Abstract
Horticultural crops are vulnerable to diverse microbial infections, which have a detrimental impact on their growth, fruit quality, and productivity. Currently, chemical pesticides are widely employed to manage diseases in horticultural crops, but they have negative effects on the environment, human health, soil physiochemical properties, and biodiversity. Additionally, the use of pesticides has facilitated the development and spread of resistant pathovars, which have emerged as a serious concern in contemporary agriculture. Nonetheless, the adverse consequences of chemical pesticides on the environment and public health have worried scientists greatly in recent years, which has led to a switch to the use of biocontrol agents such as bacteria, fungi, and insects to control plant pathogens. Biocontrol agents (BCAs) form an integral part of organic farming, which is regarded as the future of sustainable agriculture. Hence, harnessing the potential of BCAs is an important viable strategy to control microbial disease in horticultural crops in a way that is also ecofriendly and can improve the soil health. Here, we discuss the role of the biological control of microbial diseases in crops. We also discuss different microbial-based BCAs such as fungal, bacterial, and viral and their role in disease management. Next, we discuss the factors that affect the performance of the BCAs under field conditions. This review also highlights the genetic engineering of BCAs to enhance their biocontrol efficiency and other growth traits. Finally, we highlight the challenges and opportunities of biocontrol-based disease management in horticulture crops and future research directions to boost their efficacy and applications.
1. Introduction
Horticultural crops are an important source of income with high nutritional, medicinal, and industrial significance [1]. Farmers’ adaptation to horticultural farming has significantly increased in recent times because of the huge demand, substantial income, and technological advancement. Agriculture and economic diversification can be accelerated via horticulture farming [1]. However, the production of horticulture crops is affected by both biotic and abiotic stresses [2]. Among them, phytopathogens pose serious threats to horticultural crops across the globe. For instance, fungal pathogens such as Alternaria spp., Phytophthora infestans, Septoria lycopersici, Fusarium oxysporum f. sp. lycopersici, Botrytis cinerea, and Verticillium dahliae have been found to significantly affect the quality and yield production of horticulturally important crops [3,4,5,6,7]. In addition, a large number of seed-born fungal pathogens viz., Macrophomina phaseolina, Colletotrichum, Fusarium, Ascochyta pinodes, and Sclerotinia sclerotiorum have been reported to impair crop growth and yield [6,7,8,9,10]. On the other hand, many bacterial diseases like canker, soft rot, leaf spot/spot, wilt, blight, speck, and brown spot affect the fruit quality and crop productivity [11,12]. Moreover, different types of viruses cause severe diseases in horticultural crops by hampering their physiological processes and hindering growth, leading to higher yield losses [13,14].
Currently, the majority of the farmers use pesticides as a standard method of managing disease in horticultural crops, which has huge implications on human health and the environment. The excessive application of pesticides has polluted water bodies, leading to a growing apprehension regarding the spread of toxic chemicals to humans [15]. Despite the use of antifungals, on a global scale, growers lose about 10–23% of crops due to infestations of fungal pathogens [16]. The situation is further worsened by an additional 20% crop loss during the post-harvest process [16]. On the other hand, for the management of bacterial disease, antibiotics are used, which have also become a major concern due the emergence of antibiotic resistant pathovars. For instance, strAB streptomycin-resistance genes have been found in Pseudomonas syringae, Erwinia amylovora, and Xanthomonas campestris [17]. Previous study has shown that bacterial plant pathogens have developed resistance against a wide range of antibiotics such as streptomycin and tetracycline [17]. Therefore, there is a need to find a viable alternative to address this problem and manage bacterial pathogens in horticulture. Although transgenic technology has also been adapted for improving disease resistance in horticulture crops, apart from several benefits such as developing resistant plant varieties, transgenic plants raise serious concerns regarding health risks and environmental concerns [18].
BCAs can play a vital role in disease management and increase food production in a more sustainable way [19]. However, researchers should also thoroughly investigate the range of BCAs to reduce the use of synthetic chemicals that are damaging the balance in ecosystems as well as address pathogen resistance to BCAs. The use of pesticides has expanded during the last decade due to their contribution to improving agricultural productivity by controlling the prevalence and persistence of plant pathogens [20]. Previous studies support the potential benefits of pesticides to control plant pathogens [21,22]. Eventually, there is a strong anticipation that scientific endeavors will strongly need to mitigate the use of hazardous pesticides to contain the peril they pose to human well-being and disturbance in the ecosystem. All of the above-mentioned strategies and their potential constraints have forced scientists to rely on natural methods of controlling plant pathogens. One such approach is the use of BCAs, which have a huge number of benefits and the least constraints. In addition to the use of disease-resistant cultivars, the intervention of BCAs has played a pivotal role in integrated pest management strategies to reduce the use of chemical-based pesticides. Specific BCAs systematically penetrate the target pathogen without affecting the crop plant [23]. Additionally, the public perception of using BCAs as natural and environmentally friendly makes them more convenient for agricultural use. The BCAs confer disease management by enhancing the immunity of crop plants, secreting antimicrobial compounds that inhibit pathogen prevalence and growth [23]. The importance of BCAs and the principle of synergically assessing their efficiency, efficacy, durability, precision, and environmental safety further widen the scope for their use in our agricultural systems. The current review article is a compendium to address the contribution of BCAs in controlling plant pathogens and also highlights the potential benefits of BCAs in comparison to chemical pesticides.
2. Biocontrol as a Viable Approach for Controlling Disease in Horticulture Crops
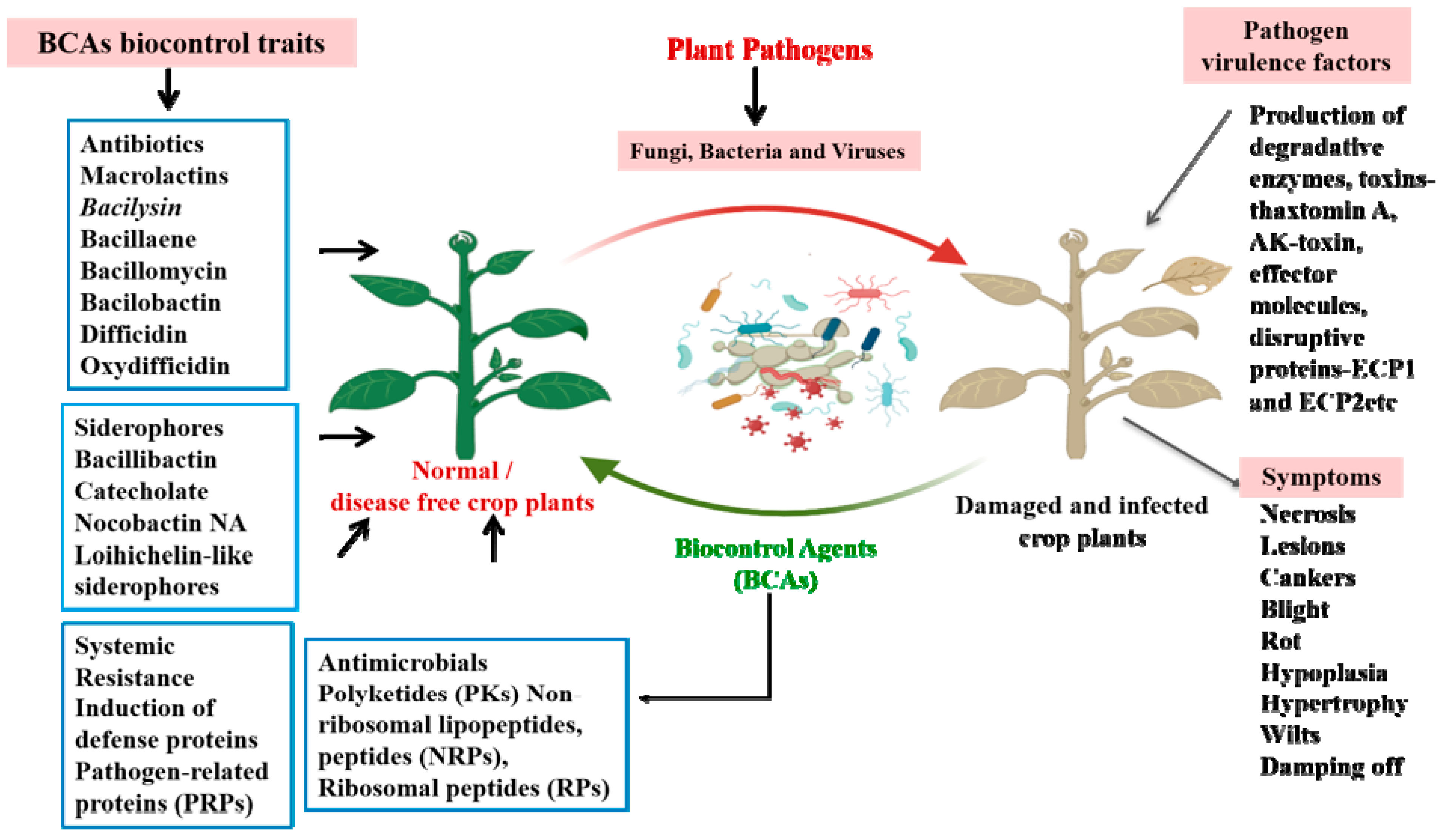
BCAs have emerged as a viable tool not only for controlling pathogens and pests, but also in improving plant growth and soil health, which make them an important tool in maintaining the one-health principle [24]. However, several enhancements such as scaling up production, optimizing formulation techniques and delivery methods, increasing cost-effectiveness, and meeting regulatory requirements are necessary before they can be successfully implemented in the field [25]. The global scientific community is at pace with exploring BCAs in terms of understanding the mechanisms behind their strategies to control pathogens [26]. BCAs include bacteria, viruses, fungi, insects, mites, nematodes, yeasts, and protozoa, which control pathogens through a wide range of biological mechanisms [27]. Upon interaction with the target pathogen, BCAs modulate their genetic machinery to produce arsenals of compounds to circumvent the pathogen dominance and disease prevalence [28,29]. Bacterial species such as Bacillus thuringiensis, Bacillus subtilis, Bacillus amyloliquefaciens, Bacillus licheniformis, Bacillus velezensis, Bacillus pumilus, and Bacillus mojavensis are used as BCAs against plant fungal pathogens [30,31,32]. The biocontrol mechanism mediated by these bacterial species includes the production of antibiotics, siderophore production, and the production of antimicrobial metabolites including lytic enzymes, and most importantly, inducing systemic resistance mechanisms against specific pathogens [33]. In addition, BCAs also trigger the production of growth phytohormones such as cytokinins (CK), indole-3-acetic acid (IAA), and gibberellins (GA) as well as the defense hormones salicylic acid (SA), abscisic acid (ABA), and jasmonic acid (JA) to improve growth and adaptive responses during stress conditions [34,35]. Moreover, some BCAs also produce metabolic intermediates such as ACC deaminase, the enzyme that degrades 1-aminocyclopropane-1-carboxylic acid (ACC) to regulate the ethylene concentration under biotic stress conditions [35]. Consequently, these reports suggest that BCAs operate multiple mechanisms to control pathogens in addition to improving the growth and metabolism in crop plants [36]. Furthermore, we show how BCAs use a multifaceted mode of action for controlling plant pathogens or pests in Figure 1.
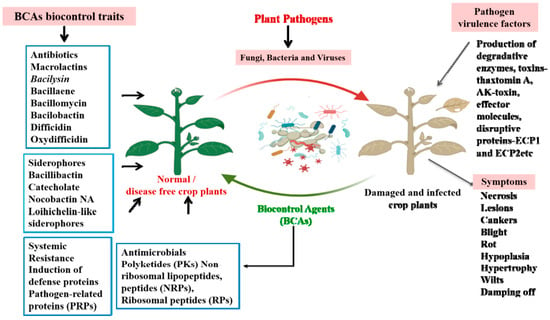
Figure 1.
Schematic diagram depicting the defense mechanism operated by BCAs against plant pathogens. The production of bioactive molecules by BCAs mediates a diverse range of defense responses against pathogens. They produce antibiotics, siderophores, antimicrobial metabolites, and induce systemic resistance mechanisms against specific pathogens. This defense response consequently improves plant growth and regulates the defense mechanisms against a wide range of pathogens.
Many antimicrobial compounds (AMCs) largely belonging to secondary metabolites are known to be secreted by microbes such as bacteria, fungi, and actinomycetes [37]. The classes of antimicrobial agents produced by bacteria include non-ribosomal lipopeptides and peptides (NRPs), ribosomal peptides (RPs), and polyketides (PKs) [38]. A large class of NRPs belongs to cyclic lipopeptides (LPs), which exert their action against a wide range of fungal and bacterial pathogens. This class of antibacterial agents mediates their action by attaching to a membrane to cause perforation, resulting in the leakage of ions, which is followed by depolarization of the membrane, where, once entering the cells, it inhibits the replication, transcription, and translation of bacterial pathogens [39]. Similarly, several types of LPs exhibit antifungal properties and exert their action by disrupting cell walls by synthesizing chitin and (1–3)-β-D-glucan synthases, which in turn disrupts osmotic regulation and the morphological architecture of fungal pathogens [40]. In addition, the Bacillus species contains three families of LPs like iturin, fengycin, and surfactin, which possess both antifungal as well as antibacterial properties [29,41]. Hussain et al. [42] recently reported that the LPs iturin A and bacillomycin F produced by the Bacillus siamensis Sh420 strain inhibited the growth of Fusarium graminearum. Similarly, previous studies have shown that iturin A and bacillomycin F from the B. siamensis JFL15 strain showed strong antifungal activity against different plant fungal pathogens such as Colletotrichum nymphaeae, Rhizoctonia solani, and Magnaporthe grisea [43]. The β-1,3-glucanase and chitinase secreted by B. siamensis QN2MO-1 contribute to the antifungal activities against Fusarium wilt in tomato plants, and also promotes growth and improves fruit quality [44]. LPs have a great potential as biocontrol elements synthesized by bacterial species to enhance agricultural productivity. For instance, the bacteriocins belonging to RP have been reported to exhibit broad-spectrum antimicrobial activities against closely related bacterial pathogens by disrupting the cell wall and damaging cytoplasmic membranes [38,45]. Several Bacillus species produce some important bacteriocins like amisin, amylolysin, ericin, entianin, thuricin, subtilin, subtilosin A, and subtilosin B, which are used as potential BCAs against a wide range of bacterial pathogens [38,46]. Bacteriocins like leuconocin S and leucocin A-UAL are produced by Gram-positive and Gram-negative bacterial species, even though their lysis mechanisms completely differ. The most widely accepted mechanism includes the absorption of bacteriocins by cell surfaces, followed by the inhibition of cell wall synthesis, enhancing the permeabilization of the cell membrane and the inhibition of RNases and DNases [45,47].
Polyketides and other peptides are another class of antimicrobial agents produced by BCAs that possess antifungal and antibacterial activities [46,48]. For instance, oxydifficidin and difficidin, produced by Bacillus methylotrophicus DR-08, exert antibacterial properties against R. solanacearum, the causative agent of bacterial wilt in tomato plants [49]. Additionally, macrolactin and bacillomycin D, isolated from B. amyloliquefaciens NJN-6, were found to possess antifungal properties against F. oxysporum and R. solanacearum in banana [50]. The polyketide viz. bacillaene, difficidin, and macrolactin, produced by B. amyloliquefaciens DSBA-11, inhibited the growth of the Ralstonia pseudosolanacearum pathogen in tomato plants [51]. Chen et al. [52] reported that the bacterial strain B. velezensis SDTB038 possessed antifungal properties against Fusarium crown and root rot in tomatoes by synthesizing metabolites like bacillaene, bactin, bacilysin, difficidin, fengycin, macrolactin H, and surfactin. A wide range of fungal species have previously been reported to be used as microbial formulations to contain the pathogenesis of pathogenic fungi Botrytis, Fusarium, Sclerotinia, and Pythium [53]. The biocontrol of these fungal species relies on mechanisms such as mycoparasitism, the synthesis of antimicrobial metabolites, and competition for nutrients and space [54,55].
The Trichoderma species-based biocontrol mechanism relies on antibiosis, mycoparasitism, competition for resources and space, and inducing defense systems. The mechanism of antibiosis relies on the production of primary and secondary metabolites that are critical in controlling the growth and division of pathogens. Reports suggest that Trichoderma spp., releases around 390 non-volatile metabolites, some of which include caffeic acid, cathequin, ferulic acid, gliotoxin, gliovirin, heptelidic acid, nematolin, sepedonin, 3,4,15-scirpenetriol, and viridian [56]. For instance, Trichoderma spp. helps in nutrient and water uptake, enhances the metabolism of plants, and induces defense signaling pathways by establishing a positive interaction with crop plants [57]. Strains of the fungal species Trichoderma harzianum have been characterized and identified as potential candidates to control F. oxysporum f. sp. lactucae (Fol) in baby lettuce (Lactuca sativa L. var acephala) [58].
3. Factors Affecting the BCAs Activities and Strategies to Overcome Limitations of BCA Effectiveness
Despite huge efforts, the impact of biocontrol has remained reasonably insignificant in managing to control diseases in crops in comparison to its synthetic counterparts [23]. Most importantly, BCAs face greater challenges when transforming their roles from experimental conditions to crop fields exposed to various environmental factors [59]. For instance, the type of plant species and genotype have a significant role in the effectiveness of BCAs [60]. Umer et al. [61] reported that the production of antimicrobials from BCAs as well as their colonization and induction of systematic resistance is dependent on the type of plant and varies from species to species. Consequently, for a targeted application of BCAs, scientists must uncover the complexities of host plants, BCAs, and pathogens to avoid less effectiveness and failure [62]. The pathogen also greatly influences the biocontrol potential of specific types of BCAs. Reports suggest that each pathogen interacts with the host plant differently in terms of pathogenesis and prevalence due to the ecological fitness and genetic variability in specific pathogens [63,64]. Additionally, a large number of pests have developed resistance to BCA antimicrobials. For example, the resistance of several pests such as Diabrotica virgifera, Busseola fusca, Helicoverpa zea, Spodoptera frugiperda, and Pectinophora gossypiella has been reported toward Bt toxins [59]. The nature of BCAs and the class of biocontrol agents they release is another factor that is critical for efficacy as potential BCAs. Consequently, to understand the nature of BCAs, it is critical to investigate the distribution pattern of BCAs in the rhizosphere [63,64]. Furthermore, the mode of action of BCAs, pathogen selection, and the ability to resist harsh climatic conditions play defining factors in the success of BCAs in disease management [65].
