Abstract
Tomato (Solanum lycopersicum L.) is widely grown in the tropics, where its production is subjected to heavy disease losses. A goal of tomato breeders is genetic improvement of early maturity genotypes with higher fruit quality under challenging environmental conditions, such as the presence of multiple pathogens, is the goal of tomato breeders. In Mexico, tomato is one of the main exported vegetables, grown in most of the northwestern states of the country, with the state of Sinaloa as one of the largest producers. In this study, we evaluated fruit quality parameters in 16 tomato hybrids (14 hybrids under development in Sinaloa and 2 as commercial lines), which were previously analyzed with molecular markers to detect gene resistance. The hybrids were harvested at the “red ripe” stage at three different harvest dates. Total soluble solids (TSS), titratable acidity, pH, color, firmness, and the TSS/acidity ratio were evaluated. Of the 16 hybrids analyzed, 2 showed the presence of genes for resistance to TYLCV, 7 for resistance to TSWV and Fol race 3, 15 for resistance to Fol race 2, and all 16 for resistance to Fol race l. Results show that most of the tomato hybrids analyzed during the three harvest dates met market standards reported in the USDA’s fresh tomato import regulations and Mexico Supreme Quality 2005 (MCS Mexico Calidad Suprema for its acronym in Spanish). However, two of the advanced developmental hybrids better met the market requirements and are also maturing early.
1. Introduction
Tomato (Solanum lycopersicum L.) is a diploid plant that belongs to the Solanaceae family, originated in South America, and its domestication occurred in Mexico. The Aztecs first cultivated it. Its name, “tomato”, from the Nahuatl language spoken in the region of Mexico, corresponds to Physalis philadelphica; the tomato itself, “jitomatl”, corresponds to S. lycopersicum [1]. Section Lycopersicon of the genus Solanum contains 13 subspecies: the cultivated tomato, S. lycopersicum, the only domesticated species, and 12 wild species [2]. During its domestication, the species S. lycopercicum lost much of the genetic diversity present in its wild ancestor [3]. Several factors probably contributed to this, such as migration out of the area of origin, gene selection and introgression to obtain a high number of fruits under self-fertilization, and genetic bottlenecks associated with domestication and crop improvement [4]. Crossing between the cultivated variety and wild species with the genetic traits of interest is necessary to recover these lost genes. However, the chances of these favorable genetic combinations occurring by chance and the probability of identifying them without the use of molecular markers could be higher. Therefore, the use of molecular markers for both the selection of wild species and the development of new hybrids is of utmost importance.
Globally, there are both private and public germplasm banks with mandates to collect accessions (wild species and developed cultivars) of tomatoes with specific characteristics such as disease resistance, adaptation to agroecosystems, and regional agricultural systems for subsequent use in the genetic improvement of the cultivated species. Older landraces are also collected to conserve the countries’ national biocultural heritage [5,6]. Germplasm characterization in Mexico is very scarce despite being the center of domestication and high germplasm production. Particularly in northwestern Mexico, there needs to be more material collections.
Tomato crop production and exporting to the United States are two main agricultural activities in Mexico. The United States is a country demanding a quality product, which represents a challenge for Mexican production companies [7]. The maturity date of tomato fruits is an essential factor in defining the price of these fruits in the market. Fruits of ultra-early and early maturing tomato varieties (60–75 days after sowing) with good quality characteristics tend to have higher prices because they are harvested on time when the product is scarce [8]. Tomatoes can only be stored for a short period, forcing producers to meet the demand for them with current production. This causes prices to increase when supply is scarce (winter months) and decrease when supply is abundant (summer months) [9,10]. In addition to seasonal trends, tomato prices can also be highly variable throughout the harvest season due to crop affectations by some pathogens, such as Tomato yellow leaf curl virus (TYLCV), tomato spotted wilt virus (TSWV), and Fusarium oxysporum f. sp. lycopersici (Fol) [11,12,13]. These variations in markets and their effects on grower incomes cause growers to seek pathogen-resistant tomato hybrids that produce early-maturity fruits with good quality.
