Nutrient Dynamics and Resource-Use Efficiency in Greenhouse Strawberries: Effects of Control Variables in Closed-Loop Hydroponics
Abstract
:1. Introduction
2. Materials and Methods
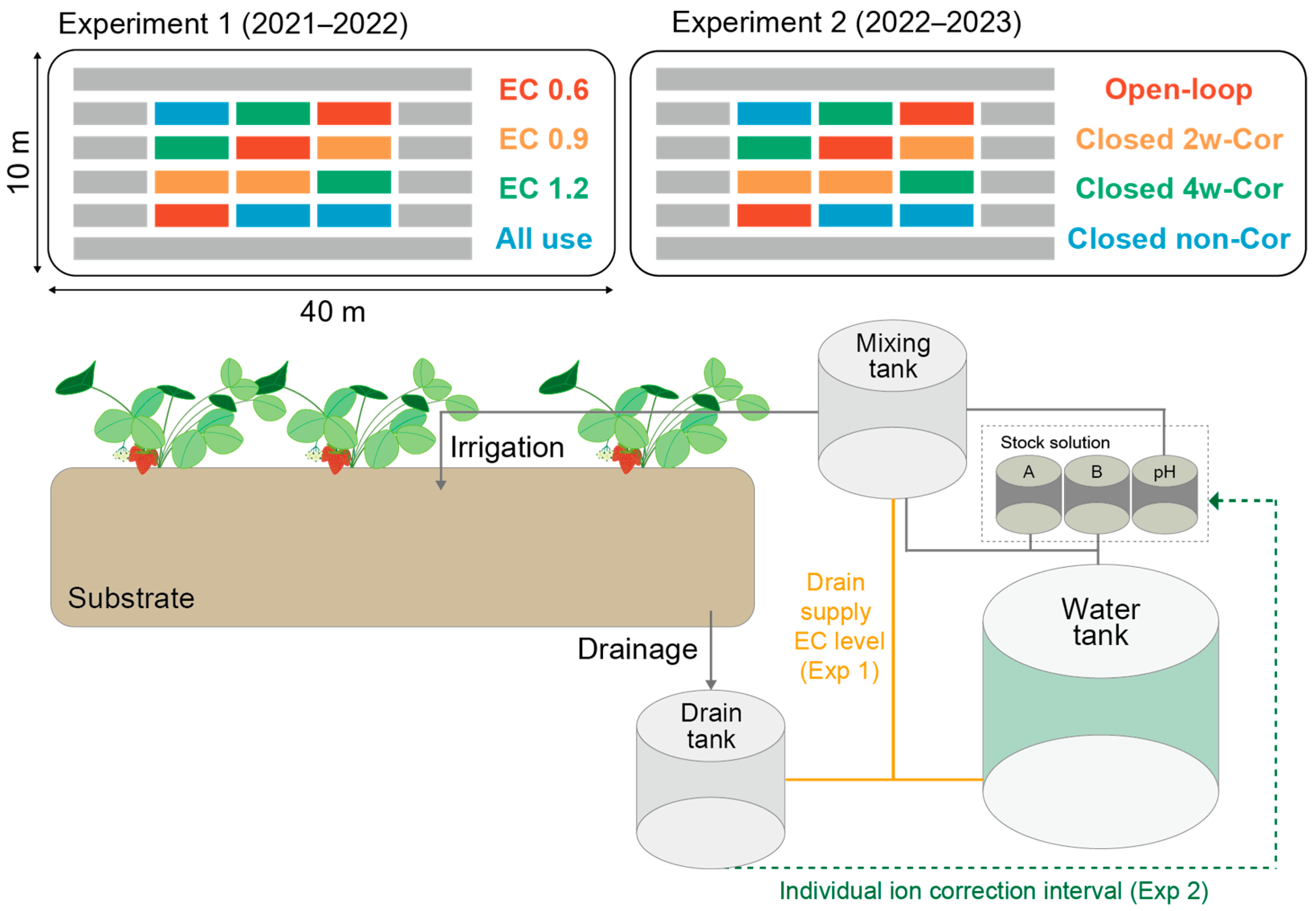
2.1. Closed-Loop Hydroponics Treatment of Experiments 1 and 2
2.2. Preparation of Water Source
2.3. Plant Materials and Growth Conditions
