Effects of Low-Phosphorus Stress on Use of Leaf Intracellular Water and Nutrients, Photosynthesis, and Growth of Brassica napus L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
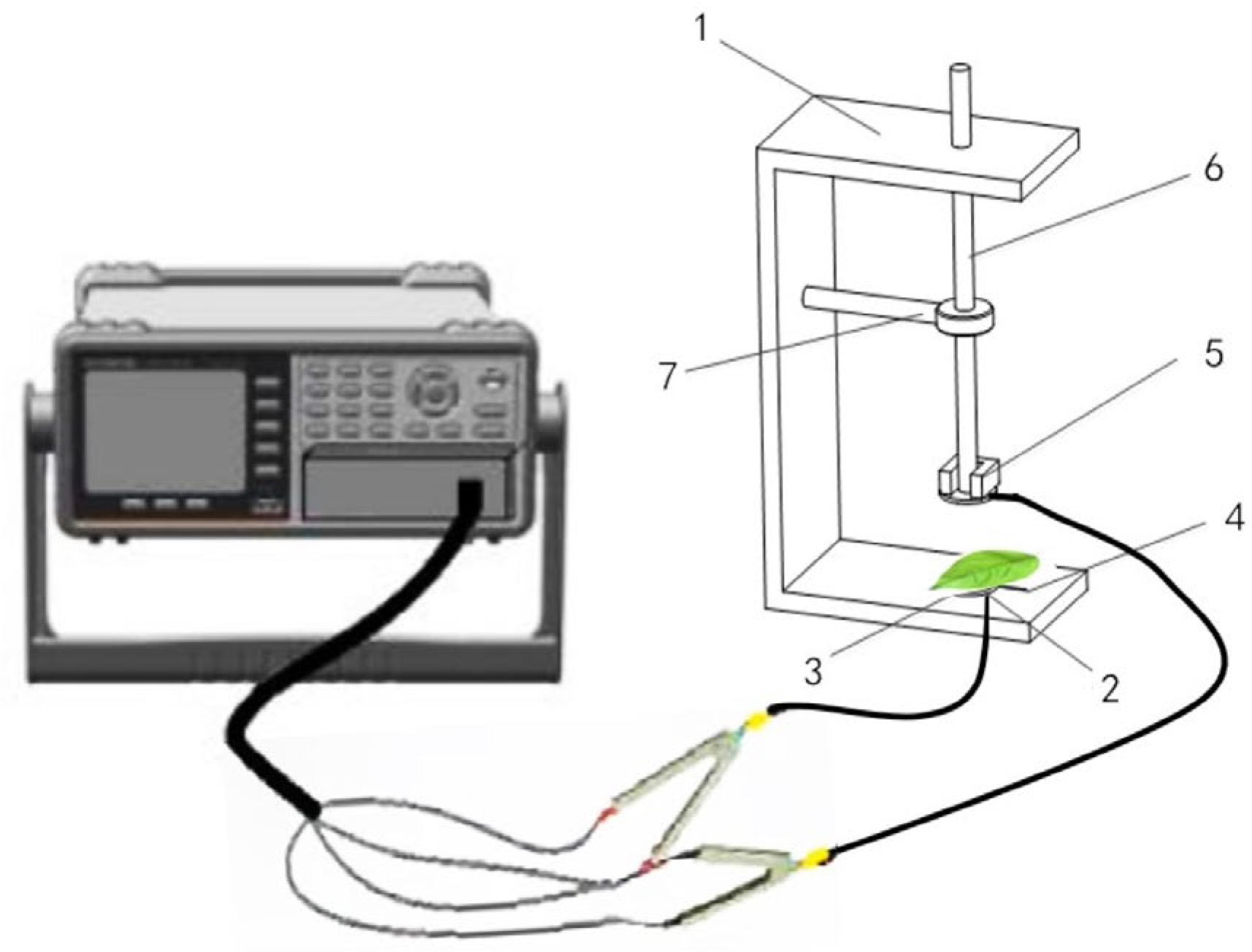
2.2. Determination of Electrophysiological Parameters
2.3. Determination of Leaf Anatomical Structure
2.4. Leaf Elastic Modulus Measurement
2.5. Measurement of Leaf Nutrient Content
2.6. Determination of Photosynthetic Parameters
2.7. Using the 4-Parameter Logistics Model to Fit the Growth Index
2.8. Data Statistics and Analysis
3. Results
3.1. Effects of Low-P Stress on Electrophysiological Parameters
3.2. Effects of Low-P Stress on Leaf Anatomical Structure
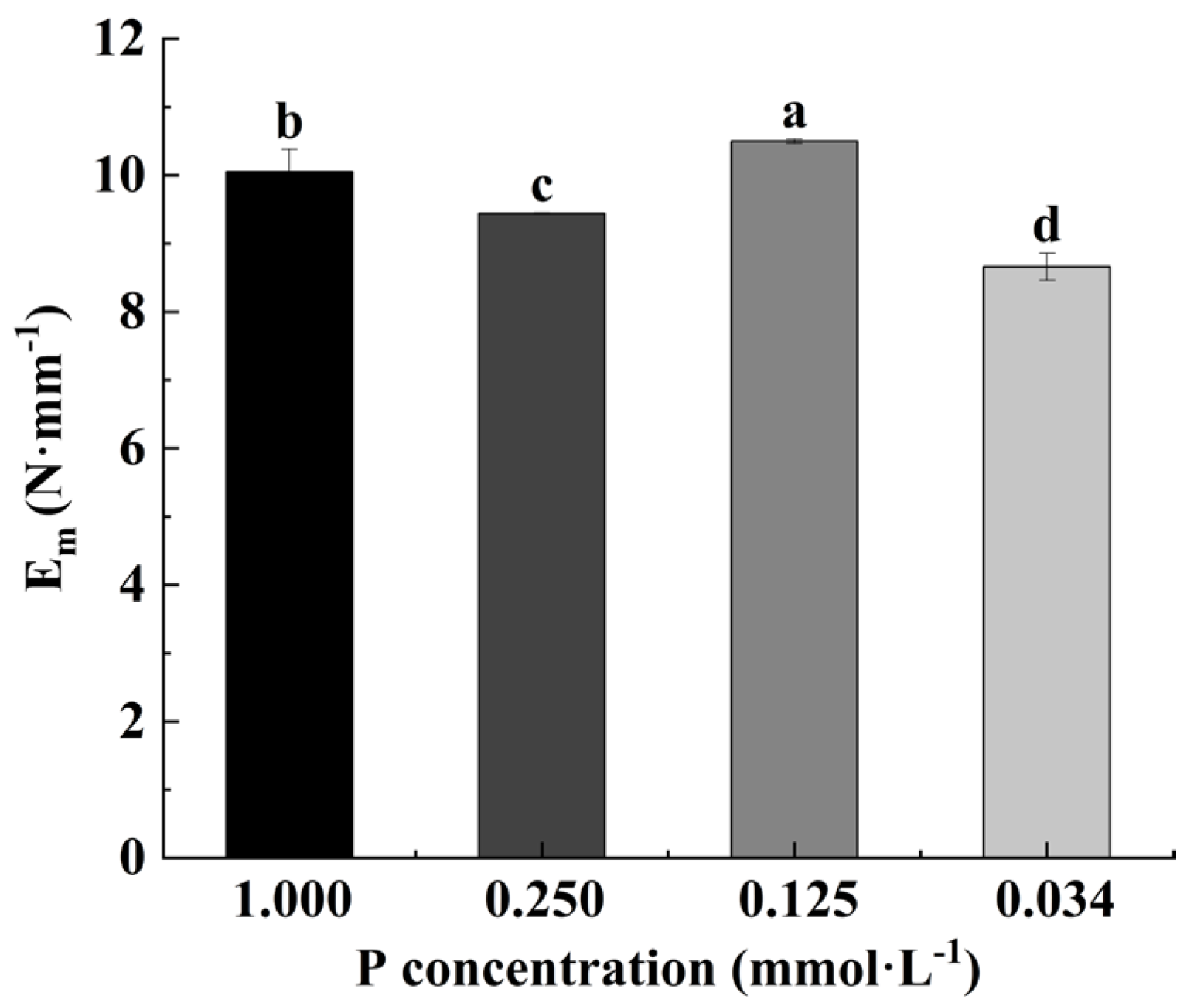
3.3. Effects of Low-P Stress on Leaf Elastic Modulus
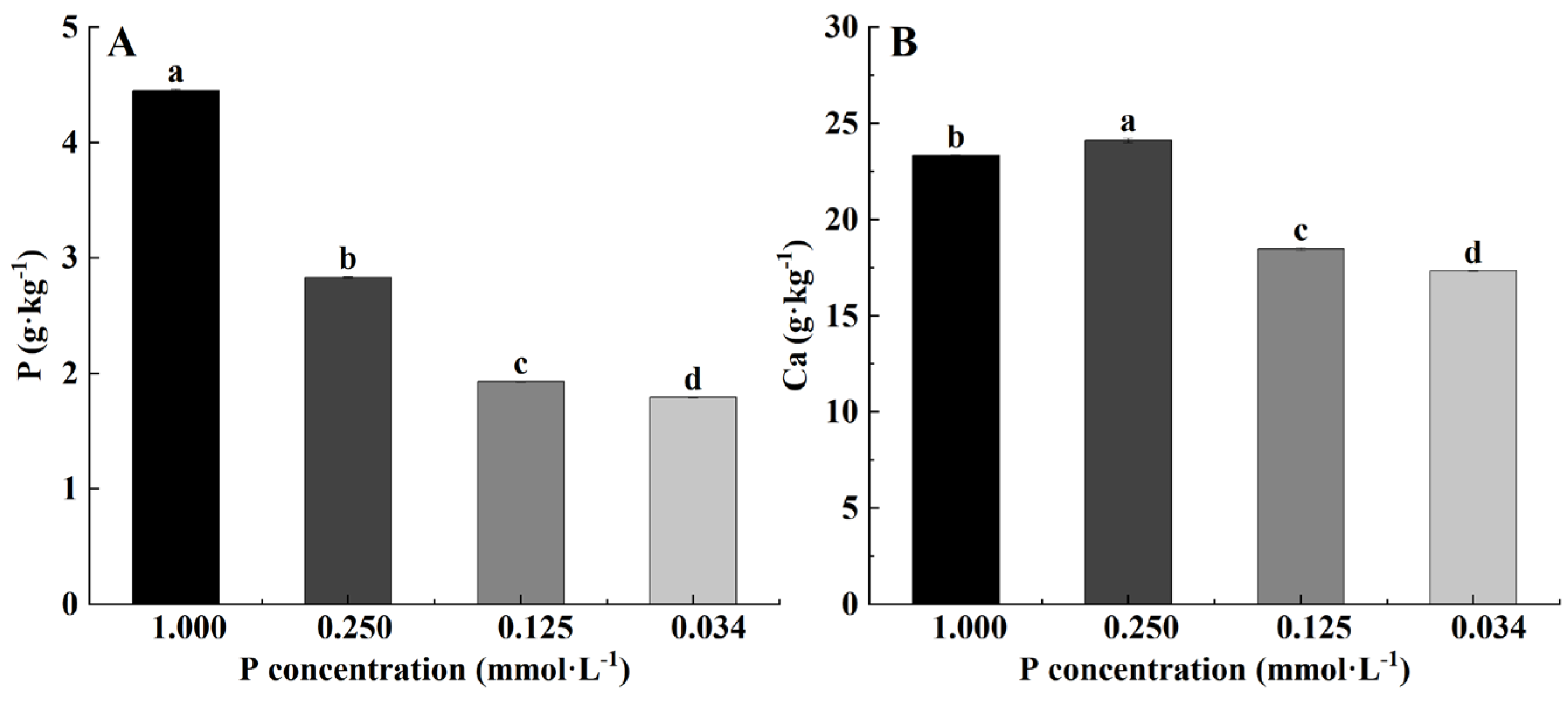
3.4. Effects of Low-P Stress on Leaf Nutrient Content
3.5. Effects of Low-P Stress on Photosynthetic Parameters
3.6. Effects of Low-P Stress on Growth Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schiessl, S.; Iniguez-Luy, F.; Wei, Q.; Snowdon, R.J. Diverse regulatory factors associate with flowering time and yield responses in winter-type Brassica napus. BMC Genom. 2015, 16, 737. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Li, H.; Xu, K.; Cui, X.; Liu, Z.; Wang, X. Genetic variation in the glycine-rich protein gene BnGRP1 contributes to low phosphorus tolerance in Brassica napus. J. Exp. Bot. 2023, 74, 3531–3543. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, J.; You, J.; Li, J.; Qian, C.; Leng, S.; Yang, G.; Zuo, Q. Effects of Phosphorus Supply on the Leaf Photosynthesis, and Biomass and Phosphorus Accumulation and Partitioning of Canola (Brassica napus L.) in Saline Environment. Agronomy 2021, 11, 1918. [Google Scholar] [CrossRef]
- Hu, Z.; Ding, Z.; Al-Yasi, H.M.; Ali, E.F.; Eissa, M.A.; Abou-Elwafa, S.F.; Sayed, M.A.; Said, M.T.; Said, A.A.; Ibrahim, K.A.M.; et al. Modeling of Phosphorus Nutrition to Obtain Maximum Yield, High P Use Efficiency and Low P-Loss Risk for Wheat Grown in Sandy Calcareous Soils. Agronomy 2021, 11, 1950. [Google Scholar] [CrossRef]