All the parameters that define the quality of tomatoes are vital for their value in the market. According to [14], the external color is one of the essential characteristics of the quality of any product since the first thing consumers consider when buying a product is the visual aspect; in the case of tomato, for the consumer, the red color is an indicator of taste quality. For this reason, markets usually adopt measures to improve the external quality of the products, such as wax coating, excellent and innovative packaging, etc. Although these measures can be of great help in the first sales, the increase or decrease in subsequent sales depends on the internal quality of the fruit [15], such as internal color, pH, titratable acidity, total soluble solids (TSS), TSS/acidity ratio and pulp firmness [16]. In this research, we first evaluated the fruit quality of 16 tomato hybrids, 14 under development in Sinaloa, Mexico and 2 as commercial lines, which contained genes resistant to the abovementioned pathogens using some PCR protocols optimized by [17]. We evaluated the quality of both external and internal factors (internal and external fruit colors, firmness, total soluble solids, pH, and titratable acidity) of tomato fruits at three maturity dates of harvest to select early ripening varieties that maintain acceptable fruit quality.
2. Materials and Methods
2.1. Plant Materials and Resistant Genes Experiment
Seeds of 16 round tomato hybrids, 14 hybrids under development, and 2 commercial hybrids were used for the trials. Dr. Juan Lopez of Tricar company provided both the developing hybrids and the commercial hybrids in Sinaloa, Mexico. The seeds were sown in 128-cavity polypropylene trays for germination using peat moss as substrate. After that, the trays were covered with black plastic bags to protect the seeds from light during germination. Five days after sowing, the seeds were germinated. The seedlings were kept in the trays until they were sufficiently developed to be transplanted into the greenhouse. They were then grown under greenhouse conditions (daytime temperatures of 21 °C to 27 °C and nighttime temperatures of 16 °C to 18 °C) and irrigated as needed using coconut fiber as a growing medium.
Leaf samples were taken to identify resistance genes present in these hybrids, and optimized PCR protocols validated by [17] were used.
Fruits were harvested at 75, 105, and 140 days after sowing (March, April, and May 2022) and immediately transported to the experimental laboratory (quality laboratory of CIAD-Culiacán), presenting uniform characteristics in color (red stage maturity or color six according to “Color Classification Requirement in United States Standards for Grades of Fresh Tomatoes” chart, published by USDA) and size. Three fruits per hybrid were taken for each sampling date for quality analysis. External factors were evaluated on the same day, and parts of the samples were stored at −20 °C until all internal factors were assessed.
2.2. Harvest Quality Analysis
2.2.1. External and Internal Colors
The external color was measured on the surface or epidermis of the tomato fruit (2 longitudinal readings/sample). The fruits were split in half for the internal color, and the measurement was made on the fruit’s pulp (2 equatorial readings/sample). Three fruits were analyzed for each hybrid. The color of tomato fruits was determined using a Konica CM-700d spectrophotometer (Minolta Inc., Tokyo, Japan), recording the components of hue angle (h°) and chromaticity (chroma) with the program OnColor QC version 5. The chroma has values ranging from 0, corresponding to dark colors, to 100, representing light colors or those with greater luminosity. In the hue angle (h°), the values range from 0 to 360° representing the color of the fruit; for example, counterclockwise, red = 0°, yellow = 90°, green = 180°, blue = 270° and from purple to red in 360°. The chromaticity of the color ranges from 0 to 60, where low values represent impure colors (towards the center of the color circle), while high values represent pure or more intense colors. To interpret the value of the equipment in a* and b* coordinates, the following formulas were used to calculate the chromaticity.
