Abstract
Tulips are among the most famous plants that are used mainly as ornamental cut flowers. Although widely cultivated, they are mainly reproduced asexually, and knowledge of their sexual reproduction is imperfect, especially in regard to botanical tulips. The aim of the present study was to investigate the temperature effect on seed germination of the Near Threatened wild-growing Greek subendemic plant Tulipa saxatilis (Liliaceae) and to define its seed dormancy type. Seed germination was facilitated by bioclimatic profiling generated with R software (version 4.3.3) connecting its natural distribution points with open access precipitation and temperature data, and it was assessed upon incubation in controlled growth chambers at five constant temperatures (5, 10, 15, 20 and 25 °C) under a 12:12 light–dark photoperiod. The seeds were dependent on temperature for germination and germinated only within a very narrow range of relatively low temperatures (5–15 °C) and optimally at 10 °C (93%), corresponding to natural winter temperatures and precipitation profiles. Increase in the incubation temperature at 20 and 25 °C resulted in no germination. The treatment of seeds with gibberellic acid (GA3) at two different concentrations (500, 1000 mg·L−1) did not widen the temperature range for seed germination. The seeds of T. saxatilis initially presented an underdeveloped embryo, and significant embryo development was detected only at low temperatures (10 and 15 °C) with almost triple embryo length after one month, thus confirming the existence of complex morphophysiological seed dormancy. Moreover, germinated seeds (with and without GA3) were planted in small-sized pots under greenhouse conditions, and the growth of bulblets was investigated in response to the application of commercially available chemical fertilizers, an integrated nutrient management scheme, and a biostimulant. The fertilization schemes affected the weight and length of the produced bulblets, whereas initial seed treatment with GA3 negatively affected the growth (weight, length, and width) of the produced bulblets.
1. Introduction
Tulips are considered worldwide to be among the most appreciated ornamental plants and cut flowers. The genus Tulipa consists of about 100 species which are naturally distributed in southern Europe, North Africa, the Middle East, and Western and Central Asia [1]. Botanical tulips encompass wild-growing species commonly used in or appreciated by the horticultural industry because of their peculiar features (small size and uncommon flower forms and/or colors) combined with special origin/identity (endemics to geographically remote regions) and uniqueness or rarity (local or range-restricted endemics with limited natural populations threatened with extinction); as such, botanical tulips are also related to biodiversity, localized geographical occurrence, and limited commercial availability [2]. Recently, such wild types of tulips have attracted growing research attention, particularly in regard to the creation of new cultivars of tulips [1,3].
Propagation-wise, seed germination is considered the most common and most effective method used for plant production. breeding, or plant conservation efforts, as it is always associated with high genetic diversity [4,5]. Although the tulip’s asexual propagation is well documented in the literature, there is very imperfect knowledge about the germination requirements of the seeds of different wild-growing species of tulips [5]. Nonetheless, it is widely known that many species have certain limitations on sexual propagation due to inherited mechanisms of seed dormancy and low seed viability [6,7]. Seed dormancy is a natural survival mechanism that allows plants to survive in habitats characterized by distinct seasonal changes. Molecular mechanisms that regulate dormancy ensure that germination occurs in a favorable season for both the germination process and seedling survival [8]. Among several dormancy types, there are three basic types of endogenous dormancy: physiological, morphological and morphophysiological [9]. The first one is caused by a physiological inhibition mechanism in the embryo that is associated with low growth potential; the second type is due to an underdeveloped embryo and appropriate conditions for embryonic growth and germination are required; and the third one is a combination of underdeveloped and physiologically dormant embryos [9]. Various studies have argued that some tulip seeds have combined physiological and morphological dormancy, namely “morphophysiological dormancy” (MPD) (e.g., [5]). For a seed with MDP to germinate, the embryo must grow to a certain critical size to be released from morphological dormancy, and it should meet the appropriate conditions (especially those related to temperature) to be able to overcome physiological dormancy [7]. Depending on the temperatures required for germination and dormancy release, as well as the seed’s reaction to gibberellic acid, it has been reported in the literature that MPD has many different levels (e.g., non-deep simple, intermediate simple, deep simple, non-deep simple epicotyl, deep simple epicotyl, deep simple double, non-deep complex, intermediate complex, and deep complex) [6]. All previously mentioned seed dormancy levels have been divided into two main subclasses: complex MPD, where the embryo can develop at an appropriate temperature for cold stratification (0 to 10 °C), and simple MPD, where the development of the embryo occurs in an environment suitable for warm stratification (≥15 °C) [5,6,7].
In Greece, there are 15 recognized wild-growing species in the genus Tulipa which are all nationally protected according to the Greek Presidential Decree 67/1981 [10,11], including the herein focal species Tulipa saxatilis Sieber ex Spreng, which is also traded over the internet by several nurseries as an attractive and non-improved botanical tulip [11]. Tulipa saxatilis is physically a local subendemic species of the Aegean Archipelago found only in the Cretan area (scattered throughout Crete but being rare in Karpathos Island), Rodos Island, and adjacent Anatolia in Turkey [12] which has recently been assessed as Near Threatened [13]. Previous studies have shown that many Chinese native wild-growing tulip species have a non-deep complex MPD [14], and other studies related to Greek endemic species have shown a type of complex MPD [5,15]. In addition, previous studies on several wild-growing Greek tulips (T. bakeri A.D. Hall, T. australis Link, T. clusiana DC., T. undulatifolia Boiss., T. goulimyi Sealy and Turrill, T. hageri Heldr., T. orphanidea Heldr.) have shown that seed germination usually takes place at relatively low temperatures [5,15], indicating that this pattern should be envisaged as a natural adaptation of native plants to the Mediterranean climate. Although the existence of dormancy was confirmed, it was not possible to determine the exact type of dormancy associated with these species. Thus, the aim of this study is to determine as accurately as possible the type of dormancy of T. saxatilis and to investigate the development of first-year bulblets, two areas that only a small amount of research has covered to date.
To bridge the above-mentioned information gaps in benefit of the effective conservation of T. saxatilis and its sustainable cultivation, we presumed that its seeds would be dormant because of the undeveloped embryo, needing relatively low temperatures to germinate [5,15]. Thus, the present study focused on defining the type of dormancy and examining the potential spectrum of different temperatures required for dormancy breaking, with the aim being to develop a species-specific seed germination protocol for the studied species. Moreover, the seedlings of germinated seeds were examined under a series of different fertilization treatments to determine the effect on the size of the first-year’s produced bulblets in a step facilitating their potential ex situ cultivation in man-made settings as well as their subsequent sustainable utilization as a promising botanical tulip.
2. Materials and Methods
2.1. Plant Material
Mature fruits (capsules) of T. saxatilis Sieber ex Spreng. were collected from 10 individuals at Kakopetros, the Chania district, Crete Island, Greece. The selected capsules collected in the wild were not yet open but contained fully mature seeds ready for seed dispersal. This collection took place as part of the botanical expeditions conducted in 2020 and 2021 for the research project TULIPS.GR. All collections were made with special permission (182,336/879 on 16 May 2019, 64,886/2959 on 6 July 2020, and 26,895/1527 on 21 April 2021) and issued annually by the national competent authority, the Greek Ministry of Environment and Energy. The collected material after taxonomic identification was provided with the IPEN (International Plant Exchange Network) accession number GR-1-BBGK-2242, and the seeds were cleaned by hand and stored in glass containers under dry conditions at 5 °C (Figure 1).

Figure 1.
Tulipa saxatilis plant individuals flowering in their natural habitat. (A) Flowering wild-growing individuals on rocky outcrops of Kakopetros, the Chania district, Crete, Greece; (B) vertical view of flowers; (C) collected capsules with mature and immature seeds (scale bar in red: 10 mm), and (D) Individual mature seeds of T. saxatilis (scale bar in red: 5 mm).
