tall. Its native range includes Hungary. Its typical habitat is the edges of deciduous forests. Corylus avellana and its different cultivars are also widely planted as ornamentals.
Its fruit, the hazelnut, is one of the most important nuts globally, together with walnuts and almonds [2]. The current planted area of roughly 1.07 million hectares yields approximately 1.22 million tons [2], over 60% of which originate from Turkey [3]. Other countries with significant production quantities are Italy, the USA, Azerbaijan, Chile and Georgia [2].
In Hungary, hazel has long been a crop of cottage gardens and small farms, but now it has become, from an economic point of view, one of the most important nuts in Hungary [2,4]. In addition to the cultivation of hazelnuts, a number of mycorrhizal plantations with the aim of growing truffles (mostly Tuber aestivum, and experimentally also T. macrosporum and T. borchii) are also being established [5].
A notable disease of hazel plantations in Hungary, and also in several other hazel-growing regions, is powdery mildew. At least nine species of powdery mildew fungi (Erysiphaceae, Helotiales) can infect Corylus species [6], but in Hungary, the disease is generally caused by the widespread pathogen Phyllactinia guttata [4,7]. The fungus is mostly found to infect plants belonging to the Betulaceae family, including the genus Corylus in Europe [8]. It has also been described from common lilac (Syringa vulgaris) [9] and is known to infect several other host plants as well [10]. The symptom of P. guttata infection is an effuse or evanescent, gray-white mycelial coating on the leaves of hazels [8], which is more likely to appear hypophyllously [8].
However, another species of powdery mildew fungi, Erysiphe corylacearum, is also known to infect hazels [11,12]. Its symptoms develop in early spring on young shoots, leaves and young fruits [12]. The symptoms of infection mostly appear on the upper surface of the hazel leaf, but the fungus can also colonize the back of the leaf [8,11]. The leaves turn brown during infection, and symptoms also include necrosis of the leaves or leaf drop [12]. More importantly, hazelnut fruits can also be colonized by the powdery mildew fungus [12,13], rendering them shriveled and underdeveloped. The prevalence of symptoms can be as high as 100%, potentially leading to significant yield losses.
Erysiphe corylacearum is indigenous to Asia, where it appears on C. heterophylla (Asian hazel) and C. sieboldiana (Japanese hazel) [6]. This species was known to occur only in the Americas and Asia [8] until the beginning of the 2010s. The first reported outbreak outside of these regions was observed in Turkey in 2013 [12]. Then, the pathogen was observed on C. avellana in Azerbaijan [14]. The emergence of powdery mildew has also been reported in Iran [15], followed by its identification in Ukraine [16,17] and Georgia [18]. Thereafter, the fungus seemed to spread rapidly through Europe, appearing in different locations in Switzerland [19], Austria [20], Germany [21], Italy [13,22], Spain [23], Romania [24] and Hungary [4,11], where its reported hosts include C. avellana and C. colurna. Recent outbreaks of powdery mildew have also been reported in Slovenia, Bulgaria, Poland and the Czech Republic [25,26,27,28].
The two powdery mildew-causing species P. guttata and E. corylacearum can be differentiated by observing chasmothecia (fruiting bodies), as those of P. guttata are about 155–225 μm in diameter, with bristle-like appendages [8]. The chasmothecia of E. corylacearum are significantly smaller in size (80–115 μm) and have branched appendages with recurved tips [4,8]. However, when fruiting bodies are not available, differentiating the two species needs tedious preparation of microscopic slides and observation of anamorphic features.
In this study, we aimed to develop a species-specific PCR (ssPCR) for the molecular-based differentiation of E. corylacearum and P. guttata. Additionally, we aimed to investigate the occurrence of the fungus in Hungary using both the newly developed ssPCR method and morphological analysis.
2. Materials and Methods
2.1. Sampling and Morphological Analysis
A total of 59 hazel leaves showing PM symptoms were collected from Hungary from 12 counties and Budapest from 2021 to 2023. Details of these samples are shown in Table A1. The presence or absence of chasmothecia was noted, and the chasmothecial morphology of PM fungi was observed under a Zeiss Stemi 2000C stereomicroscope (Jena, Germany). The powdery mildew species present was identified based on descriptions of chasmothecial morphology [8,11,29].
2.2. DNA Extraction
Twelve control DNA extracts (three for both species, and the experiment was conducted twice, hence twelve) were created from the chasmothecia of E. corylacaearum and P. guttata. To create these control DNA extracts, five individual chasmothecia were carefully selected and collected individually from each species. The chasmothecia were then crushed and subjected to DNA extraction by incubating them in TE buffer at 97 °C [30]. The control DNA extracts were used to test species-specific primers.
From field samples not carrying chasmothecia, DNA was extracted with a Qiagen Plant Mini Kit (Qiagen GmbH; Hilden, Germany). For this, an approximately 1 cm2 piece was cut from the leaf with powdery mildew symptoms and was used for DNA extraction. DNA extraction was conducted according to the manufacturer’s instructions. From the samples with chasmothecia, DNA extracts were created from the fruiting bodies. For this, randomly chosen chasmothecia were collected and subjected to DNA extraction by incubating them in TE buffer as described above for the control DNA extracts.