- Cadot, S.; Bélanger, G.; Ziadi, N.; Morel, C.; Sinaj, S. Critical plant and soil phosphorus for wheat, maize, and rapeseed after 44 years of P fertilization. Nutr. Cycl. Agroecosys. 2018, 112, 417–433. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Stewart, B.A. Differences of Some Leguminous and Nonleguminous Crops in Utilization of Soil Phosphorus and Responses to Phosphate Fertilizers. Adv. Agron. 2011, 110, 125–249. [Google Scholar] [CrossRef]
- Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus Plays Key Roles in Regulating Plants’ Physiological Responses to Abiotic Stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Hasan, M.M.; Teixeira da Silva, J.A.; Li, X. Regulation of phosphorus uptake and utilization: Transitioning from current knowledge to practical strategies. Cell. Mol. Biol. Lett. 2016, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for Using Phosphate-Solubilizing Microorganisms as Natural Fertilizers in Agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, Z.; Liao, X.; Guo, Q. Effect of phosphorus on yield, absorption and accumulation of mineral nutrients in Brassica napus (L.). Acta Agron. Sin. 2006, 8, 1231–1235. (In Chinese) [Google Scholar]
- Pan, Y.; Ding, G.; Cai, H.; Jin, K.; Broadley, M.R.; Xu, F.; Shi, L. A novel Brassica–rhizotron system to unravel the dynamic changes in root system architecture of oilseed rape under phosphorus deficiency. Ann. Bot. 2016, 118, 173–184. [Google Scholar] [CrossRef]
- Yu, L.; Tian, L.; Zhang, C.; Ma, N.; Li, J. Effects of Low Phosphorus Stress on Photosynthesis of Rapeseed Leaves in Different Periods. Chin. Agric. Sci. Bull. 2008, 24, 232–236. (In Chinese) [Google Scholar]
- Zhang, H.; Huang, Y.; Ye, X.; Shi, L.; Xu, F. Genotypic differences in phosphorus acquisition and the rhizosphere properties of Brassica napus in response to low phosphorus stress. Plant Soil 2009, 320, 91–102. [Google Scholar] [CrossRef]