2.2. Ecological Profiling of Tulipa saxatilis
To gain insight about the abiotic conditions that prevail in the natural habitats across the Greek distribution range of T. saxatilis, we first recreated the natural distribution of T. saxatilis, employing data from the BBGK and WU herbaria (https://www.jacq.org/#database, accessed on 9 April 2024; acronyms of herbaria according to Index Herbariorum) in combination with georeferencing of a species-specific distribution dot map [12]. Detailed climatic information regarding temperature, precipitation (including minimum, maximum, and average values), and 19 bioclimatic variables prevalent in the native habitats of the studied species was extracted from the WorldClim database (https://www.worldclim.org/data/worldclim21.html, accessed on 9 April 2024). The historic climate data had a pixel size of 30 sec and a spatial resolution of 1 km2. These datasets were then correlated in R [16] with the natural distribution of T. saxatilis using the R’s raster package [17].
2.3. Effect of Temperature on Seed Germination
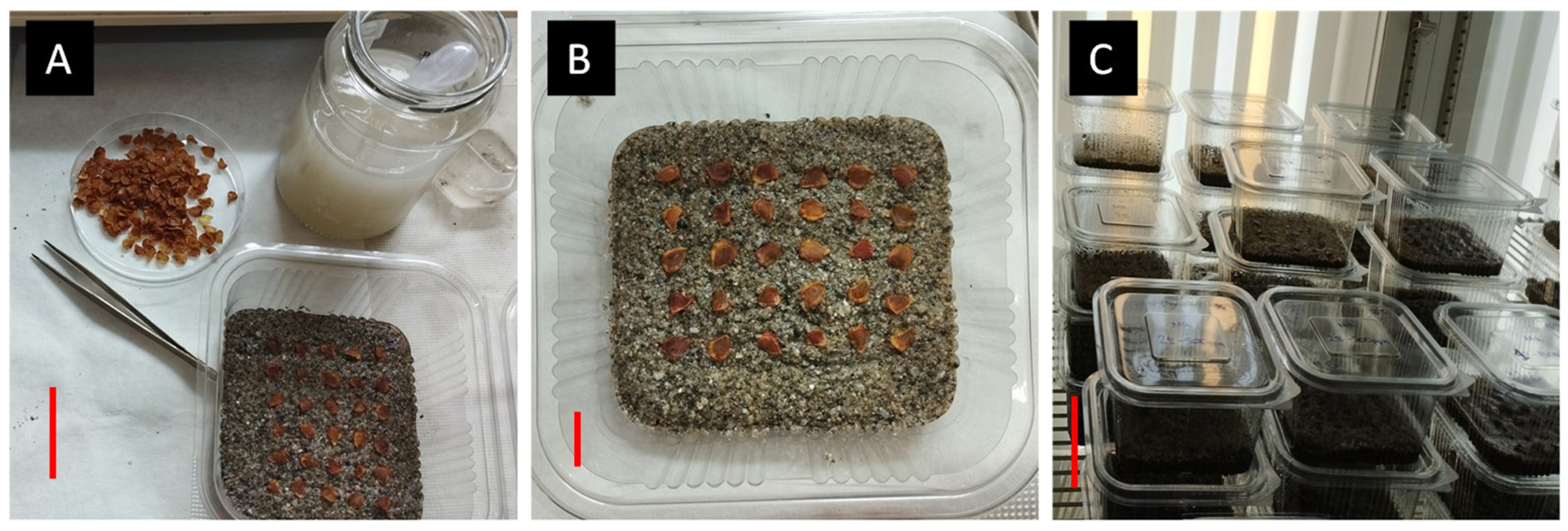
The species-specific seed germination experiments started in 6 December 2022, and took place in the Laboratory of Floriculture, School of Agriculture, Aristotle University of Thessaloniki. At first, experiments were conducted to determine how different temperatures affected the germination of the seeds. Data mining in the R-derived bioclimatic profile indicated the natural range of extreme temperatures in the original habitats and locations of the wild-growing populations of T. saxatilis in Greece (n = 26). As these temperatures may range from 5 to 30 °C year-round, the species’ natural adaptation can be deduced, including summer estivation as lethargic bulbs and seeds (ca. 25 °C), and appropriate temperature partial ranges were introduced to investigate the temperature effect in the experimental trials (5, 10, 15, 20, 25 °C), excluding temperatures > 25 °C due to natural dormancy in the wild. Specifically, we tested how the seeds germinate at 5, 10, 15, 20, 25 °C in controlled temperature growth chambers using four replications of 25 seeds at each incubation temperature. Due to the large size of the seeds, we were unable to use Petri dishes, so they were placed in transparent plastic containers on top of sterile sand which was steeped with a mixture of two commercial fungicides (Switch: 25% w/w fludioxonil + 37.5% w/w cyprodinil, Syngenta, Basel, Switzerland, and Merpan: 80% captan, Adama agricultural solutions, Basel, Switzerland) in a ratio of 1:1 (w/w) and diluted in water (0.2% w/v) to maintain the moisture and prevent future infections. Subsequently, after the containers were filled, they were placed in the growth chambers and were allowed to germinate (Figure 2). The substrate was kept moist throughout the whole experimental period. Once every week, the germinated seeds were counted and removed for a period of eleven weeks. We considered a seed as being germinated when radicle protrusion ≥ 2 mm long appeared [18,19].
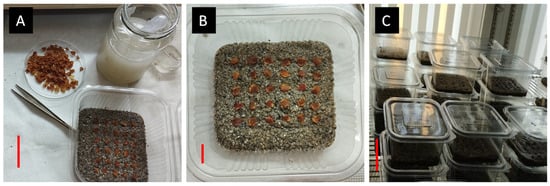
Figure 2.
The seed management process for germination tests in a controlled environment employing Tulipa saxatilis seeds placed in plastic containers with sterilized sand (A,B) and put in a growth chamber at a controlled temperature (C). Scale bars are indicated with red lines: A = 3 cm, B = 1 cm, C = 5 cm.
A second similar experiment was conducted with seeds immersed for 24 h in gibberellic acid (GA3) solutions of 500 and 1000 mg·L−1. Following that, the seeds were placed in plastic containers with the same substate as previously mentioned. Four replications of 25 seeds each were placed in each incubation temperature (5, 10, 15, 20 and 25 °C). The moisture of the substrate and the germination of the seeds were carefully monitored, as previously mentioned.
At the eleventh week, after the beginning of the germinated test, the non-germinated seeds in the growth chambers of 15, 20 and 25 °C were transferred at the incubation temperature of 10 °C, and the germinated seeds were then counted and removed each week for a period of ten weeks.
2.4. Seed Embryo Testing and Effect of Temperature on Embryonic Growth
A further experiment was performed for T. saxatilis using a randomly picked sample of 200 seeds divided into four replications of 50 seeds. In this experiment, the goal was to determine the growth status of the seed embryo with the help of a stereoscope.
Τhe ideal temperature for embryonic growth was assessed via measuring of the length of the embryo within the seed using a random seed sample of T. saxatilis. Firstly, the seeds were divided into random groups of 15 seeds, and then the size of the embryo was measured with the help of a stereoscope equipped with a built-in micrometer. These seeds were put in plastic containers lined with moistened filter paper and were moist stratified at 5, 10, 15, and 20 °C. After 30 days, the embryo’s length from each seed was measured for all applied temperatures. These measurements allowed us to calculate the embryo to seed (E–S) ratio and the standard deviation for each group of 15 seeds.
2.5. Seedling Production
In parallel with the germination experiments, a fertilization experiment was conducted. The germinated seeds of the previous experiments were used and placed in small plastic pots (6 × 6 × 6.5 cm in dimension and 0.33 mL in volume) filled with a mixture of enriched peat (TS1) and perlite in a 3:1 ratio (v/v). Lastly, they were covered with a thin layer of sterile sand to keep the seedlings moist (Figure 3). The filled pots were moved in special trays for easy transportation, and the trays were put in an unheated bench inside the greenhouse (Figure 3). To maintain the moisture of the pots and the proper growth of the seedlings, watering was applied every three to four days per week.
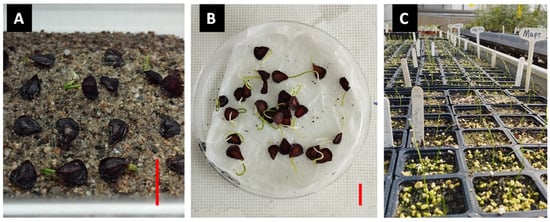
Figure 3.