2.3. Species-Specific PCR
An ssPCR was developed as follows: Sequences of the internal transcribed spacer (ITS) region of E. corylacearum (isolate EC; MW031866 [20]) and P. guttata (voucher WTUF72463; MT162617 [31]) were downloaded from GenBank. The sequences were aligned with MEGA7 [32] and dissimilar regions of the ITS of the two species were identified. Primers E_coryl-f (CAGAGTGTGAGGCTCACTC) and E_coryl-r (TCCATGTGACTGGAGCAAAAG) were designed based on the E. corylacearum sequence to bind to the 5′ end and 3′ end of the ITS region, respectively, with the aid of SnapGene Viewer software (version 6.0.2; GSL Biotech, San Diego, CA, USA). A Phyllactinia guttata-specific primer PG-r3 (AAACGTGACTACGCGGAGAG [29]) was used along with a modified version of PG-f2 [29]. The latter primer was modified to avoid potential mismatches revealed after alignment of the original PG-f2 primer sequence to the ITS of P. guttata with MEGA7, resulting in PG-f2-alt (ACCCGTGTCGATTGTATCTTCTGT).
The control DNA extracts (see above), presumably containing DNA from one of the two species only, were assayed with both primer pairs. The ssPCR was started in a thermocycler (Tianlong; Xi’an, China) preheated to 95 °C to ensure specificity. PCRs were run with the following protocol: 95 °C for 3 min followed by 35 cycles of 94 °C for 20 s, 56 °C for 20 s and 72 °C for 1 min, and at the end, 72 °C for 5 min. Initially, a thermal gradient PCR (56–64 °C) was used to identify the optimal annealing temperature for providing sufficient yields and specificity at the same time. The reaction mix composition included 10 µL of DreamTaq Green PCR MasterMix (Thermo Fisher Scientific, Waltham, MA, USA), 0.6 µL of 10 µM forward and reverse primers, and 1 µL of DNA template, resulting in a final volume of 20 µL. To assure consistent results and avoid byproducts, a frozen PCR rack was used when setting up reaction mixes. A negative control (molecular biology grade water) was always included in each reaction. The resulting PCR products were run on 1% sodium borate [33] agarose gel and visualized under UV light.
To test the effectiveness of the method on DNA extracts containing both species, eleven artificial mixtures were created. These contained DNA extracts from each of the species: single chasmothecial DNA from E. corylacearum and P. guttata from the samples HPM19 and HPM28 were mixed in a 1:1 (v/v) ratio; and DNA from E. corylacearum chasmothecia from the samples HPM10, HPM24 and HPM25 and DNA from P. guttata chasmothecia from the samples HPM33, HPM40 and HPM41 were mixed in a 1:1 ratio in all nine possible combinations. These were then used as targets in the ssPCR. The experiment was conducted twice.
After the development of the ssPCR, the assay was used on all DNA samples originating from PM-infected Corylus leaf samples to determine which species of the two is present on each sample.
2.4. DNA Sequencing
To verify whether the ssPCR amplified the ITS of the targeted species only, and to verify the morphology-based identifications and the specificity of the ssPCR, six amplified ITS fragments from both species were sent for sequencing to LGC Genomics GmbH (Berlin, Germany). Sequencing was performed with the same primers used for the amplifications. Electropherograms were processed and individually checked using the CodonCode Aligner 8.0.2 (CodonCode Corporation; Centerville, MA, USA). The resulting sequences were analyzed using BLASTn to identify the most similar sequences in GenBank. If a sequence was identical or almost identical to reference sequences of E. corylacearum or P. guttata, it was considered to verify species identity. Representative ITS sequences determined in this study were deposited in GenBank (accession numbers PP825796-PP825800).
3. Results
In the morphological analysis, two types of chasmothecia could be distinguished (Figure 1). Parts of the chasmothecia, typical of P. guttata, were flattened and spherical, approximately 200 μm in diameter with bristle-like (acicular) appendages of about 400 μm. Other chasmothecia were distinctly smaller (less than 100 μm), bearing appendages with branched apices. These were characteristic of E. corylacearum. After observing 59 samples, chasmothecia were detected in 36 samples, and 23 samples did not have chasmothecia (Table A1). We identified only E. corylacearum fruiting bodies in 17 samples, those of P. guttata only were found in 6 samples and we had 13 samples with chasmothecia of both species (Table A1; Figure 1). Thus, a total of 30 samples had chasmothecia of E. corylacearum and 19 had those of P. guttata. Mostly, but not exclusively, P. guttata was found on the back (abaxial), while E. corylacearum was found on the upper (adaxial) side of the leaves.

Figure 1.
Infection of Corylus avellana by two powdery mildew fungi on same leaf. Sample HPM18 with chasmothecia of E. corylacearum (upper left) and P. guttata (lower right) is shown. Bar: 1 cm.
