Potentially Bioactive Compounds and Sensory Compounds in By-Products of Several Cultivars of Blackberry (Rubus fruticosus L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reference Substances
2.2. Blackberry Pomace
2.3. Analysis of Total Polyphenolic Compounds (TPC)
2.4. Determination of Total Flavonoid Content (TFC)
2.5. Assay for Antioxidant Activity ABTS•+ Discoloration Method
2.6. Analysis of DPPH• Radical-Scavenging Activity (DPPH•-RSA)
2.7. Determination of Polyphenolic Compounds by HPLC
2.8. Anthocyanins Determination by HPLC
2.9. Organic Acids and Sugars Determination by HPLC
2.10. Analysis of Volatile Compounds
2.11. Statistical Analysis
3. Results
3.1. Total Polyphenol Levels, Flavonoid Concentrations, and Antioxidant Activities (DPPH• and ABTS•+) in Blackberry Pomace
3.2. Phenolics Profile in Blackberry Pomace
3.3. Organic Acids and Sugars in Blackberry Pomace
3.4. VOCs Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Di Vittori, L.; Mazzoni, L.; Battino, M.; Mezzetti, B. Pre-harvest factors influencing the quality of berries. Sci. Hortic. 2018, 233, 310–322. [Google Scholar] [CrossRef]
- Vaillant, F. Blackberries. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Academic Press: Cambridge, MA, USA, 2020; Volume 25, pp. 407–422. [Google Scholar] [CrossRef]
- Rios de Souza, V.; Pimenta Pereira, P.A.; Marques Pinheiro, A.C.; de Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Analysis of the Subtropical Blackberry Cultivar Potential in Jelly Processing. J. Food Sci. 2014, 79, S1776–S1781. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Stobiecka, A.; Bonikowski, R.; Krajewska, A.; Sikora, M.; Kula, J. A comparative study on composition and antioxidant activities of supercritical carbon dioxide, hexane and ethanol extracts from Blackberry (Rubus fruticosus) growing in Poland. Sci. Food Agric. 2017, 97, 3576–3583. [Google Scholar] [CrossRef]
- Kaume, L.; Howard, L.R.; Devareddy, L. The Blackberry fruit: A review onits composition and chemistry, metabolism and bioavailability, and health benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef]
- Van Hoed, V.; De Clercq, N.; Echim, C.; Andjelkovic, M.; Leber, E.; Dewettinck, K.; Verhé, R. Berry seeds: A source of specialty oils with high content of bioactives and nutritional value. J. Food Lipids 2009, 16, 33–49. [Google Scholar] [CrossRef]
- Struck, S.; Plaza, M.; Turner, C.; Rohm, H. Berry pomace—A review of processing and chemical analysis of its polyphenols. Int. J. Food Sci. Technol. 2016, 51, 1305–1318. [Google Scholar] [CrossRef]
- Krivokapić, S.; Vlaović, M.; Damjanović Vratnica, B.; Perović, A.; Perović, S. Biowaste as a Potential Source of Bioactive Compounds—A Case Study of Raspberry Fruit Pomace. Foods 2021, 10, 706. [Google Scholar] [CrossRef]