Environmental conditions such as biotic and abiotic factors play a pivotal role in the ability of BCAs to kill specific pathogens [59]. It is very important to select a specific type of BCA that has higher stability and efficacy in a wide range of environmental conditions like heat stress, drought stress, salinity stress, type of soil, and competition with other biotic species [66]. Understanding the factors that influence the BCAs and their efficacy is critical before their application in crop fields. Currently, the efficacy, viability, and susceptibility of the BCAs to stressful environmental conditions is one of the major problems that has limited its expansion [23]. Considering this limitation, the efficacy of biocontrol BCAs can be increased by preparing small liquid formulations. For instance, to control the devastating impact of Sclerotium rolfsii, Sutthisa et al. [67] prepared 5% trehalose-Trichoderma asperellum MSU007 as a liquid formulation. Another strategy to enhance the efficacy of BCAs is to combine them with fungicides in order to weaken and stress the pathogens to render them more susceptible to subsequent attacks by BCAs [68]. For instance, the combination of Fusarium sp. isolates with a benzimidazole fungicide improved the effectiveness of Fusarium sp. in controlling the Fusarium wilt of carnation or cyclamen [69]. Consequently, by employing a combination of BCAs and synthetic chemicals, the duration of effectiveness will be increased, and therefore, the use of fungicides or antibacterial chemicals will be reduced, leading to the sustainable agricultural production of crops. The combinatorial approach of using nanoparticles and BCAs is another versatile method for enhancing the efficacy of BCAs. For example, Vehapi et al. [70] used a formulation based on nanoparticles (BpNPs), polyvinyl alcohol (PVA), and sodium alginate (SA) in combination with B. pumilus as plant protection against Colletotrichum gloeosporioides infestation to inhibit postharvest fungal decay disease in crop plants. The huge number of inclusive studies demonstrates the potential of BCAs to enhance agricultural productivity at a global scale [71]. However, the affectivity of BCAs is determined by the number of crops per farm, the size of the farms, and the cultivation strategies [72,73]. Our existing agricultural practices must be redesigned to favor biocontrol applications. Apart from the use of BCAs, monitoring measures are also important to make decisions regarding the time of application, preventive measures, and the use of pathogen-resistant varieties are crucial to decrease the use of synthetic chemicals for pest control. Additional considerations include the choice of cropping system and specific environmental conditions that help avoid pest pressure and disease progression.
4. Fungal Species as BCAs: Mechanisms and Applications
Fungal species are one of the important groups of BCAs that are commonly used to control pests and pathogens mainly due to their target specificity, quick generation time, and relatively high reproduction rate [74]. Many genera of fungi have been utilized extensively as efficient BCAs against fungal phytopathogens such as Alternaria [75] Penicillium [76], Aspergillus [77], Fusarium [78], Rhizoctonia [79], Colletotrichum [80], and other devastating pests such as insects and nematodes [81]. Trichoderma spp. is the most well-known fungal antagonist being studied and employed as a microbial fungicide. Their integration promotes sustainable agricultural practices and aids in the efficient management of plant diseases [82].
Numerous fungi, particularly Trichoderma, have been found to have broad-spectrum antagonistic activity against a variety of phytopathogens [75,76,77,78,79,80]. Trichoderma is a common genus of fungus organisms that live in soil and function as mycoparasites, saprotrophs, and plant symbionts [82]. Filamentous fungi of this genus have been studied in detail and used as BCAs against agricultural plant diseases [83]. In order to provide effective plant disease management, a great deal of research has been conducted in the last 10 years on the direct and indirect control potential of BCAs against phytopathogens. In 1794, Trichoderma was initially isolated from soil and decaying organic materials [84]. Nowadays, Trichoderma is the source of more than 60% of effective biofungicides used worldwide [85]. Several antibacterial secondary metabolites including viridiofungin, trichokonin, and lysozyme have been identified from T. harzianum. These compounds have shown an antibacterial activity against bacterial plant pathogens such as Clavibacter michiganensis and Erwinia amylovora [86]. R. solanacearum, a causing agent of bacterial wilt disease in tomato, chilli, and other crops, is responsible for enormous agricultural losses globally [87]. Many approaches have been studied for the management of R. solanacearum. The crude extract of T. harzianum could inhibit the growth of R. solanacearum when in vitro and in planta experiments were conducted on tomato plants. Disruption of bacterial cells was confirmed by scanning electron microscopy [88]. Applications of the T. asperellum isolates T4 and T8 in the field delayed the onset of wilt, reduced the incidence of R. solanacearum-caused illness, and improved tomato plant growth and yield [89].
The fungal strain BCP, also called Acremonium strictum, has the ability to hinder B. cinerea growth by severely lysing the host hyphae [90]. Using light microscopy, the lysis of B. cinerea mycelia was verified [90]. Fusarium wilt was found to be effectively controlled by the Fo47 strain of F. oxysporum, which was recognized as nonpathogenic. It was also discovered that Fo47 could raise the bioactivity of chitinase, β-1,3-glucanase, and β-1,4-glucosidase in tomato plants, indicating that Fo47 could cause resistance in tomatoes [91]. Upon applying a mixture of T. harzianum and rice straw (RST) and oil palm (EFB) compost to okra plants, the plants exhibited resilience against Choanephora wet rot disease. Up to 85.04% less illness severity was observed when T. harzianum was suspended in water [92]. Jaihan et al. [93] used the extracts of entomopathogenic fungus Ophiocordyceps sobolifera against Colletotrichum spp. in chili plants and found that it significantly inhibited the mycelial growth and conidial germination of all the tested Colletotrichum spp. under in vitro conditions. A previous study showed that the coexpression of β-1,3- and β-1,6-glucanase genes in T. virens significantly inhibited pathogens such as R. solani, Rhizopus oryzae, and Pythium ultimum (Oomycota, Chromista) [94]. In a field study, commercial formulations of T. virens (Soilgard) and T. harzianum (Rootshield) were used to prevent Fusarium wilt in tomato plants, where it was found that these formulations prevented 62 and 68% of the disease, respectively [95]. Fusarium wilt of potatoes (F. oxysporum) was better controlled by T. harzianum in a greenhouse setting compared to a field setting, but a higher yield was obtained in the field [96]. Previous study has reported that gliotoxin, an important secondary metabolite of Trichoderma virens T23, suppressed Sclerotium rolfsii, which causes destructive soilborne disease in many plants [97]. Isolates of T. harzianum, T. viride, and T. spirale showed different inhibitory effects against the mycelial growth of R. solani and F. oxysporum f. sp. phaseoli of bean in laboratory, greenhouse, and field conditions. T. harzianum formulation-treated plots had a yield comparable to the healthy control. Boureghda and Bouznad [98] reported that three strains of T. atroviride and T. harzianum (Th. 16) were the most effective isolates in protecting chickpea seedlings against Fusarium wilt among several isolates of T. atroviride, T. harzianum, and T. longibrachiatum. They also observed that an increase in the vegetative growth of the tested plants was correlated with a decrease in the severity of the disease. Tsai et al. [99] reported that among five Trichoderma strains isolated from Anoectochilus rhizospheres, a conidial formulation of a strain of T. asperellum mixed with carboxymethyl cellulose (CoCMC) had an excellent ability in controlling the stem rot disease (F. oxysporum) of Taiwan Anoectochilus and could completely protect the tested plants against this disease for 9 weeks. Schubert et al. [100] examined the potential of Trichoderma spp. as a wound treatment for controlling wood decay fungi in urban trees and reported that T. atroviride (T-15603.1) could be successfully applied as a biological wound treatment against wood decay fungi. Another previous study reported that Penicillium citrinum and Aspergillus terreus showed biocontrol activity against the Sclerotium rolfsii pathogen by inducing jasmonic acid and salicylic accumulation in sunflower plants [101]. Among the fungal BCAs, Trichoderma is the most widely used BCA across the globe. However, there is need to explore novel fungal BCAs with growth promoting traits as well as effective biocontrol activity that will boost agricultural productivity.
Mechanism of Action
Fungal BCAs can employ various mechanisms such as parasitism, competition for nutrients and space, prevention of pathogen colonization in specific host tissues, antibiosis, and the induction of plant resistance against diseases to target pathogens, thereby inhibiting their growth, sporulation, and spread within infected plants [45,55]. Multiple approaches have been developed to investigate the activity mechanisms of selected BCAs [102]. Interestingly, some BCAs initially thought to hinder pathogen development through mycoparasitism or antibiotic production were later found to induce systemic resistance in plants against pathogens [82]. Besides protecting plants from diseases, certain fungal BCAs have the ability to increase plant growth, leading to increased biomass and yield. Hyperparasitism is a fungal propensity characterized by direct antagonistic interactions, whereby other microorganisms eliminate a pathogen [59]. Sometimes, a fungal species can be parasitic on different species of fungi; this tendency is called mycoparasitism [103]. When cucumbers in greenhouse conditions were treated with conidial suspensions of the hyperparasite Ampelomyces quisqualis, they were found to be effective against Sphaerotheca fuliginea, a causative agent of powdery mildew of cucumber. A. quisqualis parasitized S. fuliginea extensively in studies with commercial cucumber crops [104]. Previous studies have reported that Trichoderma spp. are useful in the biocontrol of R. solani, the main fungus that causes damping-off and root rot in many crops [105,106]. Due to their hyperparasitic nature, Trichoderma spp. are among the most studied fungi as BCAs because they effectively protect crops against a wide range of plant pathogens. On the other hand, antagonistic relationships, or antibiosis, are biological interactions between two or more organisms that are harmful to one or more of them. Antagonistic fungi, for instance, produce antimicrobial compounds to inhibit the growth of pathogenic fungi in their vicinity [107]. Fungi produce one or more antimicrobial compounds and other secondary metabolites with antibiotic activity [108]. The most studied fungal species for antibiosis is Trichoderma [109], and Gliocladium spp. [110] and T. virens (syn. Gliocladium virens) secrete gliotoxin and gliovirin [111,112]. A previous study reported that the inoculation of strawberry plants with Trichoderma spp. controlled B. cinerea infection as well as improved growth [113].
Competition occurs when two organisms vie for nutrients or space. Certain species of fungi and yeasts can impede phytopathogens through competitive mechanisms, diminishing nutrient availability. Fungus can inhibit the pathogens through the suppression of the spore germination rates and decrease the germ tube development of pathogens [114]. Endophytic fungi can reside inside the plant tissues; this can be either cellular interspace or intracellular space [115]. Competition for essential nutrients often leads to pathogen starvation, a common cause of microorganism mortality, consequently facilitating the biological control of fungal phytopathogens [116]. Trichoderma spp. produce various secondary metabolites including nonribosomal peptides, polyketides, peptaibols, pyrones, siderophores, and volatile and non-volatile terpenes, which possess pharmaceutical and biotechnological significance [117]. The symbiotic association of Trichoderma with plant root systems enhances mineral and water uptake while conferring resistance against pathogenic organisms. Under iron-deficient conditions, many fungi secrete siderophores, iron-binding ligands facilitating the mobilization of environmental iron [118]. These siderophores enhance the rhizosphere competence of T. asperellum strain T34, rendering it effective as a BCA against F. oxysporum f. sp. lycopersici (Fol) on tomato plants [100]. For instance, the efficient biocontrol of Pythium and F. oxysporum mediated by Trichoderma was found to be associated with the bioavailability of iron [119].
Trichoderma spp. has gained significant attention in the field of agriculture due to its potential as a BCA against various phytopathogens. Its effectiveness lies not only in its direct antagonistic action against pathogens, but also in its ability to trigger various regulatory mechanisms in plants, leading to enhanced disease resistance [82]. The T. asperelloides PSU-P1 strain was able to inhibit the growth of Stagonosporopsis cucurbitacearum by expressing PR genes, chitinase, and the glucanase enzyme. S. cucurbitacearum is responsible for disease in muskmelon. Scanning electron microscopy (SEM) analysis confirmed that T. asperelloides PSU-P1 mediated the destruction of S. cucurbitacearum hyphae [120]. The biocontrol of Sorghum anthracnose, caused by Colletotrichum graminicola, with T. asperellum and T. harzianum was demonstrated in one study. In this study, seeds bio-primed using the T3 (T. asperellum) isolate resulted in the highest increased activities of the antioxidant enzymes, with superoxide dismutase showing a 36.63% increase, peroxidase exhibiting a 43.59% increase, and polyphenol oxidase showing a 40.96% increase at 48 h post pathogen inoculation. Following the 15th day of pathogen inoculation, lignification was increased in the sorghum roots in most treatments, indicating a reinforcement of the defense mechanism. The field trials with T3 were found to be most effective [80]. Many other species of Trichoderma have been reported for their prominent plant growth promotion and biocontrol properties. The noticeable species of Trichoderma are T. atroviride [121], T. harzianum [122], T. koningii [123], T. hebeiense [124], T. longibrachiatum [125], T. polysporum [126], T. reesei [127], T. virens [128], T. viride [129]. T. koningii, and B. megaterium, either alone or in combination have been tested for controlling root-knot nematodes and F. oxysporum in potato plants [130].
Induced resistance (IR) stands out as a crucial mechanism of biocontrol in plants and is effective against both soilborne and foliar pathogens, as highlighted by Hossainet al. [131]. This resistance mechanism hampers pathogen growth and spread by triggering the secretion of defense-related enzymes like chitinases, proteases, and peroxidases [132]. The fungi Penicillium and Trichoderma have been shown to induced systemic resistance (ISR against pathogens. Previous study has reported that the inoculation of plant growth-promoting fungi (PGPF) in tobacco plants not only increased their growth, but also induced immunity against potato virus Y (PVY) infection. In comparison to non-inoculated plants, the treated plants exhibited better growth, reduced virus concentrations, and activated genes associated with defense, indicating the efficacy of PGPF in boosting plant protection [133].
Salicylic acid (SA), produced by the T. harzianum T39 strain, has been shown to induce resistance against B. cinerea in bean plants [134]. Applying a BCA directly to a diseased plant part exhibited ISR, while using dead cells of the BCA showcased local induced resistance (IR). For instance, the use of dead cells of T39 can hinder powdery mildew infection in cucumber and B. cinerea infection in tobacco, pepper, and beans. Additionally, inoculating the cucumber seedlings’ leaves, and roots with T. harzianum led to increased peroxidase and chitinase activity [135]. Root endophytic fungal isolates KB2S2-15 (ectomycorrhiza) and KA2S1-42 (Pleosporales) showed the complete suppression of P. infestans sporangia germination, with a slightly lower inhibition with KB1S1-4. Pre-treating leaflets with a 5% extract from these endophytes resulted in the total suppression of P. infestans mycelial development and late blight symptoms. This suggests that these biocontrol candidates could be used to control late blight disease [136]. Different fungal BCAs for disease management in sustainable agriculture are shown in Table 1.

Table 1.
List of fungal BCAs for disease management in plants.
In the past two decades, there has been significant progress in utilizing fungi as biological control agents (BCAs), with several commercially available BCA products already on the market. The future expansion of fungal BCAs hinges on the successful development of resting spores and robust mycelia. However, BCAs alone may not suffice to control all types of plant diseases across diverse conditions. While the mechanisms of action for some BCAs are becoming clearer, further research and development are necessary to better understand their behavior. The genetic transformation of fungi holds promise for enhancing BCA performance under varying environmental conditions, however, potential risks related to their application into the environment require thorough investigation to establish acceptable implementation guidelines.
5. Bacteria as BCAs for Plant Disease Management
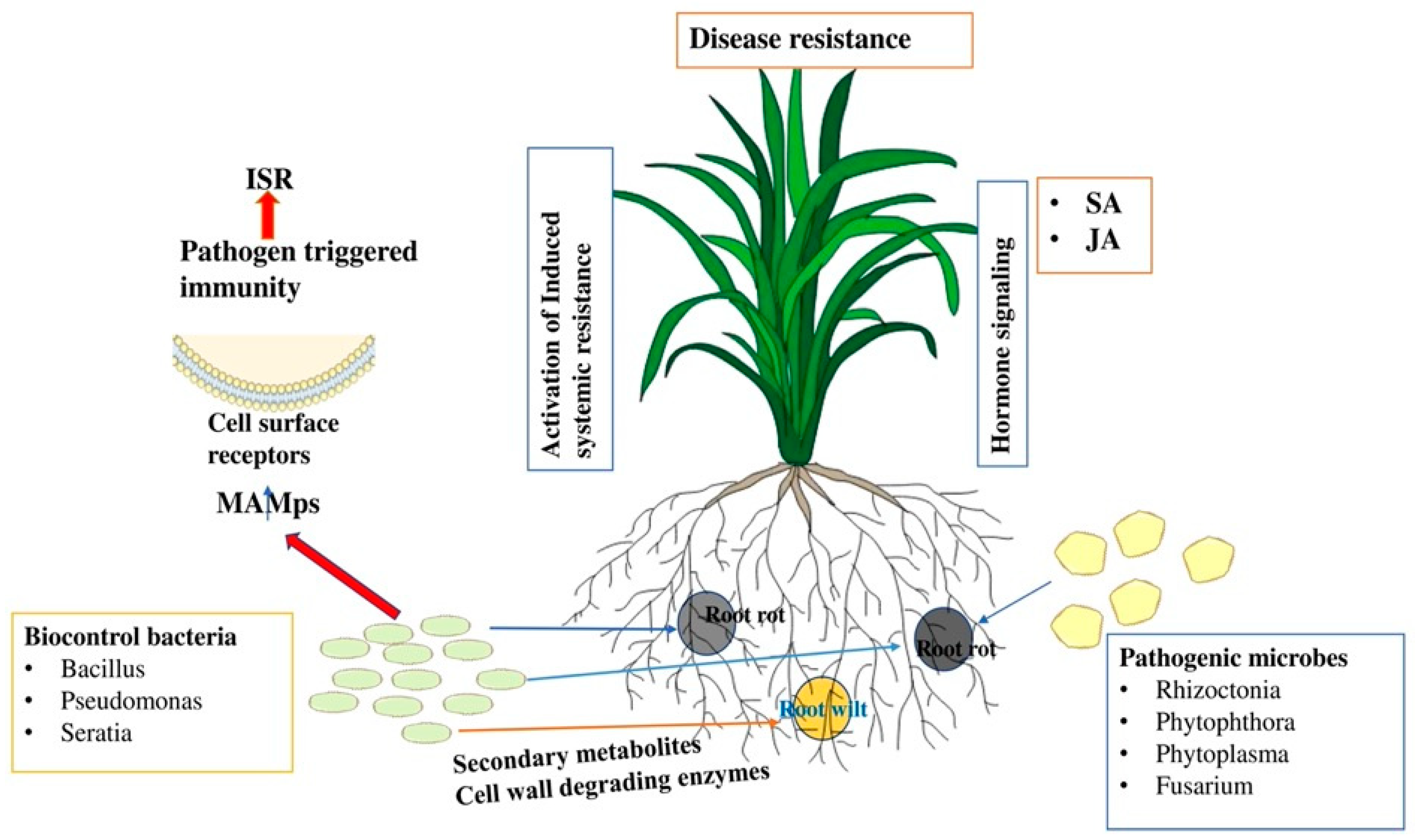
Beneficial bacteria such as Bacillus, Bradyrhizobium, Paenibacillus, Pseudomonas, Acinetobacter, Azotobacter, Azospirillum, Rhizobium, and Streptomyces are known to promote not only plant growth, but also act as portent BCAs [171,172] that use different strategies to combat pathogen attack. For example, they can secrete diverse antimicrobial compounds such as antibiotics, lipopeptides, biosurfactants, microbial volatile compounds, bacteriocins, and cell-wall degrading enzymes against different bacterial and fungal pathogens [29,172,173,174]. On the one hand, they can trigger the host immune system by acting as elicitors or priming agents. Additionally, BCAs may potentially disrupt the pathogens’ quorum sensing (QS) system by preventing or enzymatically breaking down the formation of signal molecules that initiate infections [174,175]. When it comes to nutrition acquisition, highly competitive bacterial BCA can outcompete the pathogen by using low-molecular-weight siderophores that have an affinity for ferrous iron to colonize and persist in the infected site [175]. The first use of bacteria as a BCA for plant protection was Bacillus thuringiensis [176]. Similarly, after the discovery of penicillin as an antibiotic to control bacterial pathogens, Staphylococcus was the first to revolutionize the scientific community [177]. The primary bacteria utilized as BCAs in horticulture crops are strains of Bacillus, Pseudomonas, and Serratia [173]. The significant contribution of microbial pathogens in controlling major diseases in horticulture crops is widely recognized. The bacteria release secondary metabolites to inhibit the growth of fungal pathogens and serve as potential biopesticides. Bacillus species are the most commonly used biopesticides and they also enhance plant defense by activating the induced systemic response (ISR) (Figure 2). Ongena et al. [178] reported that bean and tomato plants with a high concentration of Bacillus in their rhizosphere exhibited enhanced disease resistance against B. cinerea.