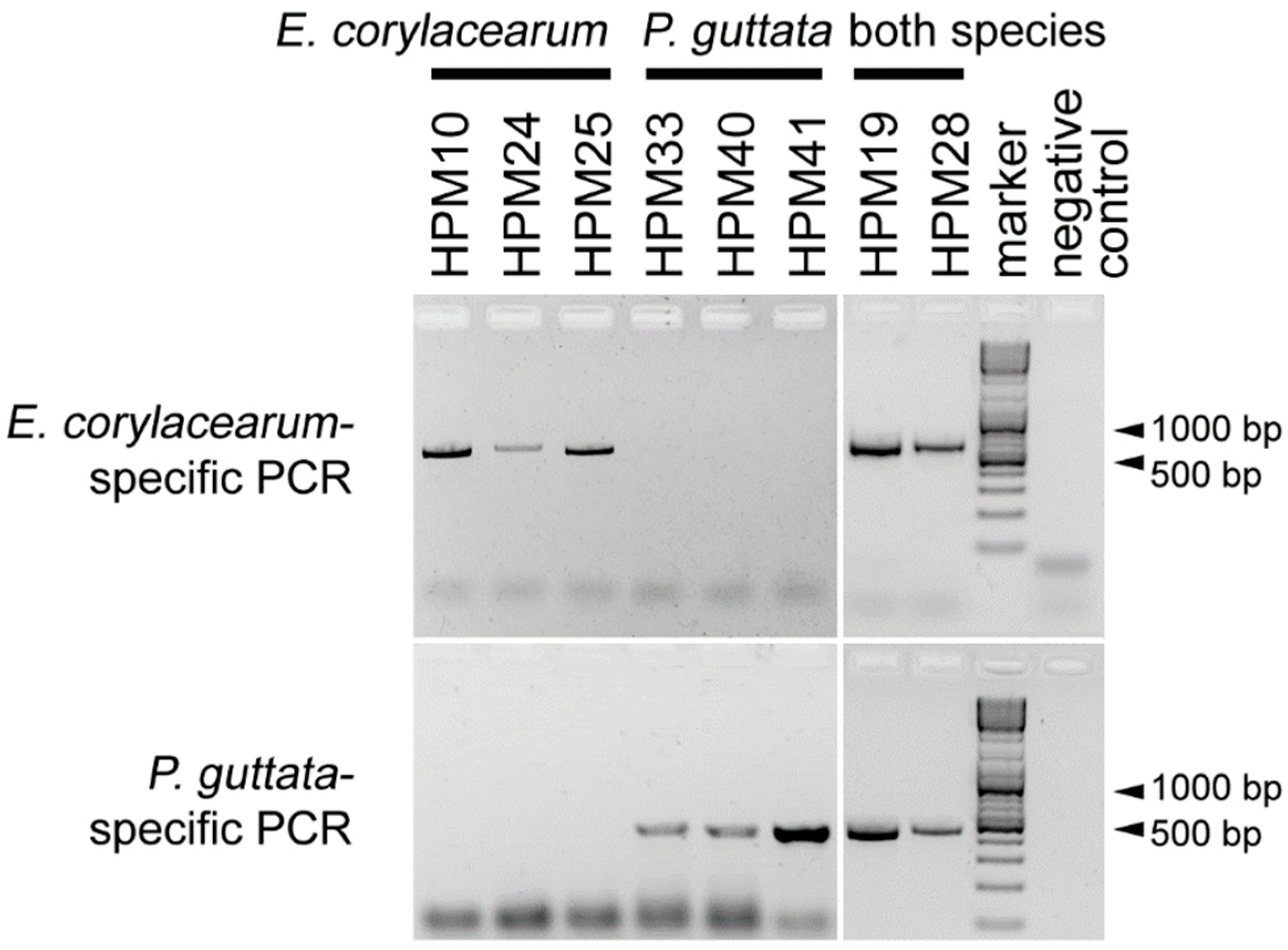
The developed ssPCR successfully differentiated P. guttata and E. corylacearum; by using DNA extracts obtained from E. corylacearum, the first primer set resulted in PCR amplicons, but DNA extracts from P. guttata did not, and vice versa for the second primer pair (Figure 2). All artificial targets resulting from mixing the DNA of both species gave positive results for both. Thus, the identity of the fungi present in the leaf samples could be determined based on the amplified DNA fragments’ presence and size. The E. corylacearum-specific primer pair resulted in a product of ~550 bp, and the product of the P. guttata-specific primer pair was ~420 bp long. BLASTn analysis of the sequenced fragments showed 100% identity to other ITS sequences of E. corylacearum and 99–100% similarity to other P. guttata samples, respectively.
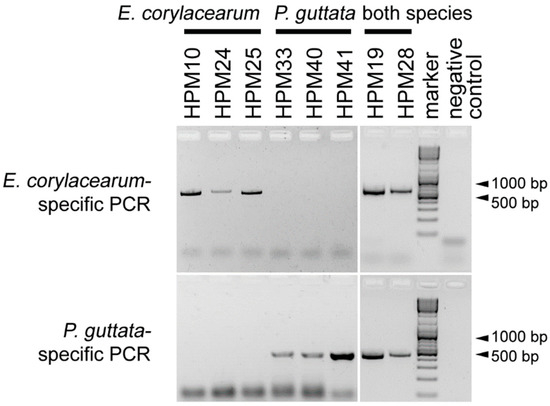
Figure 2.