2.4. Root-Zone Ion Component Analysis and Solution Sampling
2.5. Analyses of Plant Growth, Yield, and Fruit Quality
2.6. Calculation of Water and Nutrient Use Efficiency
2.7. Statistical Analyses
3. Results
3.1. Quality of Raw Water in Exp 1 and 2
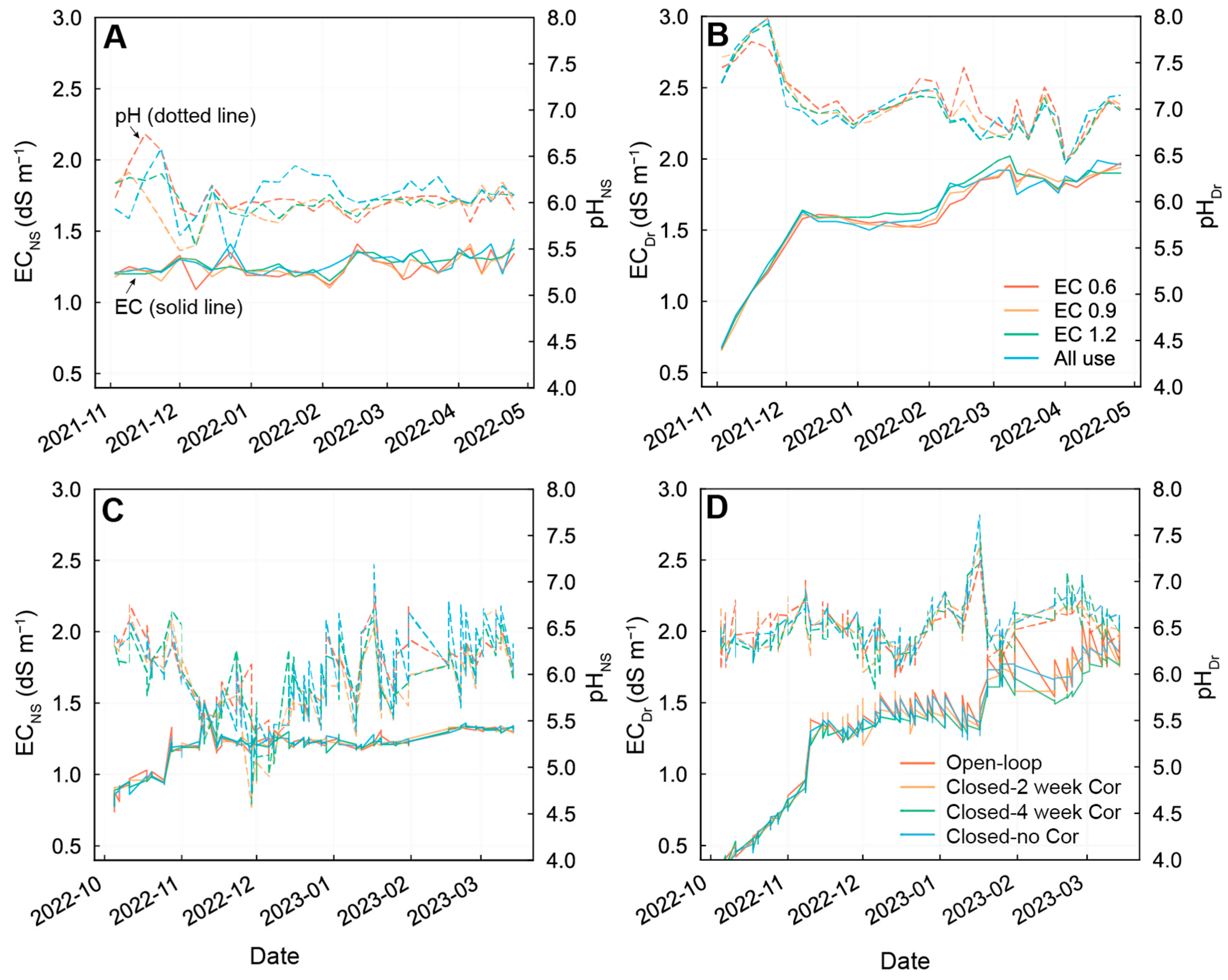
3.2. EC and pH Changes during Cultivation in Exp 1 and 2