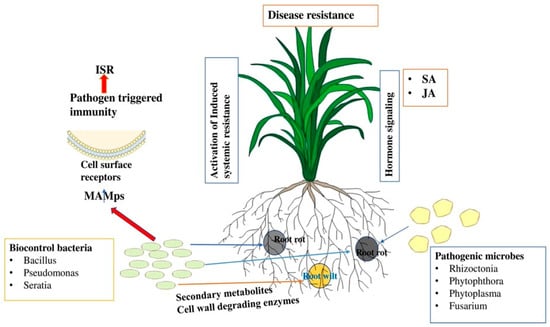
Figure 2.
Schematic diagram showing the role of bacterial biocontrol agents in plant disease resistance. The induction of defense hormones triggers the production of SA and jasmonic acid (JA), which in turn confers resistance to fungal infections. The role of secondary metabolites secreted by beneficial microbes leads to the activation of induced systemic resistance (ISR). They can also produce diverse antimicrobial compounds such as antibiotics, lipopeptides, biosurfactants, microbial volatile compounds, bacteriocins, and cell-wall degrading enzymes to inhibit pathogen growth and disease progression.
Biological control agents show great promise in managing diseases and reducing the spread of pathogens at the site of infection. BCAs are integrated into the soil to improve the diversity of beneficial microorganisms in the vicinity of the rhizosphere. It restricts pathogen growth through various processes including parasitism and the production of lytic enzymes. Some bacteria emit volatile organic compounds such as acetoin, 2,3-butadial, and 3-hydroxy-2butanone that stimulate defense enzymes and restrict pathogen growth [179]. Similarly, the VOCs produced by P. fluorescens WR-1 have restricted the growth of R. solanacearum in tomato plants [180]. Applying beneficial microorganisms (BCAs) to root exudate enhances plant defense by activating the induced systemic resistance (ISR) pathway and promoting the expression of defense-related genes. The root colonization by BCAs is an important feature in the delivery of secondary metabolites and cell-wall degrading enzymes. Additionally, the application of Pseudomonas strain PCL1751 to tomato plants improves their resistance to the root rot pathogen F. oxysporum [181]. In another study conducted by Schuhegger et al. [182], it was found that Serratia liquefaciens and Pseudomonas putida have the ability to stimulate induced systemic resistance (ISR) in tomato plants, providing defense against Alternaria alternata. Induced systemic resistance is a crucial aspect of disease resistance as it facilitates the activation of defense hormones and ultimately results in the production of resistance genes. In Table 2, we summarize the list of potential bacterial BCAs that have been used against different pathogens in sustainable agriculture.

Table 2.
Role of bacterial BCAs to control pathogens in different crop systems.
6. Viruses as Biocontrol Agents
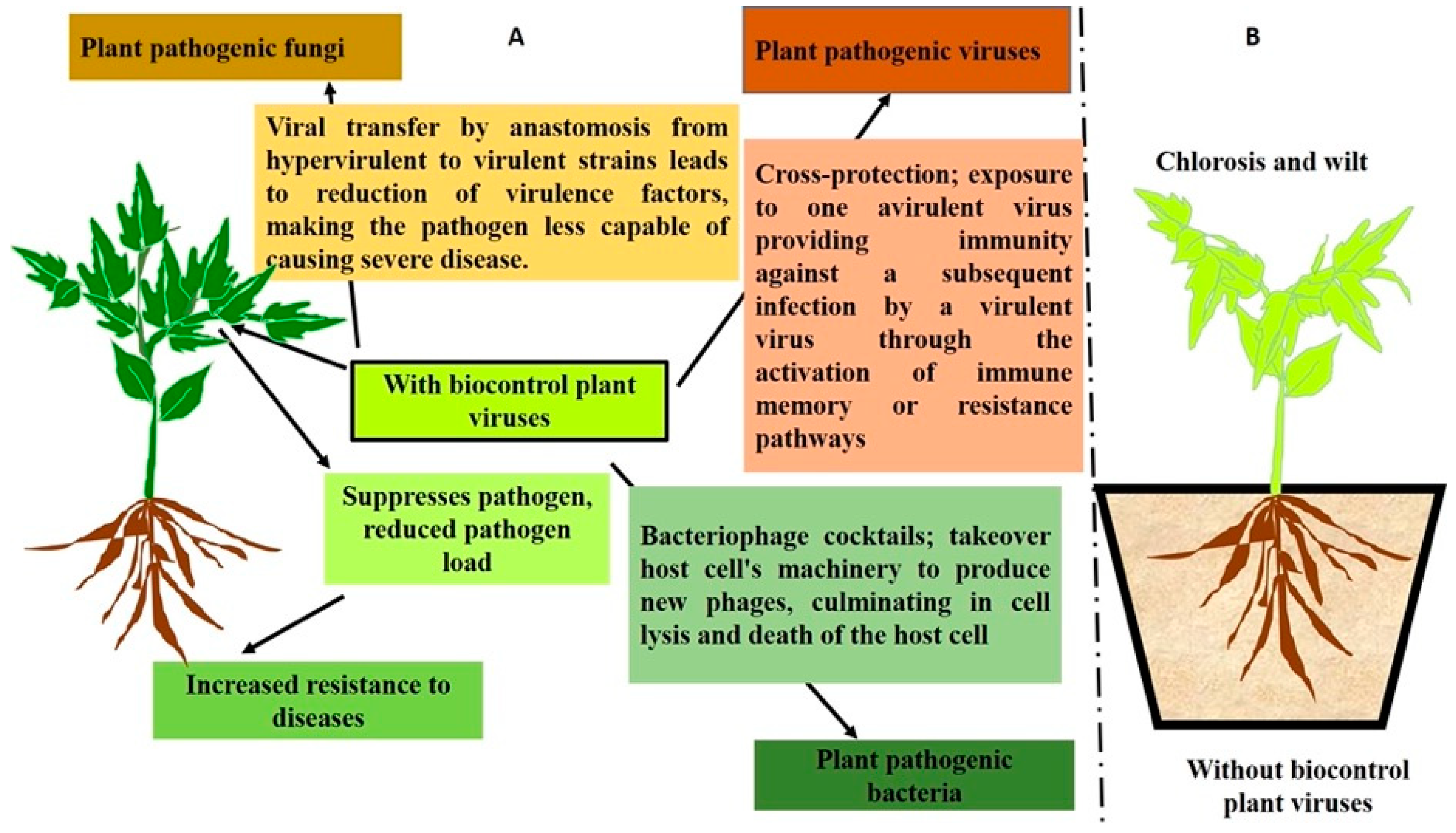
Viruses are important BCAs that act against a wide range of microbial pathogens and pests [193]. Viruses often carry a negative reputation due to their association with diseases and pandemics. However, it is important to note that the majority of viral species are harmless or even helpful, playing crucial roles in ecosystems [194]. As the most abundant entities on Earth, viruses are believed to have a significant ecological impact in maintaining the balance of organism populations. All three domains of life are susceptible to viral infection; however, many endocellular bacteria such as phytoplasmas are conspicuously immune to viral infection. Since viruses are frequently regarded as the ultimate parasites and do not usually cause detectable harm to their hosts, they are appealing candidates for BCAs [195]. A century ago, Mallmann and Hemstreet [196] initially proposed the use of bacteriophages as BCAs against bacteria. Currently, researchers are interested in investigating phages as BCAs for bacterial disease control, primarily because of the fast development of antibiotic resistance in bacterial pathogens that has resulted in serious agricultural loss [197]. Interestingly, phages are viruses that only infect bacteria; they do not directly harm plants or mammals. On the other hand, the utilization of the mycovirus Cryphonectria hypovirus 1 (CHV1), which was found to induce hypovirulence in the ascomycetous fungus Cryphonectria parasitica, was the first instance of a virus being a successful BCA in fungi [193]. Since then, CHV1 has been used to impede the spread of chestnut blight in forests and orchards [198]. The first instances of virus biocontrol in insects were the applications of baculoviruses to manage populations of lepidopteran insects [199]. Viruses can also be employed to manage viral disease in plants via cross protection. Here, attenuated plant virus strains are used as a BCA in order to safeguard crops from pathogenic strains of the same or similar viral species. The basis for this crop protection technique is the cross protection/interference phenomena, which was initially studied in tobacco plants infected with a yellow strain of the tobacco mosaic virus (TMV). Figure 3, shows viral BCAs employ different strategies to safeguard plants from pathogen attacks.
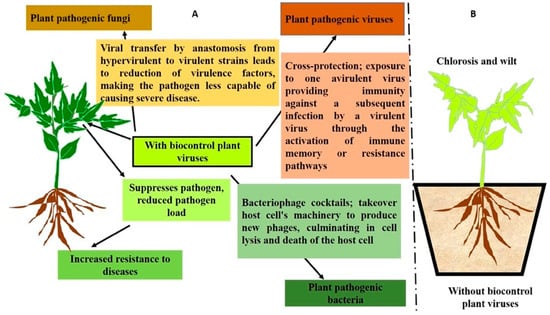
Figure 3.
Schematic diagram showing the role of viruses as BCAs in controlling plant pathogens and pests. (A) Depicts plants with viral biocontrol agents that protect them from pathogens by using diverse strategies such as activating immune signaling pathways, infecting pathogens to alter their virulence, triggering pathogen cell lysis, and cross infection by activating memory resistance. (B) Depicts plants without viral BCAs being challenged by pathogens, which leads to disease development and ultimately host death.
6.1. Biocontrol Potential of Mycoviruses against Fungal Plant Pathogens
Mycoviruses are obligate parasites found in the majority of economically significant plant-pathogenic fungi. Latent infections are often linked to mycovirus–fungus interactions; however, mycovirus infection can potentially be advantageous or deleterious to the fungal host. They have a variety of genome types, but the most common ones are double-stranded RNA (dsRNA) or positive- and negative-sense single-stranded RNA (ssRNA+/−) genomes. Single-stranded DNA (ssDNA) genomes are far less common and are conspicuously absent from dsDNA viruses [200,201]. These have a mono- or multi-segmented genome, naked or encapsidated [202]. Mycovirus-infected hypovirulent Cryphonectria parasitica was effectively used in the past to control chestnut blight, which led to an increased investigation into mycoviruses and their potential for the biocontrol of pathogenic fungus in plants across different pathosystems [203]. Based on artificial transection methods, it was found that mycoviruses have broad host ranges and induce hypovirulence. For instance, species that are phylogenetically close to C. parasitica such as C. radicalis, C. havanensis, C. cubensis, and Endothia gyrosa were artificially infected using the synthetic transcript of CHV1-EP713, which showed that CHV1 was not only able to multiply, but also induced hypovirulence as well as other phenotypic changes in all of these fungal strains [204,205]. Previous reports have shown that CHV1 can also reduce the virulence and growth of important pant pathogens such as Valsa ceratosperma and Phomopsis G-type, highlighting their potential as BCAs [206]. The infection of C. parasitica, Glomerella cingulate, and V. ceratosperma with mycovirus Rosellinia necatrix partitivirus 1 (RnPV1) also inhibited growth and reduced their virulence [207]. On the other hand, Xiao et al. [208] used purified virions of mycovirus Sclerotinia sclerotiorum partitivirus 1 (SsPV1) to infect B. cinerea, which also showed growth alteration, stable persistent infection, and the induction of reduced virulence. Lee et al. [209] used protoplast fusion to transmit mycovirus FgV1-DK21 to F. graminearum, F. asiaticum, F. oxysporum f. sp. lycopercisi, and C. parasitica. All of these hosts allowed FgV1-DK21 to proliferate, even the distantly related C. parasitica, where it resulted in growth changes and decreased pathogenicity, which were different from those seen in CHV1 infections. These studies further support the notion that mycoviruses are potential BCAs and could be applied to the control of a wide range of plant fungal pathogens.
6.2. Phage-Based Biobactericides
Plant pathogenic bacteria also pose a serious threat to crop productivity. Currently, many bacterial pathogens have developed antibiotic resistance, which is a major concern to the environment and human health. For example, Xanthomonas campestris, Erwinia amylovora, and Pseudomonas syringae have been found to harbor antibiotic resistance genes (strAB) that confer resistance against streptomycin [210]. To overcome this problem, harnessing phage-based BCAs is a viable strategy for controlling bacterial pathogens in sustainable agriculture. Bacteriophages, often known as phages, are viruses that cause bacterial hosts to degrade by selectively infecting and multiplying within them as antimicrobial agents. In order to control bacterial disease and increase crop yields, phage therapy is a viable, effective strategy against resistant bacterial plant pathogens [211]. The U.S. Environmental Protection Agency (EPA) has approved four distinct phages from Omnilytics as active ingredients for bactericides since 2005. The first cocktail, known as AgriPhage, guards tomato and pepper plants in both greenhouses and open fields from bacterial spot and speck by including bacteriophages against X. campestris pv. vesicatoria and P. syringae. Similarly, Omnilytics has created concoctions to combat fire blight on pear and apple trees as well as bacterial canker disease on citrus and tomatoes [195]. Unfortunately, the literature lacks specific information regarding the phage composition and anticipated reductions in symptom development of the current cocktails; however, preliminary formulations of the X. campestris pv. vesicatoria phage cocktail were found to reduce tomato plant bacterial spot disease severity by an average of 17% [212]. Five greenhouse trials found that a cocktail against X. citri subsp. citri comprising of at least some of the Omnilytics phages might lower citrus canker disease severity by an average of 59% [213]. A&P Inphatec, a subsidiary of Otsuka Pharmaceutical, developed XylPhi-PD, a phage product that the EPA licensed in 2021 to guard grapevines in California against Pierce’s disease, which is brought on by Xylella fastidiosa [194]. In a four-site field study conducted in California, the business revealed that the constant injection of XylPhi-PD in the xylem reduced disease incidence by 57%. The Hungarian government has given Enviroinvest permission to distribute the phage cocktail Erwiphage Plus domestically as an anti-E. amylovora bactericide. This medicine is modified every year to prevent the development of resistance, and it can only be used under very specific circumstances during the flowering season. Each year, emergency authorization is granted for as few as 120 days, spanning from mid-March to mid-July [214]. An engineered cocktail of four lytic phages has been reported as an effective BCA of X. fastidiosa and its associated infections including PD, olive-quick decline syndrome, and oleander, coffee, and almond leaf scorch [215,216]. Ahmad et al. [217] showed that the filamentous phage XacF1 caused loss of virulence, reduced EPS production, restricted motility, and delayed growth rate in Xanthomonas axonopodis pv. citri, the causative agent of citrus canker disease, which further highlights its biocontrol potential against bacterial pathogens. On the other hand, phages such as vRsoP-WF2, vRsoP-WR2, and vRsoP-WM2 were also identified as effective BCAs against R. solanacearum [218]. Two particular phage species, E. amylovora Siphoviridae phage (PhiEaH1) [214], and PhiEaH2 [219], were isolated locally and effectively decreased the incidence of fire blight cases in field studies.
6.3. Cross-Protection
Cross-protection is a strategy that pre-immunizes plants against mild virus strains in order to fight viral infections [195]. Plants are shielded against secondary infection by other viral strains by cross protection. McKinney was the first to demonstrate the cross-protection effect in tobacco plants chronically infected with a tobacco mosaic virus (TMV) light green isolate, which decreased the onset of yellow symptoms caused by a TMV yellow mosaic isolate [220]. There has been some success using it to manage viral illnesses such as cross-protecting tomatoes against mild strains of the tobacco mosaic virus, papaya against minor strains of the papaya ring spot virus, and citrus against moderate strains of the citrus tristeza virus [221]. On the other hand, yellow symptoms were not suppressed by the TMV moderate dark green isolate. Many viruses such as the potato virus X, potato leafroll virus, and citrus tristeza virus (CTV) have been shown to exhibit cross protection [222]. “Plant vaccines” are another term for attenuated isolates. Sap from appropriate leaves can be made to proliferate an attenuated strain in order to provide a real plant vaccination. Next, approximately ten day old seedlings are mechanically given this sap along with carborundum in their cotyledons [223]. An attenuated virus cannot infect another viral species that is closely related to it once it has infected a whole plant. As a result, attenuated viruses are crucial agents of biological control. Salaman [224] initially looked into the cross-protective effects of plant viruses in 1933 and discovered that a moderate isolate of potato virus X (PVX) may prevent a severe PVX isolate from becoming infected. Holmes subsequently created a mild isolate of TMV by heating a virulent strain and observed that in plants infected with the mild isolate, the symptoms brought on by infection with the virulent isolate were reduced [225]. For field studies involving over 2000 citrus rootstocks, a mild isolate of CTV was created in 1951 [226]. The findings suggested that citrus trees protected against more severe CTV isolates were infected with milder isolates. Posnette and Todd [227] employed a similar study approach when they tested a mild isolate of cacao virus 1A in an African area where swollen shoot disease was common. In that study, only 35 out of 416 trees infected with the mild isolate exhibited severe symptoms of infection compared to 273 out of 387 uninfected trees that displayed severe symptoms. This outcome was interpreted as proof that inoculating cacao trees with a moderate viral isolate may successfully protect them in the real world. As a result, attenuated viruses have been the subject of both fundamental and applied research for many years. In addition, cross-protection against the pepino mosaic virus has been recently created and widely used in Europe [228]. These strains of the virus also received separate authorization from the European Food Safety Agency in 2015. Since then, cross-protection has been used in open-field settings to guard against significant viral diseases caused by the papaya ringspot virus in papaya [229] and the citrus tristeza virus (CTV) in citrus species [229]. In South Africa, Peru, and the U.S., cross protection has been proven to be an effective management strategy for the citrus stem pitting disease caused by CTV [230].