The representative results of the species-specific PCR developed to distinguish Erysiphe corylacearum and Phyllactinia guttata. Six DNA extracts and two DNA mixtures were assayed with E. corylacearum-specific (upper panel) and P. guttata-specific primers (lower panel). The first three samples were infected by E. corylacearum, and the following three samples were infected by P. guttata. In the next two wells, PCR products resulting from the amplifications of mixtures of DNA extracts from both species (mixtures of extracts originating from HPM19 and HPM28) are shown. The last sample was the negative control (molecular biology grade water).
The molecular-based identification of the 59 samples was carried out using the developed ssPCR. According to this identification, 12 samples carried solely P. guttatta, 22 were infected solely by E. corylacearum and 25 samples harbored both species. These results confirmed the results of the identification based on morphological analysis. All 30 samples carrying E. corylacearum chasmothecia as observed by microscopy were also positive for E. corylacearum in the ssPCR. Similarly, all samples observed to have P. guttata chasmothecia were positive in the ssPCR (Table A1).
The alien fungus E. corylacearum was found in all but one of the sampled regions (Budapest and eleven counties) based on observations of the chasmothecia and the results of the ssPCR and ITS sequence data (Table A1). Altogether, it was present on more than three-quarters (47 of 59) of the leaves. Erysiphe corylacearum was found on C. avellana and also on C. colurna (e.g., samples HPM12 and HPM37 in Table A1) but was not present on any of the C. maxima samples. In about half (25) of all of the samples (47) infected by E. corylacearum, the fungus was found together with P. guttata (Table A1).
Of the samples without any chasmothecia, eleven were positive for E. corylacearum and six for P. guttata. There were six samples (HPM5, HPM15, HPM43-HPM45 and HPM51; Table A1) that did not contain any fruiting bodies but were diagnosed as positive for both species in the ssPCR.
In some cases, fruiting bodies of only E. corylacearum were detected; however, both species were present according to the ssPCR results (samples HPM22, HPM46-HPM49 and HPM54; Table A1).
4. Discussion
Our morphological analysis and the developed ssPCR distinguished P. guttata and E. corylacearum on hazel leaves. Notably, E. corylacearum was prevalent across sampled regions, not only on C. avellana but also on C. colurna. To our knowledge, the fungus was not detected on the latter host in Hungary earlier.
Erysiphe corylacearum has been spreading from its region of origin into Europe in the last decade. The spread is remarkably fast, as judged by the first reports of the fungus in different countries. The process was called an “epidemic spread” [21]. In Hungary, it has been present since 2020 at least, and by 2023, it was found in all but one of the sampled regions in the present work. Most probably, by now, its area of occurrence includes the whole country. As shown by our data and by other studies, the symptoms of E. corylacearum usually occur earlier in the vegetation period ([12], Table A1). As we also found in several samples, the fungus also readily co-occurs with P. guttata ([4], Table A1). These traits are characteristic of an invasive pathogen [16,23], possibly contributing to its spreading potential. It was also assumed, however, that the spread of the fungus is facilitated by human activities, such as the import of infected propagating material [13]. Erysiphe corylacearum also infects Corylus species which are predominantly native to Asia. As some of the species, such as C. colurna, are also widely planted in Hungary as ornamentals, and rootstocks of Corylus species for hazelnut growing are imported, it is reasonable to hypothesize that trade plays a significant role in the spread of the fungus.
In general, powdery mildew fungi are among the most commonly reported alien species in European countries [34,35]. Indeed, the occurrence of E. corylacearum, a species from Asia, on native hazels shows remarkable similarities to that of other newly reported alien species, such as E. salmonii, which infects Fraxinus trees [36], E. syringae-japonicae on lilacs [37] and E. kenjiana occurring on Ulmus sp. [38]. In all of these cases, there were established or native powdery mildew fungi regularly occurring on the respective host plants, and new pathogens originating from Asia infecting the same hosts were introduced.
Unlike P. guttata, E. corylacearum is also able to infect hazelnuts [7,12], which makes it potentially significantly more devastating than P. guttata [13]. Therefore, it may be essential to identify the pathogen quickly, preferably using a cost-effective method. Our ssPCR technique allows for the rapid and accurate identification of E. corylacearum, even in cases when fruiting bodies are not present or when P. guttata is also infecting the plant. This latter is a clear advantage of our method over another assay [39], which was not tested with samples containing both species. Thus, it is unknown how it performs in detecting P. guttata and, consequently, whether it detects both species in cases of coinfections. Our ssPCR method can aid the development of effective management strategies and allow for the monitoring of E. corylacearum in the affected regions.
The emergence and spread of E. corylacearum highlight the importance of continued research to find a suitable management strategy and assaying the susceptibility of different varieties to mitigate the impact of powdery mildew on hazelnut cultivation.
Author Contributions
Conceptualization, M.Z.N., É.P.; sampling, K.K., O.M., J.Á., M.Z.N.; methodology, all authors; validation, K.B., O.M., M.Z.N.; investigation, all authors; writing—original draft preparation, K.K.; writing—review and editing, all authors; visualization, K.B. and M.Z.N.; funding acquisition, É.P., M.Z.N. All authors have read and agreed to the published version of the manuscript.