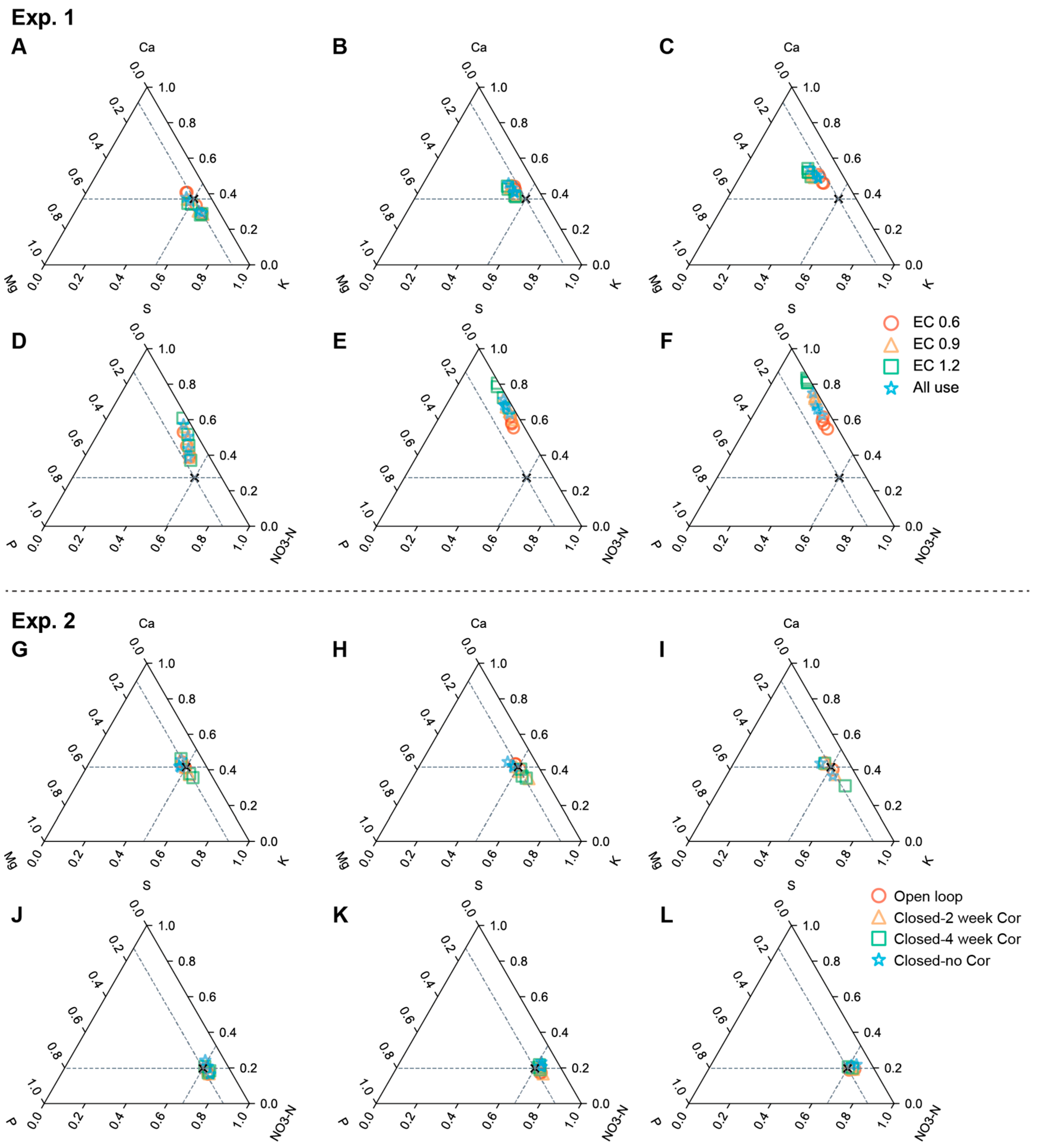
3.3. Ion Balance of NSs in Exp 1 and 2
3.4. Drainage Ion Changes during Cultivation in Exp 1 and 2
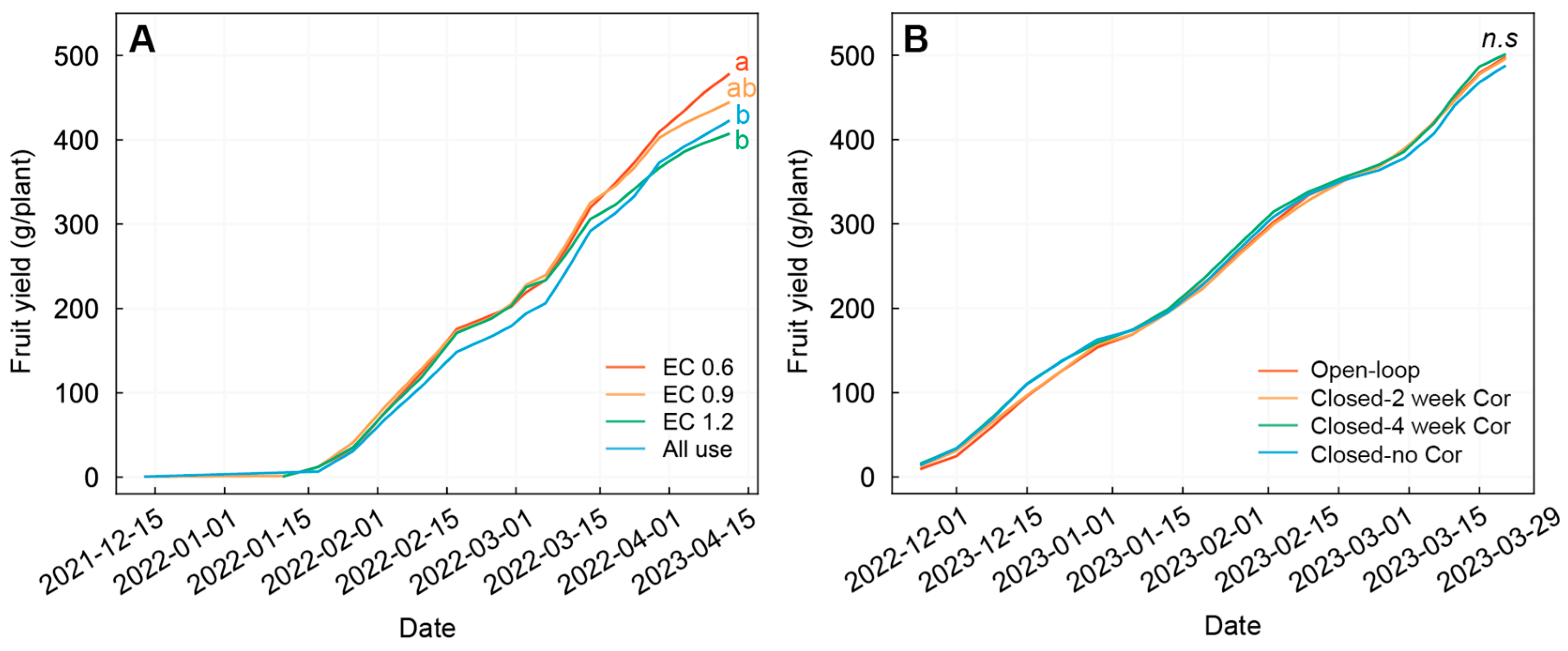
3.5. Strawberry Fruit Yield and Quality
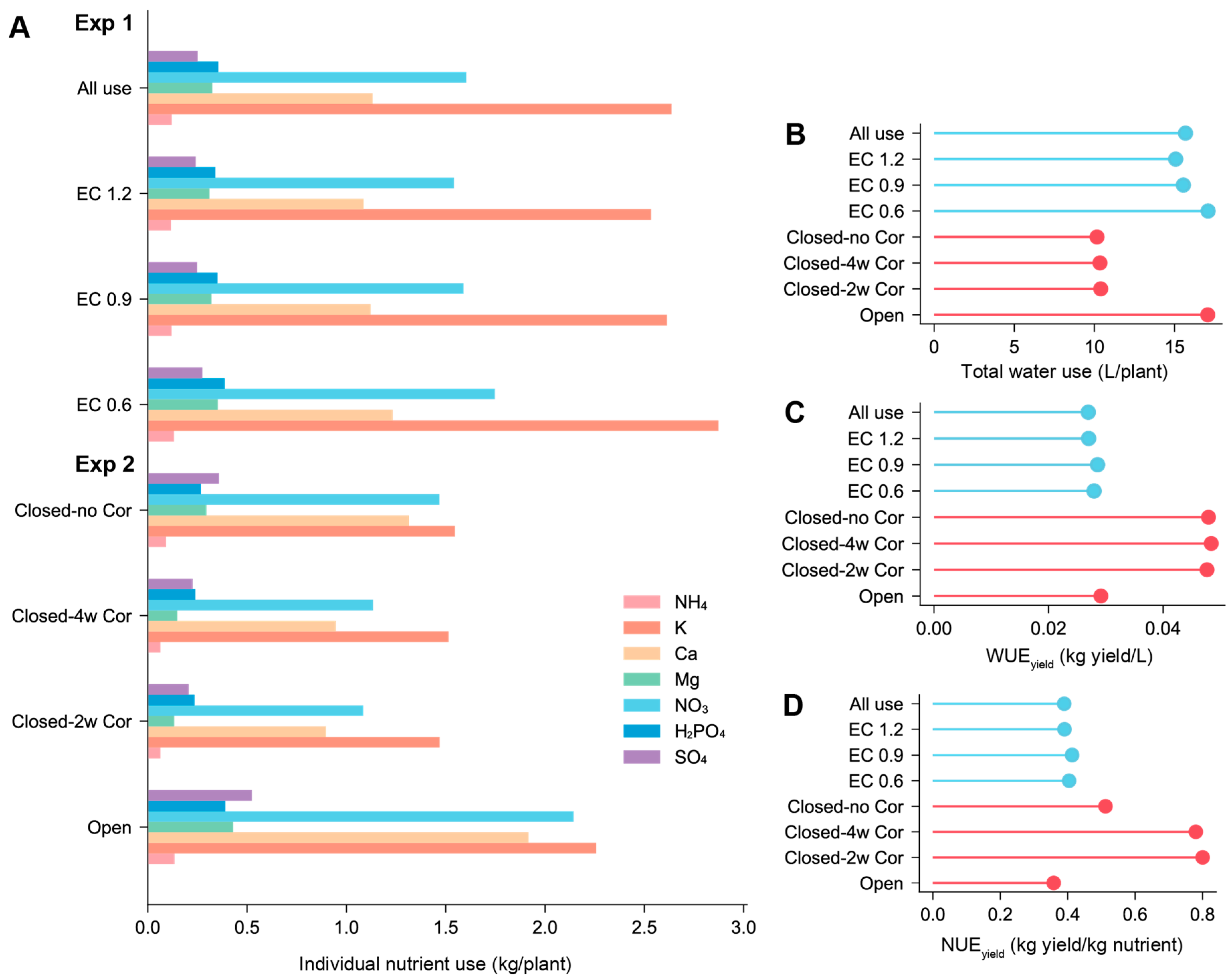
3.6. Accumulated Water and Nutrient Use Based on Closed-Loop Hydroponics
4. Discussion
4.1. Plant Growth According to Hydroponic Treatments
4.2. Root-Zone Ion Variations and Ion-Correction Intervals
4.3. Water and Nutrient Use Efficiency According to Each Closed-Loop Hydroponics Regime
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Massa, D.; Magán, J.J.; Montesano, F.F.; Tzortzakis, N. Minimizing water and nutrient losses from soilless cropping in southern Europe. Agric. Water Manag. 2020, 241, 106395. [Google Scholar] [CrossRef]
- MAFRA (Ministry of Agriculture, Food and Rural Affairs). 2022 Greenhouse Vegetable Status and Vegetable Production. 2023. Available online: https://www.mafra.go.kr/bbs/home/789/568302/artclView.do (accessed on 24 April 2024).