Chromaticity = √a2 + b2
hue angle (h°) = tan −1 (b/a)
2.2.2. pH and Titratable Acidity
The determinations of pH and titratable acidity were carried out according to the methodology proposed by [16]. An extract was obtained by liquefying 10 g of the sample with 50 mL of distilled water previously neutralized to pH seven and filtered through an organza cloth. An aliquot of 50 mL of the extract was taken and titrated with a 0.1 N NaOH solution in a Mettler Toledo Model T-50 automatic titrator until a pH of 8.2 was reached, which indicates acid–base neutralization. The pH was expressed directly prior to titration as hydrogen ions were present in the sample. Three repetitions were made for each hybrid. The results of the titratable acidity were expressed as a percentage of citric acid present in the sample, according to the following formula:
where 0.064 is the milliequivalent of citric acid.
%A.T. = (mL NaOH spent) (N of NaOH) (0.064) (100)/mL of concentrate
2.2.3. Total Soluble Solids
All tomato fruits were harvested at a late stage of ripening (full red color) because the physiological maturity of the fruit at the time of harvest is a determinant of quality as TSS content. As for pH and acidity, the same extract was obtained by liquefying 10 g of the sample with 50 mL of distilled water, which had been previously neutralized to pH seven and filtered through an organza cloth. One to two drops of clear juice were placed on the prism of a Mettler Toledo RM-40 refractometer (Columbus, OH, USA) previously calibrated with distilled water with a range of 0 to 32 °Brix, a resolution of 0.2 °Brix, and a compensated temperature. Between samples, the prism of the refractometer was washed with distilled water and dried before use. The results obtained were multiplied by the dilution factor (water and pulp) and expressed in °Brix. Three repetitions were made for each hybrid. The TSS/acidity ratio was calculated by dividing the TSS percentage by the acidity percentage.
2.2.4. Firmness
For the determination of firmness by puncture, a tetrameter (Chatillon DFGS100, AMETEK Measurement and Calibration Technologies division, Somerset Drive Largo, FL, USA) was used, equipped with a round steel tip of 8 mm diameter, whose force to penetrate the fruit tissue was expressed in Newton (N). A 10 mm insertion was applied on the mesocarp side, using two sides of each tomato fruit (in equidistant zones). Three fruits and two readings per fruit were analyzed for each tomato hybrid.
2.3. Statistical Analysis
A two-factor, completely randomized design with one covariate, was used. The factors were 16 hybrids used in the study and three harvesting dates (75, 105, and 140 days after sowing). The covariate was five resistance genes (I, I-3, Sw-5, Ty-3, I-2). The response variables were total soluble solids (°Brix), firmness (N), internal and external colors (hue angle and chromaticity), pH, and titratable acidity. Three replications (fruits) were analyzed for each hybrid on each harvest date.
3. Results
3.1. Genes Detections
Of the 16 hybrids (14 in development and 2 commercial) analyzed, 2 showed the presence of genes for resistance to TYLCV, 7 for resistance to TSWV and Fol race 3, 15 for resistance to Fol race 2, and all 16 for resistance to Fol race 1 (Table 1).

Table 1.
Commercial and non-commercial tomato materials were used in this study.
3.2. Analysis of Internal and External Color
In all the hybrids, the external color values of the hue angle were found between 30 and 60°, which means that the tomato fruit’s color is red, and the values of chromaticity were between 30 and 40, which means that the red color is intense (Figure 1A–C). In the case of the internal color, the values of the hue angle were between 18 and 32, and the values of the chromaticity were between 18 and 30 (Figure 1D–F). These data indicate that the internal color is also red but less intense than the peel or external color. The closer the chromaticity values are to 60, the purer or more intense the color. The developing hybrid 117 had the highest external chromaticity values during the three harvest dates between 39 and 35, and the developing hybrid 118 had the highest internal chromaticity values during the three harvest dates between 31 and 27, while the commercial hybrid T142 had the lowest external and internal chromaticity values between 31 and 28 and 21 and 18, respectively (Figure 2A,C). On the first and second harvest dates, the hue angle values were lower compared to the third harvest date. In the third harvest date for most of the hybrids, the external and internal hue angle values were the highest, which means that during this harvest date, the tomato fruits were less red than in the previous harvest date (Figure 2B,D).
