Seed Morpho-Anatomy, Dormancy and Germination Requirements in Three Schizanthus Species (Solanaceae) with Ornamental Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
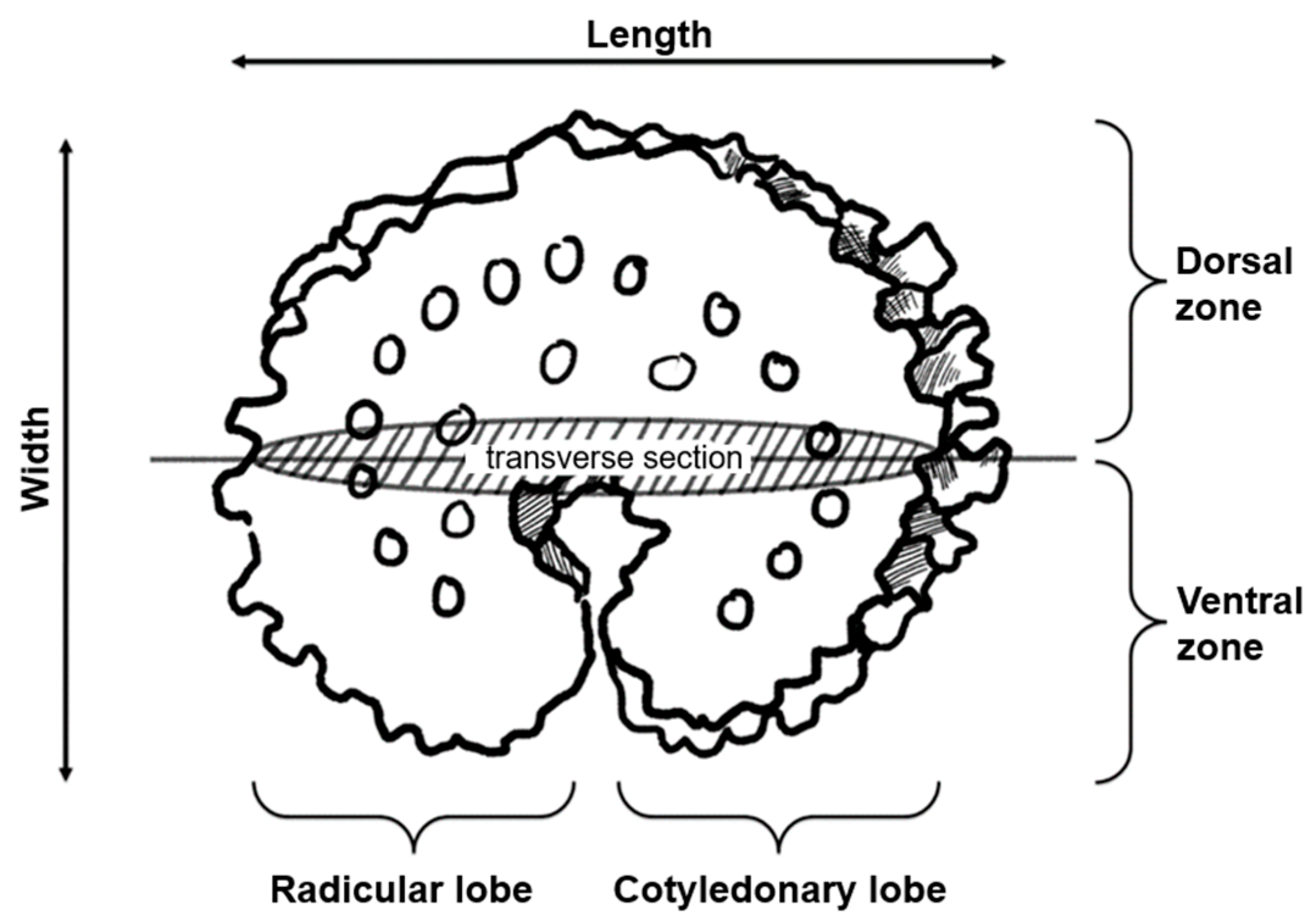
2.2. Morpho-Anatomical Characterization of Schizanthus spp. Seeds
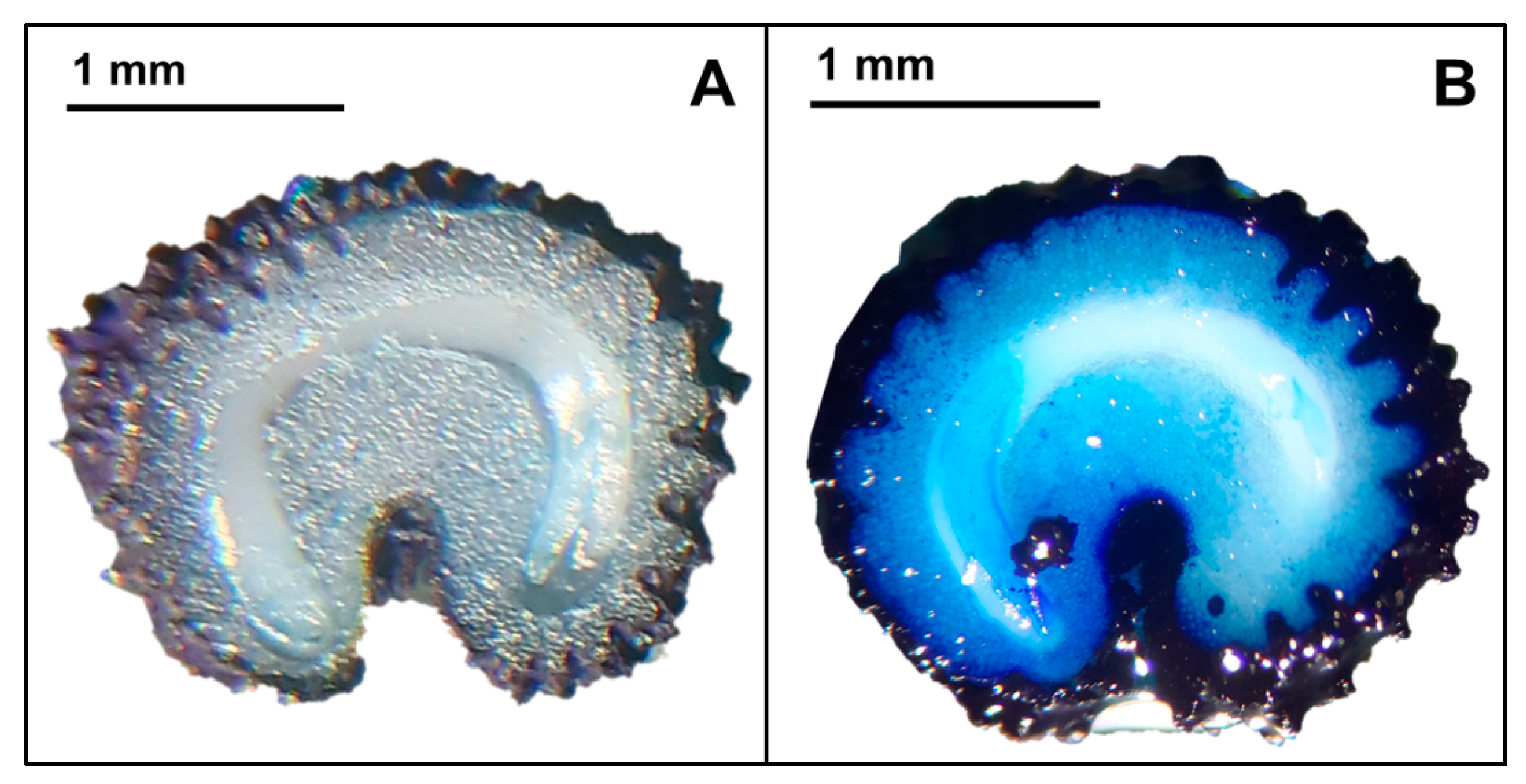
2.3. Characterization of Seed Dormancy and Germination Requirements
2.4. Statistical Analysis
3. Results
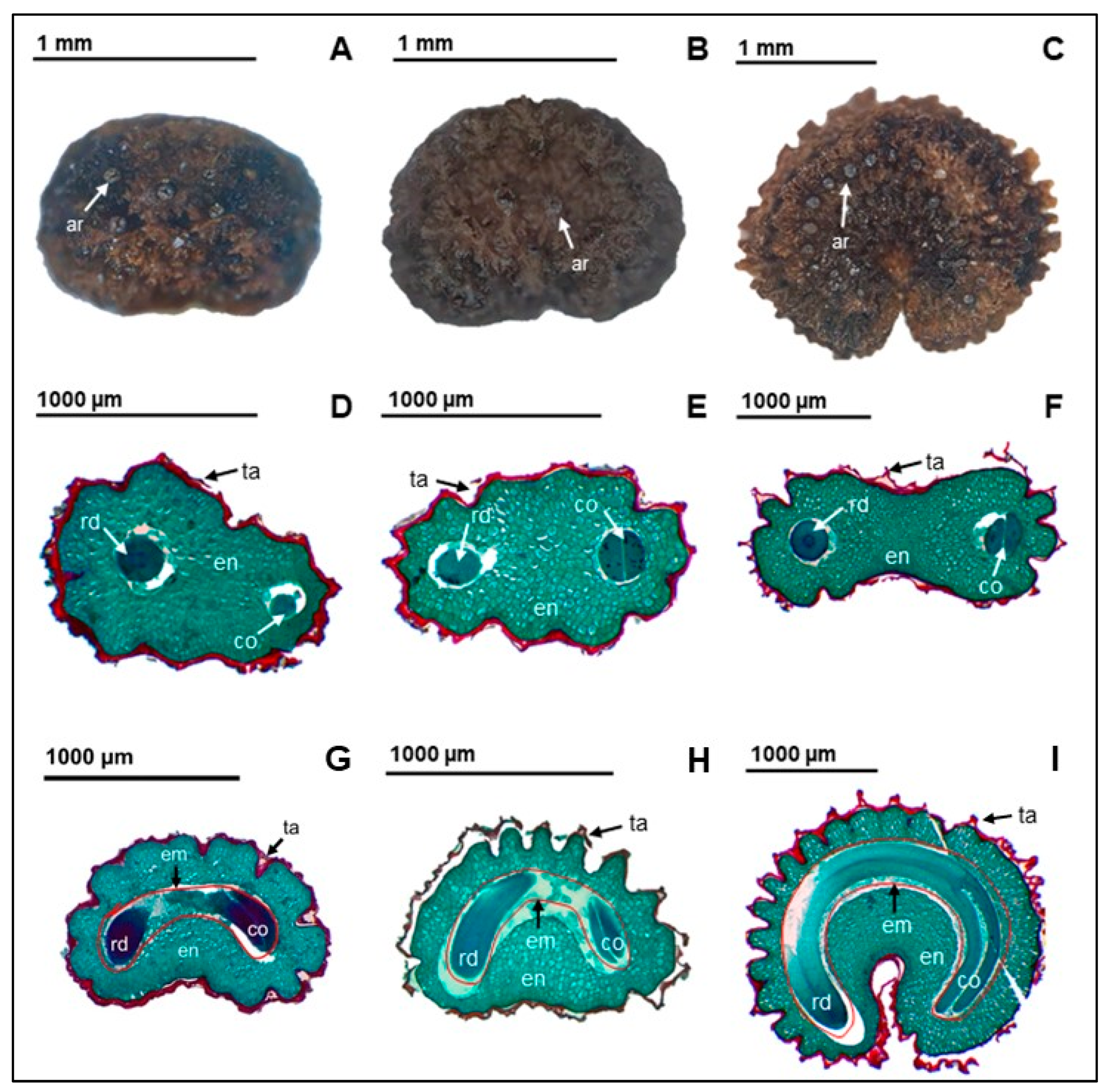
3.1. Seed Morphology and Anatomy