- Pinto, T.; Vilela, A.; Pinto, A.; Nunes, F.M.; Cosme, F.; Anjos, R. Influence of cultivar and of conventional and organic agricultural practices on phenolic and sensory profile of blackberries (Rubus fruticosus). Sci. Food Ant. Agric. 2018, 98, 12. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Santisteban, A.; Gordillo, B.; Hernanz, D.; Heredia, F.J.; Escudero-Gilete, M.L. Comparative study of red berry pomaces (Blueberry, red Raspberry, red Currant and Blackberry) as source of antioxidants and pigments. Eur. Food Res. Technol. 2019, 245, 1–9. [Google Scholar] [CrossRef]
- Chanjirakul, K.; Wang, S.Y.; Wang, C.Y.; Siriphanich, J. Natural volatile treatments increase free-radical scavenging capacity of strawberries and blackberries. J. Sci. Food Agric. 2007, 87, 1463–1472. [Google Scholar] [CrossRef]
- Ali, L.; Svensson, B.; Alsanius, B.W.; Olsson, M.E. Late season harvest and storage of Rubus berries—Major antioxidant and sugar levels. Sci. Hortic. 2011, 129, 376–381. [Google Scholar] [CrossRef]
- Klavins, M.; Kukela, A.; Kviesis, J.; Klavins, L. Valorisation of Berry Pomace: From Waste to Bioactive Compounds. In Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions; Springer Nature: Berlin/Heidelberg, Germany, 2018; pp. 1145–1146. [Google Scholar] [CrossRef]
- Akin, M.; Eyduran, S.P.; Ercisli, S.; Kapchina-Toteva, V.; Eyduran, E. Phytochemical profiles of wild blackberries, black and white mulberries from southern Bulgaria. Biotechnol. Biotechnol. Equip. 2016, 30, 899–906. [Google Scholar] [CrossRef]
- Milivojević, J.; Maksimović, V.; Nikolić, M.; Bogdanović, J.; Maletić, R.; Milatović, D. Chemical and Antioxidant Properties of Cultivated and Wild Fragaria and Rubus Berries. J. Food Qual. 2011, 34, 1–9. [Google Scholar] [CrossRef]
- Kafkas, E.; Koşar, M.; Türemiş, N.; Başer, K.H.C. Analysis of sugars, organic acids and vitamin C contents of Blackberry genotypes from Turkey. Food Chem. 2006, 97, 732–736. [Google Scholar] [CrossRef]
- Ganganelli, I.; Molina Agostini, M.C.; Galatro, A.; Gergoff Grozeff, G.E. Specific wavelength LED light pulses modify vitamin C and organic acids content in Raspberry and Blackberry fruit during postharvest. J. Hortic. Sci. Biotechnol. 2023, 98, 649–661. [Google Scholar] [CrossRef]
- Turemis, N.; Kafkas, E.; Kafkas, S. Determination of Aroma Compounds in Blackberry by GC/MS Analysis. Chem. Nat. Compd. 2003, 39, 174–176. [Google Scholar] [CrossRef]
- Qian, M.C.; Wang, Y. Seasonal Variation of Volatile Composition and Odor Activity Value of ‘Marion’ (Rubus spp. hyb) and Thornless Evergreen (R. laciniatus L.) Blackberries. J. Food Sci. 2006, 70, 13–20. [Google Scholar] [CrossRef]