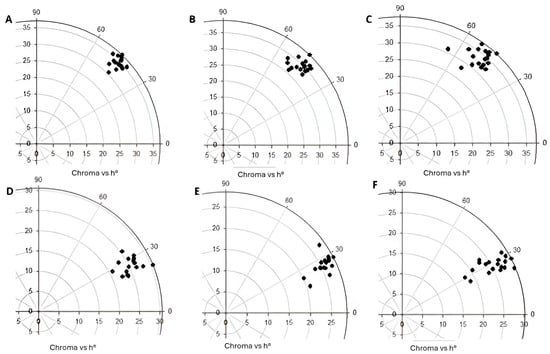
Figure 1.
Polar plot of the external and internal colors (h° & chroma) for the three harvest dates. (A) An external color of the first harvest date; (B) the external color of the second harvest date; (C) the external color of the third harvest date; (D) the internal color of the first harvest date; (E) the internal color of the second harvest date; (F) the internal color of the third harvest date.
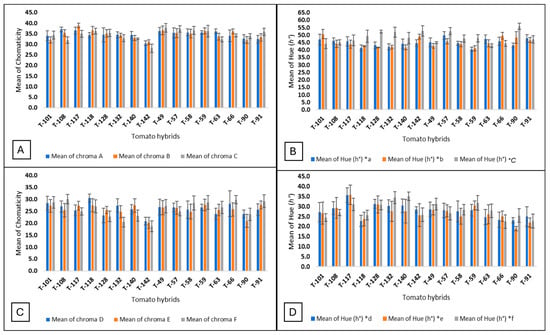
Figure 2.
Bar plots of the external and internal chromaticity and hue angle for the three harvest dates. (A) External chromaticity; (B) external hue angle; (C) internal chromaticity; (D) internal hue angle (chroma A, external chromaticity of the harvest date; chroma B, external chromaticity of the second harvest date; chroma C, external chromaticity of the third harvest date; chroma D, internal chromaticity of harvest date; chroma E, internal chromaticity of the second harvest date; chroma F, internal chromaticity of the third harvest date) (hue (h°) a*, external angle hue of the first harvest date; hue (h°) b*, external angle hue of the second harvest date; hue (h°) C*, external angle hue of the third harvest date; hue (h°) *d, internal angle hue of the first harvest date; hue (h°) *e, internal angle hue of the second harvest date; hue (h°) *f, internal angle hue of the third harvest date).
3.3. Analysis of Total Soluble Solids, Firmness, pH and Titratable Acidity
Even though no statistically significant differences were observed in the amount of TSS among the three dates of harvest (Table 2), in the T-128, T-132, and T-140 hybrids, an increased TSS content was observed on the second harvest date from 6 to 7 °Brix, but on the third harvest date the TSS of T-132 and T-140 was reduced to 6 °Brix except for T-128, which remained at seven °Brix (Figure 3A (Supplementary Table S1). Fruit firmness was the parameter that showed the greatest variation during the three harvesting dates. On the first date, it ranged from 4.7 to 9.5 N; on the second, from 5.6 to 10.8 N; and on the third, from 7.0 to 14.8 N. The hybrids T-128, T-140, and T-142 were the ones with the highest firmness values on the third harvesting date, with 14.8, 14.3, and 14.2 N, respectively (Figure 3B) (Supplementary Table S2).

Table 2.
Analysis of variance titratable acidity, total soluble solid, firmness, pH, external and internal chromaticity, and hue angle.
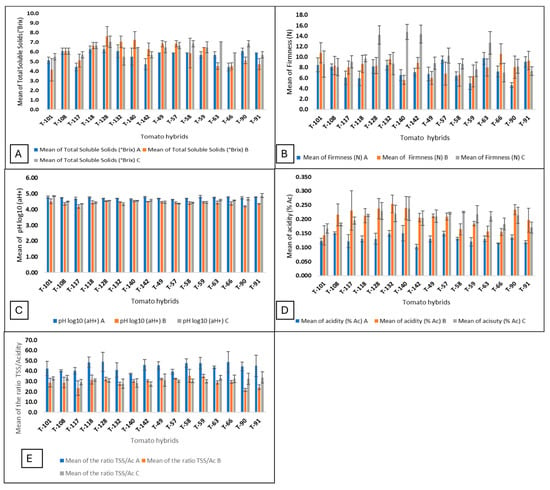
Figure 3.