7. Biocontrol Agents’ Production and Formulations
7.1. Production of BCAs
A critical factor that must be considered when selecting a BCA for commercial development is the availability of a cost-effective manufacturing and stabilization technology that yields an optimally effective form of the antagonist [23]. More studies on the practical aspects of mass production and formulation need to be undertaken to make new biocontrol products stable, effective, safer, and more cost effective [231]. One essential stage in creating effective BCAs for market use is the production of BCAs. The basic objective of industrial scale-up processes, which involve either liquid or solid phase fermentation, is to produce a high amount of biomass at the lowest feasible cost [231]. This is typical procedure to create bacteria and yeasts by liquid fermentation in a continuously stirred tank in order to attain a high aeration. However, a lot of molds are mostly made in a solid state. Regardless of the mass production method, the main objective is to have the highest yield at the lowest cost [232]. The practical efficacy of a BCA greatly depends on the quality of the inoculant, itself a function of the production and formulation processes [233]. The two main processes in production optimization are determining the composition of the medium and enhancing the growing conditions. To manage the BCA production operation and attain an excessive number of cells, create secondary metabolites, or both, it is vital to comprehend their mode of action. Erlenmeyer flasks are typically used in a low-volume laboratory setting for the first phase, which involves evaluating a huge number of substances and concentrations. After that, 2 to 5 L laboratory bioreactors are used to properly calibrate the growth settings. Ultimately, 100–300 L pilot plant bioreactors will be used for the initial large-scale manufacturing, and it will not be hard to scale up the operation to commercial requirements if the pilot plant results are satisfactory. In specifically made plastic bags (VALMIC®) encompassing turba:vermiculite (1:1 w/w), the generation of Penicillium frequentans strain Pf909, a BCA in the management of brown rot in stone fruit, was developed [234]. By applying potato extract, V-8 juice, molasses, and wheat fiber, the BCA Trichoderma spp. was grown in both liquid form [235] and on solid media containing various cereals such as sorghum and millet [236]. As an example, the formation of the conidia of the antagonist Ampelomyces quisqualis has been shown in a variety of liquid media including a sugar-based medium enhanced with shrimp shell powder [237] and potato dextrose broth adjusted with 2.5% glycerol [238]. In order to guarantee a high, strong, and productive microbial population, careful research is required to enhance the growth conditions (temperature, pH, agitation, aeration, initial inoculum, and process duration) after the optimal growth medium for each microorganism has been determined [239]. A valuable product can be recovered after fermentation by focusing on acquiring cells (or spores), the supernatant containing the released metabolites, or even both, depending on the BCA. One instance of BCA that is recommended is the recovery and formulation of B. amyloliquefaciens [240], as both forms are involved in its mode of action to manage diseases. This includes both the vegetative and endospore cells as well as the produced metabolites. Microorganisms are typically present in low concentrations and are combined with other molecules from which they must be removed through a number of steps. These processes include centrifugation, flocculation, and filtering (pressure, rotary vacuum drum, and flocculation) [241].
7.2. BCA Formulation
When developing a formulation, a number of factors need to be considered including the cost of production and acquisition and compatibility with agricultural machinery [242]. As the link between fermentation and field application, the formulation is sometimes seen as the main obstacle to the commercialization of BCAs [243,244]. Both liquid and solid BCA formulations come in a wide range of varieties. Solid formulations can increase the BCA stability, although liquid formulations are typically simple and less expensive to produce. A formulated product is primarily made up of, or may contain: (1) the active ingredient, which is synthesized microorganism cells and secondary metabolites, (2) carriers, which are often inert materials that carry and deliver the active ingredient in the target site, and (3) adjuvants, which are substances that protect the active ingredient from high temperatures, desiccation, ultraviolet radiation, and other environmental stresses while also facilitating the product’s spread and dispersal in the intended environment. The microbe must be compatible with all adjuvants employed in the formulation process and not negatively affected by any of them [245].
7.2.1. Liquid Formulation
In order to maximize the product’s viability and improve the BCA’s adhesion, surfactant, and dispersion capabilities, liquid formulations combine whole cultures or cell suspensions with additives like stabilizers, colorants, and extra nutrients [246]. Compared to formulations based on solids, these formulations are less expensive and simpler to process. Particles can be suspended in a variety of liquids such as oil or water, dispersants, surfactants, suspender components, or a carrier liquid in some liquid compositions. When the processed culture is combined with emulsifiers, surfactants, and/or mineral or vegetable oils that facilitate their subsequent dispersion in water, the mixture is referred to as oil-based. The oils that are utilized must not be harmful to humans, plants, microorganisms, and animals. Since oils provide a defensive effect that lengthens the shelf life of microorganisms, it is generally thought that oil-based formulations are appropriate for foliar applications in dry ambient circumstances [247]. The development of the filamentous fungus T. harzianum, an efficient opponent to prevent Botrytis rot in apples, is an illustration of this system and was conducted by emulsion [248]. The yeast Hanseniaspora guilliermondii isolate YBB3 was developed as a liquid formulation based on glycerol to manage Aspergillus rot in grapes. This formulation proved to be more effective than solid formulations that mixed the biomass produced with talc/kaolin powder alone or modified with yeast extract, sucrose, and sodium alginate [249].
7.2.2. Solid Formulation
Although dried products have short viability rates due to both the thermal and dehydration stresses that occur during the drying operation, they are nevertheless a practical solution because of their low production costs and ease of storage and transportation [250]. As a result, every isolate requires a technique that is experimentally tailored to it. The following methods of dehydration are efficient: fluidized bed drying, spray drying, and freeze-drying. Vacuum desiccation, often known as freeze-drying or lyophilization, is a technique for dehydrating labile goods. In short, the sampled liquid needs to be chilled until the solutes crystallize, then the non-crystallizing solutes create an amorphous matrix, and the freezable solution water finally turns into ice. Next, the water is removed from the amorphous matrix by sublimating the ice in vacuum-controlled conditions. Ultimately, the product’s moisture content is disported [251]. This method is frequently employed for the conservation of microorganisms, particularly those kept in microbiology collections. According to Prakash et al. [252], it keeps microorganisms viable for over twenty years and does not require specific low-temperature settings for continued management. By eliminating the moisture from the liquid droplets, a product can be transformed from a liquid to a solid state in the form of powder through spray-drying, also known as atomization. The scattered droplets (10–200 m), which are mostly created by pressure nozzles, pneumatic type atomizers, or rotary wheel/disc atomizers, are combined with low-humidity hot air (150–170 °C) inside the chamber. Subsequently, rapid mass transfer and related heat reactions cause the moisture to swiftly escape as vapor from the suspended droplets. Until the required particle properties are attained, the droplets continue to dry inside the drying chamber. Ultimately, external apparatus like cyclones and/or bag-filter houses are used to separate the dried particles from the drying air and collect them afterward [253]. The only BCAs that can be created with this method are those that can withstand high temperatures such as those that can produce thermoresistant endospores. B. amyloliquefaciens strain CPA-8, created to suppress Monilinia spp. in nectarines and peaches, has shown some promising effects [253]. Powders, granules, and spheres are dried, granulated, and coated using fluidized bed technology [254]. Its foundation is the strong interactions that occur between particles. Via a perforated plate, heated air that has been filtered and perhaps dehumidified (between 35 and 45 °C) enters the product, counteracting gravity and fluidizing the material that has been previously sucked or placed into the granulator. However, in order to use this product, it must first be extruded and then chopped into short rods or “pellets”. Filters retain small particles that are transported by the air flow to the cylindrical expansion chamber, allowing them to return to the conical product chamber. As the process proceeds, this cycle is mostly controlled by changes in the product’s temperature and the fluidization air flow [254]. Because this technology uses relatively mild temperatures, takes little time, and is inexpensive, it is especially well-suited for BCAs that are sensitive to heat. Numerous BCAs including Epicoccum nigrum and Penicillium oxalicum have been successfully conserved using this technique [255].
The process of encapsulation involves the microorganism being placed inside a specific matrix or capsule that may be biodegradable if it is made of polyacrylamides, guar gum, gum arabic, sodium alginate, chitosan, or other biocomposites [256]. Vemmer et al. [227,257] demonstrated that these matrices are effective carriers because they enclose living cells and shield them from harsh environmental elements like UV rays and rain. Additionally, González et al. [258] reported that these microcapsules are easily manipulable, can be kept long-term in room temperature storage, and are excellent for BCAs that require a delayed release such those placed in soil. The alginate encapsulation of Gliocladium virens (Soil Gard) [259] and the encapsulations of B. thuringiensis in shell-core hydrocapsules, which have a liquid center surrounded by a polymer membrane [260], are two examples of this type of formulation.
8. Role of Genetic Engineering for Improving BCAs Traits
The use of BCAs for disease and pest management has significantly increased in recent times because of the negative impact of chemical pesticides on soil fertility, the environment, and human health. However, there are many challenges while using BCAs such as their environmental stability, efficacy, pest or disease suppression ability, host range, target selection, reducing the ecological risk, and improving the mass production or storage [261,262]. Recent developments in genomics and biotechnology have facilitated improvements in the above traits in BCAs and also aids in the identification of novel BCAs, their characterization, and the genetic by-products [261,262,263]. The availability of microbial genome data has opened new dimensions for the discovery of a large array of BCAs [263]. The integrated approaches of omics technologies will provide a strong foundation for in-depth insights regarding the interactions between BCAs and their hosts. Through the application of omics approaches such as the genomics, transcriptomics, and proteomics of BCAs, pathogens, and crop plants, a specific set of interactome could reveal the key mechanisms and factors influencing the efficacy and applicability of an organism as a BCA [264,265]. The efficacy of BCAs can be increased using genetic engineering. For instance, in Pseudomonas fluorescens F113, the loss of the sadB, wspR, and kinB genes results in increased motility and improved root colonization, demonstrating good biocontrol activity against Phytophthora cactorum in strawberry plants and Fusarium oxysporum f. sp. Radicis-lycopersici in tomato plants [266]. Conversely, significant mycosubtilin production resulting from the overexpression of the mycosubtilin gene utilizing the Staphylococcus aureus constitutive promoter in Bacillus subtilis strain ATCC 6633 enhances Pythium aphaniderm suppression [267]. Introduction of the chitinase gene in Bacillus thuringiensis 3023 from Serratia marcescens showed broad biocontrol activity against various pests [268]. Genetically modified Pseudomonas fluorescens strain BL915 with high antipathogenic substances (APS) like pyrrolnitrin showed strong biocontrol activity against soilborne and seedling pathogens [269]. Similarly, the overexpression of Cry genes in B. thuringiensis showed strong insecticide activity against susceptible and Bt-resistant insects in tobacco plants [270]. Genetically-modified Pseudomonas spp., through the introduction a translational enhancer from Bacillus confers strong biocontrol activity. Genome shuffling has been used in Bacillus subtilis, Streptomyces melanosporofaciens, and Streptomyces bikiniensis for the improvement in biocontrol traits against Fusarium oxysporum f. sp. melonis, Phytophthora infestans, Fusarium oxysporum f. sp. Cucumerinum [271], Streptomyces melanosporofaciens [272], and Streptomyces bikiniensis [273]. Recently, the Sarocladium oryzae mutant strain was found to efficiently control Rhizoctonia solani and Sclerotinia sclerotiorum pathogens [274]. Transformation of the Serratia marcescens chitinase gene into Pseudomonas fluorescens improved the biocontrol efficiency against R. solani on bean seedlings [275]. Similarly, the introduction of pyrrolnitrin coding genes from Pseudomonas protegens Pf-5 to P. synxantha 2–79 showed strong biocontrol activity against wheat pathogens. It has been demonstrated that T. virens cotton strains containing two copies of the ech42 gene exhibited increased antagonistic activity against R. solani [276]. Sun et al. [277] genetically modified the Clonostachys rosea strain, which showed strong biocontrol activity against Sclerotinia rot of soybean. To increase its effectiveness against soilborne infections, a genetically engineered strain of Pseudomonas putida was developed with phz or phl biosynthesis gene loci, which produced constitutively phenazine-1-carboxylate (PCA) or 2,4-diacetylphloroglucinol (DAPG) [278]. Another study found that once the trichodermin gene (tri5-trichodiene synthase) was cloned and overexpressed in T. brevicompactum, it produced higher trichodermin, which exhibited strong biocontrol activity against Aspergillus fumigatus and Fusarium spp. Ma et al., 2003. A previous study showed that T. virens Δtvk1 mutants showed improved biocontrol efficacy against R. solani along with the overproduction of lytic enzymes and the increased expression of genes linked to mycoparasitism [279]. These studies further support the notion that genetic engineering is the key to developing long-term effective BCAs. Although genetic engineering has been a major driver for BCA development, it has some possible health risks and lateral gene flow to non-target animals, which might pose threats to the environment. In this regard, genome editing is an alternative for improving the traits of BCAs more precisely and effectively without endangering the environment. The use of CRISPR and Cas genes has expedited the creation of versatile and economical genomic engineering toolkits that rely on the programmable targeting of CRISPR–Cas technologies [280]. Genome editing using CRISPR/Cas has been used for improving diverse biocontrol traits in Trichoderma species [281]. In future, CRISPR/Cas based gene editing for the improvement of biocontrol traits is the most promising tool for developing future climate resilient, efficient, and ecofriendly BCAs in sustainable agriculture.
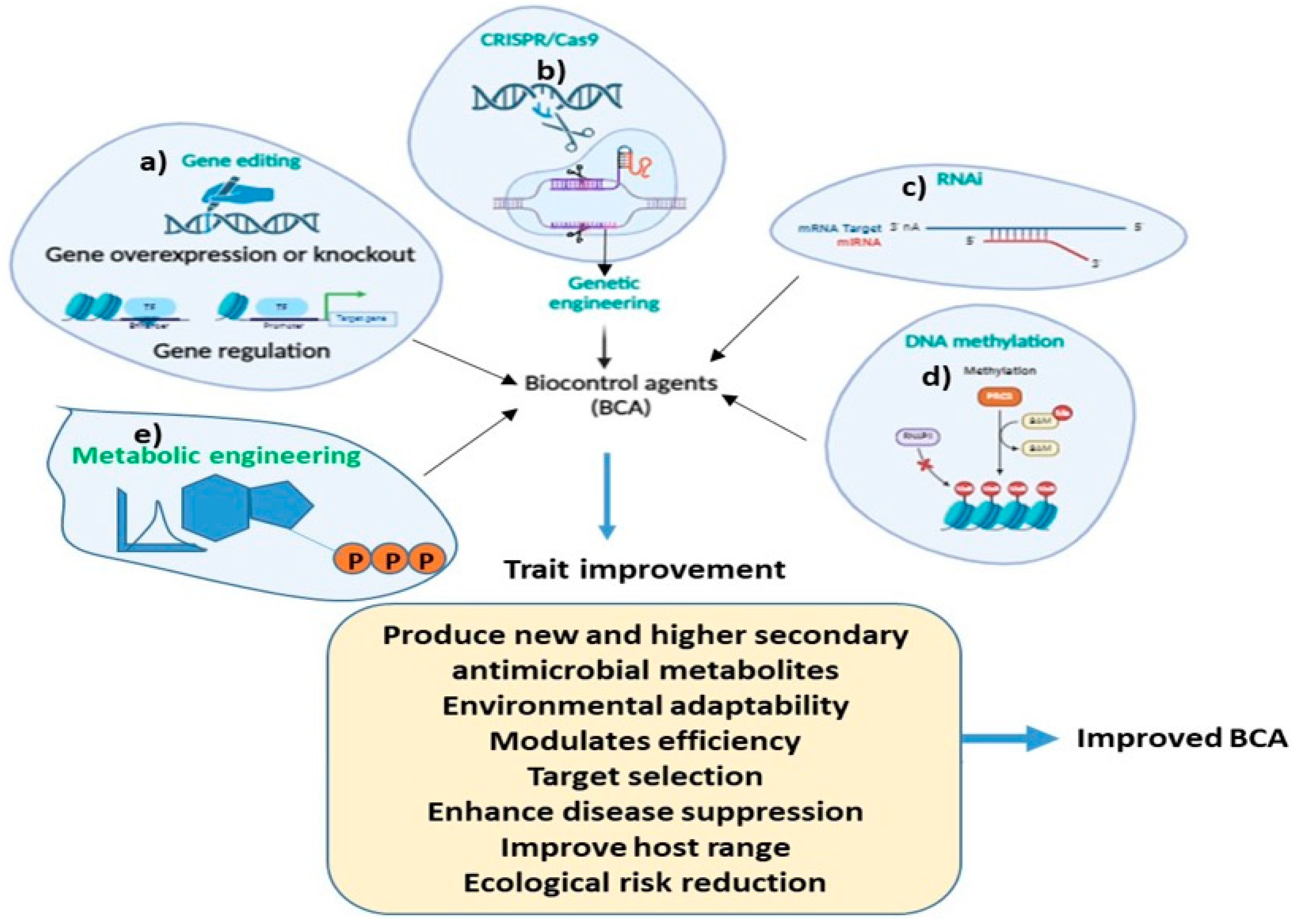
An understanding of the biosynthetic pathway of antimicrobial metabolites in BCAs is important for improving their biocontrol efficiency. For example, the antibiosis action of BCAs is often achieved by the synthesis of low-molecular-weight chemicals that either directly or indirectly impede the development of pathogens. These encompass a wide range of chemical groups including polyketides [282], terpenes [283], and peptides [284,285]. Thus, metabolic engineering is needed to increase the secondary metabolite production of BCAs, which may potentially result in the synthesis of new antimicrobial compounds. Metabolic engineering holds great promise for the development of future BCAs, offering innovative strategies to enhance their efficacy, specificity, and environmental sustainability. Modern techniques such as plasmid engineering, RNAi interference, and CRISPR-based genome editing utilized to modify the T. hazrium strain for strain enhancement seems promising (Figure 4).
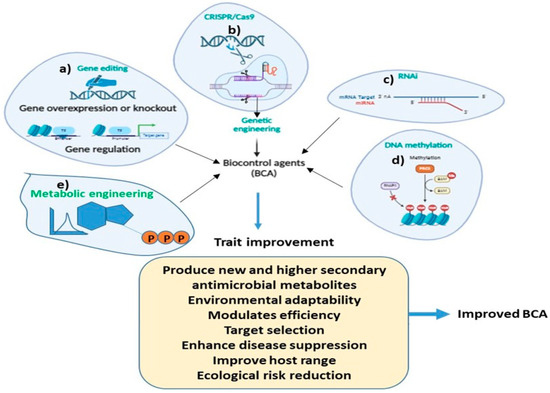
Figure 4.
Schematic diagram highlighting the role of different molecular methods for improving the biocontrol traits in BCAs for disease and pest management. (a) gene editing, (b) CRISPR/Cas9, (c) RNAI, (d) DNA methylation and (e) Metabolic engineering.
9. Conclusions
In sustainable agriculture, BCAs are currently being examined as alternatives to synthetic pesticides to manage plant pathogens and pests, mainly due to their safety and ecofriendly nature. These include naturally occurring microorganisms such as fungus, bacteria, and viruses, which are commercially produced as biopesticides and have a variety of agricultural applications in biological and integrated pest management programs. BCAs use diverse mechanisms such as parasitism, antibiosis, or competition and induce the plant defense system to manage diseases or pests. They also play a vital role in improving plant growth and soil fertility. However, the identification and development of a novel class of biocontrol agents possessing increased potency, high productivity in fermenters, extended shelf life, room temperature storage capacity, and excellent compatibility with other control strategies are required for the sustainable management of plant diseases. On the other hand, BCAs must be evaluated for any negative impacts on crops, consumers, and the environment before they can be approved and commercially produced. To further clear the regulatory road and guarantee biosafety, it is also necessary to comprehend how BCAs affect the natural microbiome, which includes the soil and plant microbiomes. Before they may be effectively used in the field, a number of modifications must be made including improving the formulation and delivery strategies, scaling up manufacturing, adhering to regulatory standards, and enhancing cost-effectiveness. Further investigations on certain underdeveloped areas such as the creation of next generation BCAs and the application of biotechnology in conjunction with “omics” approaches to enhance biocontrol efficacy are also necessary for the future development of successful biological control strategies. In this regard, genetic engineering using CRISPR genome editing have the potential to decrease undesirable traits in BCAs and introduce new, desirable traits, which can act against a broad spectrum of pathogens and pests in different crop systems. In climate change scenarios, biological control agents may lose their efficacy if there is a higher fluctuation in temperature, humidity, frequency of rain, and other weather conditions. Therefore, it is important to develop climate resilient biocontrol agents in sustainable agriculture.