Funding
Project no. FK142735 has been implemented with support provided by the Ministry of Culture and Innovation of Hungary from the National Research, Development and Innovation Fund, financed under the FK_22 funding scheme. This study was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (awarded to Márk Z. Németh; BO/00221/21/4), and by the ÚNKP-23-5 New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund. The research of the HUN-REN-SZE PhatoPlant-Lab was supported by the Hungarian Research Network TKI (HUN-REN TKI) (project number: 3200107).
Data Availability Statement
All relevant data except for the determined sequences are available in the manuscript. The determined sequences are deposited in GenBank under accession numbers PP825796-PP825800.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
List of samples collected during this study. “+” denotes chasmothecia present, “−“ denotes chasmothecia not detected.
Table A1.
List of samples collected during this study. “+” denotes chasmothecia present, “−“ denotes chasmothecia not detected.
| Sample Identifier | Fruiting Bodies of E. corylacearum | Fruiting Bodies of P. guttata | Powdery Mildew Fungus | Host Plant Species | Place of Collection (City, County) | GPS Coordinates | Date of Collection |
|---|---|---|---|---|---|---|---|
| HPM1 | − | − | E. corylacearum | Corylus avellana | Budapest | 47.505302, 19.138174 | 4 June 2021 |
| HPM2 | + | + | E. corylacearum, P. guttata | C. avellana | Érd, Pest | 47.345625, 18.859505 | 8 August 2021 |
| HPM3 | + | − | E. corylacearum | C. avellana | Dobogókő, Pest | 47.719439, 18.892749 | 30 October 2021 |
| HPM4 | − | − | E. corylacearum | C. avellana | Kerepes, Pest | 47.576529, 19.264852 | 23 June 2022 |
| HPM5 | − | − | E. corylacearum, P. guttata | C. colurna | Budapest | 47.500904, 19.031150 | 24 June 2022 |
| HPM6 | − | − | E. corylacearum | C. avellana | Kerepes, Pest | 47.576572, 19.265041 | 27 June 2022 |
| HPM7 | − | − | E. corylacearum | C. avellana | Szekszárd, Tolna | 46.330652, 18.666095 | 5 July 2022 |
| HPM8 | − | − | E. corylacearum | C. avellana | Szekszárd, Tolna | 46.330609, 18.665910 | 5 July 2022 |
| HPM9 | − | − | E. corylacearum | C. avellana | Szekszárd, Tolna | 46.330496, 18.665554 | 5 July 2022 |
| HPM10 | + | − | E. corylacearum | C. avellana | Budakeszi, Pest | 47.511878, 18.933464 | 7 July 2022 |
| HPM11 | − | − | E. corylacearum | C. avellana | Budakeszi, Pest | 47.508907, 18.931212 | 7 July 2022 |
| HPM12 | − | − | E. corylacearum | C. colurna | Budakeszi, Pest | 47.511938, 18.930508 | 7 July 2022 |
| HPM13 | − | − | E. corylacearum | C. avellana | Budapest | 47.505037, 19.138470 | 11 July 2022 |
| HPM14 | − | − | E. corylacearum | C. avellana | Mende, Pest | 47.434124, 19.457578 | 15 July 2022 |
| HPM15 | − | − | E. corylacearum, P. guttata | C. avellana | Garáb, Nógrád | 47.963556, 19.746401 | 23 July 2022 |
| HPM16 | + | − | E. corylacearum | C. avellana | Szada, Pest | 47.635653, 19.333720 | August 2022 |
| HPM17 | − | − | E. corylacearum | C. avellana | Jánossomorja, Győr-Moson-Sopron | 47.787065, 17.150850 | 30 August 2022 |
| HPM18 | + | + | E. corylacearum, P. guttata | C. avellana | Gyula, Békés | 46.664682, 21.263174 | 19 September 2022 |
| HPM19 | + | + | E. corylacearum, P. guttata | C. avellana | Püspökladány, Hajdú-Bihar | 47.335130, 21.091024 | 24 September 2022 |
| HPM20 | + | + | E. corylacearum, P. guttata | C. avellana | Püspökladány, Hajdú-Bihar | 47.334187, 21.090440 | 24 September 2022 |
| HPM21 | + | − | E. corylacearum | C. avellana ‘Heterophylla’ | Püspökladány, Hajdú-Bihar | 47.335080, 21.091447 | 24 September 2022 |
| HPM22 | + | − | E. corylacearum, P. guttata | C. avellana | Dobogókő, Pest | 47.719446, 18.892284 | 2 October 2022 |
| HPM23 | + | − | E. corylacearum | C. avellana | Dobogókő, Pest | 47.719278, 18.895112 | 2 October 2022 |
| HPM24 | + | − | E. corylacearum | C. avellana | Dobogókő, Pest | 47.718488, 18.904377 | 2 October 2022 |
| HPM25 | + | − | E. corylacearum | C. avellana | Vál, Fejér | 47.359523, 18.681418 | 3 October 2022 |
| HPM26 | + | − | E. corylacearum | C. avellana | Diósd, Pest | 47.411191, 18.936779 | 3 October 2022 |