3.2. Seed Dormancy and Germination Requirements
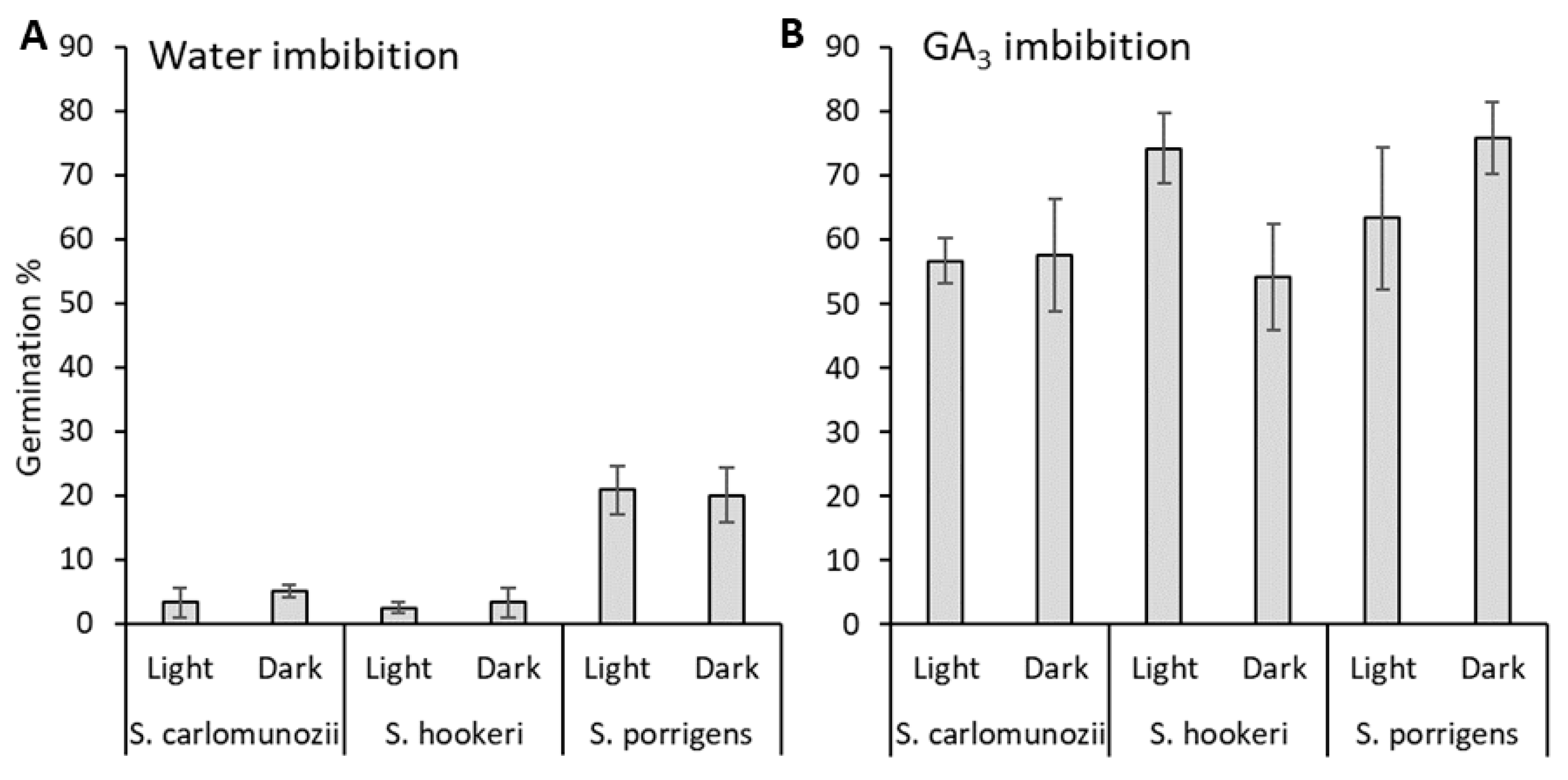
3.2.1. Seed Dormancy Characterization
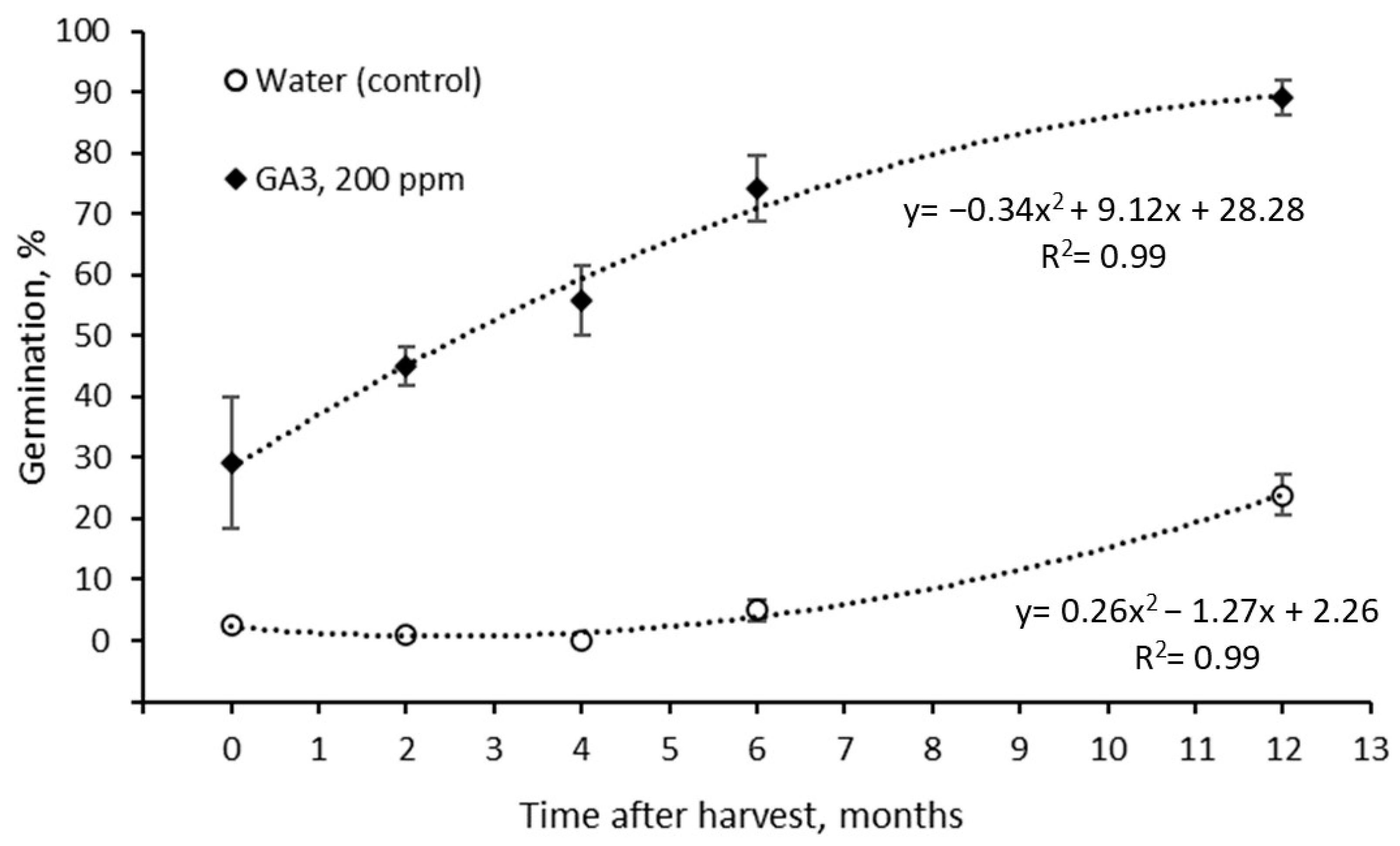
3.2.2. Germination Changes in S. hookeri Seeds during Storage
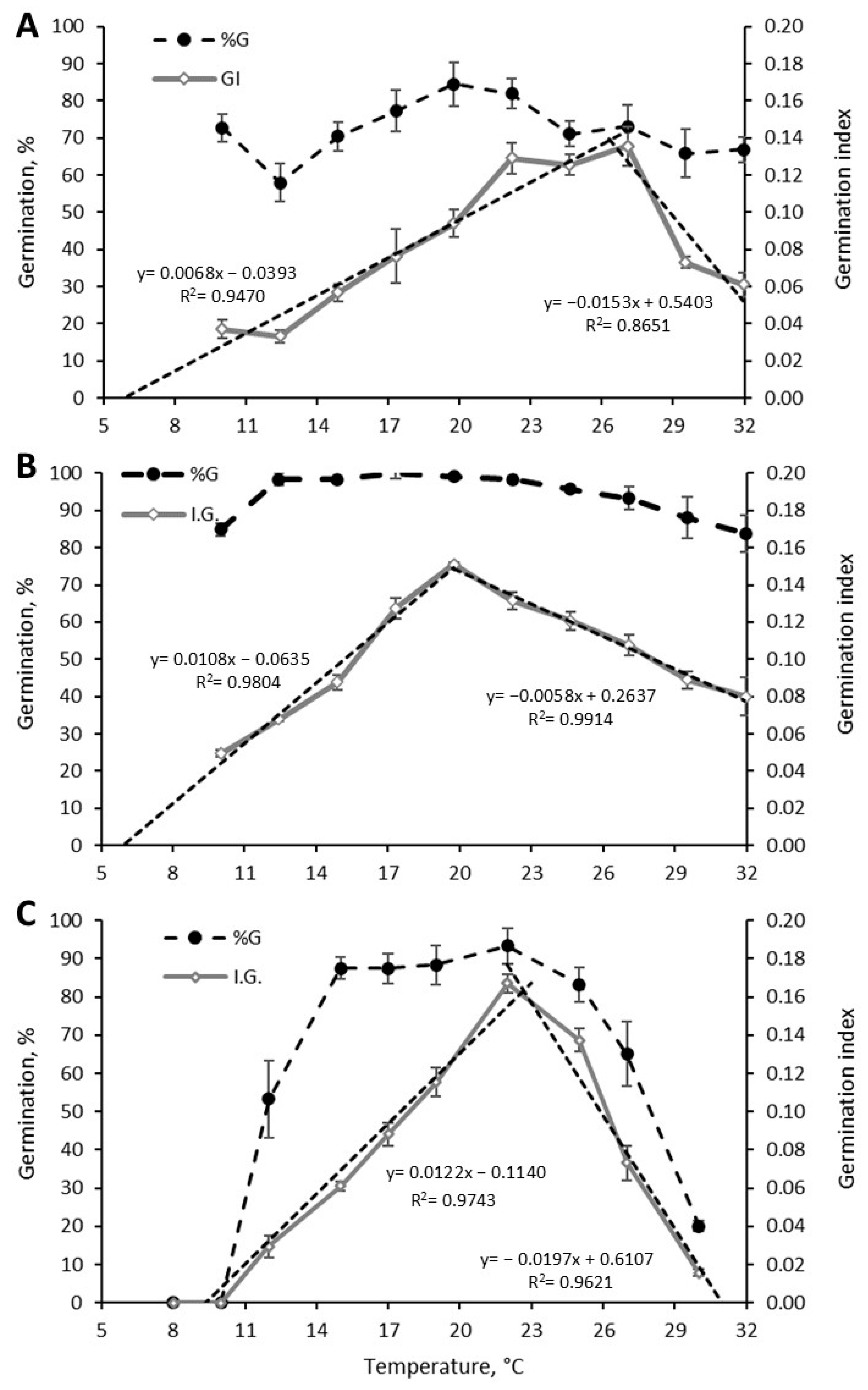
3.2.3. Germination Response to Temperature
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morales-Fierro, V.; Muñoz-Schick, M.; Moreira-Muñoz, A. Synopsis of Schizanthus Ruiz y Pav. (Solanaceae), a genus endemic to the southern Andes. PhytoKeys 2020, 154, 57–102. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Schick, M.; Moreira-Muñoz, A. El género Schizanthus (Solanaceae) en Chile. Rev. Chagual 2008, 6, 21–32. [Google Scholar]
- Gold, K.; León, P.; Way, M. Manual de Recolección de Semillas Silvestres para la Conservación a Largo Plazo y Restauración Ecológica; Boletín INIA N° 110; Instituto de Investigaciones Agropecuarias, Centro Regional Intihuasi: La Serena, Chile, 2004. [Google Scholar]
- Jara, P.; Arancio, G.; Moreno, R.; Carmona, M. Factores abióticos que influencian la germinación de seis especies herbáceas de la zona árida de Chile. Rev. Chil. Hist. Nat. 2006, 79, 309–319. [Google Scholar] [CrossRef][Green Version]
- Riedemann, P.; Aldunate, G. Flora Nativa de Valor Ornamental, Chile, Zona Centro, Identificación y Propagación; Editorial Antártica: Santiago, Chile, 2006. [Google Scholar]
- Riedemann, P.; Aldunate, G.; Teillier, S. Flora Nativa de Valor Ornamental, Chile, Zona Norte, Identificación y Propagación; Editorial Flora de Chile: Santiago, Chile, 2006. [Google Scholar]
- Lohengrin, C.; Sierra-Almeida, A. Assessing the importance of cold-stratification for seed germination in alpine plant species of the High-Andes of central Chile. Perspect. Plant Ecol. Evol. Syst. 2018, 30, 125–131. [Google Scholar] [CrossRef]
- Gunn, C.R.; Gaffney, F.B. Seed Characteristics of 42 Economically Important Species of Solanaceae in the United States; Technical Bulletins 160049; United States Department of Agriculture, Economic Research Service: Washington, DC, USA, 1974. [Google Scholar] [CrossRef]
- Ramírez, B.; Goyes, R. Botánica. Generalidades, Morfología y Anatomía de Plantas Superiores; Editorial Universidad del Cauca: Popayán, Colombia, 2004. [Google Scholar]
- Navas, L. Flora de la Cuenca de Santiago de Chile, Tomo III; Ediciones de la Universidad de Chile: Santiago, Chile, 1979. [Google Scholar]
- Albornoz, P. Técnicas Histológicas Vegetal. HISTO-NOA; Universidad Nacional de Tucuman: San Miguel de Tucumán, Argentina, 2010. [Google Scholar]
- Albornoz, F.; Vilches, I.; Contreras, S. Managing lettuce seed quality through nitrogen nutrition in soilless production. Sci. Hortic. 2019, 252, 169–175. [Google Scholar] [CrossRef]
- González, J.A.; Buedo, S.E.; Bruno, M.; Prado, F.E. Quantifying cardinal temperatures in quinoa (Chenopodium quinoa) cultivars. Lilloa 2017, 54, 179–194. [Google Scholar] [CrossRef]
- InfoStat. InfoStat, Versión 2019; Grupo InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2019.