- Padilla-Jimenez, S.; Angoa-Pérez, M.V.; Mena-Violante, H.G.; Oyoque-Salcedo, G.; Renteria-Ortega, M.; Oregel-Zamudio, E. Changes in the Aroma of Organic Blackberries (Rubus fruticosus) During Ripeness. Anal. Chem. Lett. 2019, 9, 64–73. [Google Scholar] [CrossRef]
- Yamashita, C.; Chung, M.M.S.; dos Santos, C.; Mayer, C.R.M.; Moraes, I.C.F.; Branco, I.G. Microencapsulation of an anthocyanin-rich Blackberry (Rubus spp.) by-product extract by freeze-drying. LWT 2017, 84, 256–262. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from Blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- Kitrytė, V.; Narkevičiūtė, A.; Tamkutė, L.; Syrpas, M.; Pukalskienė, M.; Venskutonis, P.R. Consecutive high-pressure and enzyme assisted fractionation of Blackberry (Rubus fruticosus L.) pomace into functional ingredients: Process optimization and product characterization. Food Chem. 2020, 312, 126072. [Google Scholar] [CrossRef]
- Llobera, A.; Cañellas, J. Dietary fibre content and antioxidant activity of Manto Negro red grape (Vitis vinifera): Pomace and stem. Food Chem. 2007, 101, 659–666. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Viškelis, P.; Venskutonis, P.R. Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various Raspberry (Rubus spp.) cultivars. Food Chem. 2012, 132, 1495–1501. [Google Scholar] [CrossRef]
- Urbonavičienė, D.; Bobinas, Č.; Bobinaitė, R.; Raudonė, L.; Trumbeckaitė, S.; Viškelis, J.; Viškelis, P. Composition and Antioxidant Activity, Supercritical Carbon Dioxide Extraction Extracts, and Residue after Extraction of Biologically Active Compounds from Freeze-Dried Tomato Matrix. Processes 2021, 9, 467. [Google Scholar] [CrossRef]
- Albert, C.; Codin, G.G.; Héjja, M.; András, C.D.; Chetrariu, A.; Dabija, A. Study of Antioxidant Activity of Garden Blackberries (Rubus fruticosus L.) Extracts Obtained with Different Extraction Solvents. Appl. Sci. 2022, 12, 4004. [Google Scholar] [CrossRef]
- Głowacka, A.; Rozpara, E.; Hallmann, E. The dynamic of polyphenols concentrations in organic and conventional sour cherry fruits: Results of a 4-year field study. Molecules 2020, 25, 3729. [Google Scholar] [CrossRef]
- Ponder, A.; Frąckowiak, M.; Kruk, M.; Hallmann, E. Estimation of chemical compounds in selected Italian and French wines produced through organic and conventional methods. Appl. Sci. 2024, 14, 2466. [Google Scholar] [CrossRef]
- Xie, R.; Tu, M.; Wu, Y.; Adhikari, S. Improvement in HPLC separation of acetic acid and levulinic acid in the profiling of biomass hydrolysate. Bioresour. Technol. 2011, 102, 4938–4942. [Google Scholar] [CrossRef]