| HPM27 | + | + | E. corylacearum, P. guttata | C. avellana ‘Pendula’ | Balatonvilágos, Somogy | 46.962783, 18.163501 | 6 October 2022 |
| HPM28 | + | + | E. corylacearum, P. guttata | C. avellana | Balatonboglár, Somogy | 46.754358, 17.665625 | 6 October 2022 |
| HPM29 | + | − | E. corylacearum | C. avellana | Szekszárd, Tolna | 46.330563, 18.666124 | 12 October 2022 |
| HPM30 | + | + | E. corylacearum, P. guttata | C. avellana | Szekszárd, Tolna | 46.330320, 18.665476 | 12 October 2022 |
| HPM31 | + | − | E. corylacearum | C. avellana | Szekszárd, Tolna | 46.329513, 18.665134 | 12 October 2022 |
| HPM32 | + | + | E. corylacearum, P. guttata | C. avellana | Szekszárd, Tolna | 46.329911, 18.665134 | 12 October 2022 |
| HPM33 | − | + | P. guttata | C. avellana | Szekszárd, Tolna | 46.329359, 18.665081 | 12 October 2022 |
| HPM34 | + | + | E. corylacearum, P. guttata | C. avellana | Budapest | 47.513955, 19.010434 | 14 October 2022 |
| HPM35 | + | + | E. corylacearum, P. guttata | C. avellana | Budakeszi, Pest | 47.511878, 18.933464 | 15 October 2022 |
| HPM36 | + | + | E. corylacearum, P. guttata | C. avellana | Budakeszi, Pest | 47.508907, 18.931212 | 15 October 2022 |
| HPM37 | + | + | E. corylacearum, P. guttata | C. colurna | Budakeszi, Pest | 47.511938, 18.930508 | 15 October 2022 |
| HPM38 | − | + | P. guttata | C. colurna | Budakeszi, Pest | 47.519600, 18.929327 | 15 October 2022 |
| HPM39 | − | + | P. guttata | C. avellana | Budapest | 47.512486, 19.014810 | 2 November 2022 |
| HPM40 | − | + | P. guttata | C. avellana | Budapest | 47.514257, 19.010399 | 17 November 2022 |
| HPM41 | − | + | P. guttata | C. avellana | Budapest | 47.501734, 19.034448 | 21 November 2022 |
| HPM42 | + | − | E. corylacearum | C. avellana | Galgahévíz, Pest | 47.620339, 19.543198 | 21 November 2022 |
| HPM43 | − | − | E. corylacearum, P. guttata | C. avellana | Budapest | 47.513344, 19.011459 | 20 June 2023 |
| HPM44 | − | − | E. corylacearum, P. guttata | C. avellana | Nagyrákos, Vas | 46.822309, 16.460806 | 14 July 2023 |
| HPM45 | − | − | E. corylacearum, P. guttata | C. avellana | Dunaújváros, Fejér | 46.954716, 18.949894 | 3 September 2023 |
| HPM46 | + | − | E. corylacearum, P. guttata | C. avellana | Szalkszentmárton, Bács-Kiskun | 46.924567, 19.118414 | 4 September 2023 |
| HPM47 | + | − | E. corylacearum, P. guttata | C. avellana | Táborfalva, Pest | 47.135971, 19.477080 | 4 September 2023 |
| HPM48 | + | − | E. corylacearum, P. guttata | C. avellana | Szigetújfalu, Pest | 47.234927, 18.937678 | 4 September 2023 |
| HPM49 | + | − | E. corylacearum, P. guttata | C. avellana | Zalaháshágy, Zala | 46.882798, 16.631994 | 8 September 2023 |
| HPM50 | − | − | P. guttata | C. avellana | Nagyrákos, Vas | 46.822472, 16.460779 | 8 September 2023 |
| HPM51 | − | − | E. corylacearum, P. guttata | C. avellana | Kerekegyháza, Bács-Kiskun | 46.930966, 19.473140 | 8 September 2023 |
| HPM52 | − | − | P. guttata | C. avellana | Nagykarácsony, Fejér | 46.872177, 18.767247 | 8 September 2023 |
| HPM53 | − | − | P. guttata | C. avellana | Berettyóújfalu, Hajdú-Bihar | 47.213776, 21.541546 | 10 September 2023 |
| HPM54 | + | − | E. corylacearum, P. guttata | C. avellana | Érd, Pest | 47.394408, 18.931385 | 11 September 2023 |
| HPM55 | − | − | P. guttata | C. maxima ‘Purpurea’ | Csemő, Pest | 47.147483, 19.712353 | 11 September 2023 |
| HPM56 | − | + | P. guttata | C. maxima ‘Purpurea’ | Szentes, Csongrád-Csanád | 46.695962, 20.211840 | 12 September 2023 |
| HPM57 | − | − | P. guttata | C. avellana | Szentes, Csongrád-Csanád | 46.727203, 20.227526 | 12 September 2023 |
| HPM58 | − | − | P. guttata | C. avellana | Kiskunfélegyháza, Bács-Kiskun | 46.669697, 19.815522 | 12 September 2023 |
| HPM59 | + | + | E. corylacearum, P. guttata | C. avellana | Decs, Tolna | 46.282690, 18.760560 | 25 September 2023 |
References
- Yang, Z.; Wang, G.; Ma, Q.; Ma, W.; Liang, L.; Zhao, T. The complete chloroplast genomes of three Betulaceae species: Implications for molecular phylogeny and historical biogeography. PeerJ 2019, 7, e6320. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT—Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 15 July 2024).