- Hong, S.J.; Eum, H.L. Determination of the harvest date and ripening phase of ‘Seolhyang’strawberry. J. Bio-Environ. Control. 2020, 29, 62–72. [Google Scholar] [CrossRef]
- Lee, Y.H.; Yoon, C.S.; Park, N.I.; Yeoung, Y.R. Influence of various nutrient concentrations on the growth and yield of summer strawberry cultivars cultivated in a hydroponic system. Hortic. Environ. Biotechnol. 2015, 56, 421–426. [Google Scholar] [CrossRef]
- Blok, C.; Voogt, W.; Barbagli, T. Reducing nutrient imbalance in recirculating drainage solution of stone wool grown tomato. Agric. Water Manag. 2023, 285, 108360. [Google Scholar] [CrossRef]
- Massa, D.; Incrocci, L.; Maggini, R.; Bibbiani, C.; Carmassi, G.; Malorgio, F.; Pardossi, A. Simulation of crop water and mineral relations in greenhouse soilless culture. Environ. Model. Softw. 2011, 26, 711–722. [Google Scholar] [CrossRef]
- Massa, D.; Incrocci, L.; Maggini, R.; Carmassi, G.; Campiotti, C.A.; Pardossi, A. Strategies to decrease water drainage and nitrate emission from soilless cultures of greenhouse tomato. Agric. Water Manag. 2010, 97, 971–980. [Google Scholar] [CrossRef]
- Incrocci, L.; Massa, D.; Pardossi, A. New Trends in the Fertigation Management of Irrigated Vegetable Crops. Horticulturae 2017, 3, 37. [Google Scholar] [CrossRef]
- Zekki, H.; Gauthier, L.; Gosselin, A. Growth, Productivity, and Mineral Composition of Hydroponically Cultivated Greenhouse Tomatoes, with or without Nutrient Solution Recycling. J. Am. Soc. Hortic. Sci. 1996, 121, 1082–1088. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G.; Passam, H.C. Plant nutrition and physiological disorders in greenhouse grown tomato, pepper and eggplant. Eur. J. Plant Sci. Biotechnol. 2008, 2, 45–61. [Google Scholar]
- Roosta, H.R.; Afsharipoor, S. Effects of different cultivation media on vegetative growth, ecophysiological traits and nutrients concentration in strawberry under hydroponic and aquaponic cultivation systems. Adv. Environ. Biol. 2012, 6, 543–555. [Google Scholar]
- Neocleous, D.; Savvas, D. Assessment of different strategies to balance high Mg levels in the irrigation water when preparing nutrient solution for soilless strawberry crops. Eur. J. Hortic. Sci. 2013, 78, 267–274. [Google Scholar]
- Neocleous, D.; Savvas, D. Response of hydroponically-grown strawberry (Fragaria × ananassa Duch.) plants to different ratios of K: Ca: Mg in the nutrient solution. J. Hortic. Sci. Biotechnol. 2013, 88, 293–300. [Google Scholar] [CrossRef]
- Savvas, D. SW—Soil and Water: Automated replenishment of recycled greenhouse effluents with individual nutrients in hydroponics by means of two alternative models. Biosyst. Eng. 2002, 83, 225–236. [Google Scholar] [CrossRef]
- Liao, C.F.H. Devarda’s Alloy Method for Total Nitrogen Determination. Soil Sci. Soc. Am. J. 1981, 45, 852–855. [Google Scholar] [CrossRef]
- Ahn, T.I.; Son, J.E. Application of an Alternative Nutrient Replenishment Method to Electrical Conductivity-Based Closed-Loop Soilless Cultures of Sweet Peppers. Horticulturae 2022, 8, 295. [Google Scholar] [CrossRef]