- Wojtasik-Kalinowska, I.; Guzek, D.; Gorska-Horczyczak, E.; Głabska, D.; Brodowska, M.; Sun, D.W.; Wierzbicka, A. Volatile compounds and fatty acids profile in Longissimus dorsi muscle from pigs fed with feed containing bioactive components. LWT 2016, 67, 112–117. [Google Scholar] [CrossRef]
- Blejan, A.M.; Nour, V.; Păcularu-Burada, B.; Popescu, S.M. Wild Bilberry, Blackcurrant, and Blackberry by-products as a source of nutritional and bioactive compounds. Int. J. Food Prop. 2023, 26, 1579–1595. [Google Scholar] [CrossRef]
- Miodrag, J.R.; Vulić, J.; Kukrić, Z.; Topalić-Trivunović, L.N.; Aleksandar, S.V. Chemical composition, biological potentials and antimicrobial activity of wild and cultivated blackberries. Acta Period. Technol. 2018, 49, 65–79. [Google Scholar] [CrossRef]
- Paun, N.; Botoran, O.R.; Niculescu, V.-C. Total Phenolic, Anthocyanins HPLC-DAD-MS Determination and Antioxidant Capacity in Black Grape Skins and Blackberries: A Comparative Study. Appl. Sci. 2022, 12, 936. [Google Scholar] [CrossRef]
- Zafra-Rojas, Q.; Cruz-Cansino, N.; Delgadillo-Ramírez, A.; Alanís-García, E.; Añorve-Morga, J.; Quintero-Lira, A.; Castañeda-Ovando, A.; Ramírez-Moreno, E. Organic Acids, Antioxidants, and Dietary Fiber of Mexican Blackberry (Rubus fruticosus) Residues cv. Tupy. J. Food Qual. 2018, 9, 5950761. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Sariburun, E.; Şahin, S.; Demir, C.; Türkben, C.; Uylaşer, V. Phenolic Content and Antioxidant Activity of Raspberry and Blackberry Cultivars. J. Food Sci. 2010, 75, C328–C335. [Google Scholar] [CrossRef]
- Gulcin, I.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Lee, K.J.; Oh, J.C.; Cho, W.K.; Ma, J.Y. Antioxidant and Anti-Inflammatory Activity Determination of One Hundred Kinds of Pure Chemical Compounds Using Offline and Online Screening HPLC Assay. Evid. Based Complement. Altern. Med. 2015, 13, 165457. [Google Scholar] [CrossRef]
- Kalušević, A.; Salević, A.; Đorđević, R.; Veljović, M.; Nedović, V.A. Raspberry and Blackberry pomaces as potential sources of bioactive compounds. Ukr. Food J. 2016, 5, 485–491. [Google Scholar] [CrossRef]
- Gil-Martínez, L.; Mut-Salud, N.; Ruiz-García, J.A.; Falcón-Piñeiro, A.; Maijó-Ferré, M.; Baños, A.; De la Torre-Ramírez, J.M.; Guillamón, E.; Verardo, V.; Gómez-Caravaca, A.M. Phytochemicals Determination, and Antioxidant, Antimicrobial, Anti-Inflammatory and Anticancer Activities of Blackberry Fruits. Foods 2023, 12, 1505. [Google Scholar] [CrossRef]
- Herrmann, K.; Nagel, C.W. Occurrence and content of hydroxycinnamic and hydroxybenzoic acid compounds in foods. Crit. Rev. Food Sci. Nutr. 1989, 28, 315–347. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of Blackberry and Raspberry fruits. J. Food Comp. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.H.; El-Mahdy, M.A.; Abd-Ellah, M.F.; Helal, G.K.; Khalifa, F.; Hamada, F.M.A. Influence of p-coumaric acid on doxorubicin-induced oxidative stress in rat’s heart. Pharmacol. Res. 2003, 48, 461–465. [Google Scholar] [CrossRef]