- Foehse, D.; Jungk, A. Influence of phosphate and nitrate supply on root hair formation of rape, spinach and tomato plants. Plant Soil 1983, 74, 359–368. [Google Scholar] [CrossRef]
- Hoffland, E.; Findenegg, G.R.; Nelemans, J.A. Solubilization of rock phosphate by rape. Plant Soil 1989, 113, 161–165. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, J.; Zhou, H.; Wang, M.; Liu, M.; Ke, Y.; Li, P.; Li, J.; Du, H. Global Survey and Expressions of the Phosphate Transporter Gene Families in Brassica napus and Their Roles in Phosphorus Response. Int. J. Mol. Sci. 2020, 21, 1752. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Sun, H.; Xu, F.; Zhang, X.; Liu, S. Comparative proteome analysis of metabolic changes by low phosphorus stress in two Brassica napus genotypes. Planta 2011, 233, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Kohli, P.S.; Pazhamala, L.T.; Mani, B.; Thakur, J.K.; Giri, J. Root Hair-Specific Transcriptome Reveals Response to Low Phosphorus in Cicer Arietinum. Front. Plant Sci. 2022, 13, 983969. [Google Scholar] [CrossRef] [PubMed]
- Maejima, E.; Watanabe, T.; Osaki, M.; Wagatsuma, T. Phosphorus deficiency enhances aluminum tolerance of rice (Oryza sativa) by changing the physicochemical characteristics of root plasma membranes and cell walls. J. Plant Physiol. 2014, 171, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Wang, W.; Wu, Y.; Qin, X.; Li, M.; Chen, X.; Yu, R. Translocation and Utilization Mechanisms of Leaf Intracellular Water in Karst Plants Orychophragmus violaceus (L.) O. E. Schulz and Brassica napus L. Horticulturae 2022, 8, 1082. [Google Scholar] [CrossRef]
- Guo, Z.; Gong, J.; Luo, S.; Zuo, Y.; Shen, Y. Role of Gamma-Aminobutyric Acid in Plant Defense Response. Metabolites 2023, 13, 741. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Verstraeten, I.; Roosjen, M.; Takahashi, K.; Rodriguez, L.; Merrin, J.; Chen, J.; Shabala, L.; Smet, W.; Ren, H.; et al. Cell surface and intracellular auxin signalling for H+ fluxes in root growth. Nature 2021, 599, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Zang, J.; Shao, T.; Jiang, Q.; Li, Y.; Zhang, W.; Liu, M. Cadmium Distribution and Transformation in Leaf Cells Involved in Detoxification and Tolerance in Barley. Ecotoxicol. Environ. Saf. 2023, 249, 114391. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zhang, Z.; Wang, Z.; Zhang, P.; Xiong, C.; Kuang, Y.; Peng, X.; Yu, M.; Qian, Y. Compositional Variations in Algal Organic Matter during Distinct Growth Phases in Karst Water. Front. Environ. Sci. 2023, 11, 1112522. [Google Scholar] [CrossRef]
- Jócsák, I.; Végvári, G.; Vozáry, E. Electrical Impedance Measurement on Plants: A Review with Some Insights to Other Fields. Theor. Exp. Plant Physiol. 2019, 31, 359–375. [Google Scholar] [CrossRef]
- Galvani, L. Aloysii Galvani De Viribus Electricitatis in Motu Musculari Commentarius, 1st ed.; Typographia Instituti Scientiarum: Bologna, Italy, 1791; pp. 363–418. [Google Scholar] [CrossRef]
- Malone, M. Wound-induced hydraulic signals and stimulus transmission in Mimosa pudica L. New Phytol. 1994, 128, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, Y.; Xing, D.; Li, H.; Zhang, F. Water Metabolism of Lonicera japonica and Parthenocissus quinquefolia in Response to Heterogeneous Simulated Rock Outcrop Habitats. Plants 2023, 12, 2279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Su, Y.; Wu, Y.; Li, H.; Zhou, Y.; Xing, D. Comparison on the Nutrient Plunder Capacity of Orychophragmus violaceus and Brassica napus L. Based on Electrophysiological Information. Horticulturae 2021, 7, 206. [Google Scholar] [CrossRef]
- Priveé, J.; Zhang, M.I.N. Estimating Cold Stress in ‘Beautiful Arcade’ Apple Roots using Electrical Impedance Analysis. Horttechnology 1996, 6, 54–58. [Google Scholar] [CrossRef]
- Xing, D.; Chen, L.; Wu, Y.; Zwiazek, J.J. Leaf Physiological Impedance and Elasticity Modulus in Orychophragmus Violaceus Seedlings Subjected to Repeated Osmotic Stress. Sci. Hortic. 2021, 276, 109763. [Google Scholar] [CrossRef]