- Ehret, D.; Ho, L. The effects of salinity on dry matter partitioning and fruit growth in tomatoes grown in nutrient film culture. J. Hortic. Sci. 1986, 61, 361–367. [Google Scholar] [CrossRef]
- Trajkova, F.; Papadantonakis, N.; Savvas, D. Comparative Effects of NaCl and CaCl2 Salinity on Cucumber Grown in a Closed Hydroponic System. HortSci 2006, 41, 437–441. [Google Scholar] [CrossRef]
- Carmassi, G.; Incrocci, L.; Maggini, R.; Malorgio, F.; Tognoni, F.; Pardossi, A. Modeling Salinity Build-Up in Recirculating Nutrient Solution Culture. J. Plant Nut. 2005, 28, 431–445. [Google Scholar] [CrossRef]
- Giannothanasis, E.; Spanoudaki, E.; Kinnas, S.; Ntatsi, G.; Voogt, W.; Savvas, D. Development and validation of an innovative algorithm for sodium accumulation management in closed-loop soilless culture systems. Agric. Water Manag. 2024, 301, 108968. [Google Scholar] [CrossRef]
- Le Deunff, E.; Tournier, P.H.; Malagoli, P. The Thermodynamic Flow-Force Interpretation of Root Nutrient Uptake Kinetics: A Powerful Formalism for Agronomic and Phytoplanktonic Models. Front. Physiol. 2016, 7, 243. [Google Scholar] [CrossRef]
- Ahn, T.I.; Shin, J.H.; Son, J.E. Theoretical and Experimental Analyses of Nutrient Control in Electrical Conductivity-Based Nutrient Recycling Soilless Culture System. Front. Plant Sci. 2021, 12, 656403. [Google Scholar] [CrossRef]
- Lee, J.Y.; Rahman, A.; Azam, H.; Kim, H.S.; Kwon, M.J. Characterizing nutrient uptake kinetics for efficient crop production during Solanum lycopersicum var. cerasiforme Alef. growth in a closed indoor hydroponic system. PLoS ONE 2017, 12, 0177041. [Google Scholar] [CrossRef] [PubMed]
- Vardar, G.; Altıkatoğlu, M.; Ortaç, D.; Cemek, M.; Işıldak, İ. Measuring calcium, potassium, and nitrate in plant nutrient solutions using ion-selective electrodes in hydroponic greenhouse of some vegetables. Biotechnol. Appl. Bioc. 2015, 62, 663–668. [Google Scholar] [CrossRef]
- Tan, X.W.; Ikeda, H.; Oda, M. The absorption, translocation, and assimilation of urea, nitrate or ammonium in tomato plants at different plant growth stages in hydroponic culture. Sci. Hortic. 2000, 84, 275–283. [Google Scholar] [CrossRef]
- Xu, X.; Du, X.; Wang, F.; Sha, J.; Chen, Q.; Tian, G.; Zhu, Z.; Ge, S.; Jiang, Y. Effects of potassium levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Front. Plant Sci. 2020, 11, 904. [Google Scholar] [CrossRef]
- Rufí-Salís, M.; Calvo, M.J.; Petit-Boix, A.; Villalba, G.; Gabarrell, X. Exploring nutrient recovery from hydroponics in urban agriculture: An environmental assessment. Resour. Conserv. Recycl. 2020, 155, 104683. [Google Scholar] [CrossRef]
- Rufí-Salís, M.; Petit-Boix, A.; Villalba, G.; Sanjuan-Delmás, D.; Parada, F.; Ercilla-Montserrat, M.; Arcas-Pilz, V.; Muñoz-Liesa, J.; Rieradevall, J.; Gabarrell, X. Recirculating water and nutrients in urban agriculture: An opportunity towards environmental sustainability and water use efficiency? J. Clean. Prod. 2020, 261, 121213. [Google Scholar] [CrossRef]