- Hakyemez, I.N.; Cevizci, M.N.; Aksoz, E.; Yilmaz, K.; Uysal, S.; Altun, E. Protective effects of p-coumaric acid against gentamicin-induced nephrotoxicity in rats. Drug Chem. Toxicol. 2021, 45, 2825–2832. [Google Scholar] [CrossRef]
- Ntamo, Y.; Jack, B.; Ziqubu, K.; Mazibuko-Mbeje, S.E.; Nkambule, B.B.; Nyambuya, T.M.; Mabhida, S.E.; Hanser, S.; Orlando, P.; Tiano, L. Epigallocatechin gallate as a nutraceutical to potentially target the metabolic syndrome: Novel insights into therapeutic effects beyond its antioxidant and anti-inflammatory properties. Crit. Rev. Food Sci. Nutr. 2022, 64, 87–109. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Latos, M.; Zaborski, M.; Podsedek, A. Antioxidant and Antiradical Properties of Green Tea Extract Compounds. Int. J. Electrochem. Sci. 2017, 12, 6600–6610. [Google Scholar] [CrossRef]
- Kim, H.-S.; Quon, M.J.; Kim, J.-A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Schulz, M.; Seraglio, S.K.T.; Betta, F.D.; Nehring, P.; Valese, A.C.; Daguer, H.; Gonzaga, L.V.; Oliveira Costa, A.C.; Fett, R. Blackberry (Rubus ulmifolius Schott): Chemical composition, phenolic compounds and antioxidant capacity in two edible stages. Food Res. Int. 2019, 122, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Jacques, A.C.; Chaves, F.C.; Zambiazi, R.C.; Brasil, M.C.; Caramão, E.B. Bioactive and volatile organic compounds in Southern Brazilian Blackberry (Rubus fruticosus) fruit cv. Tupy. Food Sci. Technol. 2014, 34, 636–643. [Google Scholar] [CrossRef]
- Li, J.; Shi, C.; Shen, D.; Han, T.; Wu, W.; Lyu, L.; Li, W. Composition and Antioxidant Activity of Anthocyanins and Non-Anthocyanin Flavonoids in Blackberry from Different Growth Stages. Foods 2022, 11, 2902. [Google Scholar] [CrossRef]
- Fan-Chiang, H.J.; Wrolstad, R.E. Anthocyanin pigment composition of blackberries. J. Food Sci. 2005, 70, 198–202. [Google Scholar] [CrossRef]
- Vega, E.N.; Molina, A.K.; Pereira, C.; Dias, M.I.; Heleno, S.A.; Rodrigues, P.; Fernandes, I.P.; Barreiro, M.F.; Stojković, D.; Soković, M.; et al. Anthocyanins from Rubus fruticosus L. and Morus nigra L. Applied as Food Colorants: A Natural Alternative. Plants 2021, 10, 1181. [Google Scholar] [CrossRef]
- Petruskevicius, A.; Viskelis, J.; Urbonaviciene, D.; Viskelis, P. Anthocyanin Accumulation in Berry Fruits and Their Antimicrobial and Antiviral Properties: An Overview. Horticulturae 2023, 9, 288. [Google Scholar] [CrossRef]