Author Contributions
Conceptualization, M.A.A. and S.A.; Methodology, A.T., T.L.T., H.K., R.A.M., Z.A.M., S.M., N.M., G.G., S.K.V., M.A.A. and S.A.; Software, A.T., T.L.T., H.K., R.A.M., Z.A.M., S.M., N.M., G.G., S.K.V., M.A.A. and S.A.; Validation, A.T., T.L.T., H.K., R.A.M., Z.A.M., S.M., N.M., G.G., S.K.V., M.A.A. and S.A.; Formal analysis, A.T., T.L.T., H.K., R.A.M., Z.A.M., S.M., N.M., G.G., S.K.V., M.A.A. and S.A.; Investigation, M.A.A. and S.A.; Resources, A.T., T.L.T., H.K., R.A.M., Z.A.M., S.M., N.M., G.G., S.K.V., M.A.A. and S.A.; Data curation, A.T., T.L.T., H.K., R.A.M., Z.A.M., S.M., N.M., G.G., S.K.V., M.A.A. and S.A.; Writing—original draft preparation, A.T., T.L.T., H.K., R.A.M., Z.A.M., S.M., N.M., G.G., S.K.V., M.A.A. and S.A.; Writing—review and editing, A.T., T.L.T., H.K.; R.A.M., Z.A.M., S.M., N.M.; G.G., S.K.V., M.A.A. and S.A.; Visualization, A.T., T.L.T., H.K., R.A.M., Z.A.M., S.M., N.M., G.G., S.K.V., M.A.A. and S.A.; Supervision, M.A.A. and S.A.; Project administration, M.A.A. and S.A.; Funding acquisition, M.A.A. and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mall, M.; Kumar, R.; Akhtar, M.Q. Horticultural crops and abiotic stress challenges. In Stress Tolerance in Horticultural Crops; Woodhead Publishing: Cambridge, UK, 2021; pp. 1–19. [Google Scholar]
- Xu, J.; Zhang, N.; Wang, K.; Xian, Q.; Dong, J.; Chen, X. Exploring new strategies in diseases resistance of horticultural crops. Front. Sustain. Food Syst. 2022, 6, 1021350. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Rajarammohan, S.; Mir, Z.A.; Bae, H. Revisiting Alternaria-host interactions: New insights on its pathogenesis, defense mechanisms and control strategies. Sci. Hortic. 2023, 322, 112424. [Google Scholar] [CrossRef]
- Chaerani, R.; Voorrips, R.E. Tomato early blight (Alternaria solani): The pathogen, genetics, and breeding for resistance. J. Gen. Plant Pathol. 2006, 72, 335–347. [Google Scholar] [CrossRef]
- Utkhede, R.S.; Mathur, S. Preventive and curative biological treatments for control of Botrytis cinerea stem canker of greenhouse tomatoes. BioControl 2006, 51, 363–373. [Google Scholar] [CrossRef]
- Charoenporn, C.; Kanokmedhakul, S.; Lin, F.C.; Poeaim, S.; Soytong, K. Evaluation of bio-agent formulations to control Fusarium wilt of tomato. Afr. J. Biotechnol. 2010, 9, 5836–5844. [Google Scholar]
- Panno, S.; Davino, S.; Caruso, A.G.; Bertacca, S.; Crnogorac, A.; Mandić, A.; Noris, E.; Matić, S. A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the mediterranean basin. Agronomy 2021, 11, 2188. [Google Scholar] [CrossRef]
- Khodadadi, F.; González, J.B.; Martin, P.L.; Giroux, E.; Bilodeau, G.J.; Peter, K.A.; Doyle, V.P.; Aćimović, S.G. Identification and characterization of Colletotrichum species causing apple bitter rot in New York and description of C. noveboracense sp. nov. Sci. Rep. 2020, 10, 11043. [Google Scholar] [CrossRef]
- Marquez, N.; Giachero, M.L.; Declerck, S.; Ducasse, D.A. Macrophomina phaseolina: General Characteristics of Pathogenicity and Methods of Control. Front. Plant Sci. 2021, 12, 634397. [Google Scholar] [CrossRef]
- Tripathi, A.N.; Singh, B.P. Evaluation of seed health of seeds of different vegetable crops. Veg. News Letter. 2022, 9, 7. [Google Scholar]
- Sundin, G.W.; Wang, N. Antibiotic Resistance in Plant-Pathogenic Bacteria. Annu. Rev. Phytopathol. 2018, 25, 161–180. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; González-García, V.; Martín-Gil, J.; Lorenzo-Vidal, B.; Palacio-Bielsa, A.; Martín-Ramos, P. Phytochemical Screening and Antibacterial Activity of Taxus baccata L. against Pectobacterium spp. and Dickeya chrysanthemi. Horticulturae 2023, 9, 201. [Google Scholar] [CrossRef]
- Hull, R. Symptoms and Host Range. In Plant Virology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 145–198. [Google Scholar]
- Mehetre, G.T.; Leo, V.V.; Singh, G.; Sorokan, A.; Maksimov, I.; Yadav, M.K.; Upadhyaya, K.; Hashem, A.; Alsaleh, A.N.; Dawoud, T.M.; et al. Current Developments and Challenges in Plant Viral Diagnostics: A Systematic Review. Viruses 2021, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-Onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in drinking water—A review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef] [PubMed]
- Stukenbrock, E.; Gurr, S. Address the growing urgency of fungal disease in crops. Nature 2023, 617, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Verhaegen, M.; Bergot, T.; Liebana, E.; Stancanelli, G.; Streissl, F.; Mingeot-Leclercq, M.P.; Mahillon, J.; Bragard, C. On the use of antibiotics to control plant pathogenic bacteria: A genetic and genomic perspective. Front. Microbiol. 2023, 14, 1221478. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, A.D.; Sarkale, P.S.; Patil, P.A. Transgenic Plants and Animals in Agriculture: Assessing the Risks and Benefits. Nat. Camp. 2024, 28, 95–102. [Google Scholar]
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of pesticides on environment. In Plant Soil and Microbes; Springer: Berlin/Heidelberg, Germany, 2016; pp. 253–269. [Google Scholar]
- Sandy, Y.A.; Zahro, F.A.; Rizky, D.R.; Fajarwati, S.K.; Effendi, M. Knowledge Level of Farmers regarding the Use of Pesticide for Pest and Disease Control. J. Agrinika J. Agroteknologi Agribisnis 2024, 8, 12–22. [Google Scholar]
- Dalavayi, H.M.; Bala, S.; Choudhury, D. Eco-friendly plant based on botanical pesticides. Plant Arch. 2021, 21, 2197–2204. [Google Scholar]
- He, D.C.; He, M.H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Jung, H.; Koo, B.K.; Han, J.A.; Lee, H.S. Exploiting bacterial genera as biocontrol agents: Mechanisms, interactions and applications in sustainable agriculture. J. Plant Biol. 2023, 66, 485–498. [Google Scholar] [CrossRef]
- Theresa, M.; Radhakrishnan, E.K. Microbial biocontrol formulations for commercial applications. In Microbiome Stimulants for Crops; Woodhead Publishing: Cambridge, UK, 2021; pp. 179–192. [Google Scholar]
- Galli, M.; Feldmann, F.; Vogler, U.K.; Kogel, K.-H. Can biocontrol be the game-changer in integrated pest management? A review of definitions, methods and strategies. J. Plant Dis. Prot. 2024, 131, 265–291. [Google Scholar] [CrossRef]
- Stenberg, J.A.; Sundh, I.; Becher, P.G.; Björkman, C.; Dubey, M.; Egan, P.A.; Friberg, H.; Gil, J.F.; Jensen, D.F.; Jonsson, M.; et al. When is it biological control? A framework of definitions, mechanisms, and classifications. J. Pest Sci. 2021, 94, 665–676. [Google Scholar] [CrossRef]
- Funck Jensen, D.; Dubey, M.; Jensen, B.; Karlsson, M. Clonostachys rosea for the control of plant diseases. In Microbial Bioprotectants for Plant Disease Management; BDS Publishing: Cambridge, UK, 2021; pp. 429–471. [Google Scholar]
- Köhl, J.; Kolnaar, R.; Ravensberg, J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shao, J.; Li, B.; Yan, X.; Shen, Q.; Zhang, R. Contribution of bacillomycin D in Bacillus amyloliquefaciens SQR9 to antifungal activity and biofilm formation. Appl. Environ. Microbiol. 2013, 79, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kamle, M.; Borah, R.; Mahato, D.K.; Sharma, B. Bacillus thuringiensis as microbial biopesticide: Uses and application for sustainable agriculture. Egypt. J. Biol. Pest Control 2021, 31, 95. [Google Scholar] [CrossRef]
- Salazar, B.; Ortiz, A.; Keswani, C.; Minkina, T.; Mandzhieva, S.; Singh, S.P.; Rekadwad, B.; Borriss, R.; Jain, A.; Singh, H.B.; et al. Bacillus spp. as bio-factories for antifungal secondary metabolites: Innovation beyond whole organism formulations. Microb. Ecol. 2023, 86, 1–24. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Z.; Shao, J.; Xu, Z.; Liu, Y.; Xun, W.; Miao, Y.; Shen, Q.; Zhang, R. Biocontrol mechanisms of Bacillus: Improving the efficiency of green agriculture. Microb. Biotechnol. 2023, 16, 2250–2263. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Abd_Allah, E.F. Bacillus subtilis: A plant-growth-promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting Rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Karačić, V.; Miljaković, D.; Marinković, J.; Ignjatov, M.; Milošević, D.; Tamindžić, G.; Ivanović, M. Bacillus Species: Excellent Biocontrol Agents against Tomato Diseases. Microorganisms 2024, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Berdi, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Müller, A.; Miess, H.; Gross, H. Cyclic lipopeptides as antibacterial agents-Potent antibiotic activity mediated by intriguing mode of actions. Int. J. Med. Microbiol. 2014, 304, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Helmy, N.M.; Parang, K. Cyclic peptides with antifungal properties derived from bacteria, fungi, plants, and synthetic sources. Pharmaceuticals 2023, 16, 892. [Google Scholar] [CrossRef] [PubMed]
- Meena, K.R.; Kanwar, S.S. Lipopeptides as the antifungal and antibacterial agents: Applications in food safety and therapeutics. BioMed Res. Int. 2015, 2015, 473050. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Tai, B.; Ali, M.; Jahan, I.; Sakina, S.; Wang, G.; Zhang, X.; Yin, Y.; Xing, F. Antifungal potential of lipopeptides produced by the Bacillus siamensis Sh420 strain against Fusarium graminearum. Microbiol. Spectr. 2024, 12, 4008–4023. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.H.; Lu, Y.Q.; Ye, Z.W.; Zheng, Q.W.; Wei, T.; Lin, J.F.; Guo, L.Q. Genomics. guided discovery and structure identification of cyclic lipopeptides from the Bacillus siamensis JFL15. PLoS ONE 2018, 13, e0202893. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Pan, Y.; Qi, D.; Zhou, D.; Chen, Y.; Feng, J.; Wei, Y.; Zhao, Y.; Li, K.; et al. Biocontrol mechanism of Bacillus siamensis sp. QN2MO.1 against tomato fusarium wilt disease during fruit postharvest and planting. Microbiol. Res. 2024, 283, 127694. [Google Scholar] [CrossRef]
- Epparti, P.; Eligar, S.M.; Sattur, A.; Kumar, B.S.G.; Halami, P.M. Characterization of dual bacteriocins producing Bacillus subtilis SC3.7 isolated from fermented food. Food Sci. Technol. 2022, 154, 112854. [Google Scholar] [CrossRef]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Gillor, O.; Benjamin, C.K.; Margaret, A.R. Colicins and Microcins: The next generation antimicrobials. Adv. Appl. Microbiol. 2004, 54, 129–146. [Google Scholar] [PubMed]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef] [PubMed]
- Im, S.M.; Yu, N.H.; Joen, H.W.; Kim, S.O.; Park, H.W.; Park, A.R.; Kim, J.C. Biological control of tomato bacterial wilt by oxydifficidin and difficidin-producing Bacillus methylotrophicus DR-08. Pestic. Biochem. Physiol. 2020, 163, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Li, B.; Zhang, N.; Waseem, R.; Shen, Q.; Huang, Q. Production of bacillomycin- and macrolactin-type antibiotics by Bacillus amyloliquefaciens NJN-6 for suppressing soilborne plant pathogens. J. Agric. Food Chem. 2012, 60, 2976–2981. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Devappa, V.; Yadav, D.K. Suppression of tomato bacterial wilt incited by Ralstonia pseudosolanacearum using polyketide antibiotic-producing Bacillus spp. isolated from rhizospheric soil. Agriculture 2022, 12, 2009. [Google Scholar] [CrossRef]
- Chen, Q.; Qiu, Y.; Yuan, Y.; Wang, K.; Wang, H. Biocontrol activity and action mechanism of Bacillus velezensis strain SDTB038 against Fusarium crown and root rot of tomato. Front. Microbiol. 2022, 13, 994716. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guzmán, P.; Kumar, A.; de Los Santos-Villalobos, S.; Parra-Cota, F.I.; Orozco-Mosqueda, M.D.C.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma Species: Our Best Fungal Allies in the Biocontrol of Plant Diseases-A Review. Plants 2023, 12, 432. [Google Scholar] [CrossRef]
- Elad, Y. Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action. Crop Prot. 2000, 19, 709–714. [Google Scholar] [CrossRef]
- Howell, C.R. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef]
- Guo, Q.; Shi, L.; Wang, X.; Li, D.; Yin, Z.; Zhang, J.; Ding, G.; Chen, L. Structures and biological activities of secondary metabolites from the Trichoderma genus (covering 2018–2022). J. Agric. Food Chem. 2023, 71, 13612–13632. [Google Scholar] [CrossRef]
- Asad, S.A. Mechanisms of action and biocontrol potential of Trichoderma against fungal plant diseases—A review. Ecol. Complex. 2022, 49, 100978. [Google Scholar] [CrossRef]
- Manganiello, G.; Nicastro, N.; Ortenzi, L.; Pallottino, F.; Costa, C.; Pane, C. Trichoderma Biocontrol Performances against Baby-Lettuce Fusarium Wilt Surveyed by Hyperspectral Imaging-Based Machine Learning and Infrared Thermography. Agriculture 2024, 14, 307. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Brévault, T.; Carrière, Y. Insect resistance to Bt crops: Lessons from the first billion acres. Nat. Biotechnol. 2013, 31, 510–521. [Google Scholar] [CrossRef]
- Timper, P. Conserving and Enhancing Biological Control of Nematodes. J. Nematol. 2014, 46, 75–89. [Google Scholar]
- Umer, M.; Mubeen, M.; Iftikhar, Y.; Shad, M.A.; Usman, H.M.; Sohail, M.A.; Atiq, M.N.; Abbas, A.; Ateeq, M. Role of Rhizobacteria on Plants Growth and Biological Control of Plant Diseases: A Review. Plant Prot. 2021, 5, 59–73. [Google Scholar]
- Matsumoto, A.; Takahashi, Y. Endophytic Actinomycetes: Promising Source of Novel Bioactive Compounds. J. Antibiot. 2017, 70, 514–519. [Google Scholar] [CrossRef]
- Farzand, A.; Moosa, A.; Zubair, M.; Khan, A.R.; Massawe, V.C.; Tahir, H.A.S.; Sheikh, T.M.M.; Ayaz, M.; Gao, X. Suppression of Sclerotinia sclerotiorum by the Induction of Systemic Resistance and Regulation of Antioxidant Pathways in Tomato Using Fengycin Produced by Bacillus amyloliquefaciens FZB42. Biomolecules 2019, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Ezrari, S.; Mhidra, O.; Radouane, N.; Tahiri, A.; Polizzi, G.; Lazraq, A.; Lahlali, R. Potential Role of Rhizobacteria Isolated from Citrus Rhizosphere for Biological Control of Citrus Dry Root Rot. Plants 2021, 10, 872. [Google Scholar] [CrossRef]
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal Volatiles from Bacillus atrophaeus GBSC56 Promote Growth and Stimulate Induced Systemic Resistance in Tomato against Meloidogyne incognita. Int. J. Mol. Sci. 2021, 22, 5049. [Google Scholar] [CrossRef]
- Zubair, M.; Hanif, A.; Farzand, A.; Sheikh, T.M.M.; Khan, A.R.; Suleman, M.; Ayaz, M.; Gao, X. Genetic Screening and Expression Analysis of Psychrophilic Bacillus spp. Reveal Their Potential to Alleviate Cold Stress and Modulate Phytohormones in Wheat. Microorganisms 2019, 7, 337. [Google Scholar] [CrossRef]
- Sutthisa, W.; Hompana, W.; Yutthasin, R. Enhancing Biocontrol Potential: Development and Efficacy Assessment of a Liquid Formulation of Trichoderma Asperellum MSU007 against Sclerotium Rrolfsii. Trends Sci. 2024, 21, 7550. [Google Scholar] [CrossRef]
- Hjeljord, L.; Tronsmo, A. Trichoderma and Gliocladium in biological control: An overview. In Trichoderma & Gliocladium—Enzymes, Biological Control and Commercial Applications; Harman, G.E., Kubicek, C.P., Eds.; Taylor & Francis Ltd.: London, UK, 1998; pp. 131–151. [Google Scholar]
- Minuto, A.; Migheli, Q.; Garibaldi, A. Evaluation of antagonistic strains of Fusarium spp. in the biological and integrated control of Fusarium wilt of cyclamen. Crop Prot. 1995, 14, 221–226. [Google Scholar] [CrossRef]
- Vehapi, M.; İnan, B.; Kayacan-Cakmakoglu, S.; Sagdic, O.; Özçimen, D.B. Preparation of Bacillus pumilus loaded electrosprayed nanoparticles as a plant protective against postharvest fungal decay. Eur. J. Plant Pathol. 2024, 168, 121–136. [Google Scholar] [CrossRef]
- Goulet, F.; Aulagnier, A.; Fouilleux, E. Moving beyond pesticides: Exploring alternatives for a changing food system. Environ. Sci. Policy 2023, 147, 177–187. [Google Scholar] [CrossRef]
- Galluzzo, N. How does eliminating the use of pesticides affect technical efficiency in Italian farms? Bulg. J. Agric. Sci. 2023, 29, 14–23. [Google Scholar]
- Palmisano, T. Narratives and practices of pesticide removal in the Andean valleys of Chile and Argentina. Environ. Sci. Policy 2023, 139, 149–156. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. Fungi in Biocontrol: An Overview of Fungal Antagonists Applied Against Fungal Plant Pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef]
- Sánchez-Cruz, R.; Mehta, R.; Atriztán-Hernández, K.; Martínez-Villamil, O.; del Rayo Sánchez-Carbente, M.; Sánchez-Reyes, A.; Folch-Mallol, J.L. Effects on Capsicum annuum plants colonized with Trichoderma atroviride P. karst strains genetically modified in Taswo1, a gene coding for a protein with Expansin-like activity. Plants 2021, 10, 1919. [Google Scholar] [CrossRef]