- Baskin, J.; Baskin, C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Hepp, J.; Gómez, M.; León-Lobos, P.; Montenegro, G.; Vilalobos, L.; Contreras, S. Characterization of seed dormancy of 12 Chilean species of Nolana (Solanaceae) from the coastal Atacama Desert. Seed Sci. Res. 2021, 31, 20–29. [Google Scholar] [CrossRef]
- Ni, B.R.; Bradford, K.J. Germination and dormancy of abscisic acid-and gibberellin-deficient mutant tomato (Lycopersicon esculentum) seeds (sensitivity of germination to abscisic acid, gibberellin, and water potential). Plant Phys. 1993, 101, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Shin, S.L.; Kim, Y.R.; Kim, S.Y. Effects of temperature, gibberellic acid, and KNO3 treatments on seed germination of the wild plant Maesa japonica. Seed Sci. Technol. 2020, 48, 65–72. [Google Scholar] [CrossRef]
- Hernández, J.A.; Díaz-Vivancos, P.; Acosta-Motos, J.R.; Barba-Espín, G. Potassium nitrate treatment is associated with modulation of seed water uptake, Antioxidative Metabolism and Phytohormone Levels of Pea Seedlings. Seeds 2021, 1, 5–15. [Google Scholar] [CrossRef]
- Ricote, N.; Bastias, C.C.; Valladares, F.; Pérez, F.; Bozinovic, F. Selfing and drought-stress strategies under water deficit for two herbaceous species in the South American Andes. Front. Plant Sci. 2019, 10, 1595. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.J.; Sobreira, A.R.; de Lima, M.; Aparecida, P.; de Oliveira, L.S.; de Arauco, P.; de Oiviera, C.d.M.B.; Lopes, J.C. Luminosity, temperature, and substrates on the germination of Solanum capsicoides. Biosci. J. 2022, 38, e38061. [Google Scholar] [CrossRef]
- Ozaslan, C.; Farooq, S.; Onen, H.; Ozcan, S.; Bukun, B.; Gunal, H. Germination biology of two invasive Physalis species and implications for their management in arid and semi-arid regions. Sci. Rep. 2017, 7, 16960. [Google Scholar] [CrossRef] [PubMed]
- Yu Sung, Y.S.; Cantliffe, D.J.; Nagata, R.T. Seed developmental temperature regulation of thermotolerance in lettuce. J. Am. Soc. Hortic. Sci. 1998, 123, 700–705. [Google Scholar] [CrossRef]
- Contreras, S.; Bennett, M.; Metzger, J.; Tay, D.; Nerson, H. Red to far-red ratio during seed development affects lettuce seed germinability and longevity. HortScience 2009, 44, 130–134. [Google Scholar] [CrossRef]
- Bewley, J.; Bradford, K.; Hilhorst, H.; Nonogaki, H. Dormancy and de control of germination. In Seeds, Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013; pp. 247–297. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Elsevier: San Diego, CA, USA, 2014. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, J.; Gómez, M.; Contreras, S. Seed Morpho-Anatomy, Dormancy and Germination Requirements in Three Schizanthus Species (Solanaceae) with Ornamental Potential. Horticulturae 2024, 10, 867. https://doi.org/10.3390/horticulturae10080867
Moreno J, Gómez M, Contreras S. Seed Morpho-Anatomy, Dormancy and Germination Requirements in Three Schizanthus Species (Solanaceae) with Ornamental Potential. Horticulturae. 2024; 10(8):867. https://doi.org/10.3390/horticulturae10080867
Chicago/Turabian StyleMoreno, Joaquín, Miguel Gómez, and Samuel Contreras. 2024. "Seed Morpho-Anatomy, Dormancy and Germination Requirements in Three Schizanthus Species (Solanaceae) with Ornamental Potential" Horticulturae 10, no. 8: 867. https://doi.org/10.3390/horticulturae10080867
APA StyleMoreno, J., Gómez, M., & Contreras, S. (2024). Seed Morpho-Anatomy, Dormancy and Germination Requirements in Three Schizanthus Species (Solanaceae) with Ornamental Potential. Horticulturae, 10(8), 867. https://doi.org/10.3390/horticulturae10080867