- Ştefănuţ, M.N.; Căta, A.; Pop, R.; Moşoarcă, C.; Zamfir, A.D. Anthocyanins HPLC-DAD and MS Characterization, Total Phenolics, and Antioxidant Activity of Some Berries Extracts. Anal. Lett. 2011, 44, 2843–2855. [Google Scholar] [CrossRef]
- Čechovičienė, I.; Šlepetienė, A.; Gumbytė, M.; Paulauskienė, A.; Tarasevičienė, Ž. Composition and Physicochemical Properties of Pomace of Various Cultivars of Blackberry (Rubus fruticosus L.). Horticulturae 2024, 10, 38. [Google Scholar] [CrossRef]
- Hasnaoui, N.; Jbir, R.; Mars, M.; Trifi, M.; Kamal-Eldin, A.; Melgarejo, P.; Hernandez, F. Organic Acids, Sugars, and Anthocyanins Contents in Juices of Tunisian Pomegranate Fruits. Int. J. Food Prop. 2011, 14, 741–757. [Google Scholar] [CrossRef]
- Famiani, F.; Battistelli, A.; Moscatello, S.; Cruz-Castillo, J.G.; Walker, R.P. The organic acids that are accumulated in the flesh of fruits: Occurrence, metabolism and factors affecting their contents—A review. Rev. Chapingo Ser. Hortic. 2015, 21, 97–128. [Google Scholar] [CrossRef]
- Akamatsu, F.; Jomura, N.; Tsuchida, Y.; Igi, Y.; Hisatsune, Y.; Teramoto, S.; Yamada, O. Effect of water deficit stress during fruit cultivation on the carbon stable isotopes of organic acids in Japanese apricots and liqueur prepared from these fruits. Isot. Environ. Health Stud. 2023, 60, 1–12. [Google Scholar] [CrossRef]
- Abeysuriya, H.I.; Bulugahapitiya, V.P.; Pulukkuttige, J.L. Total Vitamin C, Ascorbic Acid, Dehydroascorbic Acid, Antioxidant Properties, and Iron Content of Underutilized and Commonly Consumed Fruits in Sri Lanka. Int. J. Food Sci. 2020, 13, 4783029. [Google Scholar] [CrossRef]
- Fenoll, J.; Martínez, A.; Hellín, P.; Flores, P. Simultaneous determination of ascorbic and dehydroascorbic acids in vegetables and fruits by liquid chromatography with tandem-mass spectrometry. Food Chem. 2011, 127, 340–344. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Zia, H.; Fischbach, N.; Hofsommer, M.; Slatnar, A. Simultaneous analysis of ascorbic and dehydroascorbic acid in fruit juice using HILIC chromatography coupled with mass spectrometry. J. Food Compos. Anal. 2023, 124, 105714. [Google Scholar] [CrossRef]
- Ungureanu, M.C.R.; Lupitu, A.I.; Moisa, C.; Rivis, A.; Copolovici, L.O.; Poiana, M.-A. Investigation on High-Value Bioactive Compounds and Antioxidant Properties of Blackberries and Their Fractions Obtained by Home-Scale Juice Processing. Sustainability 2020, 12, 5681. [Google Scholar] [CrossRef]
- Granucci, N.; Harris, P.J.; Villas-Boas, S.G. Chemical Compositions of Fruit and Vegetable Pomaces from the Beverage Industries. Waste Biomass Valor. 2023, 14, 3841–3856. [Google Scholar] [CrossRef]
- Benvenutti, L.; Bortolini, D.G.; Fischer, T.E. Bioactive compounds recovered from apple pomace as ingredient in cider processing: Monitoring of compounds during fermentation. J. Food Sci. Technol. 2022, 59, 3349–3358. [Google Scholar] [CrossRef]