- Qin, X.; Xing, D.; Wu, Y.; Wang, W.; Li, M.; Solangi, K. Diurnal Variation in Transport and Use of Intracellular Leaf Water and Related Photosynthesis in Three Karst Plants. Agronomy 2022, 12, 2758. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Y.; Su, Y.; Xing, D.; Dai, Y.; Wu, Y.; Fang, L. A Plant’s Electrical Parameters Indicate Its Physiological State: A Study of Intracellular Water Metabolism. Plants 2020, 9, 1256. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. Calif. Agric. Exp. Stn. 1950, 347, 1–32. Available online: http://www.cabdirect.org/abstracts/19500302257.html (accessed on 15 May 2024).
- Li, H.; Lv, J.; Su, Y.; Wu, Y. Appropriate Sodium Bicarbonate Concentration Enhances the Intracellular Water Metabolism, Nutrient Transport and Photosynthesis Capacities of Coix lacryma-jobi L. Agronomy 2023, 13, 1790. [Google Scholar] [CrossRef]
- Ali Solangi, K.; Wu, Y.; Xing, D.; Ahmed Qureshi, W.; Hussain Tunio, M.; Ali Sheikh, S.; Shabbir, A. Can electrophysiological information reflect the response of mangrove species to salt stress? A case study of rewatering and Sodium nitroprusside application. Plant Signal Behav. 2022, 17, 2073420. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L. Plant Section Technique; Science Press: Beijing, China, 1978. [Google Scholar]
- Xing, D.; Chen, X.; Wu, Y.; Li, Z.; Khan, S. Changes in elastic modulus, leaf tensity and leaf density during dehydration of detached leaves in two plant species of Moraceae. Chil. J. Agric. Res. 2021, 81, 434–447. [Google Scholar] [CrossRef]
- Guo, X.; Jiang, Y. Spatial characteristics of ecological stoichiometry and their driving factors in farmland soils in Poyang Lake Plain, Southeast China. J. Soils Sediments 2019, 19, 263–274. [Google Scholar] [CrossRef]
- Schade, J.D.; Kyle, M.; Hobbie, S.E.; Fagan, W.F.; Elser, J.J. Stoichiometric tracking of soil nutrients by a desert insect herbivore. Ecol. Lett. 2003, 6, 96–101. [Google Scholar] [CrossRef]