Bar plots of the three harvest dates. (A) Total soluble solid; (B) firmness; (C), pH; (D) acidity; (E) ratio TSS/acidity (A, first harvest date; B, second harvest date; C, third harvest date).
The value of pH decreased in almost all hybrids on the second date of harvest. pH intervals varied between 4.62 to 4.79 and 4.18 to 4.53. The T-140 hybrid was the only one that maintained a constant pH in the three harvesting dates (Figure 3C) (Supplementary Table S3). An increased percentage of acidity was also observed on the second and third harvesting dates, ranging between 0.119 and 0.151% of citric acid to a range between 0.143 and 0.245% (Figure 3D) (Supplementary Table S4). The ratio of total soluble solids to titratable acidity is important in defining flavor differences between tomato hybrids and affects consumer acceptability. The highest values of the TSS/titratable acidity ratio were observed at the first harvesting date, and all developing hybrids had higher values than the commercial hybrid T-140 (Figure 3E) (Supplementary Table S5).
4. Discussion
In the case of tomatoes, the red color is an indicator of taste quality for the consumer [19]. Based on the hue angle values ranging from 0 to 360° representing the color of the fruit where the red color is represented by values below 50°, the closer the hue angle values of tomato fruit are to 0, the redder it is [17]. In the third harvest date for most of the hybrids, both external and internal hue angle values were highest, which means that during this harvest date, the tomato fruits were less red than in the previous harvest dates. That could be because the plants are no longer producing the same amounts of ethylene due to their age [20]. Our values of hue angle and chromaticity were like the values observed by other authors [21]. The increase in TSS in tomato fruit is due to the breakdown of the starch, pectin, and sucrose chain present in the fruit at the green to ripe stage, where this phenomenon provides individual glucose and fructose structures in ripe fruits originating the sweet taste [22,23]. Ref. [24] mentioned that the titratable acidity value of tomato ranges between 0.34 and 0.35%; however, this value tends to decrease as the ripening process advances. In this work, we obtained a lower acidity value than the above-mentioned, which could be due to the ripening stage at which we harvested the tomato fruits, which were red-ripe. Taste and flavor are increasingly recognized as key components of tomato marketability, so more emphasis is now being placed on improving traits such as TSS, acidity, and pH content in tomatoes. TSS is one of the most important factors that determines fruit quality and affects the preferences of consumers [16,23]. That is why, regardless of the date of harvest, TSS should be stable in hybrids that are going to the fresh market. For tomatoes, several authors indicate an average pH between 3.78 and 5.25 [25,26,27,28,29]. Ref. [30] mentions that the pH of ripe tomatoes can exceed 4.6. In our study, no significant differences were observed between the TSS of three harvesting dates. Our pH values exceed 4.6 but are within the range mentioned by those authors.