Illustration of the process from germinated seeds to seedlings of Tulipa saxatilis: (A) Germinated T. saxatilis seeds (scale bar in red: 10 mm); (B) germinated seeds removed from the plastic containers (scale bar in red: 10 mm); (C) seedlings of T. saxatilis growing in small plastic containers under greenhouse conditions 20 days after germination.
Germinated seeds of each treatment with GA3 (0, 500 and 1000 mg·L−1) were sown separately. Subsequently, the seedlings obtained from each seed treatment were divided into four separate groups, each group receiving a different type of fertilization treatment. Group A was the control group, with no fertilization; Group B received integrated nutrient management (INM) as fertilization; Group C received inorganic fertilization (ChF); and Group D had a biostimulant applied. In each of the four treatments, there were different subgroups of plants pre-treated during germination tests with 500 mg·L−1 and 1000 mg·L−1 GA3 in addition to plants without GA3 treatment. For Group B, the fertilization consisted of a nutrition solution of PRECISE 0.6 mL/L, THEOCOPPER 3 mL/L, THEOCAL 0.75 g/L, and HYPER PHOS 0.3 mL/L. For Group C, K2SO4 0.2 g/L, CuSO4 0.11 g/L, Ca(NO3)2 0.83 g/L, HYPER PHOS 0.3 mL/L, and NH4NO3 13.5 g/L was utlized, while, for Group D, THEOMASS 3 mL/L was employed (all products of Theofrastos fertilizers, Korinth, Greece). The experiment started on 3 March 2023, and the fertilizations were applied every 15 days for three months (last application on 12 May 2023); every time, a consistent amount of 5 mL for each fertilizer was added to each group, and the pots were watered one day before the application of the fertilization.
On 11 April 2023, the trays with the seedlings that were in the greenhouse were repositioned on specially designed benches outdoors to continue their growth in an area with a lower temperature than that of the greenhouse. Once the cotyledonary leaf was deceased, the first year bulblets were removed from the pots and set to dry for two days. Subsequently, the weight of each bulblet was measured with precision laboratory equipment (Adam equipment, Milton Keynes, UK) and scaled with four decimals (Figure 4). The bulblets were also photographed digitally, and their length and width were calculated with ImageJ (Wayne Rasband and contributors, National Institutes of Health, Bethesda, MD, USA).

Figure 4.
Collection, sorting, and measuring of one-year-old bulblets of Tulipa saxatilis: (A) first year bulblets collected from the experimental substrate; (B) bulblets weighted on the precision scale after collection; (C) one-year-old bulblets of T. saxatilis produced from different seedlings. Red lines indicate scale bars: A = 2 cm, C = 1 cm.
2.6. Statistical Analysis
For germination experiments, a completely randomized design was used. The data were subjected to analysis of variance (ANOVA). Prior to the ANOVA, only the germination percentage data were transformed to arc-sine square root values [20]. The germination data linked to incubation temperatures of 20 and 25 °C were not analyzed since none of the seeds germinated.
The experiment regarding the effects of fertilization and GA3 pre-treatment of T. saxatilis seeds on bulblet growth employed a completely randomized design with two factors. The factors were the fertilization (four levels; control, INM, ChF, Biostimulant) and GA3 pre-treatment of T. saxatilis seeds (three levels; 0, 500, 1000 mg·L−1). The data were analyzed using the ANOVA in the frame of the procedure GLM (General Linear Model) [19]. The comparisons of the means were made using the Duncan’s test and the LSD test at a significance level of p ≤ 0.05 [21].
3. Results
3.1. Bioclimatic Profiling
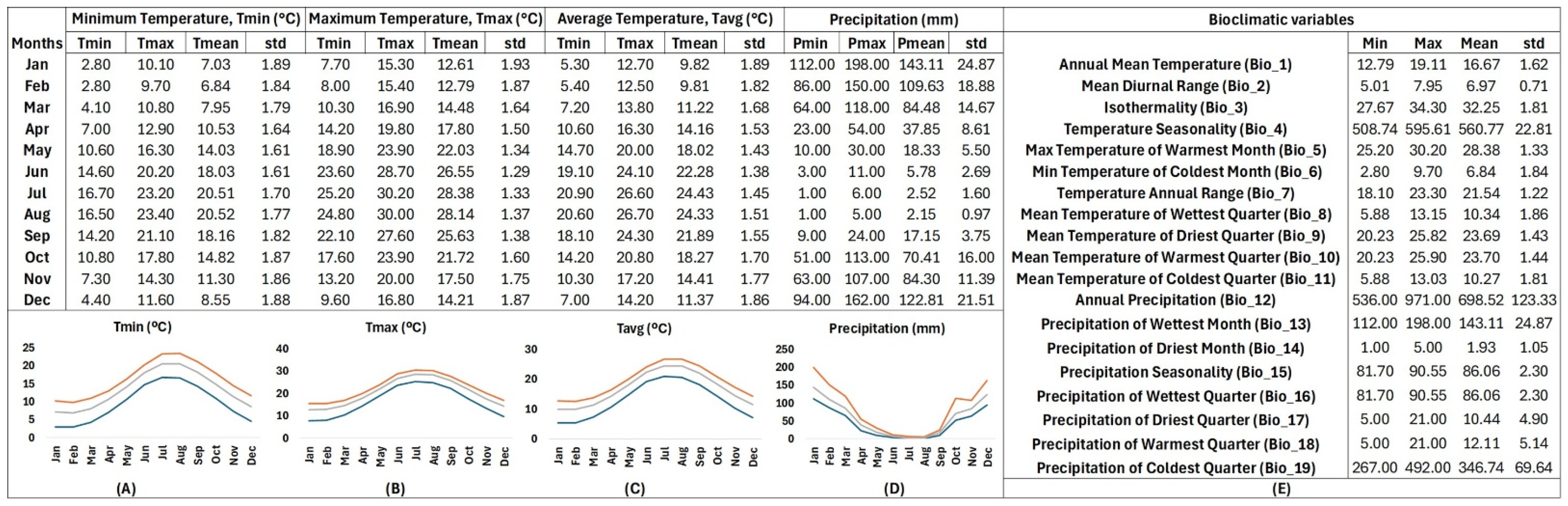
The R-derived bioclimatic data from the natural sites where T. saxatilis thrives in the wild employed an annual mean temperature at 16.67 ± 1.62 °C and indicated a mild winter profile with mean temperatures of the coldest quarter (10.27 ± 1.81 °C) ranging from 9.82 ± 1.89 °C in January to 9.81 ± 1.82 °C in February and 11.22 ± 1.68 °C in March. From April, temperature was shown to rise (14.16 ± 1.53 °C) until the warmest quarter of the year (mean temperature of the warmest quarter: 23.70 ± 1.44 °C), ranging from 22.28 ± 1.38 °C in June or 24.43 ± 1.45 °C in July to 24.33 ± 1.51 °C in August. After the warm season, temperature was shown to decline steadily from September (21.89 ± 1.55 °C) to November (14.41 ± 1.77 °C), marking the onset of the cold season. The bioclimate data indicated that the abiotic environment in the sites where T. saxatilis naturally thrives is not characterized by extremes values either in winter (Tmin of Tmin = 2.80 °C in January) or in summer (Tmax of Tmax = 30.20 °C in July) (Figure 5).
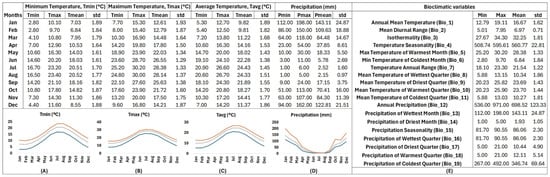
Figure 5.
R-derived bioclimatic profiling of Tulipa saxatilis based on 26 Greek localities corresponding to wild-growing populations. The columns in different sections include minimum, maximum, average values and standard deviation for (A) the minimum temperature, (B) the maximum temperature, (C) the average temperature, (D) the precipitation, and (E) another 19 bioclimatic variables. The graphs illustrate the yearly fluctuation patterns of minimum (blue), maximum (orange) and mean (gray) temperatures and precipitation (mm).