- Shafie, N.A.; Aris, A.Z.; Puad, N.H.A. Influential factors on the levels of cation exchange capacity in sediment at Langat River. Arabian J. Geosci. 2013, 6, 3049–3058. [Google Scholar] [CrossRef]
- Javed, Q.; Wu, Y.; Xing, D.; Azeem, A.; Ullah, I.; Zaman, M. Re-watering: An effective measure to recover growth and photosynthetic characteristics in salt-stressed Brassica napus L. Chilean J. Agric. Res. 2017, 77, 78–86. [Google Scholar] [CrossRef]
- Yu, R.; Wu, Y.; Xing, D. Can Electrophysiological Parameters Substitute for Growth, and Photosynthetic Parameters to Characterize the Response of Mulberry and Paper Mulberry to Drought? Plants 2021, 10, 1772. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Li, H.; Zhang, X.; Yu, K.; Chao, M.; Han, S.; Zhang, D. Physiological and Proteomics Analyses Reveal Low-Phosphorus Stress Affected the Regulation of Photosynthesis in Soybean. Int. J. Mol. Sci. 2018, 19, 1688. [Google Scholar] [CrossRef] [PubMed]
- Sarker, B.C.; Rashid, P.; Karmoicer, J.L. Anatomical Changes of Lentil (Lens Culinaris Medik.) Under Phosphorus Deficiency Stress. Bangladesh J. Bot. 2015, 44, 73–78. [Google Scholar] [CrossRef]
- Choi, H.-I.; Park, H.-J.; Park, J.H.; Kim, S.; Im, M.-Y.; Seo, H.-H.; Kim, Y.-W.; Hwang, I.; Kim, S.Y. Arabidopsis Calcium-Dependent Protein Kinase AtCPK32 Interacts with ABF4, a Transcriptional Regulator of Abscisic Acid-Responsive Gene Expression, and Modulates Its Activity. Plant Physiol. 2005, 139, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Ren, T.; Li, J.; Hu, W.; Zhang, J.; Yan, J.; Li, X.; Cong, R.; Guo, S.; Lu, J. Nutrition-mediated cell and tissue-level anatomy triggers the covariation of leaf photosynthesis and leaf mass per area. J. Exp. Bot. 2020, 71, 6524–6537. [Google Scholar] [CrossRef] [PubMed]
- Zambrosi, F.C.B.; Mesquita, G.L.; Tanaka, F.A.O. Assessment of leaf ultrastructure offers insights into mechanisms regulating sugarcane performance under low-phosphorus stress. Acta Physiol. Plant 2020, 42, 54. [Google Scholar] [CrossRef]