- Shah, H.M.S.; Singh, Z.; Kaur, J.; Hasan, M.U.; Woodward, A.; Afrifa-Yamoah, E. Trends in maintaining postharvest freshness and quality of Rubus berries. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4600–4643. [Google Scholar] [CrossRef]
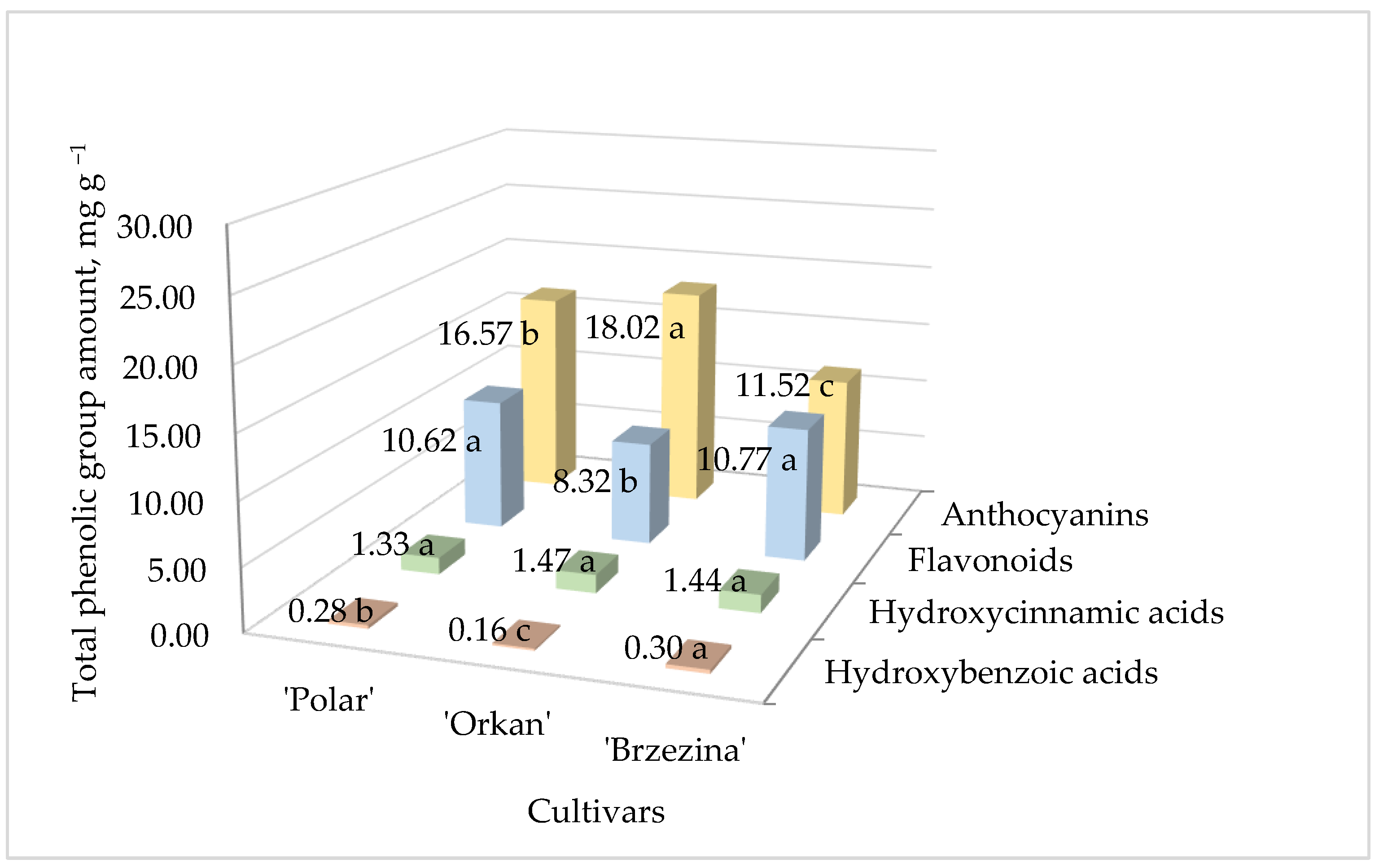
| Blackberry Cultivar | TPC mg 100 g−1 DW | TFC mg 100 g−1 DW | DPPH• µmol TE/g | ABTS•+ µmol TE/g |
|---|---|---|---|---|
| ‘Polar’ | 2380.60 ± 15.10 a * | 161.29 ± 1.80 a | 223.96 ± 5.30 ab | 343.78 ± 11.20 c |
| ‘Orkan’ | 2347.20 ± 19.20 b | 154.25 ± 4.30 b | 227.36 ± 9.10 a | 373.44 ± 6.30 b |
| ‘Brzezina’ | 2088.00 ± 10.30 c | 148.10 ± 0.90 c | 220.59 ± 7.40 b | 390.81 ± 9.30 a |
| Polyphenols/Cultivar | ‘Polar’ | ‘Orkan’ | ‘Brzezina’ |
|---|---|---|---|
| Flavonoids | |||
| Epigallocatechin | 9.72 ± 0.40 a | 7.28 ± 0.58 b | 9.60 ± 0.24 a |
| Catechin | 0.33 ± 0.01 c | 0.48 ± 0.04 a | 0.42 ± 0.01 b |
| Epigallocatechin gallate | 0.13 ± 0.02 a | 0.12 ± 0.02 a | 0.13 ± 0.00 a |
| Quercetin-3-O-rutinoside | 0.26 ± 0.01 b | 0.30 ± 0.01 a | 0.25 ± 0.01 b |
| Kaempferol-3-O-glucoside | 0.10 ± 0.01 b | 0.04 ± 0.01 c | 0.26 ± 0.01 a |
| Myricetin | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.02 ± 0.00 a |
| Quercetin | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| Kaempferol | 0.03 ± 0.00 b | 0.05 ± 0.01 a | 0.05 ± 0.00 a |
| Quercetin-3-O-glucoside | 0.02 ± 0.00 b | 0.03 ± 0.00 a | 0.02 ± 0.00 b |
| Phenolic acids | |||
| Chlorogenic acid | 0.27 ± 0.01 b | 0.30 ± 0.01 a | 0.26 ± 0.01 b |
| Ellagic acid | 0.28 ± 0.01 b | 0.16 ± 0.00 c | 0.30 ± 0.00 a |
| p-coumaric acid | 1.05 ± 0.04 a | 1.17 ± 1.14 a | 1.16 ± 0.02 a |
| Anthocyanins | |||
| Cyanidin-3-O-glucoside | 8.93 ± 0.02 b | 9.79 ± 0.05 a | 6.19 ± 0.02 c |
| Cyanidin-3-O-rutinoside | 7.65 ± 0.03 b | 8.23 ± 0.03 a | 5.33 ± 0.08 c |
| ‘Polar’ | ‘Orkan’ | ‘Brzezina’ | |
|---|---|---|---|
| Organic acids | |||
| Dehydroascorbic acid | 107.92 ± 3.15 c | 174.58 ± 0.63 a | 118.34 ± 0.91 b |
| L-ascorbic acid | 2.38 ± 0.12 c | 4.80 ± 0.05 a | 3.40 ± 0.02 b |
| Citric acid | 33.66 ± 1.14 c | 47.98 ± 0.08 b | 50.21 ± 0.39 a |
| Malic acid | 17.94 ± 0.68 c | 28.99 ± 0.27 b | 30.14 ± 0.17 a |
| Propionic acid | 8.89 ± 0.32 c | 16.96 ± 0.98 a | 13.28 ± 1.42 b |
| Butyric acid | 0.17 ± 0.04 c | 0.52 ± 0.03 a | 0.38 ± 0.05 b |
| Sugars | |||
| Glucose | 84.57 ± 1.04 c | 126.31 ± 0.84 b | 147.66 ± 0.51 a |
| Fructose | 77.13 ± 0.83 c | 117.78 ± 2.53 b | 145.23 ± 1.37 a |
| Name | Formula | MW | Class | ‘Polar’ | ‘Orkan’ | ‘Brzezina’ |
|---|---|---|---|---|---|---|
| Heptanal | C7H14O | 114.19 g/mol | Ketone | + | + | + |