Historical precipitation data from the distribution points of T. saxatilis revealed intermediate to high levels of rainfall (annual precipitation 698.52 ± 123.33 mm) in the sites where T. saxatilis naturally thrives with considerable year to year fluctuations, as indicated by the high standard deviation (Figure 5). The highest quantity of rainfall mainly was shown to occur in December (122.81 ± 21.51 mm), January (143.11 ± 24.87 mm), and February (109.63 ± 18.88 mm), gradually decreasing from March (84.48 ± 14.67 mm) until May (18.33 ± 5.50 mm). The driest quarter of the year (on average 10.44 ± 4.90 mm) was shown to span from June (5.78 ± 2.69 mm) to August (2.15 ± 0.97 mm), while precipitation was shown to remain low until September (17.15 ± 3.75 mm). From October (70.41 ± 16.00 mm) onwards, rainfall was shown to start increasing again until reaching its peak during the wettest quarter of the year, especially in January (143.11 ± 24.87 mm) (Figure 5).
3.2. Seed Germination Tests
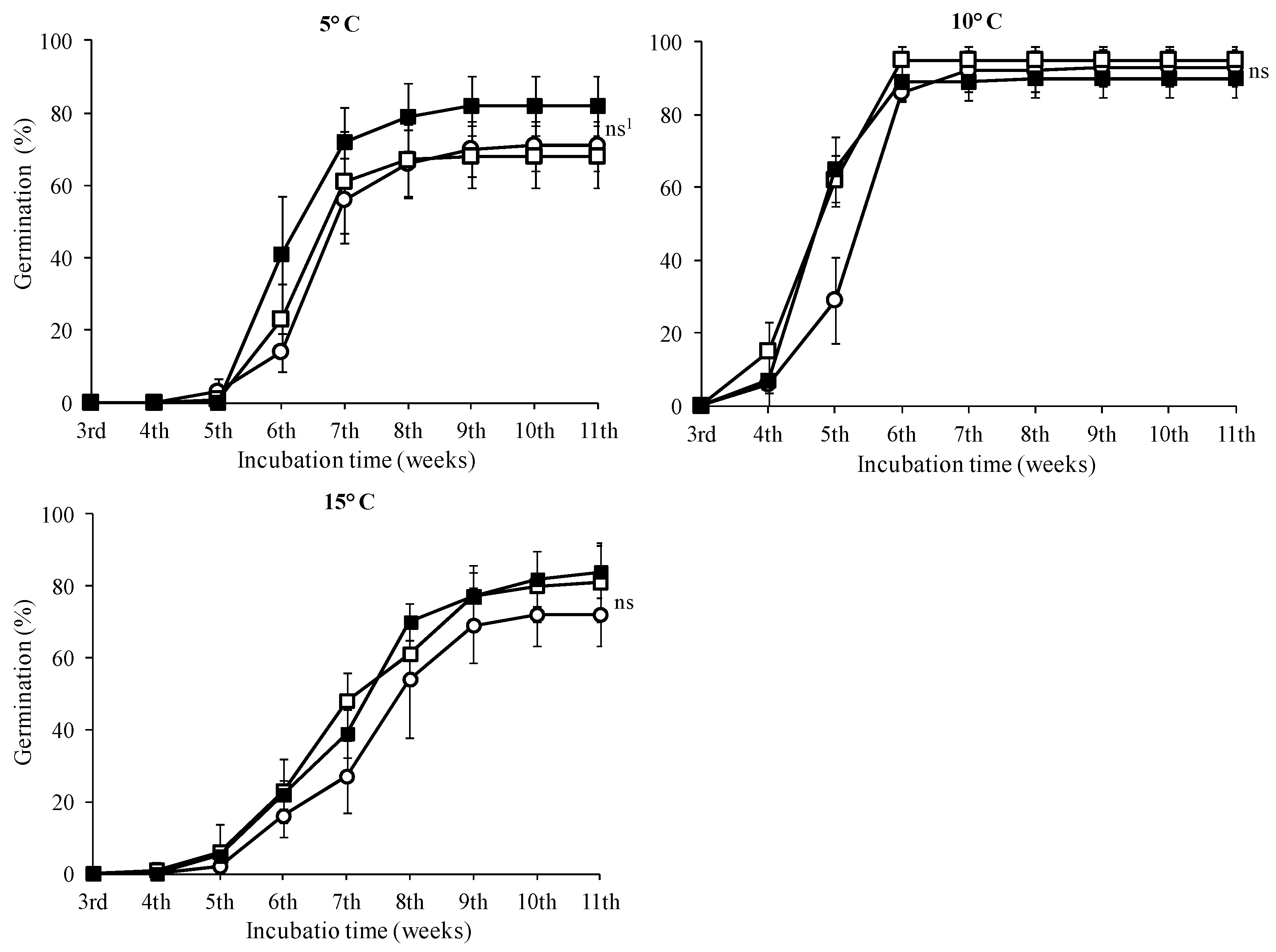
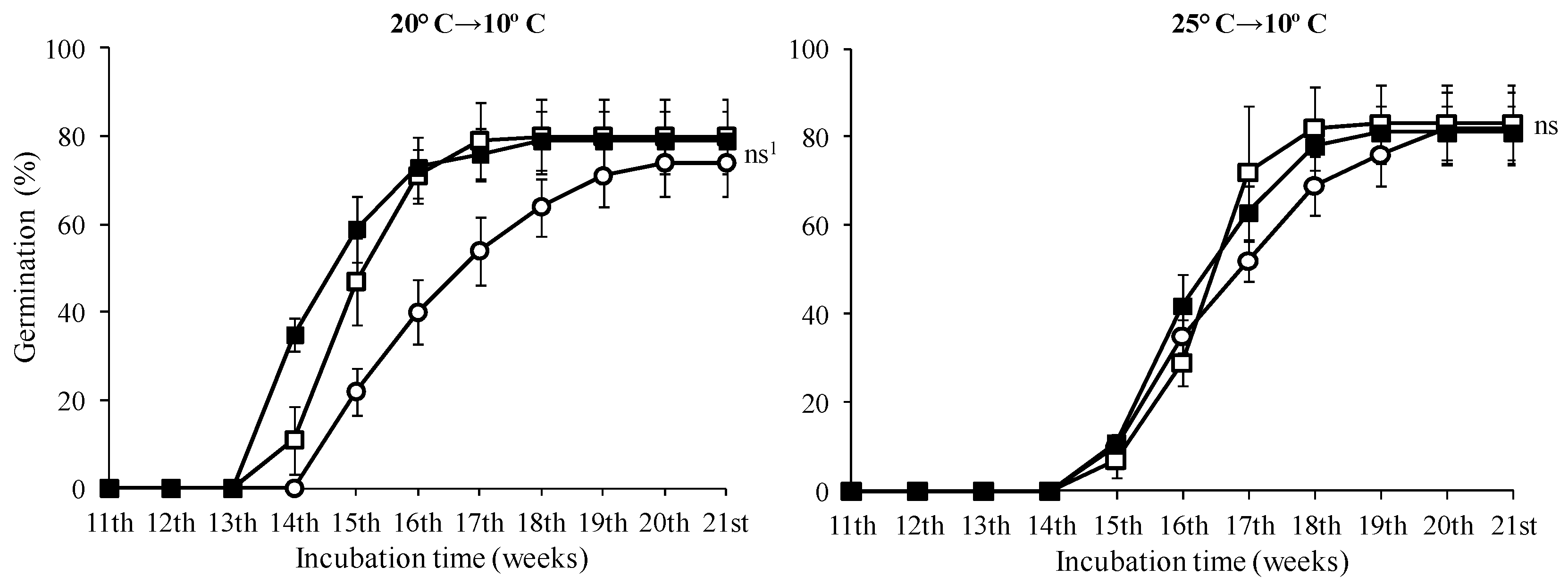
In seeds treated with 0 and 500 mg·L−1, the highest germination rates were observed in seeds incubated at 10 °C with no statistically significant difference in this temperature among the GA3 treatments (Figure 6, p < 0.05). No seeds of T. saxatilis incubated at 20 and 25 °C germinated, regardless of the treatment with GA3 solutions. The seed germination started during the fourth week and was completed two weeks later for all GA3 treatments (completion on the sixth week) (Figure 6). At the 5 and 15 °C temperatures, a similar germination pattern was observed (Figure 6). For these seeds, there was no statistically significant difference between the treatments without GA3 and the ones with 500 or 1000 mg·L−1 (Figure 6); however, in seeds pre-treated with 1000 mg·L−1 GA3, no significant differences were observed among the three incubation temperatures (5, 10 and 15 °C) (Table 1, p < 0.05). For the seeds incubated at 20 °C and 25 °C for eleven weeks and then transferred at 10 °C, the germination started in the third or fourth week, after the placement of seeds at 10 °C, and continued for about five weeks; when germination of the seeds was completed, all three treatments reached a germination rate of about 80% (Figure 7).