Fruit firmness is another of the most important factors in determining tomato quality. The USDA mentions that tomato fruits must be firm to be marketable [29]. We confirmed that most of the hybrid tomatoes analyzed in this study met market standards in this respect, as most of our firmness values were similar or higher than those found by other authors [31,32]. It is very difficult to determine the texture of the inner structure of the fruits by hand, even if some fruit looks very firm when touched by hand, so to determine the exact firmness of the fruits, a tetrameter is needed [17]. The fruit firmness values of this study were higher than those obtained by [32], who found an increase in firmness from 4.71 to 7.28 N when they analyzed three different hybrids in five different maturity stages. According to [33], ripe tomato fruits with a firmness equal to or greater than 11 Newtons are considered very firm and, consequently, have a longer shelf life. In reference to the TSS/acidity ratio, it resulted in higher values in fruits with red aril; this factor is determined by the sweet and sour balance of the fruit [34]. An increase in these two organic constituents results in a consequent increase in flavor intensity. Particularly when sugar concentration increases, flavor acceptability increases significantly. A high value of this ratio correlates with a mild flavor, while low values correlate with a sour flavor. Consumer acceptability tends to decrease with acid added at a given sugar concentration [35]. Many countries have developed different quality standards to facilitate and ensure the exchange of products and to define with precision the main aspects involved. For example, the United States government reports its standards for the importation of fresh tomatoes [29]. In the case of Mexico, the MCS tomato standard [28] indicates the different requirements of the product for sale to the consumer. Based on these two standards, the tomato hybrids that we analyzed during the three harvest dates met market standards. Statistically significant differences were observed among the three dates of harvest for these parameters: firmness, pH, titratable acidity, and external color. No statistically significant differences were observed in the number of pathogen resistance genes present in the hybrids, nor was there any possibility that these genes would affect the quality of tomato fruit produced by these hybrids on the three harvest dates. The tomato hybrids analyzed maintain high fruit quality regardless of the number of resistance genes. This result was expected because these hybrids are advanced lines that were already genetically cleaned up to maintain quality after gene introgression.
5. Conclusions
The results show that hybrids T-128 and T-132 best meet the requirements for the fresh market and are in early maturation. This study confirmed that most of the tomato hybrids analyzed during the three harvesting dates met the United States and Mexico standards to be sold to consumers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10080839/s1.
Author Contributions
Conceptualization, R.L., C.V. and J.L.-F.; Methodology, R.L., J.B.V.-T., R.S.G.-E. and M.A.B.S.; Software, R.L. and J.B.V.-T.; Validation, R.L., J.B.V.-T., R.S.G.-E. and M.A.B.S.; Formal analysis, R.L., J.B.V.-T., M.A.B.S. and J.L.-F.; Investigation, R.L. and M.J.E.-A.; Resources, R.S.G.-E., M.A.B.S. and J.L.-F.; Data curation, R.L., J.B.V.-T. and M.A.B.S.; Writing—original draft, R.L.; Writing—review and editing, R.L., C.V., J.B.V.-T., R.S.G.-E., M.A.B.S., M.J.E.-A. and J.L.-F.; Visualization, C.V., M.A.B.S., M.J.E.-A. and J.L.-F.; Supervision, C.V., J.B.V.-T., R.S.G.-E., M.J.E.-A. and J.L.-F.; Project administration, J.L.-F.; Funding acquisition, J.L.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Centro de Investigación en Alimentación y Desarrollo, A. C. Culiacán, grant number 2018110477.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to Juan Lopez Santiago for providing infrastructure and technical support for the development of this research. They also thank Rosalba Contreras Martínez and Jesús Héctor Carrillo Yáñez for their support in the technical procedures.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Jenkins, J.A. The origin of the cultivated tomato. Econ. Bot. 1948, 2, 379–392. [Google Scholar] [CrossRef]
- Blancard, D.; Laterrot, H.; Marchoux, G.; Candresse, T. Enfermedades del Tomate: Identificar, Conocer, Controlar; Mundi-Prensa México S.A de C.V.: Mexico City, México, 2011. [Google Scholar]
- Peralta, I.; Spooner, D.M.; Knapp, S. Taxonomy of wild tomatoes and their relatives (Solanum sections lycopersicoides, Juglandilolia, Lycopersicon; Solanaceae). Syst. Bot. Monogr. 2008, 84, 1–186. [Google Scholar]
- Tanksley, S.D.; McCouch, S.R. Seed banks and molecular maps: Unlocking genetic potential from the wild. Science 1997, 277, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, H. Directorio de Colecciones de Germoplasma en América Latina y el Caribe, 1st ed.; Colectar las razas nativas más antiguas, con el fin de conservar el patrimonio nacional biocultural; International Plant Genetic Resources Institute (IPGRI): Rome, Italy, 2000. [Google Scholar]
- Engels, J.M.; Visser, L. Guía para el Manejo Eficaz de un Banco de Germoplasma; Manuales para Bancos de Germoplasma No. 6. (eds); Bioversity International: Rome, Italy, 2007. [Google Scholar]
- SIAP. 2022. Available online: https://nube.siap.gob.mx/gobmx_publicaciones_siap/pag/2022/Panorama-Agroalimentario-2022 (accessed on 10 May 2023).