| n-Nonanal | C9H18O | 142.24 g/mol | Ketone | + | − | − |
| 2-Nonanone | C9H18O | 142.24 g/mol | Ketone | − | − | + |
| Decanal | C10H20O | 156.26 g/mol | Aldehyde | + | + | + |
| Octanal | C8H16O | 128.21 g/mol | Aldehyde | + | + | + |
| Hexyl acetate | C8H16O2 | 144.21 g/mol | Ester | + | − | − |
| 1-Propanol | C4H10O | 74.12 g/mol | Ester | + | + | − |
| 1-Butanol | C4H10O | 74.12 g/mol | Ester | − | − | + |
| Ethyl propanoate | C5H10O2 | 102.13 g/mol | Ester | + | + | − |
| Isopropyl acetate | C5H10O2 | 102.13 g/mol | Ester | − | − | + |
| Methyl 2-methylbutanoate | C6H12O2 | 116.16 g/mol | Ester | + | + | − |
| Butyl acetate | C6H12O2 | 116.16 g/mol | Ester | − | − | + |
| Ethyl 3-(methylthio)propanoate | C6H12O2S | 148.23 g/mol | Ester | + | + | + |
| Ethyl hexanoate | C8H16O2 | 144.21 g/mol | Ester | + | + | + |
| Ethyl octanoate | C10H20O2 | 172.26 g/mol | Ester | + | + | − |
| Isoamyl acetate | C7H14Oc | 130.18 g/mol | Ester | + | + | − |
| 1-Hexanol | C6H14O | 102.17 g/mol | Alcohol | + | + | + |
| 1-Nonanol | C9H20O | 144.25 g/mol | Alcohol | + | + | + |
| 2-Heptanol | C7H16O | 116.2 g/mol | Alcohol | − | − | + |
| 3-Heptanol | C7H16O | 116.2 g/mol | Alcohol | + | + | − |
| cis-3-Hexen-1-ol | C6H12O | 100.16 g/mol | Alcohol | + | + | + |
| Pentanal | C5H10O | 86.13 g/mol | Alcohol | + | + | + |
| Maltol | C6H6O3 | 126.11 g/mol | Alcohol/Aldehyde | + | + | + |
| 1-Octanol | C8H18O | 130.229g/mol | Alcohol | − | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čechovičienė, I.; Viškelis, J.; Viškelis, P.; Hallman, E.; Kruk, M.; Tarasevičienė, Ž. Potentially Bioactive Compounds and Sensory Compounds in By-Products of Several Cultivars of Blackberry (Rubus fruticosus L.). Horticulturae 2024, 10, 862. https://doi.org/10.3390/horticulturae10080862
Čechovičienė I, Viškelis J, Viškelis P, Hallman E, Kruk M, Tarasevičienė Ž. Potentially Bioactive Compounds and Sensory Compounds in By-Products of Several Cultivars of Blackberry (Rubus fruticosus L.). Horticulturae. 2024; 10(8):862. https://doi.org/10.3390/horticulturae10080862
Chicago/Turabian StyleČechovičienė, Indrė, Jonas Viškelis, Pranas Viškelis, Ewelina Hallman, Marcin Kruk, and Živilė Tarasevičienė. 2024. "Potentially Bioactive Compounds and Sensory Compounds in By-Products of Several Cultivars of Blackberry (Rubus fruticosus L.)" Horticulturae 10, no. 8: 862. https://doi.org/10.3390/horticulturae10080862
APA StyleČechovičienė, I., Viškelis, J., Viškelis, P., Hallman, E., Kruk, M., & Tarasevičienė, Ž. (2024). Potentially Bioactive Compounds and Sensory Compounds in By-Products of Several Cultivars of Blackberry (Rubus fruticosus L.). Horticulturae, 10(8), 862. https://doi.org/10.3390/horticulturae10080862