- Liu, P.; Luo, L.; Long, C.A. Characterization of competition for nutrients in the biocontrol of Penicillium italicum by Kloeckera apiculata. Biol. Control 2013, 67, 157–162. [Google Scholar] [CrossRef]
- Ren, X.; Branà, M.T.; Haidukowski, M.; Gallo, A.; Zhang, Q.; Logrieco, A.F.; Altomare, C. Potential of Trichoderma spp. for biocontrol of aflatoxin-producing Aspergillus flavus. Toxins 2022, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Matarese, F.; Sarrocco, S.; Gruber, S.; Seidl-Seiboth, V.; Vannacci, G. Biocontrol of Fusarium head blight: Interactions between Trichoderma and mycotoxigenic Fusarium. Microbiology 2012, 158, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Mubeen, M.; Zheng, H.; Sohail, M.A.; Shakeel, Q.; Solanki, M.K.; Zhou, L. Trichoderma spp. genes involved in the biocontrol activity against Rhizoctonia solani. Front. Microbiol. 2022, 13, 884469. [Google Scholar] [CrossRef] [PubMed]
- Manzar, N.; Singh, Y.; Kashyap, A.S.; Sahu, P.K.; Rajawat, M.V.S.; Bhowmik, A.; Saxena, A.K. Biocontrol potential of native Trichoderma spp. against anthracnose of great millet (Sorghum bicolor L.) from Tarai and hill regions of India. Biol. Control 2021, 152, 104474. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Garrido-Jurado, I.; Yousef-Yousef, M.; González-Mas, N. Multitrophic Interactions of Entomopathogenic Fungi. Biol. Control 2022, 67, 457–472. [Google Scholar]
- Manzar, N.; Kashyap, A.S.; Goutam, R.S.; Rajawat, M.V.S.; Sharma, P.K.; Sharma, S.K.; Singh, H.V. Trichoderma: Advent of Versatile Biocontrol Agent, Its Secrets and Insights into Mechanism of Biocontrol Potential. Sustainability 2022, 14, 12786. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol. Control 2018, 117, 147–157. [Google Scholar] [CrossRef]
- Persoon, C.H. Disposita methodical fungorum. Romers. Neues. Mag. Bot. 1794, 1, 81–128. [Google Scholar]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)–prospects and challenges. Biocontrol Sci. Technol. 2019, 29, 207–228. [Google Scholar] [CrossRef]
- El-Hasan, A.; Walker, F.; Schone, J.; Buchenauer, H. Detection of viridiofungin A and another antifungal metabolites excreted by Trichoderma harzianum active against different plant pathogens. Eur. J. Plant Pathol. 2009, 124, 457–470. [Google Scholar] [CrossRef]
- Kashyap, A.S.; Manzar, N.; Rajawat, M.V.S.; Kesharwani, A.K.; Singh, R.P.; Dubey, S.C.; Pattanayak, D.; Dhar, S.; Lal, S.K.; Singh, D. Screening and Biocontrol Potential of Rhizobacteria Native to Gangetic Plains and Hilly Regions to Induce Systemic Resistance and Promote Plant Growth in Chilli against Bacterial Wilt Disease. Plants 2021, 10, 2125. [Google Scholar] [CrossRef] [PubMed]
- del Carmen, H.; Rodríguez, M.; Evans, H.C.; de Abreu, L.M.; de Macedo, D.M.; Ndacnou, M.K.; Bekele, K.B.; Barreto, R.W. New species and records of Trichoderma isolated as mycoparasites and endophytes from cultivated and wild coffee in Africa. Sci. Rep. 2021, 11, 5671. [Google Scholar] [CrossRef]
- Sundheim, L. Control of Cucumber Powdery Mildew by the Hyperparasite Ampelomyces quisqualis and Fungicides. Plant Pathol. 1982, 31, 209–214. [Google Scholar] [CrossRef]
- Gurung, M. Evaluation of Trichoderma asperellum as Biocontrol Agent for Phytophthora Foot and Root Rot of Citrus. Master’s Thesis, Texas A&M University, Kingsville, TX, USA, 2018. [Google Scholar]
- Contina, J.B. Biological Control of Fusarium solani, Rhizoctonia solani and the Pale Cyst Nematode Globodera pallida with Trichoderma harzianum ThzID1; University of Idaho: Pocatello, ID, USA, 2016. [Google Scholar]
- Di Francesco, A.; Martini, C.; Mari, M. Biological control of postharvest diseases by microbial antagonists: How many mechanisms of action? Eur. J. Plant Pathol. 2016, 145, 711–717. [Google Scholar] [CrossRef]
- Jaihan, P.; Sangdee, K.; Sangdee, A. Disease suppressive activity of extracts from entomopathogenic fungus Ophiocordyceps sobolifera against chili anthracnose fungi Colletotrichum spp. in a pot experiment. J. Gen. Plant Pathol. 2018, 84, 237–242. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.; Abdel-Hafez, S.I.; Abdel-Rahim, I.R. Isolation of Trichoderma and evaluation of their antagonistic potential against Alternaria porri. J. Phytopathol. 2014, 162, 567–574. [Google Scholar] [CrossRef]
- Kalimutu, P.K.; Mahardika, I.B.K.; Sagung, P.R.A.A.A. Antagonism Test of Trichoderma atroviride and Gliocladium sp. Bali Local Isolates As a Disease Control of Blendok Disease (Botryodiplodia theobromae) in Grapefruit (Citrus grandis L. Osbeck). Sustain. Environ. Agric. Sci. 2020, 4, 102–110. [Google Scholar] [CrossRef]
- Sherkhane, P.D.; Bansal, R.; Banerjee, K.; Chatterjee, S.; Oulkar, D.; Jain, P.; Mukherjee, P.K. Genomics-Driven Discovery of the Gliovirin Biosynthesis Gene Cluster in the Plant Beneficial Fungus Trichoderma Virens. ChemSelect 2017, 2, 3347–3352. [Google Scholar] [CrossRef]
- Hua, L.; Zeng, H.; He, L.; Jiang, Q.; Ye, P.; Liu, Y.; Zhang, M. Gliotoxin Is an Important Secondary Metabolite Involved in Suppression of Sclerotium rolfsii of Trichoderma virens T23. Phytopathology 2021, 111, 1720–1725. [Google Scholar] [CrossRef]
- Boureghda, H.; Bouznad, Z. Biological control of Fusarium wilt of chickpea using isolates of Trichoderma atroviride, T. harzianum and T. longibrachiatum. Acta Phytopathol. Entomol. Hung. 2009, 44, 25–38. [Google Scholar] [CrossRef]
- Tsai, C.C.; Tzeng, D.S.; Hsieh, S.P.Y. Biological control of Fusarium stem rot of Anoectochilus formosanus Hayata by Trichoderma asperellum TA strain. Plant Pathol. Bull. 2008, 17, 243–254. [Google Scholar]
- Schubert, M.; Fink, S.; Schwarze, F.W.M.R. Field experiments to evaluate the application of Trichoderma strain (T.15603.1) for biological control of wood decay fungi in trees. Part II. Arboric. J. 2008, 31, 249–268. [Google Scholar] [CrossRef]
- Waqas, M.; Khana, A.L.; Hamayuna, M.; Shahzad, R.; Kang, S.M.; Kim, J.G.; Lee, I.J. Endophytic Fungi Promote Plant Growth and Mitigate the Adverse Effects of Stem Rot: An Example of Penicillium citrinum and Aspergillus terreus. J. Plant Interact. 2015, 10, 280–287. [Google Scholar] [CrossRef]
- Gressel, J. Four pillars are required to support a successful biocontrol fungus. Pest Manag. Sci. 2024, 80, 5–39. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Álvarez, P.; Fernández-González, R.A.; Sanz-Ros, A.V.; Pando, V.; Diez, J.J. Two fungal endophytes reduce the severity of pitch canker disease in Pinus radiata seedlings. Biol. Control 2016, 94, 1–10. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; AbuQamar, S.F. Plant Growth-Promoting Microorganisms as Biocontrol Agents of Plant Diseases: Mechanisms, Challenges and Future Perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef]
- Kumari, N.; Srividhya, S. Secondary Metabolites and Lytic Tool Box of Trichoderma and Their Role in Plant Health. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Academic Press: Cambridge, MA, USA, 2020; pp. 305–320. [Google Scholar]
- Segarra, G.; Casanova, E.; Avilés, M.; Trillas, I. Trichoderma asperellum Strain T34 Controls Fusarium Wilt Disease in Tomato Plants in Soilless Culture through Competition for Iron. Microb. Ecol. 2010, 59, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Nigro, M.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Lorito, M. Harzianic Acid: A Novel Siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129. [Google Scholar] [CrossRef]
- Yan, L.; Khan, R.A.A. Biological Control of Bacterial Wilt in Tomato through the Metabolites Produced by the Biocontrol Fungus, Trichoderma harzianum. Egypt. J. Biol. Pest Control 2021, 31, 1–9. [Google Scholar]
- Konappa, N.; Krishnamurthy, S.; Siddaiah, C.N.; Ramachandrappa, N.S.; Chowdappa, S. Evaluation of biological efficacy of Trichoderma asperellum against tomato bacterial wilt caused by Ralstonia solanacearum. Egypt J. Biol. Pest Control 2018, 28, 63. [Google Scholar] [CrossRef]
- Choi, G.J.; Kim, J.C.; Jang, K.S.; Cho, K.Y.; Kim, H.T. Mycoparasitism of Acremonium strictum BCP on Botrytis cinerea, the Gray Mold Pathogen. J. Microbiol. Biotechnol. 2008, 18, 167–170. [Google Scholar] [PubMed]
- Fuchs, J.G.; Moënne-Loccoz, Y.; Défago, G. Nonpathogenic Fusarium oxysporum strain Fo47 induces resistance to Fusarium wilt in tomato. Plant Dis. 1997, 81, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Y.; Sariah, M.; Ismail, M.R.; Ali, A. Trichoderma.fortified compost extracts for the control of choanephora wet rot in okra production. Crop Prot. 2008, 27, 385–390. [Google Scholar] [CrossRef]
- Kowalska, J. Effects of Trichoderma asperellum [T1] on Botrytis cinerea [Pers.: Fr.], growth and yield of organic strawberry. Acta Scientiarum Polonorum. Hortorum Cultus 2011, 10, 107–114. [Google Scholar]
- Djonovic, S.; Vargas, W.A.; Kolomiets, M.V.; Horndeski, M.; Wiest, A.; Kenerley, C.M. A proteinaceous elicitor Sm1 from the beneficial fungus Trichoderma virens is required for induced systemic resistance in maize. Plant Physiol. 2007, 145, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.M.; Al-Masri, M.I. Effect of Trichoderma harzianum in combination with fungicides in controlling gray mould disease (Botrytis cinerea) of strawberry. Am. J. Plant Sci. 2017, 8, 651–665. [Google Scholar] [CrossRef]
- Sallam, N.; Eraky, A.M.I.; Sallam, A. Effect of Trichoderma spp. on Fusarium wilt disease of tomato. Mol. Biol. Rep. 2019, 46, 4463–4470. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Urbano, M.; Goicoechea, N.; Velasco, P.; Poveda, J. Development of agricultural bio-inoculants based on mycorrhizal fungi and endophytic filamentous fungi: Co-inoculants for improve plant-physiological responses in sustainable agriculture. Biol. Control 2023, 182, 105223. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, F.; Zhang, Y.; Zhang, J. Involvement of Trichoderma asperellum strain T6 in regulating iron acquisition in plants. J. Basic Microbiol. 2014, 54, S115–S124. [Google Scholar] [CrossRef]
- Půža, V.; Tarasco, E. Interactions between entomopathogenic fungi and entomopathogenic nematodes. Microorganisms 2023, 11, 163. [Google Scholar] [CrossRef]
- Intana, W.; Wonglom, P.; Suwannarach, N.; Sunpapao, A. Trichoderma asperelloides PSU-P1 Induced Expression of Pathogenesis-Related Protein Genes against Gummy Stem Blight of Muskmelon (Cucumis melo) in Field Evaluation. J. Fungi 2022, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Gezgin, Y.; Maral Gül, D.; Sözer Şenşatar, S.; Kara, C.U.; Sargın, S.; Sukan, F.V.; Eltem, R. Evaluation of Trichoderma atroviride and Trichoderma citrinoviride growth profiles and their potentials as biocontrol agent and biofertilizer. Turk. J. Biochem. 2020, 45, 163–175. [Google Scholar] [CrossRef]
- Zhang, F.; Ge, H.; Zhang, F.; Guo, N.; Wang, Y.; Chen, L.; Li, C. Biocontrol potential of Trichoderma harzianum isolate T-aloe against Sclerotinia sclerotiorum in soybean. Plant Physiol. Biochem. 2016, 100, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.H.; Javaid, A. In Vitro Biocontrol Potential of Trichoderma pseudokoningii against Macrophomina phaseolina. Int. J. Agric. Biol. 2020, 24, 730–736. [Google Scholar]
- Chen, K.; Zhuang, W.Y. Three new soil-inhabiting species of Trichoderma in the Stromaticum clade with test of their antagonism to pathogens. Curr. Microbiol. 2017, 74, 1049–1060. [Google Scholar] [CrossRef]
- Degani, O.; Rabinovitz, O.; Becher, P.; Gordani, A.; Chen, A. Trichoderma longibrachiatum and Trichoderma asperellum confer growth promotion and protection against late wilt disease in the field. J. Fungi 2021, 7, 444. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Ma, Y.; Guo, Q.; Xu, B. Biological weed control using Trichoderma polysporum strain HZ-31. Crop Prot. 2020, 134, 105161. [Google Scholar] [CrossRef]
- Hinterdobler, W.; Li, G.; Spiegel, K.; Basyouni-Khamis, S.; Gorfer, M.; Schmoll, M. Trichoderma reesei Isolated from Austrian Soil with High Potential for Biotechnological Application. Front. Microbiol. 2021, 12, 552301. [Google Scholar] [CrossRef] [PubMed]
- Hyder, S.; Inam-ul-Haq, M.; Bibi, S.; Humayun, A.; Ghuffar, S.; Iqbal, S. Novel Potential of Trichoderma spp. as Biocontrol Agent. J. Entomol. Zool. Stud. 2017, 5, 214–222. [Google Scholar]
- Kumar, P.; Misra, A.K.; Modi, D.R.; Gupta, V.K. Biocontrol Potential of Trichoderma Species against Mango Malformation Pathogens. Arch. Phytopathol. Plant Prot. 2012, 45, 1237–1245. [Google Scholar] [CrossRef]
- El-Shennawy, M.Z.; Khalifa, E.Z.; Ammar, M.M.; Mousa, E.M.; Hafez, S.L. Biological Control of the Disease Complex on Potato Caused by Root-Knot Nematode and Fusarium Wilt Fungus. Nematol. Mediterr. 2012, 40, 169–172. [Google Scholar]
- Hossain, M.M.; Sultana, F.; Islam, S. Plant Growth-Promoting Fungi (PGPF): Phytostimulation and Induced Systemic Resistance. In Plant-Microbe Interactions in Agro-Ecological Perspectives: Volume 2: Microbial Interactions and Agro-Ecological Impacts; Springer: Singapore, 2017; pp. 135–191. [Google Scholar]
- Patel, Z.M.; Mahapatra, R.; Jampala, S.S.M. Role of Fungal Elicitors in Plant Defense Mechanism. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Academic Press: Cambridge, MA, USA, 2020; pp. 143–158. [Google Scholar]
- Elsharkawy, M.M. Suppression of Potato Virus Y Infection in Tobacco by Plant Growth Promoting Fungi. Egypt. J. Biol. Pest Control 2016, 26, 695–700. [Google Scholar]
- De Meyer, G.; Bigirimana, J.; Elad, Y.; Hofte, M. Induced systemic resistance in Trichoderma harzianum T39 biocontrol of Botrytis cinerea. Eur. J. Plant Pathol. 1998, 104, 279–286. [Google Scholar] [CrossRef]
- Yedidia, I.; Benhamou, N.; Chet, I. Induction of Defense Responses in Cucumber Plants (Cucumis sativus L.) by the Biocontrol Agent Trichoderma harzianum. Appl. Environ. Microbiol. 1999, 65, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- El-Hasan, A.; Ngatia, G.; Link, T.I.; Voegele, R.T. Isolation, Identification, and Biocontrol Potential of Root Fungal Endophytes Associated with Solanaceous Plants against Potato Late Blight (Phytophthora infestans). Plants 2022, 11, 1605. [Google Scholar] [CrossRef] [PubMed]
- Malviya, D.; Thosar, R.; Kokare, N.; Pawar, S.; Singh, U.B.; Saha, S.; Rai, J.P.; Singh, H.V.; Somkuwar, R.G.; Saxena, A.K. A comparative analysis of microbe-based technologies developed at ICAR-NBAIM against Erysiphe necator causing powdery mildew disease in grapes (Vitis vinifera L.). Front. Microbiol. 2022, 13, 871901. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.; Sánchez, C.P.; Candela, M.E. Evaluation of Induction of Systemic Resistance in Pepper Plants (Capsicum annuum) to Phytophthora capsici using Trichoderma harzianum and its Relation with Capsidiol Accumulation. Eur. J. Plant Pathol. 2000, 106, 817–824. [Google Scholar] [CrossRef]
- Santos, M.; Diánez, F.; Sánchez-Montesinos, B.; Huertas, V.; Moreno-Gavira, A.; Esteban García, B.; Garrido-Cárdenas, J.A.; Gea, F.J. Biocontrol of Diseases Caused by Phytophthora capsici and P. parasitica in Pepper Plants. J. Fungi 2023, 9, 360. [Google Scholar] [CrossRef] [PubMed]
- Segarra, G.; Avilés, M.; Casanova, E.; Borrero, A.; Trillas, I. Effectiveness of biological control of Phytophthora capsici in pepper by Trichoderma asperellum strain T34. Phytopathol. Mediterr. 2013, 52, 77–83. [Google Scholar]
- Adikaram, N.K.; Joyce, D.C.; Terryc, L.A. Biocontrol activity and induced resistance as a possible mode of action for Aureobasidium pullulans against grey mould of strawberry fruit. Australas. Plant Pathol. 2002, 31, 223–229. [Google Scholar] [CrossRef]
- Iqbal, M.; Andreasson, E.; Stenberg, J.A. Biological control of strawberry diseases by Aureobasidium pullulans and sugar beet extract under field conditions. J. Plant Pathol. 2023, 105, 933–941. [Google Scholar] [CrossRef]
- Ippolito, A.; Nigro, F.; Romanazzi, G.; Campanella, V. Field application of Aureobasidium pullulans against Botrytis storage rot of strawberry. In Non Conventional Methods for the Control of Post-Harvest Disease and Microbiological Spoilage; Workshop Proceedings; COST: Brussels, Belgium, 1997; pp. 127–133. [Google Scholar]
- Iqbal, M.; Jamshaid, M.; Zahid, M.A.; Andreasson, E.; Vetukuri, R.R.; Stenberg, J.A. Biological control of strawberry crown rot, root rot and grey mould by the beneficial fungus Aureobasidium pullulans. BioControl 2021, 66, 535–545. [Google Scholar] [CrossRef]