- Detweiler, A.J.; Noordijk, H.; Bell, N.N.C.; Bubl, C.E. 2014. Available online: https://extension.oregonstate.edu/sites/default/files/documents/ec1333-s.pdf (accessed on 10 May 2023).
- United States Department of Agriculture. Plants Database. 2023. Available online: http://plants.usda.gov (accessed on 5 August 2024).
- FAOSTAT; FAO. 2023. Available online: http://www.fao.org/faostat/es/#data/QC/visualize (accessed on 5 August 2024).
- Ascencio-Álvarez, A.; López-Benítez, A.; Borrego-Escalante, F.; Rodríguez-Herrera, S.; Flores-Olivas, A.; Jiménez-Díaz, F.; Gámez-Vázquez, A. Marchitez Vascular del Tomate: I. Presencia de Razas de Fusarium oxysporum f. sp. lycopersici (Sacc.) Snyder y Hansen en Culiacán, Sinaloa, México. Rev. Mex. Fitopatol. 2008, 26, 114–120. [Google Scholar]
- Al Abdallat, A.M.; Al Debei, H.S.; Asmar, H.; Misbeh, S.; Quraan, A.; Kvarnheden, A. An efficient in vitro-inoculation method for Tomato yellow leaf curl virus. Virol. J. 2010, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Melchor, O.Y.; Guzmán-Uriarte, R.; García-Estrada, R.S.; León-Félix, J.; Josefina, L.F. Geminivirus Transmitidos por Mosca Blanca (Bemisia tabaci) en Tomate, en el Valle Agrícola de Culiacán, Sinaloa. Rev. Mex. Fitopatol. 2011, 29, 109–118. [Google Scholar]
- Francis, F.J. Quality as influenced by color. Food Qual. Prefer. 1995, 6, 149–155. [Google Scholar] [CrossRef]
- Herregods, M. Postharvest market quality preferences for fruit and vegetables. In Proceedings of the XXV International Horticultural Congress, Part 8: Quality of Horticultural Products 518, Brussels, Belgium, 2–7 August 1998; pp. 207–212. [Google Scholar]
- A.O.A.C. Association of Official Analytical Chemists: Official Methods of Analysis, 16th ed.; AOAC: Washington, DC, USA, 1998. [Google Scholar]
- Lafrance, R.; Valdez-Torres, J.B.; Villicaña, C.; García-Estrada, R.S.; Esparza-Araiza, M.J.; León-Félix, J. Response Surface Methodology for Optimization of Multiplex-PCR Protocols for Detection of TYLCV, TSWV and Fol Molecular Markers: Analytical Performance Evaluation. Genes 2023, 14, 337. [Google Scholar] [CrossRef]
- Ahumada, O.; Villalobos, J.R. Operational model for planning the harvest and distribution of perishable agricultural products. Int. J. Prod. Econ. 2011, 133, 677–687. [Google Scholar] [CrossRef]
- Pesaresi, P.; Mizzotti, C.; Colombo, M.; Masiero, S. Genetic regulation and structural changes during tomato fruit development and ripening. Front. Plant Sci. 2014, 5, 82777. [Google Scholar] [CrossRef] [PubMed]
- Liñero, O.; Cidad, M.; Arana, G.; Nguyen, C.; de Diego, A. The use of a standard digital camera as an inexpensive, portable, fast and non-destructive analytical tool to measure colour: Estimation of the ripening stage of tomatoes (Solanum lycopersicum) as a case study. Microchem. J. 2017, 134, 284–288. [Google Scholar] [CrossRef]
- Wills, R.; McGlasson, B.; Graham, D.; Joyce, D. Postharvest: An Introduction to the Physiology & Handling of Fruit, Vegetables & Ornamentals, 4th ed.; Cab International: Wallingford, UK, 1998. [Google Scholar]