- Yaryura, P.; Cordon, G.; Leon, M.; Kerber, N.; Pucheu, N.; Rubio, G.; Garcia, A.; Lagorio, M.G. Effect of Phosphorus Deficiency on Reflectance and Chlorophyll Fluorescence of Cotyledons of Oilseed Rape (Brassica napus L.). J. Agron. Crop. Sci. 2009, 195, 186–196. [Google Scholar] [CrossRef]
- Asif, I.; Dong, Q.; Wang, X.; Gui, H.; Zhang, H.; Zhang, X.; Song, M. Phosphorus and carbohydrate metabolism contributes to low phosphorus tolerance in cotton. BMC Plant Biol. 2023, 23, 97. [Google Scholar] [CrossRef]
- Ellsworth, D.S.; Crous, K.Y.; De Kauwe, M.G.; Verryckt, L.T.; Goll, D.; Zaehle, S.; Bloomfield, K.J.; Ciais, P.; Cernusak, L.A.; Domingues, T.F.; et al. Convergence in phosphorus constraints to photosynthesis in forests around the world. Nat. Commun. 2022, 13, 5005. [Google Scholar] [CrossRef] [PubMed]


| P Concentration (mmol·L−1) | LIWHC | LIWUE | LIWTR | TNTC | NSUE |
|---|---|---|---|---|---|
| 1.000 | 144.235 ± 12.951 b | 0.230 ± 0.036 a | 5.842 ± 0.591 ab | 1.198 ± 0.105 a | 84.781 ± 7.680 a |
| 0.250 | 220.389 ± 24.101 ab | 0.173 ± 0.033 a | 6.295 ± 1.456 ab | 1.579 ± 0.270 a | 67.057 ± 11.080 a |
| 0.125 | 300.171 ± 51.566 a | 0.119 ± 0.055 a | 8.700 ± 0.487 a | 2.013 ± 0.509 a | 55.908 ± 12.604 a |
| 0.034 | 254.037 ± 35.185 ab | 0.176 ± 0.065 a | 4.556 ± 1.437 b | 1.660 ± 0.690 a | 79.637 ± 23.370 a |
| P Concentration (mmol·L−1) | Thickness (μm) | ||||
|---|---|---|---|---|---|
| Total Leaf Thickness | Upper Epidermis | Palisade Parenchyma | Sponge Parenchyma | Lower Epidermis | |
| 1.000 | 162.574 ± 0.869 a | 11.221 ± 0.421 c | 48.248 ± 1.364 b | 93.321 ± 1.776 a | 9.786 ± 0.343 b |
| 0.250 | 156.961 ± 0.353 b | 15.937 ± 0.484 ab | 47.581 ± 1.386 b | 81.358 ± 1.189 b | 12.085 ± 0.242 a |
| 0.125 | 128.669 ± 0.487 c | 17.077 ± 0.330 a | 57.941 ± 0.935 a | 40.597 ± 0.353 d | 13.055 ± 0.335 a |