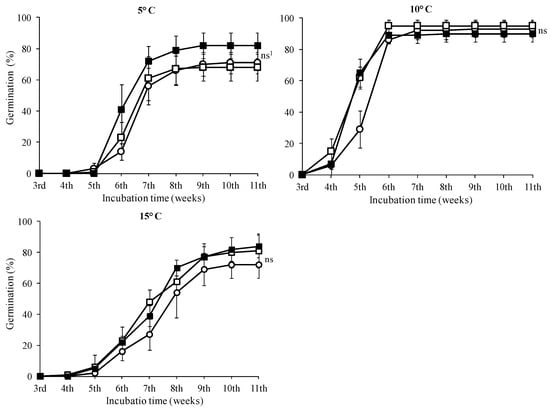
Figure 6.
Time course of germination of Tulipa saxatilis seeds at 5, 10 and 15 °C without pretreatment (○) or pretreated with 500 (□) and 1000 mg·L−1 GA3 (■). Mean and standard deviation values of four replicates are presented, each with 25 seeds. Bars indicate the standard deviation. 1 ns: means not statistically significantly different (p > 0.05), according to the Duncan’s test.

Table 1.
Effect of temperature (5, 10 and 15 °C) on the germination of seeds of Tulipa saxatilis pre-treated with GA3 solutions at 0, 500 and 1000 mg·L−1. Means and standard deviations are presented.
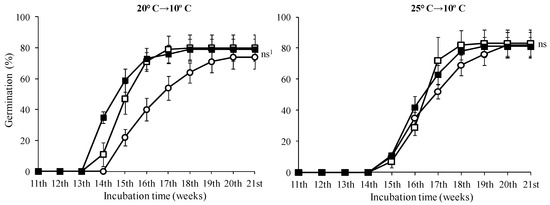
Figure 7.
Time course of germination of Tulipa saxatilis seeds placed for 11 weeks at 20 and 25 °C and then transferred to 10 °C; (○) seeds without pre-treatment, (□) or pre-treated with 500 and (■) with 1000 mg·L−1 GA3. Mean and standard deviation values of four replicates are presented, each with 25 seeds. Bars indicate the standard deviation. 1 ns: means not statistically significantly different (p > 0.05) according to the Duncan’s test.
3.3. Effect of Temperature on Embryo Growth
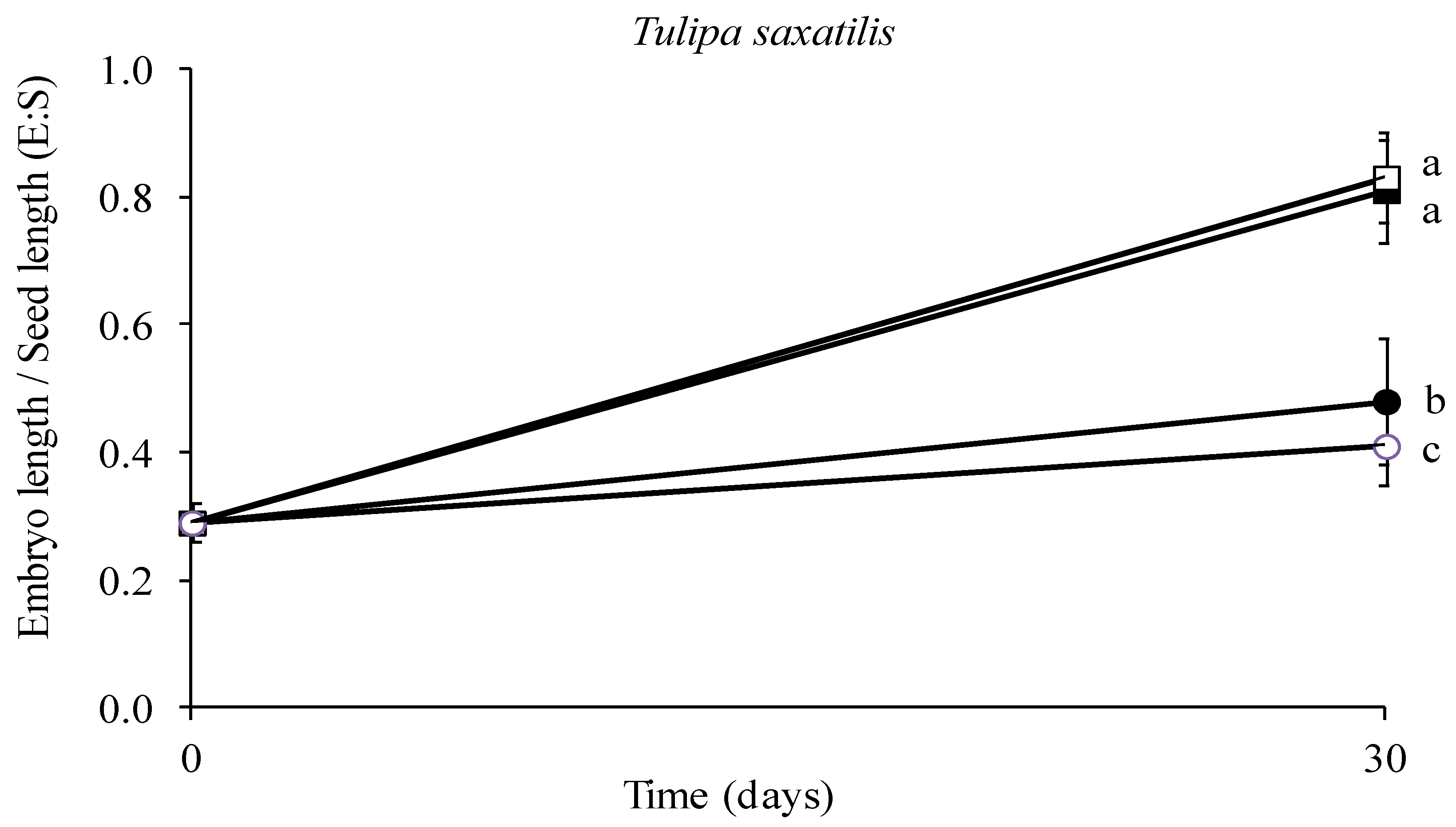
The results showed that embryo growth was greatly impacted by temperature. Specifically, the E–S ratio at the end of the test was the lowest at 5 and 20 °C, while the highest rates were observed at 15 and 10 °C, where the embryo size almost tripled in size, with no statistically significant difference between the two above-mentioned temperatures (Figure 8, p < 0.05). The results on the E–S ratio were reflected in the appearance of the embryos under the stereoscope where the size differences among the applied temperature treatments were discernible (Figure 9).
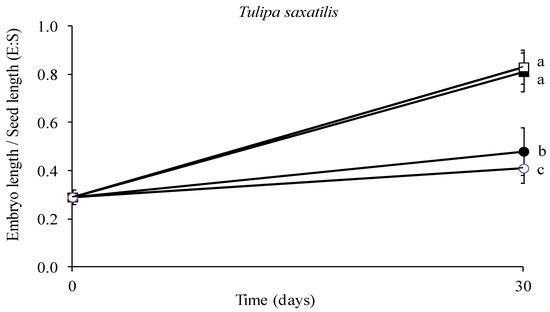
Figure 8.
Effect of one month stratification at various temperatures (● 5, ■ 10, □ 15 and ○ 20 °C) on the ratio of mean embryo length to seed length regarding the seeds of Tulipa saxatilis. Means are statistically different at p < 0.05 when not sharing a common letter (comparisons made using the LSD test). Bars indicate the standard deviation.
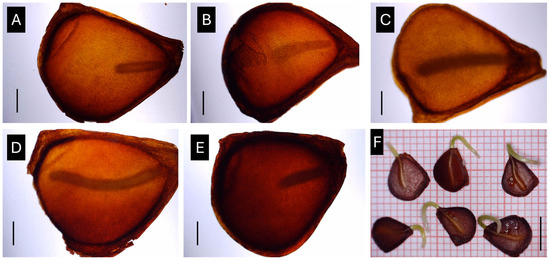
Figure 9.