- Beckles, D.M. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2012, 63, 129–140. [Google Scholar] [CrossRef]
- Casierra-Posada, F.; Aguilar-Avendaño, Ó.E. Calidad en frutos de tomate (Solanum lycopersicum L.) cosechados en diferentes estados de madurez. Agron. Colomb. 2008, 26, 300–307. [Google Scholar]
- Cantwell, M.; Stoddard, S.; LeStrange, M.; Aegerter, B. Report to the California Tomato Comimision; Tomato variety trials: Postharvest evaluations for 2006. UCCE Fresh Market Tomato Variety 2006 Trial Postharvest Evaluation; UC Davis: Davis, CA, USA, 2007; 16p. [Google Scholar]
- Chamarro, L.J. Anatomía y fisiología de la planta. In El Cultivo del Tomatee; Nuez, F., Ed.; Mundi Prensa: Madrid, Spain, 1995; pp. 43–90. [Google Scholar]
- Davies, J.N.; Huobson, G.E. The constituents of tomato fruit-the influence of environment, nutrition and genotype. CRC Crit. Rev. Food Sci. Nutr. 1981, 15, 205–280. [Google Scholar] [CrossRef] [PubMed]
- Turhan, A.; Seniz, V. Estimation of certain chemical constituents of fruits of selected tomato genotypes grown in turkey. Afr. J. Agric. Res. 2009, 4, 1086–1092. [Google Scholar]
- MCS (Mexico Calidad Suprema) Tomato 2005. PC-020-2005 Pliego de Condiciones Para el Uso de la Marca Oficial México Calidad Suprema en Tomate. SAGARPA, BANCOMEX, SECRETARIA DE ECONOMIA. México D.f. 22p. Available online: http://intranet.dif.cdmx.gob.mx/transparencia/new/art_121/52/_anexos/pliegodecondicionesmanzana.pdf (accessed on 5 August 2024).
- USDA. United States Standards for Grades of Fresh Tomatoes. 1991. Available online: https://www.ams.usda.gov/sites/default/files/media/Tomato_Standard%5B1%5D.pdf (accessed on 5 August 2024).
- García, E.; Barret, M.D. Evaluation of processing tomatoes from two consecutive growing seasons: Quality attributes, peelability and yield. J. Food Process. Preserv. 2006, 30, 20–26. [Google Scholar] [CrossRef]
- Batu, A. Determination of acceptable firmness and colour values of tomatoes. J. Food Eng. 2004, 61, 471–475. [Google Scholar] [CrossRef]
- Meza, N.; Méndez, J.M. Características del fruto de tomate de árbol (Cyphomandra betaceae [Cav.] Sendtn) basadas en la coloración del arilo, en la Zona Andina Venezolana. Rev. Científica UDO Agrícola 2009, 9, 289–294. [Google Scholar]
- Castellanos, J.Z. (Ed.) Manual de Producción de Tomate en Invernadero; Intagri, S.C.: Celaya, México, 2009; 458p. [Google Scholar]
- Martín-Hernández, S.; Ordaz-Chaparro, V.M.; Sánchez-García, P.; Beryl Colinas-Leon, M.T.; Borges-Gómez, L. Calidad de tomate (Solanum lycopersicum L.) producido en hidroponia con diferentes granulometrías de tezontle. Agrociencia 2012, 46, 243–254. [Google Scholar]
- Malundo TM, M.; Shewfelt, R.L.; Scott, J.W. Flavor quality of fresh tomato (Lycopersicon esculentum Mill.) as affected by sugar and acid levels. Postharvest Biol. Technol. 1995, 6, 103–110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).