- Bayramoglu, Z.; Ozer, O.O.; Gundogmus, E.; Tatlidil, F.F. The impact of changes in Turkey’s hazelnut policy on world markets. Afr. J. Agric. Res. 2010, 5, 7–15. [Google Scholar]
- Pintér, C.; Kövics, G.J.; Biró, G.; Csüllög, K.; Tarcali, G. Powdery mildew diseases of hazelnuts (Corylus avellana)—Phyllactinia guttata and the newly appeared Erysiphe corylacearum in Hungary. Növényvédelem 2023, 84, 93–103, (In Hungarian with an English Abstract). [Google Scholar]
- Gógán, A. Sikeres Szarvasgomba-Termesztés Magyarországon. Available online: https://agroforum.hu/lapszam-cikk/sikeres-szarvasgomba-termesztes-magyarorszagon/ (accessed on 15 July 2024).
- Bradshaw, M.; Braun, U.; Meeboon, J.; Tobin, P. Phylogeny and taxonomy of powdery mildew caused by Erysiphe species on Corylus hosts. Mycologia 2021, 113, 459–475. [Google Scholar] [CrossRef] [PubMed]
- Pintér, C.; Kövics, G.; Bíró, G.; Csüllög, K. Powdery mildew fungi on European hazelnut (Corylus avellana). Georg. Agric. 2022, 26, 147–154, (In Hungarian with an English Abstract). [Google Scholar]
- Braun, U.; Cook, R.T.A. Taxonomic Manual of the Erysiphales (Powdery Mildews); CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2012; Volume 11, pp. 1–707. [Google Scholar]
- Moesz, G. Magyarország Gombaflórája. III. Tömlősgombák. I. Rész; Országos Magyar Természettudományi Múzeum: Budapest, Hungary, 1939; Volume 32, pp. 1–61. (In Hungarian) [Google Scholar]
- Takamatsu, S.; Inagaki, M.; Niinomi, S.; Khodaparast, S.A.; Shin, H.-D.; Grigaliunaite, B.; Havrylenko, M. Comprehensive molecular phylogenetic analysis and evolution of the genus Phyllactinia (Ascomycota: Erysiphales) and its allied genera. Mycol. Res. 2008, 112, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Kalmár, K.; Desiderio, F.; Varjas, V. First report of Erysiphe corylacearum causing powdery mildew on hazelnut in Hungary. Plant Dis. 2023, 107, 579. [Google Scholar] [CrossRef]
- Sezer, A.; Dolar, F.S.; Lucas, S.J.; Köse, Ç.; Gümüş, E. First report of the recently introduced, destructive powdery mildew Erysiphe corylacearum on hazelnut in Turkey. Phytoparasitica 2017, 45, 577–581. [Google Scholar] [CrossRef]
- Matić, S.; Caruso, A.G.; D’Errico, C.; Botto, C.S.; Noris, E.; Trkulja, V.; Panno, S.; Davino, S.; Moizio, M. Powdery mildew caused by Erysiphe corylacearum: An emerging problem on hazelnut in Italy. PLoS ONE 2024, 19, e0301941. [Google Scholar] [CrossRef]
- Abasova, L.; Aghayeva, D.; Takamatsu, S. Notes on powdery mildews of the genus Erysiphe from Azerbaijan. Curr. Res. Environ. Appl. Mycol. 2018, 8, 30–53. [Google Scholar] [CrossRef]
- Arzanlou, M.; Torbati, M.; Golmohammadi, H. Powdery mildew on hazelnut (Corylus avellana) caused by Erysiphe corylacearum in Iran. For. Pathol. 2018, 48, e12450. [Google Scholar] [CrossRef]
- Heluta, V.P.; Makarenko, N.V.; Al-Maali, G.A. First records of Erysiphe corylacearum (Erysiphales, Ascomycota) on Corylus avellana in Ukraine. Ukr. Bot. J. 2019, 76, 252–259. [Google Scholar] [CrossRef]
- Heluta, V.P.; Fokshei, S.I. New records of an alien fungus Erysiphe corylacearum (Erysiphales, Ascomycota) in Ukraine. Plant Fungal Res. 2020, 1, 11–17. [Google Scholar] [CrossRef]
- Meparishvili, G.; Gur, L.; Frenkel, O.; Gorgiladze, L.; Meparishvili, S.; Muradashvili, M.; Koiava, L.; Dumbadze, R.; Reuveni, M.; Jabnidze, R. First report of powdery mildew caused by Erysiphe corylacearum on hazelnuts in Georgia. Plant Dis. 2019, 103, 2952. [Google Scholar] [CrossRef]
- Beenken, L.; Brodtbeck, T.; De Marchi, R. First record of Erysiphe corylacearum on Corylus avellana in Switzerland and in central Europe. New Dis. Rep. 2020, 41, 11. [Google Scholar] [CrossRef]
- Voglmayr, H.; Zankl, T.; Krisai-Greilhuber, I.; Kirisits, T. First report of Erysiphe corylacearum on Corylus avellana and C. colurna in Austria. New Dis. Rep. 2020, 42, 14. [Google Scholar] [CrossRef]
- Beenken, L.; Kruse, J.; Schmidt, A.; Braun, U. Epidemic spread of Erysiphe corylacearum in Europe—First records from Germany. Schlechtendalia 2022, 39, 112–118. [Google Scholar]