| 0.034 | 122.519 ± 0.194 d | 15.187 ± 0.173 b | 32.418 ± 0.173 c | 62.805 ± 0.883 c | 12.110 ± 0.363 a |
| P Concentration (mmol·L−1) | P/S Ratio (%) | CTR (%) | SR (%) |
|---|---|---|---|
| 1.000 | 51.790 ± 2.437 b | 29.675 ± 0.780 b | 57.406 ± 1.145 a |
| 0.250 | 58.556 ± 2.538 b | 30.311 ± 0.819 b | 51.837 ± 0.872 b |
| 0.125 | 142.752 ± 2.837 a | 45.029 ± 0.652 a | 31.551 ± 0.212 c |
| 0.034 | 51.662 ± 1.616 b | 26.458 ± 0.420 c | 51.264 ± 0.802 b |
| P Concentration (mmol·L−1) | a | X0 | GR50 | DTlog | Equation | R2 | p |
|---|---|---|---|---|---|---|---|
| 1.000 | 27.869 | 6.670 | 1.481 | 18.809 | 1.000 | <0.0001 | |
| 0.250 | 49.251 | 13.712 | 1.000 | 49.272 | 0.995 | <0.0001 | |
| 0.125 | 26.725 | 6.510 | 1.771 | 15.092 | 1.000 | <0.0001 | |
| 0.034 | 25.651 | 6.318 | 0.986 | 26.042 | 1.000 | <0.0001 |
| P Concentration (mmol·L−1) | a | X0 | GR50 | DTlog | Equation | R2 | p |
|---|---|---|---|---|---|---|---|
| 1.000 | 0.748 | 5.968 | 0.045 | 16.601 | 0.999 | <0.0001 | |
| 0.250 | 1.072 | 7.654 | 0.037 | 28.983 | 1.000 | <0.0001 | |
| 0.125 | 0.852 | 9.120 | 0.028 | 30.861 | 1.000 | <0.0001 | |
| 0.034 | 0.534 | 10.227 | 0.015 | 35.879 | 1.000 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Xing, D.; Wu, Y.; Zhao, K.; Wang, J.; Mao, R. Effects of Low-Phosphorus Stress on Use of Leaf Intracellular Water and Nutrients, Photosynthesis, and Growth of Brassica napus L. Horticulturae 2024, 10, 821. https://doi.org/10.3390/horticulturae10080821
Zhang Q, Xing D, Wu Y, Zhao K, Wang J, Mao R. Effects of Low-Phosphorus Stress on Use of Leaf Intracellular Water and Nutrients, Photosynthesis, and Growth of Brassica napus L. Horticulturae. 2024; 10(8):821. https://doi.org/10.3390/horticulturae10080821
Chicago/Turabian StyleZhang, Qian, Deke Xing, Yanyou Wu, Kuan Zhao, Jing Wang, and Renlong Mao. 2024. "Effects of Low-Phosphorus Stress on Use of Leaf Intracellular Water and Nutrients, Photosynthesis, and Growth of Brassica napus L." Horticulturae 10, no. 8: 821. https://doi.org/10.3390/horticulturae10080821