- Savvas, D.; Giannothanasis, E.; Ntanasi, T.; Karavidas, I.; Ntatsi, G. State of the Art and New Technologies to Recycle the Fertigation Effluents in Closed Soilless Cropping Systems Aiming to Maximise Water and Nutrient Use Efficiency in Greenhouse Crops. Agronomy 2023, 14, 61. [Google Scholar] [CrossRef]
- Katsoulas, N.; Voogt, W. Recent trends in salinity control for soilless growing systems management. In Proceedings of the International Symposium on Growing Media and Soilless Cultivation, Leiden, The Netherlands, 17–21 June 2013; Volume 1034, pp. 433–442. [Google Scholar]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef]

| Macronutrients (mmol L−1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC (dS m−1) | pH | NH4-N | K | Ca | Mg | Na | NO3-N | P | S | Cl | HCO3 | |
| Exp 1 | ||||||||||||
| Raw water 1 | 0.50 | 7.7 | 0.20 | 0.20 | 1.20 | 0.30 | 1.30 | 0.20 | 0.02 | 1.00 | 0.50 | 2.00 |
| Raw water 2 | 0.43 | 7.58 | - | 0.07 | 1.18 | 0.30 | 1.23 | 0.23 | - | 1.07 | 0.48 | 1.44 |
| Solution | 1.20 | 5.80 | 0.75 | 4.50 | 3.00 | 1.15 | - | 7.50 | 0.75 | 1.50 | - | - |
| Exp 2 | ||||||||||||
| Raw water | 0.01 | 6.54 | - | - | - | - | - | - | - | 0.01 | 0.20 | 0.10 |
| Solution | 1.20 | 5.80 | 0.75 | 3.30 | 2.75 | 1.10 | - | 8.77 | 0.75 | 1.12 | - | - |
| Treatment | Fruit Yield (g/Plant) | Individual Fruit Weight (g/Fruit) | Fruit Length (mm/Fruit) | Fruit Width (mm/Fruit) | Firmness (kg LB/Newton) | Total Soluble Sugar (Brix°) | |
|---|---|---|---|---|---|---|---|
| Exp 1 | EC 0.6 | 477 a | 31.0 a | 49.3 a | 41.2 a | 25.8 a | 10.5 a |
| EC 0.9 | 444 ab | 30.8 a | 48.8 a | 40.8 a | 26.0 a | 10.3 a | |
| EC 1.2 | 406 b | 30.3 a | 48.5 a | 40.5 a | 26.3 a | 10.5 a | |
| All use | 422 b | 30.7 a | 49.4 a | 40.3 a | 26.0 a | 10.5 a | |
| Exp 2 | Open-loop | 497 a | 36.0 a | 53.1 a | 42.6 a | 0.47 a | 10.3 a |
| Closed 2w-Cor | 495 a | 35.3 a | 53.0 a | 42.4 a | 0.46 a | 10.2 a | |
| Closed 4w-Cor | 500 a | 35.2 a | 53.1 a | 42.6 a | 0.46 a | 10.4 a | |
| Closed non-Cor | 487 a | 35.5 a | 53.7 a | 42.8 a | 0.46 a | 10.3 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, M.Y.; Kim, S.H.; Roh, M.Y.; Choi, G.L.; Kim, D. Nutrient Dynamics and Resource-Use Efficiency in Greenhouse Strawberries: Effects of Control Variables in Closed-Loop Hydroponics. Horticulturae 2024, 10, 851. https://doi.org/10.3390/horticulturae10080851
Lim MY, Kim SH, Roh MY, Choi GL, Kim D. Nutrient Dynamics and Resource-Use Efficiency in Greenhouse Strawberries: Effects of Control Variables in Closed-Loop Hydroponics. Horticulturae. 2024; 10(8):851. https://doi.org/10.3390/horticulturae10080851
Chicago/Turabian StyleLim, Mi Young, So Hui Kim, Mi Young Roh, Gyeong Lee Choi, and Dongpil Kim. 2024. "Nutrient Dynamics and Resource-Use Efficiency in Greenhouse Strawberries: Effects of Control Variables in Closed-Loop Hydroponics" Horticulturae 10, no. 8: 851. https://doi.org/10.3390/horticulturae10080851