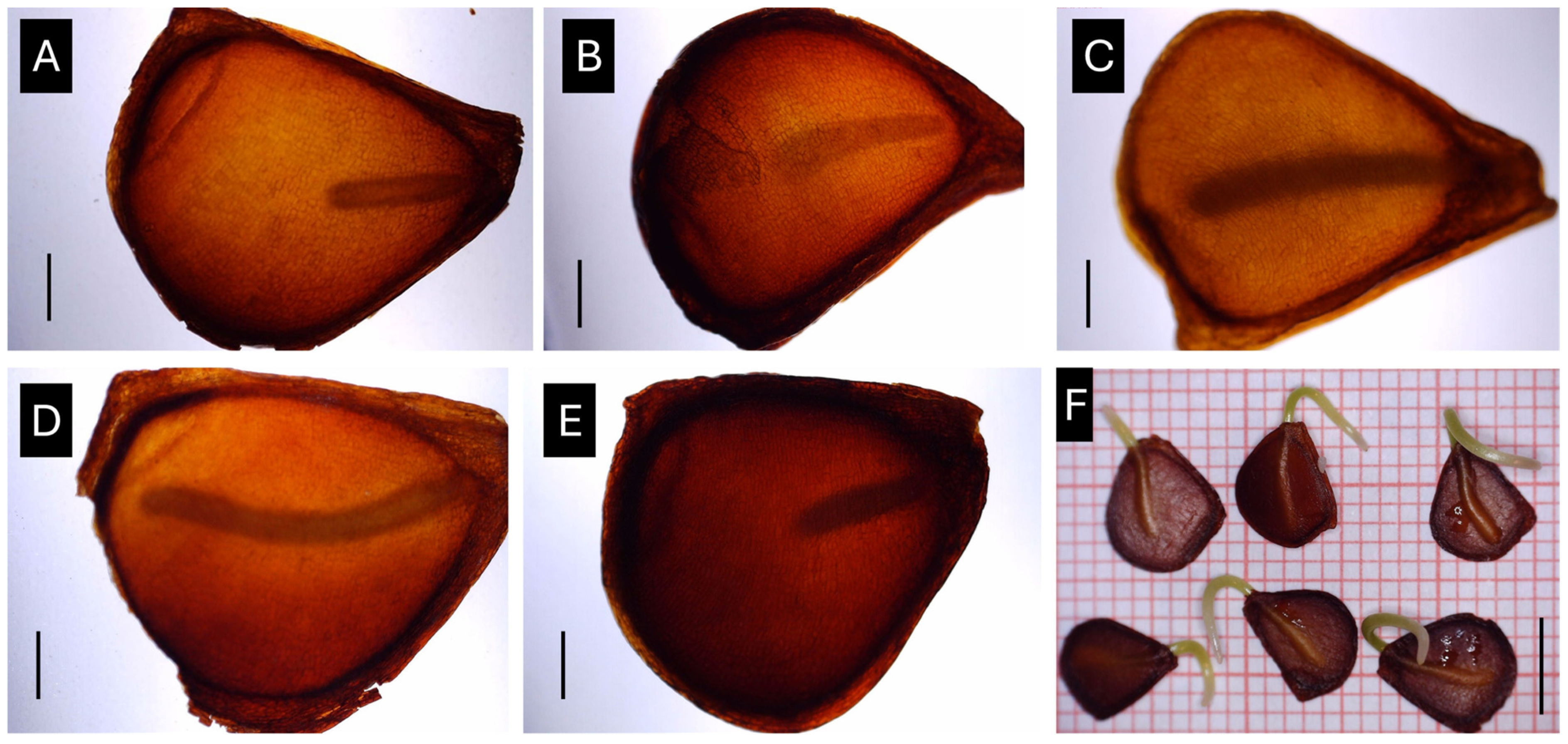
Effect of seed stratification temperature on embryonic growth of Tulipa saxatilis seeds. (A) Embryo size after collection (ripe capsules); embryo size after seed stratification for one month at different temperatures, i.e., 5 °C (B), 10 °C (C), 15 °C (D), and 20 °C (E); (F) germinated seeds of T. saxatilis following the full embryo development. Black lines indicate scale bars for (A–E) = 1 mm and for (F) = 5 mm.
3.4. Bulblet Production from Seedlings
Regarding the growth of the bulblets of T. saxatilis, it was observed that both the GA3 pretreatment of seeds and the fertilization had a significant effect on their growth in terms of length and mass, although the fertilization treatments showed no statistically significant difference in the width of the produced bulblets (Table 2, p < 0.05). The analysis of the data showed that there was no statistically significant effect of the interaction of the two factors (fertilization and GA3 pre-treatment) on the growth of the bulblets.

Table 2.
Effect of the factors fertilization (F) and GA pre-treatment (GAP) in Tulipa saxatilis seeds and their interaction on bulblet development. Means and standard errors are presented.
Regarding the length of the seedlings’ bulblets, the bulblets of control showed the lowest length, whereas no statistically significant differences among the applied fertilization schemes were observed (Table 2, p < 0.05). In the bulblets originating from seeds pre-treated with 500 mg·L−1 GA3, the length was statistically shorter than that of bulblets originated from seeds not treated with GA3 (0 mg·L−1).
Examining the width of the one-year-old bulblets produced from seedlings not pre-treated with GA3, no statistically significant differences were detected in any of the fertilization treatments (Table 2, p < 0.05). Regarding the bulblets originating from seeds pre-treated with 500 mg·L−1 GA3, it was found that the width was statistically smaller than that of bulblets originated from seeds not treated with GA3 (0 mg·L−1).
In terms of bulblet weight, INM application increased the bulblets statistically significantly, while ChF and biostimulant application reduced the weight of bulblets (Table 2, p < 0.05). Seeds pre-treated with 500 and 1000 mg·L−1 GA3 had statistically significantly reduced weights in comparison with seeds not treated with GA3.
4. Discussion
The current research complements and follows-up previous experiments related to the seed germination responses of several wild-growing tulip species of Greece which are threatened with extinction [5,10], presenting new data regarding the Near Threatened Greek subendemic T. saxatilis. Hence, the results presented herein can be considered as a contribution to the ex situ conservation efforts related to T. saxatilis [11], facilitating the future management and possible re-enforcement of its wild-growing populations, and enabling its sustainable utilization as a promising botanical tulip.