- Arras, G. Mode of action of an isolate of Candida famata in biological control of Penicillium digitatum in orange fruits. Postharvest Biol. Technol. 1996, 8, 191–198. [Google Scholar] [CrossRef]
- Droby, S.; Hofstein, R.; Wilson, C.L.; Wisniewski, M.; Fridlender, B.; Cohen, L.; Weiss, B.; Daus, A.; Timar, D.; Chalutz, E. Pilot Testing of Pichia guilliermondii: A Biocontrol Agent of Postharvest Diseases of Citrus Fruit. Biol. Control 1993, 3, 47–52. [Google Scholar] [CrossRef]
- Lahlali, R.; Hamadi, Y.; El Guilli, M.; Jijakli, M.H. Efficacy assessment of Pichia guilliermondii strain Z1, a new biocontrol agent, against citrus blue mould in Morocco under the influence of temperature and relative humidity. Biol. Control 2011, 56, 217–224. [Google Scholar] [CrossRef]
- Torres, D.E.; Rojas-Martínez, R.I.; Zavaleta-Mejía, E.; Guevara-Fefer, P.; Márquez-Guzmán, G.J.; Pérez-Martínez, C. Cladosporium cladosporioides and Cladosporium pseudocladosporioides as potential new fungal antagonists of Puccinia horiana Henn., the causal agent of chrysanthemum white rust. PLoS ONE 2017, 12, 0170782. [Google Scholar] [CrossRef] [PubMed]
- Demirci, F. Effects of Pseudomonas fluorescens and Candida famata on blue mould of citrus caused by Penicillium italicum. Aust. J. Crop Sci. 2011, 5, 341–346. [Google Scholar]
- Bandara, A.Y.; Kang, S. Trichoderma application methods differentially affect the tomato growth, rhizomicrobiome, and rhizosphere soil suppressiveness against Fusarium oxysporum. Front. Microbiol. 2024, 15, 1366690. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Ucko, O.; Chet, I. Biological control of Fusarium crown rot of tomato by Trichoderma harzianum under field conditions. Plant Dis. 1987, 71, 587–592. [Google Scholar] [CrossRef]
- Kexiang, G.; Xiaoguang, L.; Yonghong, L.; Tianbo, Z.; Shuliang, W. Potential of Trichoderma harzianum and T. atroviride to control Botryosphaeria berengeriana f. sp. piricola, the cause of apple ring rot. J. Phytopathol. 2002, 150, 271–276. [Google Scholar] [CrossRef]
- Bunker, R.N.; Kusum Mathur, K.M. Antagonism of local biocontrol agents to Rhizoctonia solani inciting dry root rot of chilli. J. Mycol. Plant Pathol. 2001, 31, 50–53. [Google Scholar]
- Trutmann, P.; Keane, P.J. Trichoderma koningii as a biological control agent for Sclerotinia sclerotiorum in Southern Australia. Soil Biol. Biochem. 1990, 22, 43–50. [Google Scholar] [CrossRef]
- Camacho-Luna, V.; Pizar-Quiroz, A.M.; Rodríguez-Hernández, A.A.; Rodríguez-Monroy, M.; Sepúlveda-Jiménez, G. Trichoderma longibrachiatum, a biological control agent of Sclerotium cepivorum on onion plants under salt stress. Biol. Control 2023, 180, 105168. [Google Scholar] [CrossRef]
- El-Komy, M.H.; Al-Qahtani, R.M.; Ibrahim, Y.E.; Almasrahi, A.A.; Al-Saleh, M.A. Soil application of Trichoderma asperellum strains significantly improves Fusarium root and stem rot disease management and promotes growth in cucumbers in semi-arid regions. Eur. J. Plant Pathol. 2022, 162, 637–653. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zhang, R.; Duan, Y.N.; Jiang, W.T.; Chen, X.S.; Shen, X.; Yin, C.M.; Mao, Z.Q. The endophytic strain Trichoderma asperellum 6S-2: An efficient biocontrol agent against apple replant disease in China and a potential plant-growth-promoting fungus. J. Fungi 2021, 7, 1050. [Google Scholar] [CrossRef] [PubMed]
- John, R.P.; Tyagi, R.D.; Prévost, D.; Brar, S.K.; Pouleur, S.; Surampalli, R.Y. Mycoparasitic Trichoderma viride as a biocontrol agent against Fusarium oxysporum f. sp. adzuki and Pythium arrhenomanes and as a growth promoter of soybean. Crop Prot. 2010, 29, 1452–1459. [Google Scholar] [CrossRef]
- Zegeye, E.D.; Santhanam, A.; Gorfu, D.; Tessera, M.; Kassa, B. Biocontrol activity of Trichoderma viride and Pseudomonas fluorescens against Phytophthora infestans under greenhouse conditions. J. Agric. Technol. 2011, 7, 1589–1602. [Google Scholar]
- Zaharia, R.; Petrisor, C.; Fatu, V.; Leveanu, I.; Botea, M.C.; Chireceanu, C. Biocontrol potential of Trichoderma viride against main phytopathogenic fungi associated with Capsicum peppers cultivated in IPM system. In Proceedings of the IX South-Eastern Europe Symposium on Vegetables and Potatoes, Bucharest, Romania, 5–9 September 2023; ISHS: Leuven, Belgium, 2023; Volume 1391, pp. 401–406. [Google Scholar]
- Djonovic, S.; Pozo, M.J.; Kenerley, C.M. Tvbgn3, a β-1, 6-glucanase from the biocontrol fungus Trichoderma virens, is involved in mycoparasitism and control of Pythium ultimum. Appl. Environ. Microbiol. 2006, 72, 7661–7670. [Google Scholar] [CrossRef]
- Etebarian, H.R.; Scott, E.S.; Wicks, T.J. Trichoderma harzianum T39 and T. virens DAR 74290 as potential biological control agents for Phytophthora erythroseptica. Eur. J. Plant Pathol. 2000, 106, 329–337. [Google Scholar] [CrossRef]
- Galli, V.; Romboli, Y.; Barbato, D.; Mari, E.; Venturi, M.; Guerrini, S.; Granchi, L. Indigenous Aureobasidium pullulans Strains as Biocontrol Agents of Botrytis cinerea on Grape Berries. Sustainability 2021, 13, 9389. [Google Scholar] [CrossRef]
- Taping, J.M.F.; Borja, B.T.; Bretaña, B.L.P.; Tanabe, M.E.N.; Cabasan, M.T.N. Fungal endophytes as potential biocontrol agent of Panama disease of banana. Egypt. J. Biol. Pest Control 2023, 33, 84. [Google Scholar] [CrossRef]
- Aggarwal, R. Chaetomium globosum: A potential biocontrol agent and its mechanism of action. Indian Phytopathol. 2015, 68, 8–24. [Google Scholar]
- Shanthiyaa, V.; Saravanakumar, D.; Rajendran, L.; Karthikeyan, G.; Prabakar, K.; Raguchander, T. Use of Chaetomium globosum for biocontrol of potato late blight disease. Crop Prot. 2013, 52, 33–38. [Google Scholar] [CrossRef]
- Di Pietro, A.; Gut-Rella, M.; Pachlatko, J.P.; Schwinn, F.J. Role of antibiotics produced by Chaetomium globosum in biocontrol of Pythium ultimum, a causal agent of damping-off. Phytopathology 1992, 82, 131–135. [Google Scholar] [CrossRef]
- Alexander, B.J.R.; Stewart, A. Glasshouse screening for biological control agents of Phytophthora cactorum on apple (Malus domestica). N. Z. J. Crop Hortic. Sci. 2001, 29, 159–169. [Google Scholar] [CrossRef]
- Utkhede, R.S. Biological Control of Apple Crown Rot and Replant Disease. In Biological Control of Plant Diseases; Tjamos, E.C., Papavizas, G.C., Cook, R.J., Eds.; NATO ASI Series; Springer: Boston, MA, USA, 1992; Volume 230, pp. 410–415. [Google Scholar]
- Nakayama, T.; Sayama, M. Suppression of potato powdery scab caused by Spongospora subterranea using an antagonistic fungus Aspergillus versicolor isolated from potato roots [Conference poster]. In Proceedings of the Ninth Symposium of the International Working Group on Plant Viruses with Fungal Vectors, Obihiro, Japan, 19–22 August 2013. [Google Scholar]
- Chin-A-Woeng, T.F.; Bloemberg, G.V.; Lugtenberg, B.J.J. Phenazines and their role in biocontrol by Pseudomonas bacteria. New Phytol. 2003, 157, 503–523. [Google Scholar] [CrossRef] [PubMed]
- Mouloud, G.; Daoud, H.; Bassem, J.; Laribi Atef, I.; Hani, B. New bacteriocin from Bacillus clausii strainGM17: Purification, characterization, and biological activity. Appl. Biochem. Biotechnol. 2013, 171, 2186–2200. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as Biological Control Agents of Plant Diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef]
- Legein, M.; Smets, W.; Vandenheuvel, D.; Eilers, T.; Muyshondt, B.; Prinsen, E.; Samson, R.; Lebeer, S. Modes of action of microbial biocontrol in the phyllosphere. Front. Microbiol. 2020, 11, 1619. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Kang, Y.C.; Lee, J.K. Quorum sensing inhibitors as antipathogens: Biotechnological applications. Biotechnol. Adv. 2019, 37, 68–90. [Google Scholar] [CrossRef]
- Savini, V.; Fazii, P. Bacillus thuringiensis insecticide properties. In The Diverse Faces of Bacillus Cereus; Academic Press: Cambridge, MA, USA, 2016; pp. 139–155. [Google Scholar]
- Lobanovska, M.; Pilla, G. Focus: Drug development: Penicillin’s discovery and antibiotic resistance: Lessons for the future? Yale J. Biol. Med. 2017, 90, 135. [Google Scholar]
- Ongena, M.; Jourdan, E.; Adam, A.; Paquot, M.; Brans, A.; Joris, B.; Arpigny, J.L.; Thonart, P. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ. Microbiol. 2007, 9, 1084–1090. [Google Scholar] [CrossRef]
- Farag, M.A.; Zhang, H.; Ryu, C.M. Dynamic chemical communication between plants and bacteria through airborne signals: Induced resistance by bacterial volatiles. J. Chem. Ecol. 2013, 39, 1007–1018. [Google Scholar] [CrossRef]
- Raza, W.; Ling, N.; Liu, D.; Wei, Z.; Huang, Q.; Shen, Q. Volatile organic compounds produced by Pseudomonas fluorescens WR-1 restrict the growth and virulence traits of Ralstonia solanacearum. Microbiol. Res. 2016, 192, 103–113. [Google Scholar] [CrossRef]
- Kamilova, F.; Validov, S.; Azarova, T.; Mulders, I.; Lugtenberg, B. Enrichment for enhanced competitive plant root tip colonizers selects for a new class of biocontrol bacteria. Environ. Microbiol. 2005, 7, 1809–1817. [Google Scholar] [CrossRef]
- Schuhegger, R.; Ihring, A.; Gantner, S.; Bahnweg, G.; Knappe, C.; Vogg, G.; Hutzler, P.; Schmid, M.; Van Breusegem, F.; Eberl, L.; et al. Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ. 2006, 29, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tan, Y.; Peng, Q.; Xiao, Y.; Xie, J.; Li, Z.; Ding, H.; Pan, H.; Wei, L. Biocontrol potential of endophytic bacterium Bacillus altitudinis GS-16 against tea anthracnose caused by Colletotrichum gloeosporioides. Peer J. 2024, 12, 16761. [Google Scholar] [CrossRef]
- Gupta, R.; Keppanan, R.; Leibman-Markus, M.; Matveev, S.; Rav-David, D.; Shulhani, R.; Elad, Y.; Ment, D.; Bar, M. Bacillus thuringiensis promotes systemic immunity in tomato, controlling pests and pathogens and promoting yield. Food Secur. 2024, 16, 675–690. [Google Scholar] [CrossRef]
- Heo, Y.; Lee, Y.; Balaraju, K.; Jeon, Y. Characterization and evaluation of Bacillus subtilis GYUN-2311 as a biocontrol agent against Colletotrichum spp. on apple and hot pepper in Korea. Front Microbiol. 2024, 14, 1322641. [Google Scholar] [CrossRef]
- Wockenfuss, A.; Chan, K.; Cooper, J.G.; Chaya, T.; Mauriello, M.A.; Yannarell, S.M.; Maresca, J.A.; Donofrio, N.M. A Bacillus velezensis strain shows antimicrobial activity against soilborne and foliar fungi and oomycetes. Front Fung Biol. 2024, 5, 1332755. [Google Scholar] [CrossRef]
- Sumera Yasmin, S.Y.; Hafeez, F.Y.; Mirza, M.S.; Rasul, M.; Arshad, H.M.I.; Muhammad Zubair, M.Z.; Mazhar Iqbal, M.I. Biocontrol of Bacterial Leaf Blight of rice and profiling of secondary metabolites produced by rhizospheric Pseudomonas aeruginosa BRp3. Front. Microbiol. 2017, 8, 1895. [Google Scholar]
- Xie, J.; Singh, P.; Qi, Y.; Singh, R.K.; Qin, Q.; Jin, C.; Wang, B.; Fang, W. Pseudomonas aeruginosa Strain 91: A Multifaceted Biocontrol Agent against Banana Fusarium Wilt. J. Fungi 2023, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, X.; Cai, Z.; Guo, L.; Chen, X.; Chen, X.; Liu, J.; Feng, M.; Qiu, Y.; Zhang, Y.; et al. A biocontrol strain of Pseudomonas aeruginosa CQ-40 promote growth and control Botrytis cinerea in tomato. Pathogens 2020, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Amaradasa, B.S.; Mei, C.; He, Y.; Chretien, R.L.; Doss, M.; Durham, T.; Lowman, S. Biocontrol potential of endophytic Pseudomonas strain IALR1619 against two Pythium species in cucumber and hydroponic lettuce. PLoS ONE 2024, 19, 0298514. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Pi, H.; Chandrangsu, P.; Li, Y.; Wang, Y.; Zhou, H.; Xiong, H.; Helmann, J.D.; Cai, Y. Antagonism of two plant-growth promoting Bacillus velezensis isolates against Ralstonia solanacearum and Fusarium oxysporum. Sci Rep. 2018, 8, 4360. [Google Scholar] [CrossRef]
- Yi, Y.; Luan, P.; Liu, S.; Shan, Y.; Hou, Z.; Zhao, S.; Jia, S.; Li, R. Efficacy of Bacillus subtilis XZ18-3 as a Biocontrol Agent against Rhizoctonia cerealis on Wheat. Agriculture 2022, 12, 258. [Google Scholar] [CrossRef]
- Myers, J.M.; James, T.Y. Mycoviruses. Curr. Biol. 2022, 32, 150–155. [Google Scholar] [CrossRef]
- Di Giallonardo, F.; Holmes, E.C. Viral biocontrol: Grand experiments in disease emergence and evolution. Trends Microbiol. 2015, 23, 83–90. [Google Scholar] [CrossRef]
- Wagemans, J.; Holtappels, D.; Vainio, E.; Rabiey, M.; Marzachì, C.; Herrero, S.; Turina, M. Going viral: Virus-based biological control agents for plant protection. Ann. Rev. Phytopathol. 2022, 60, 21–42. [Google Scholar] [CrossRef]
- Mallmann, W.; Hemstreet, C. Isolation of an inhibitory substance from plants. Agric. Res. 1924, 28, 599–602. [Google Scholar]
- Nakayinga, R.; Makumi, A.; Tumuhaise, V.; Tinzaara, W. Xanthomonas bacteriophages: A review of their biology and biocontrol applications in agriculture. BMC Microbiol. 2021, 21, 291. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.M. Check for Mycoviruses Jillian M. Myers and Timothy Y. James. Evol. Fungi Fungal-Like Org. 2023, 14, 151. [Google Scholar]
- Moscardi, F. Assessment of the application of baculoviruses for control of Lepidoptera. Annu. Rev. Entomol. 1999, 44, 257–289. [Google Scholar] [CrossRef]
- Pechinger, K.; Choo, K.M.; MacDiarmid, R.M.; Harper, S.J.; Ziebell, H. A new era for mild strain crossprotection. Viruses 2019, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Padilla, A.; Rodriguez-Romero, J.; Gomez-Cid, I.; Pacifico, D.; Ayllón, M.A. Novel mycoviruses discovered in the mycovirome of a necrotrophic fungus. mBio 2021, 12, 3705–3720. [Google Scholar] [CrossRef]
- Vainio, E.J.; Hantula, J. Fungal viruses. In Viruses of Microorganisms; Hyman, P., Abedon, S.T., Eds.; Caster Acad.: Norfolk, UK, 2018; pp. 193–209. [Google Scholar]
- Xie, J.; Jiang, D. New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu. Rev. Phytopathol. 2014, 52, 45–68. [Google Scholar] [CrossRef]
- Chen, B.; Chen, C.H.; Bowman, B.H.; Nuss, D.L. Phenotypic changes associated with wild-type and mutant hypovirus RNA transfection of plant pathogenic fungi phylogenetically related to Cryphonectria parasitica. Phytopathology 1996, 86, 301–310. [Google Scholar] [CrossRef]
- van Heerden, S.W.; Geletka, L.M.; Preisig, O.; Nuss, D.L.; Wingfield, B.D.; Wingfield, M.J. Characterization of South African Cryphonectria cubensis isolates infected with a C. parasitica hypovirus. Phytopathology 2001, 91, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Onoue, M.; Kanematsu, S.; Suzaki, K.; Miyanishi, M.; Suzuki, N.; Nuss, D.L.; Yoshida, K. Extending chestnut blight hypovirus host range within diaporthales by biolistic delivery of viral cDNA. Mol. Plant. Microbe Interact. 2002, 15, 780–789. [Google Scholar] [CrossRef]
- Kanematsu, S.; Sasaki, A.; Onoue, M.; Oikawa, Y.; Ito, T. Extending the fungal host range of a partitivirus and a mycoreovirus from Rosellinia necatrix by inoculation of protoplasts with virus particles. Phytopathology 2010, 100, 922–930. [Google Scholar] [CrossRef]
- Xiao, X.; Cheng, J.; Tang, J.; Fu, Y.; Jiang, D.; Baker, T.S.; Ghabrial, S.A.; Xie, J. A novel partitivirus that confers hypovirulence on plant pathogenic fungi. J. Virol. 2014, 88, 10120–10133. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Yu, J.; Son, M.; Lee, Y.W.; Kim, K.H. Transmission of Fusarium boothii mycovirus via protoplast fusion causes hypovirulence in other phytopathogenic fungi. PLoS ONE 2011, 6, e21629. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Haque, M.A.; Barai, H.R.; Istiaq, A.; Kim, J.J. Antibiotic Resistance in Plant Pathogenic Bacteria: Recent Data and Environmental Impact of Unchecked Use and the Potential of Biocontrol Agents as an Eco-Friendly Alternative. Plants 2024, 13, 1135. [Google Scholar] [CrossRef] [PubMed]
- Vu, N.T.; Oh, C.S. Bacteriophage usage for bacterial disease management and diagnosis in plants. Plant Pathol. J. 2020, 36, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Balogh, B.; Jones, J.B.; Momol, M.T.; Olson, S.M.; Obradovic, A. Improved efficacy of newly formulated bacteriophages for management of bacterial spot on tomato. Plant Dis. 2003, 87, 949–954. [Google Scholar] [CrossRef]
- Balogh, B.; Canteros, B.I.; Stall, K.E.; Jones, J.B. Control of citrus canker and citrus bacterial spot with bacteriophages. Plant Dis. 2008, 92, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Meczker, K.; Doemoetoer, D.; Vass, J.; Rakhely, G.; Schneider, G.; Kovacs, T. The genome of the Erwinia amylovora phage PhiEaH1 reveals greater diversity and broadens the applicability of phages for the treatment of fire blight. FEMS Microbiol. Lett. 2014, 350, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Saponari, M.; Loconsole, G.; Cornara, D.; Yokomi, R.K.; De Stradis, A.; Boscia, D.; Bosco, D.; Martelli, G.P.; Krugner, R.; Porcelli, F. Infectivity and transmission of Xylella fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae) in Apulia, Italy. J. Econ. Entomol. 2014, 107, 1316–1319. [Google Scholar] [CrossRef]