- Mezzalama, M.; Guarnaccia, V.; Martano, G.; Spadaro, D. Presence of powdery mildew caused by Erysiphe corylacearum on hazelnut (Corylus avellana) in Italy. Plant Dis. 2021, 105, 1565. [Google Scholar] [CrossRef]
- Mazzaglia, A.; Drais, M.; Turco, S.; Silvestri, C.; Cristofori, V.; Aymami, A.; Casadó, V.; Rovira, M. First report of Erysiphe corylacearum causing powdery mildew on Corylus avellana in Spain. New Dis. Rep. 2021, 44, e12035. [Google Scholar] [CrossRef]
- Rosati, M.; Bogoescu, M.; Spadaro, D. First report of Erysiphe corylacearum, agent of powdery mildew, on hazelnut (Corylus avellana) in Romania. Plant Dis. 2021, 105, 2728. [Google Scholar] [CrossRef]
- Zajc, J.; Rot, M.; Snoj, D.; Žerjav, M.; Schroers, H.-J.; Piškur, B.; Ogris, N.; Brglez, A. First report of Erysiphe corylacearum on Corylus avellana and C. colurna in Slovenia. New Dis. Rep. 2023, 47, e12160. [Google Scholar] [CrossRef]
- Boneva, D.V.; Bobev, S.G.; Van Poucke, K. First report of powdery mildew caused by Erysiphe corylacearum on hazelnut in Bulgaria. Plant Dis. 2023, 107, 4023. [Google Scholar] [CrossRef] [PubMed]
- Swiderska, U.; Wdowiak-Wróbel, S. First report of Erysiphe corylacearum on Corylus avellana in Poland. Acta Mycol. 2023, 58, 1–6. [Google Scholar] [CrossRef]
- Šafránková, I.; Holkova, L.; Michutová, M. Erysiphe corylacearum as a new pathogen of hazelnut in the Czech Republic. Plant Dis. 2024, 108, 798. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Kantartzi, S.K.; Mmbaga, M.T.; Chen, P. Differentiation of two pathogens of powdery mildew disease in flowering dogwood (Cornus florida) by PCR-mediated method based on ITS sequences. J. Phytopathol. 2009, 157, 274–279. [Google Scholar] [CrossRef]
- Pintye, A.; Németh, M.Z.; Molnár, O.; Horváth, Á.N.; Spitzmüller, Z.; Szalóki, N.; Pál, K.; Váczy, K.Z.; Kovács, G.M. Improved DNA extraction and quantitative real-time PCR for genotyping Erysiphe necator and detecting the DMI fungicide resistance marker A495T, using single ascocarps. Phytopathol. Mediterr. 2020, 59, 97–106. [Google Scholar] [CrossRef]
- Bradshaw, M.; Tobin, P.C. Sequencing herbarium specimens of a common detrimental plant disease (powdery mildew). Phytopathology 2020, 110, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Brody, J.R.; Kern, S.E. Sodium boric acid: A Tris-free, cooler conductive medium for DNA electrophoresis. BioTechniques 2004, 36, 214–216. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.-L.; Courtecuisse, R.; Robin, C.; Husson, C.; Moreau, P.-A.; Blancard, D.; Selosse, M.-A.; Lung-Escarmant, B.; Piou, D.; Sache, I. Species diversity and drivers of spread of alien fungi (sensu lato) in Europe with a particular focus on France. Biol. Invasions 2010, 12, 157–172. [Google Scholar] [CrossRef]
- Voglmayr, H.; Schertler, A.; Essl, F.; Krisai-Greilhuber, I. Alien and cryptogenic fungi and oomycetes in Austria: An annotated checklist (2nd edition). Biol. Invasions 2023, 25, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Pintye, A.; Molnár, O.; Soós, A.Z.; Seress, D.; Ágoston, J.; Németh, M.Z. Powdery mildew of ash trees caused by the non-native Erysiphe salmonii in Hungary. J. Plant. Dis. Prot. 2024, 131, 1093–1097. [Google Scholar] [CrossRef]
- Takamatsu, S.; Shiroya, Y.; Seko, Y. Geographical and spatial distributions of two Erysiphe species occurring on lilacs (Syringa spp.). Mycoscience 2016, 57, 349–355. [Google Scholar] [CrossRef]
- Molnár, O.; Seress, D.; Borostyán, K.; Pintye, A.; Németh, M.Z. Erysiphe kenjiana, a pathogen of Asian origin infecting elm trees documented first from Hungary. In Proceedings of the VII Hungarian Mycological Conference, Budapest, Hungary, 5–7 June 2024. [Google Scholar]
- Baykal, U. Detection of powdery mildew growth in hazelnut plant using PCR. Turk. J. Agric. Food Sci. Technol. 2020, 8, 1807–1810. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).