Data mining in the R-derived bioclimatic profile indicated that the natural range of extreme temperatures in the original habitats and locations of the wild-growing populations of T. saxatilis in Greece (n = 26) may range from 5 to 30 °C year-round; these temperatures (i) revealed the species’ natural adaptation, including summer estivation as lethargic bulbs and seeds (24–25 °C), and (ii) determined the selection of appropriate temperatures (5, 10, 15, 20, 25 °C) for the investigation of temperature effect in the experimental trials, excluding temperatures > 25 °C due to natural dormancy in the wild. Temperature had a significant effect on the T. saxatilis seeds’ germination process, with seeds germinating only at relatively low temperatures (5–15 °C). Furthermore, T. saxatilis was shown as naturally adapted in such temperatures in December (7 °C), January (5.3 °C), February (5.4 °C), and March (7.2 °C), inducing seed activation during winter. In addition, it was ascertained that, as the temperature increased, the germination of the seeds decreased, resulting in almost no germination at 20 and 25 °C [15]. Moreover, a delay period of around five weeks in the radicle emergence was reported herein, which is presumably associated with the expected high soil moisture for a prolonged period of time, allowing for successful seed germination in its wild habitats. It has been observed in other Liliaceae, like the Alpine rare endemic of France and Italy Fritillaria tubaeformis Gren. and Godr. subsp. moggridgei (Boiss. and Reut. ex Planch.) Rix, that a delay in radicle protrusion following seed embryo development occurs in higher, summer temperatures compared to lower, winter temperatures; for this, it has been suggested that the in situ seed germination may be facilitated via low temperatures and increased soil moisture conditions during snow melt towards the end of winter [22]. In T. saxatilis, studied herein, upon natural seed maturation and consequent dispersal, the linear embryo of the seeds was detected to be small and underdeveloped, thus indicating that it must develop within the seed coat prior to the appearance of the radicle. In other genera of the Liliaceae family, like Fritillaria, an underdeveloped embryo prior to seed radicle emergence has been reported which has been associated with the temperature-dependance of embryo seed size and within endosperm development and the existence of dormancy [22,23]. The results herein on T. saxatilis most probably confirm our initial hypothesis about the existence of some type of dormancy and are in alignment with previous studies investigating different species of Greek tulips where a low temperature range (5–10 °C for T. bakeri, T. australis, T. clusiana, T. hageri, and T. orphanidea and 5–15 °C for T. undulatifolia and T. goulimyi) is known to induce seed germination and then decrease it with rising incubation temperatures [5,15]. In addition, T. hageri, and T. orphanidea are known to demonstrate an underdeveloped embryo after seed dispersal, whereas a delay in radicle emergence is reported in T. bakeri, T. australis, T. clusiana, T. undulatifolia, and T. goulimyi, all of which are temperature-dependent for germination, thus suggesting the existence of some type of dormancy in all previously studied cases [5,15]. Furthermore, the existence of some type of seed dormancy has also been outlined in other tulip species, like the Tulipa iliensis Regel (Syn. T. thianschanica Regel) and T. kaufanniana Regel. of central Asia and the local Balkan endemics T. scardica Bornm. and T. kosovarica Kit Tan, Shuka and Krasniqi [14,24,25]. Therefore, it can be deduced that the seeds of T. saxatilis are characterized by a complex type of morphophysiological dormancy based on the following evidence: (i) the onset of germination in T. saxatilis was postponed for at least four weeks; (ii), in the meantime, the growth of the embryos was greatly increased; and (iii), in association with the latter, the seed germination process occurred only at low temperatures of 5–15 °C. Based on our results, we can rule out the non-deep complex MPD in which a period of cold stratification is necessary for embryonic growth, but it is effective only when the seeds are first subjected in a period of warm stratification [6,7,24]. Since the seeds were proven to require colder temperatures for germination and, in addition, the addition of gibberellic acid did not help in dormancy release, it can be deduced that the seeds of T. saxatilis have deep complex morphophysiological dormancy. According to personal observations in the wild, the seeds of these species become non-dormant throughout winter months as a response to cold stratification, while the germinated seedlings usually appear in March.
After a three-week period, the seeds that were removed from the 20 and 25 °C chambers and put back in the 10 °C chamber finally germinated, showing almost identical germination percentages with the seeds originally placed in the 10 °C. These results enhance our initial hypothesis that the T. saxatilis seeds require low temperatures to complete their germination.
In general, ornamental tulip hybrids and several botanical tulips are commonly reproduced by bulbs, and it is well established that bulb size affects the height of stems, leaf surface area, and root formation, which ensure full flowering. Nevertheless, all tulip bulbs need to reach a critical size for flowering to be induced [26] in corroboration with other Liliaceae genera like Lilium [27]. The size of the bulbs of the botanical tulips, such as T. saxatilis, studied herein, is rarely described in the literature, and it is only recently some publications have supplied evidence on the growth of one-year bulblets generated from seedlings of wild-growing Greek tulips [5,15].
Based on our results, T. saxatilis first year’s bulblets were found to be heavier where INM was applied. It was also detected that T. saxatilis seedlings treated with fertilizers or a biostimulant produced bulblets with significantly larger lengths compared to control ones, while no statistically significant differences among the applied fertilization schemes were observed. Furthermore, the treatment of seeds with GA3 had a negative effect on the weight, length, and width of the produced bulblets. Previous studies have reported that larger bulb size in combination with organic fertilization, in the form of farmyard manure, coupled with phosphorus-rich mineral fertilization enhance the vegetative leaf growth and flowering in the Asiatic Lilium longiflorum Schult. and Schult. f. [28], while bulb diameter is induced by organic fertilization in the cultivated germplasm of L. longiflorum [29]. On the other hand, an older study has shown that low levels of nitrogen, phosphorus, and potassium stemming from the lack of fertilization during the first year of bulblet growth in cultivated L. longiflorum is associated with reduced bulblet fresh weight, plant vegetative growth and flowering compared to the application of the above elements [30]. In another recent study, external application of nitrogen in combination with potassium has been shown to increase bulb circumference, which was accompanied with increased levels of phenolic, flavonoid, and saponin content in the edible bulbs of the highly attractive Asiatic lily namely L. lancifolium Thunb. [31]. Both the INM and chemical fertilization regimes applied herein in T. saxatilis included additional phosphorus in combination with nitrogen, potassium, calcium, and copper, among others. The current data on T. saxatilis could be helpful in future research trying to assess the optimal fertilization treatment and how many years are needed for these bulbs to flower; in other words, more research is needed to evaluate the critical size that the one-year-old bulblets need to reach for their first flowering. Projecting the present results in combination with horticultural experience, it can only be assumed that it would take around three to four years for such bulblets to reach the critical size that would allow for their first flowering. Afterall, even non-improved species of the genus Tulipa, such as the Greek subendemic T. saxatilis, studied herein, are already used for ornamental reasons—mainly but not exclusively—by plant enthusiasts and garden lovers in the UK; therefore, this species appears in the electronic commerce over the internet as already being traded, as it is supplied by the e-shops of several nurseries that send plant materials worldwide [11].
5. Conclusions
The current study outlined the R-derived bioclimatic profile for the Near Threatened and protected Greek subendemic tulip T. saxatilis, thus providing information for the first time about the abiotic conditions that prevail in the natural habitats of the studied species and its natural adaptations. Moreover, the dormancy type was determined (morphophysiologocal with embryonic growth at 10–15 °C) as well as the conditions that are required for effective seed germination in T. saxatilis (optimally 10 °C for 93% seed germination), corresponding to natural winter temperatures and precipitation profiles, thus producing an effective seed germination protocol that can facilitate future conservation efforts and can also be reproduced in man-made settings. The results acquired from these experiments revealed that the seeds of T. saxatilis have a deep complex morphophysiological dormancy and that their seed germination occurs in a small range of relatively low temperatures. In addition, new data were furnished on the development of one-year-old bulblets from seedlings using different fertilization treatments. The species-specific propagation and cultivation protocols that were developed herein for T. saxatilis can be of great help in ex situ conservation attempts going forward and may also enable its future sustainable utilization as a botanical tulip (or even for breeding purposes).
Author Contributions
Conceptualization, N.K. and G.T.; data curation, N.K., S.K., E.P., I.A., M.K., E.K., P.B., I.S. and G.T.; formal analysis, E.P., N.K., S.K., I.A., M.K., G.T. and S.H.; investigation, N.K., S.H., M.K., I.S., I.A., E.K., P.B., E.P., S.K. and G.T.; methodology, N.K., S.H., E.P., S.K., M.K. and G.T.; project administration, N.K. and G.T.; software, I.A.; resources, N.K., S.H., P.B., S.K. and G.T.; supervision, N.K., S.H., S.K., P.B. and G.T.; validation, N.K., S.H., I.A., S.K., I.S., E.K., P.B., E.P. and G.T.; visualization, M.K., E.P., I.A. and N.K.; funding acquisition, N.K. and G.T.; writing—original draft, M.K., I.A., E.P., I.S., E.K. and N.K.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-financed during 2020–2023 by the European Union and Greek national funds through the operational program Competitiveness, Entrepreneurship and Innovation under the call RESEARCH-CREATE-INNOVATE (project code: T2EDK-05115; acronym: TULIPS.GR), entitled “Value chain for Greek native tulips: development of documented propagation material and integrated conservation for sustainable exploitation”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
We are thankful for the anonymous comments made by the reviewers improving the manuscript’s quality. The authors would like to thank the staff of the Balkan Botanic Garden of Kroussia, Institute of Plant Breeding and Genetic Resources, and Hellenic Agricultural Organization-Demeter for hosting the ex situ conservation collections of TULIPS.GR.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Marasek-Ciolakowska, A.; Nishikawa, T.; Shea, D.J.; Okazaki, K. Breeding of lilies and tulips-Interspecific hybridization and genetic background. Breed. Sci. 2018, 68, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Bilias, F.; Karagianni, A.-G.; Ipsilantis, I.; Samartza, I.; Krigas, N.; Tsoktouridis, G.; Matsi, T. Adaptability of wild-growing tulips of Greece: Uncovering relationships between soil properties, rhizosphere fungal morphotypes and nutrient content profiles. Biology 2023, 12, 605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, J.; Xue, L.; Dai, H.; Lei, J. Seed morphology and germination of native Tulipa species. Agriculture 2023, 13, 466. [Google Scholar] [CrossRef]
- Mackay, A.C.; Mcgill, C.R.; Fountain, D.W.; Southward, R.C. Seed dormancy and germination of a panel of New Zealand plants suitable for re-vegetation. N. Z. J. Bot. 2002, 40, 373–382. [Google Scholar] [CrossRef]
- Pipinis, E.; Hatzilazarou, S.; Kostas, S.; Stagiopoulou, R.; Gitsa, K.; Dariotis, E.; Samartza, I.; Plastiras, I.; Kriemadi, E.; Bareka, P.; et al. Effect of temperature on breaking of morphophysiological dormancy and seed germination leading to bulblet production in two endemic tulip species from Greece. Plants 2023, 12, 1859. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Classification, biogeography, and phylogenetic relationships of seed dormancy. In Seed Conservation: Turning Science into Practice; Smith, R.D., Dickie, J.B., Simon, H., Pritchard, H.W., Probert, R.J., Eds.; The Royal Botanic Gardens, Kew: London, UK, 2003; pp. 518–544. Available online: https://www.researchgate.net/publication/265158564_Chapter_28_Classification_Biogeography_and_Phylogenetic_Relationships_of_Seed_Dormancy#fullTextFileContent (accessed on 30 May 2024).