- Ahern, S.J.; Das, M.; Bhowmick, T.S.; Young, R.; Gonzalez, C.F. Characterization of novel virulent broad.host.range phages of Xylella fastidiosa and Xanthomonas. J. Bacteriol. 2014, 196, 459–471. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Askora, A.; Kawasaki, T.; Fujie, M.; Yamada, T. The filamentous phage XacF1 causes loss of virulence in Xanthomonas axonopodis pv. citri, the causative agent of citrus canker disease. Front. Microbiol. 2014, 5, 321. [Google Scholar] [CrossRef]
- Álvarez, B.; López, M.M.; Biosca, E.G. Biocontrol of the major plant pathogen Ralstonia solanacearum in irrigation water and host plants by novel waterborne lytic bacteriophages. Front. Microbiol. 2019, 10, 2813. [Google Scholar] [CrossRef]
- Doemoetoer, D.; Becsagh, P.; Rakhely, G.; Schneider, G.; Kovacs, T. Complete genomic sequence of Erwinia amylovora phage PhiEaH2. J. Virol. 2012, 86, 10899. [Google Scholar] [CrossRef] [PubMed]
- Mckinney, H.H. Mosaic diseases in the Canary Islands, West Africa, and Gibraltar. J. Agric. Res. 1929, 39, 557–578. [Google Scholar]
- Agrios, G.N. Plant Pathology; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Gal On, A.; Shiboleth, Y.M. Cross protection. In Natural Resistance Mechanisms of Plants to Viruses; Loebenstein, G., Carr, J.P., Eds.; Springer: Berlin, Germany, 2006; pp. 261–288. [Google Scholar]
- Tomitaka, Y.; Shimomoto, Y.; Ryang, B.S.; Hayashi, K.; Oki, T.; Matsuyama, M.; Sekine, K.T. Development and Application of Attenuated Plant Viruses as Biological Control Agents in Japan. Viruses 2024, 16, 517. [Google Scholar] [CrossRef]
- Salaman, R.N. Protective inoculation against a plant virus. Nature 1933, 131, 468. [Google Scholar] [CrossRef]
- Holmes, F.O. A masked strain of tobacco mosaic virus. Phytopathology 1934, 24, 845–873. [Google Scholar]
- Grant, T.J.; Costa, A.S. A mild strain of tristeza virus of citurs. Phytopathology 1951, 41, 114–122. [Google Scholar]
- Posnette, A.F.; Todd, J.M. Viruse diseases of Cacao in West Africa IX. Strain variation and interference in virus 1A. Ann. Appl. Biol. 1955, 43, 433–453. [Google Scholar] [CrossRef]
- Aguero, J.; Gomez-Aix, C.; Sempere, R.N.; Garcia-Villalba, J.; Garcia-Nunez, J. Stable and broad spectrum cross-protection against Pepino mosaic virus attained by mixed infection. Front. Plant Sci. 2018, 9, 1810. [Google Scholar] [CrossRef]
- Yeh, S.D.; Gonsalves, D.; Wang, H.L.; Namba, R.; Chiu, R.J. Control of papaya ringspot virus by cross protection. Plant Dis. 1988, 72, 375–380. [Google Scholar] [CrossRef]
- Folimonova, S.Y. Developing an understanding of cross-protection by Citrus tristeza virus. Front. Microbiol. 2013, 4, 76. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.; Breytenbach, J.H.; Steyn, C.; de Bruyn, R.; van Vuuren, S.P.; Burger, J.T.; Maree, H.J. Grapefruit field trial evaluation of Citrus tristeza virus T68-strain sources. Plant Dis. 2021, 105, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Fravel, D.R.; Rhodes, D.J.; Larkin, R.P. Production and commercialization of biocontrol products. In Integrated Pest and Disease Management in Greenhouse Crops; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 365–376. [Google Scholar]
- Nunes, C. Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 2012, 133, 181–196. [Google Scholar] [CrossRef]
- Whipps, J.M. Developments in the biological control for soilborne plant pathogens. Adv. Bot. Res. 1997, 26, 1–134. [Google Scholar]
- Larena, I.; De Cal, A.; Melgarejo, P. Solid substrate production of Epicoccum nigrum conidia for biological control of brown rot on stone fruits. Int. J. Food Microbiol. 2004, 94, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.D.; Rangeshwaran, R.; Anuroop, C.P.; Phanikumar, P.R. Bioefficacy and shelf life of conidial and chlamydospore formulations of Trichoderma harzianum Rifai. J. Biol. Control 2002, 16, 145–148. [Google Scholar]
- Jeyarajan, R. Prospects of indigenous mass production and formulation of Trichoderma. In Current Status of Biological Control of Plant Diseases Using Antagonistic Organisms in India, Proceedings of the Group Meeting on Antagonistic Organisms in Plant Disease Management Held at Project Directorate of Biological Control, Bangalore, India, 10–11 July 2003; Project Directorate of Biological Control, Indian Council of Agricultural Research: Bangalore, India, 2006; pp. 10–11. [Google Scholar]
- Angeli, D.; Saharan, K.; Segarra, G.; Sicher, C.; Pertot, I. Production of Ampelomyces quisqualis conidia in submerged fermentation and improvements in the formualtion for increases shelf-lefe. Crop Prot. 2017, 97, 135–144. [Google Scholar] [CrossRef]
- Carbó, A.; Torres, R.; Usall, J.; Ballesta, J.; Teixidó, N. Biocontrol potential of Ampelomyces quisqualis strain CPA-9 against powdery mildew: Conidia production in liquid medium and efficacy on zucchini leaves. Sci. Hortic. 2020, 267, 109337. [Google Scholar] [CrossRef]
- Hynes, R.K.; Boyetchko, S.M. Research initiatives in the art and science of biopesticide formulations. Soil Biol. Biochem. 2006, 38, 845–849. [Google Scholar] [CrossRef]
- Gotor-Vila, A.; Usall, J.; Torres, R.; Abadias, M.; Teixidó, N. Formulation of the biocontrol agent Bacillus amyloliquefaciens CPA-8 using different approaches: Liquid, freeze-drying and fluid-bed spray-drying. BioControl 2017, 62, 545–555. [Google Scholar] [CrossRef]
- Keswani, C.; Bisen, K.; Singh, V.; Sarma, B.K.; Singh, H.B. Formulation technology of biocontrol agents: Present status and future prospects. In Bioformulations: For Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 35–52. [Google Scholar]
- Fravel, D.R.; Connick, W.J.; Lewis, J.A. Formulation of microorganisms to control plant diseases. In Formulation of Microbial Biopesticides; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 187–202. [Google Scholar]
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M.H. The science, development, and commercialization of postharvest biocontrol products. Postharvest Biol. Technol. 2016, 122, 22–29. [Google Scholar] [CrossRef]
- Usall, J.; Torres, R.; Teixidó, N. Biological control of postharvest diseases on fruit: A suitable alternative. Curr. Opin. Food Sci. 2016, 11, 51–55. [Google Scholar] [CrossRef]
- Bejarano, A.; Puopolo, G. Bioformulation of microbial biocontrol agents for a sustainable agriculture. In How Research Can Stimulate the Development of Commercial Biological Control against Plant Diseases; Springer: Berlin/Heidelberg, Germany, 2020; pp. 275–293. [Google Scholar]
- Brar, S.K.; Verma, M.; Tyagi, R.G.; Valéro, J.R. Recent advances in dowstream processing and formulations of Bacillus thuringiensis based biopestices. Process Biochem. 2006, 41, 323–342. [Google Scholar] [CrossRef]
- Batta, Y.A. Postharvest biological control of apple gray mold by Trichoderma harzianum Rifai formulated in an invert emulsion. Crop Prot. 2004, 23, 19–26. [Google Scholar] [CrossRef]
- Nandhini, M.; Harish, S.; Aiyanathan, K.E.A.; Durgadevi, D.; Beaulah, A. Glycerol-based liquid formulation of the epiphytic yeast Hanseniaspora guilliermondii isolate YBB3 with multiple modes of action controls postharvest Aspergillus rot in grapes. J. Plant Pathol. 2021, 103, 1253–1264. [Google Scholar] [CrossRef]
- Abadias, M.; Teixidó, N.; Usall, J.; Solsona, C.; Viñas, I. Survival of the postharvest biocontrol yeast Candida sake CPA-1 after dehydration by spray-drying. Biocontrol Sci. Technol. 2005, 15, 835–846. [Google Scholar] [CrossRef]
- Adams, G. The principles of Freeze-Drying. In Cryopreservation and Freeze-Drying Protocols, 2nd ed.; Day, J.G., Stacey, G.N., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2007; pp. 15–38. [Google Scholar]
- Prakash, O.; Nimonkar, Y.; Shouche, Y.S. Practice and prospects of microbial preservation. FEMS Microbiol. Lett. 2013, 339, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.S.; Machado, B.A.S.; Martin, A.R.; Bagnara, F.; Ragadalli, S.A.; Alves, A.R.C. Drying by spray drying in the food industry:Micro-encapsulation, process parameters and main carriers used. Afr. J. Food Sci. 2015, 9, 462–470. [Google Scholar]
- Yánez-Mendizábal, V.; Viñas, I.; Usall, J.; Cañamás, T.; Teixidó, N. Endospore production allows using spray-drying as a possible formulation system of the biocontrol agent Bacillus subtilis CPA-8. Biotechnol. Lett. 2012, 34, 729–735. [Google Scholar] [CrossRef]
- Strasser, S. Innovative Product Formulations Applying the Fluidised Bed Technology. Ph.D. Thesis, University of Natural Resources and Life Sciences, Vienna, Austria, 2008. [Google Scholar]
- Larena, I.; De Cal, A.; Linan, M.; Melgarejo, P. Drying of Epicoccum nigrum conidia for obtaining a shelf-stable biological product against brown rot disease. J. Appl. Microbiol. 2003, 94, 508–514. [Google Scholar] [CrossRef]
- Vemmer, M.; Patel, A.V. Review of encapsulation methods suitable for microbial biological control agents. Biol. Control 2013, 67, 380–389. [Google Scholar] [CrossRef]
- González, L.E.; Bashan, Y. Increased growth of the microalga Chlorella vulgaris when coimmobilized and cocultured in alginate beads with the plant-growth-promoting bacterium Azospirillum brasilense. Appl. Environ. Microbiol. 2000, 66, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, R.D.; Walter, J.F. Development of the biocontrol fungus Gliocladium virens: Risk assessment and approval for horticultural use. In Biological Control: Benefits and Risks; Hokkanen, H.M.T., Lynch, J.M., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 263–269. [Google Scholar]
- Kruitwagen, A.; Beukeboom, L.W.; Wertheim, B. Optimization of native biocontrol agents, with parasitoids of the invasive pest Drosophila suzukii as an example. Evol. Appl. 2018, 11, 1473–1497. [Google Scholar] [CrossRef]
- Bielza, P.; Balanza, V.; Cifuentes, D.; Mendoza, J.E. Challenges facing arthropod biological control: Identifying traits for genetic improvement of predators in protected crops. Pest Manag. Sci. 2020, 76, 3517–3526. [Google Scholar] [CrossRef]
- Leung, K.; Ras, E.; Ferguson, K.B.; Ariëns, S.; Babendreier, D.; Bijma, P.; Bourtzis, K.; Brodeur, J.; Bruins, M.A.; Centurión, A.; et al. Next-generation biological control: The need for integrating genetics and genomics. Biol. Rev. 2020, 95, 1838–1854. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, P.J.; Challis, G.L. Discovery of Microbial Natural Products by Activation of Silent Biosynthetic Gene Clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, T.; Botelho, A. Metagenomics and Other Omics Approaches to Bacterial Communities and Antimicrobial Resistance Assessment in Aquacultures. Antibiotics 2021, 10, 787. [Google Scholar] [CrossRef]
- Lahlali, R.; Ibrahim, D.S.S.; Belabess, Z.; Roni, M.Z.K.; Radouane, N.; Vicente, C.S.L.; Menendez, E.; Mokrini, F.; Barka, E.A.; de Melo e Mota, M.G.; et al. High-Throughput Molecular Technologies for Unraveling the Mystery of Soil Microbial Community: Challenges and Future Prospects. Heliyon 2021, 7, e08142. [Google Scholar] [CrossRef] [PubMed]
- Barahona, E.; Navazo, A.; Martínez-Granero, F.; Zea-Bonilla, T.; Pérez-Jiménez, R.M.; Martín, M.; Rivilla, R. Pseudomonas fluorescens F113 mutant with enhanced competitive colonization ability and improved biocontrol activity against fungal root pathogens. Appl. Environ. Microbiol. 2011, 77, 5412–5419. [Google Scholar] [CrossRef]
- Leclère, V.; Béchet, M.; Adam, A.; Guez, J.-S.; Wathelet, B.; Ongena, M.; Thonart, P.; Gancel, F.; Chollet-Imbert, M.; Jacques, P. Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl. Environ. Microbiol. 2005, 71, 4577–4584. [Google Scholar] [CrossRef]
- Okay, S.; Tefon, B.; Özkan, M.; Özcengiz, G. Expression of chitinase A (chiA) gene from a local isolate of Serratia marcescens in Coleoptera-specific Bacillus thuringiensis. J. Appl. Microbiol. 2008, 104, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, D.; Kempf, H.J.; van Pée, K.H. Natural product with antimicrobial activity from Pseudomonas biocontrol bacteria. In Pesticide Chemistry and Bioscience—The Food-Environment Challenge; Brooks, G.T., Roberts, T.R., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1999; Volume 22, pp. 179–189. [Google Scholar]
- Kota, M.; Daniell, H.; Varma, S.; Garczynski, S.F.; Gould, F.; Moar, W.J. Overexpression of the Bacillus thuringiensis (Bt) Cry2Aa2 protein in chloroplasts confers resistance to plants against susceptible and Bt-resistant insects. Proc. Natl. Acad. Sci. USA 1999, 96, 1840–1845. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Schneider, K.; Vater, J.; Süssmuth, R.; Piel, J.; Borriss, R. Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J. Biotechnol. 2009, 140, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Clermont, N.; Lerat, S.; Beaulieu, C. Genome shuffling enhances biocontrol abilities of Streptomyces strains against two potato pathogens. J. Appl. Microbiol. 2011, 111, 671–682. [Google Scholar] [CrossRef]
- Zhao, P.; Quan, C.; Wang, Y.; Wang, J.; Fan, S. Bacillus amyloliquefaciens Q-426 as a potential biocontrol agent against Fusarium oxysporum f. sp. spinaciae. J. Basic Microbiol. 2014, 54, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Côrtes, M.V.; de Sousa Oliveira, M.I.; Mateus, J.R.; Seldin, L.; Silva-Lobo, V.L.; Freire, D.M. A pipeline for the genetic improvement of a biological control agent enhances its potential for controlling soil-borne plant pathogens. Biol. Control 2021, 152, 104460. [Google Scholar] [CrossRef]
- Downing, K.J.; Thomson, J.A. Introduction of the Serratia marcescens chiA gene into an endophytic Pseudomonas fluorescens for the biocontrol of phytopathogenic fungi. Can. J. Microbiol. 2000, 46, 363–369. [Google Scholar] [CrossRef]
- Baek, J.M.; Howell, C.R.; Kenerley, C.M. The role of an extracellular chitinase from Trichoderma virens Gv29-8 in the biocontrol of Rhizoctonia solani. Curr. Genet. 1999, 35, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.B.; Sun, M.H.; Zhou, M.; Li, S.D. Transformation of the endochitinase gene Chi67-1 in Clonostachys rosea 67-1 increases its biocontrol activity against Sclerotinia sclerotiorum. AMB Express 2017, 7, 1. [Google Scholar] [CrossRef]
- Bakker, P.A.; Glandorf, D.; Viebahn, M.; Ouwens, T.W.; Smit, E.; Leeflang, P.; van Loon, L.C. Effects of Pseudomonas putida modified to produce phenazine-1-carboxylic acid and 2, 4-diacetylphloroglucinol on the microflora of field grown wheat. Antonie Leeuwenhoek 2002, 81, 617–624. [Google Scholar] [CrossRef]
- Mendoza-Mendoza, A.; Pozo, M.J.; Grzegorski, D.; Martínez, P.; García, J.M.; Olmedo-Monfil, V.; Cortés, C.; Kenerley, C.; Herrera-Estrella, A. Enhanced biocontrol activity of Trichoderma through inactivation of a mitogen-activated protein kinase. Proc. Natl. Acad. Sci. USA 2003, 100, 15965–15970. [Google Scholar] [CrossRef] [PubMed]
- Deveau, H.; Garneau, J.E.; Moineau, S. CRISPR/Cas system and its role in phage-bacteria interactions. Annu. Rev. Microbiol. 2010, 64, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, H.; Ma, L.; Gong, M.; Wu, Y.; Bao, D.; Zou, G. Use of CRISPR-Cas tools to engineer Trichoderma species. Microb. Biotechnol. 2022, 10, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhu, X.; Zhou, H.; Sun, N.; Wang, T.; Bi, C.; Zhang, X. CRISPR-based metabolic pathway engineering. Metab. Eng. 2021, 63, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.P.; Fang, S.T.; Miao, F.P.; Yin, X.L.; Ji, N.Y. Diterpenes and sesquiterpenes from the marine algicolous fungus Trichoderma harzianum X-5. J. Nat. Prod. 2018, 81, 2553–2559. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Mine, K.; Akiyama, K.; Ohki, S.; Hayashi, H. Anti-plant viral activity of peptaibols, trichorzins HA II, HA V, and HA VI, isolated from Trichoderma harzianum HK-61. J. Pestic. Sci. 2018, 43, 283–286. [Google Scholar] [CrossRef]
- van Bohemen, A.I.; Ruiz, N.; Zalouk-Vergnoux, A.; Michaud, A.; Robiou du Pont, T.; Druzhinina, I.; Atanasova, L.; Prado, S.; Bodo, B.; Pouchus, Y.F.; et al. Pentadecaibins I-V: 15-residue peptaibols produced by a marine-derived Trichoderma sp. of the harzianum clade. J. Nat. Prod. 2021, 84, 1271–1282. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).