- Finch-Savage, W.E.; Footitt, S. Seed dormancy cycling and the regulation of dormancy mechanisms to time germination in variable field environments. J. Exp. Bot. 2017, 68, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Baskin, C.C.; Baskin, J. Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014; ISBN 9780124166776. [Google Scholar]
- Samartza, I.; Tsaballa, A.; Sakellariou, M.; Mahnev, P.; Tsiripidis, I.; Krigas, N.; Tsoktouridis, G. Taxonomic and molecular characterization of 15 wild-growing tulip species of Greece using the internal transcribed spacer (ITS) nuclear marker in combination with the psbA-trnH and trnL/trnF plastid markers. Biotechnol. Biotechnol. Equip. 2024, 38, 2337694. [Google Scholar] [CrossRef]
- Krigas, N.; Lykas, C.; Ipsilantis, I.; Matsi, T.; Weststrand, S.; Havström, M.; Tsoktouridis, G. Greek tulips: Worldwide electronic trade over the internet, global ex situ conservation and current sustainable exploitation challenges. Plants 2021, 10, 580. [Google Scholar] [CrossRef]
- Strid, A. Atlas of the Aegean Flora. Part 1: Text & Plates. Part 2: Maps, 1st ed.; Englera 33 (1 & 2); Botanic Garden and Botanical Museum Berlin, Freie Universität Berlin: Berlin, Germany, 2016; ISBN 9783921800973. [Google Scholar] [CrossRef]
- Bareka, P.; Kriemadi, E.; Samartza, I.; Koutsovoulou, K.; Krigas, N. Tulipa saxatilis Sieber ex Spreng—Near Threatened (NT). The IUCN Red List of Threatened Species 2024, submitted. Available online: https://www.iucnredlist.org/ (accessed on 30 July 2024).
- Rouhi, H.R.; Shakarami, R.; Tavakkol Afshari, R. Seed Treatments to Overcome Dormancy of Waterlily Tulip (Tulipa kaufmanniana Regel.). Austr. J. Crop Sci. 2010, 4, 718–721. Available online: https://www.cropj.com/tavakol_4_9_2010_718_721.pdf (accessed on 30 July 2024).
- Hatzilazarou, S.; Pipinis, E.; Kostas, S.; Stagiopoulou, R.; Gitsa, K.; Dariotis, E.; Avramakis, M.; Samartza, I.; Plastiras, I.; Kriemadi, E.; et al. Influence of temperature on seed germination of five wild-growing Tulipa species of Greece associated with their ecological profiles: Implications for conservation and cultivation. Plants 2023, 12, 1574. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing 2021. Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 20 June 2024).
- Hijmans, R.J.; van Etten, J. Raster: Geographic Analysis and Modeling with Raster Data. 2012. Available online: http://raster.r-forge.r-project.org/ (accessed on 10 June 2024).
- International Seed Testing Association. International Rules for Seed Testing. Seed Sci. Technol. 1999, 27, 25–30. [Google Scholar]
- Thanos, C.A.; Kadis, C.C.; Skarou, F. Ecophysiology of germination in the aromatic plants thyme, savory and oregano (Labiatae). Seed Sci. Res. 1995, 5, 161–170. [Google Scholar] [CrossRef]
- Snedecor, G.; Cochran, W. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
- Klockars, A.J.; Sax, G. Multiple Comparisons; Quantitative applications in the social sciences, No. 61.; Sage Publications, Inc.: Thousand Oaks, CA, USA, 1986; ISBN 0-8039-2051-2. [Google Scholar]
- Carasso, V.; Hay, F.R.; Probert, R.J.; Mucciarelli, M. Temperature control of seed germination in Fritillaria tubiformis subsp. moggridgei (Liliaceae) a rare endemic of the South-west Alps. Seed Sci. Res. 2011, 21, 33–38. [Google Scholar] [CrossRef]
- Luo, M.; Gao, J.; Liu, R.; Wang, S.; Wang, G. Morphological and anatomical changes during dormancy break of the seeds of Fritillaria taipaiensis. Plant Signal. Behav. 2023, 18, 2194748. [Google Scholar] [CrossRef]
- Gashi, B.; Osmani, M.; Aliu, S. Breaking seed dormancy of Tulipa scardica Bornm. and Tulipa kosovarica Kit Tan, Shuka & Krasniqi by pre-chilling, plant growth regulators and some chemical treatments. Acta Agric. Slov. 2019, 113, 203–210. [Google Scholar] [CrossRef]
- Zhang, W.; Qu, L.-W.; Zhao, J.; Xue, L.; Dai, H.-P.; Xing, G.-M.; Lei, J.-J. Practical methods for breaking seed dormancy in a wild ornamental tulip species Tulipa thianschanica Regel. Agronomy 2020, 10, 1765. [Google Scholar] [CrossRef]
- van Tuyl, J.; Creij, M.G.M. Tulip: Tulipa gesneriana and Tulipa hybrids. In Flower Breeding & Genetics: Issues, Challenges and Opportunities for the 21st Century; Anderson, N.O., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 623–641. [Google Scholar] [CrossRef]
- Lazare, S.; Zaccai, M. Flowering pathway is regulated by bulb size in Lilium longiflorum (Easter lily). Plant Biol. 2016, 18, 577–584. [Google Scholar] [CrossRef]
- Alsaleh, T.; Awadh, A. The Effect of the Size of the Bulbs and the Type of Fertilizer in the Number and Length of Leaves and Flower Production in Lilium longiflorum. Marsh Bull. 2020, 15, 92–102. Available online: https://www.iasj.net/iasj/download/bd0dc098b3f5c795 (accessed on 3 July 2024).
- Kumar, N.; Prasad, V.; Kerketta, A.; Das, K. Effect of organic and inorganic fertilizer on vegetative growth parameter of Lilium (Lilium longiflorum) cv. pavia under shade net condition. Int. J. Environ. Clim. Change 2023, 13, 323–328. [Google Scholar] [CrossRef]
- Niedziela, C.E.; Kim, S.H.; Nelson, P.V.; De Hertogh, A.A. Effects of N–P–K deficiency and temperature regime on the growth and development of Lilium longiflorum ‘Nellie White’ during bulb production under phytotron conditions. Sci. Hortic. 2008, 116, 430–436. [Google Scholar] [CrossRef]
- Wang, Y.; Bu, H.; Wang, H.; Zhang, P.; Jin, L. Effects of applying nitrogen and potassium on Lilium lancifolium growth and accumulation of secondary metabolites in bulbs. Horticulturae 2023, 9, 396. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).