The Application of Carbon-Based Fertilizer Changed the Microbial Composition and Co-Occurrence Network Topological Properties of Vineyard Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Experimental Site
2.2. Fertilizer Used in the Experiment
2.3. Experimental Design
2.4. Soil Collection
2.5. DNA Extraction, PCR Amplification and Sequencing
2.6. Data Analysis
2.7. Measurement of Agronomic Traits in Grapes
3. Results
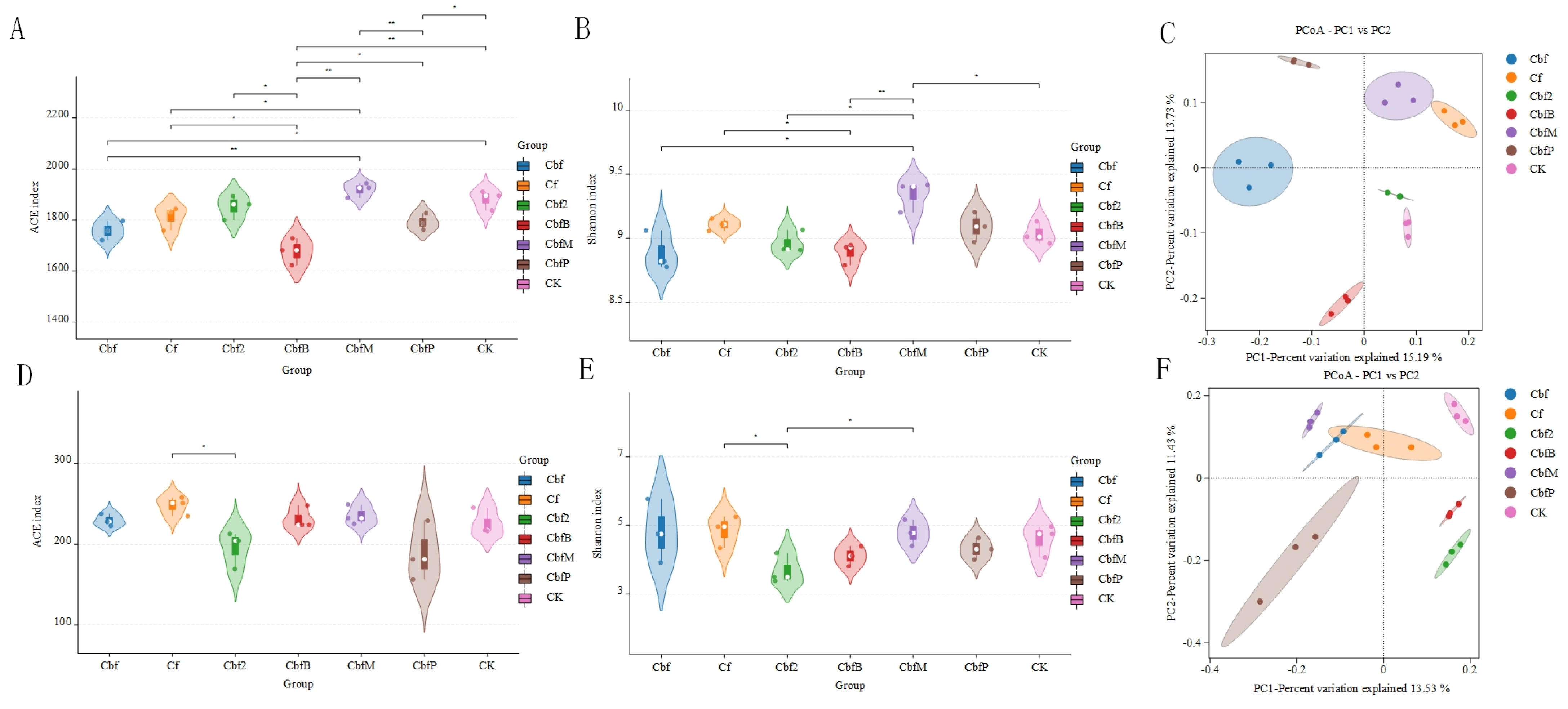
3.1. Effects of Different Fertilization Methods on the Diversity of Soil Microbial Communities in Vineyards
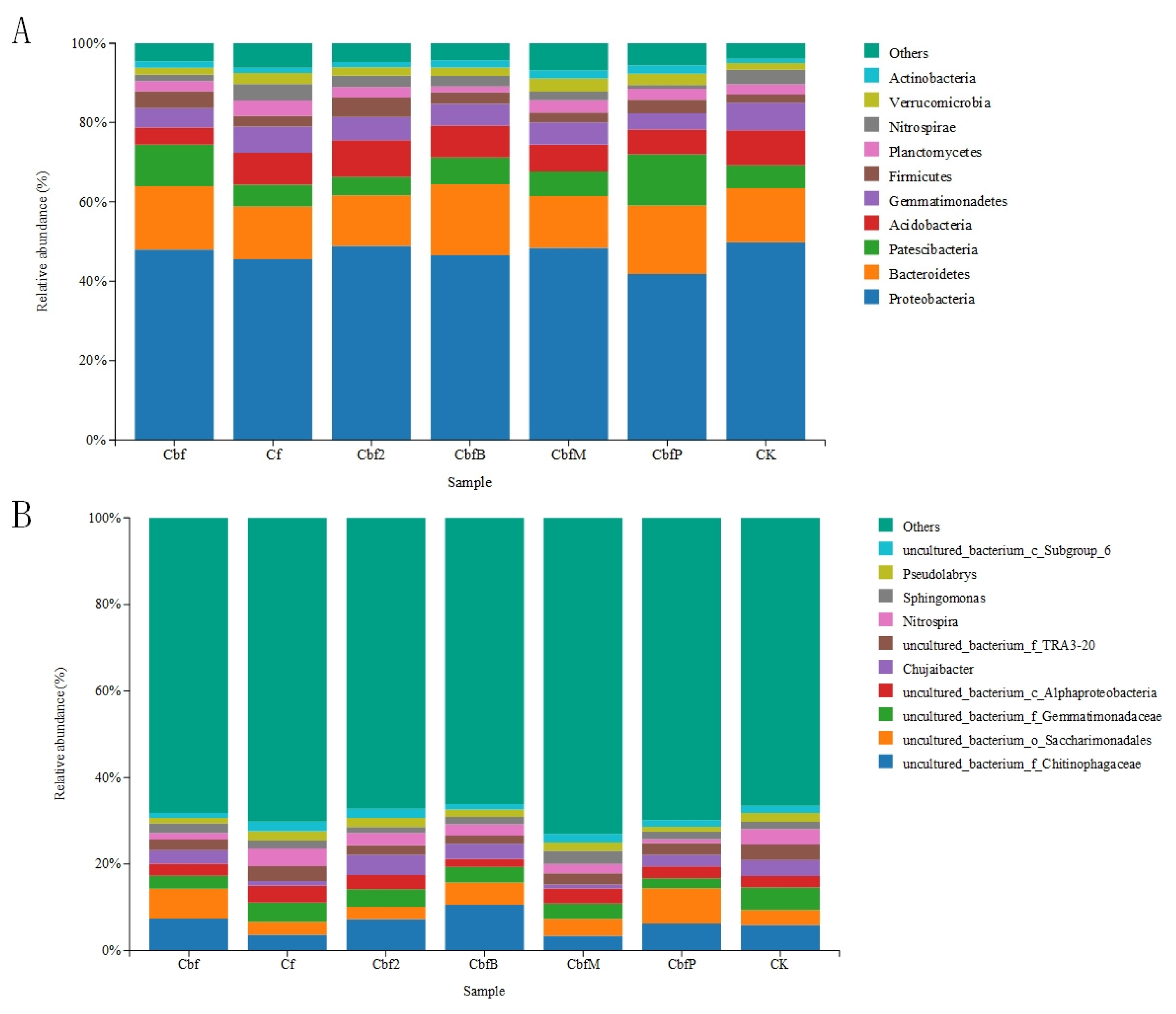
3.2. Differences in Soil Bacterial Community Fractions in Vineyards with Different Fertilization Practices
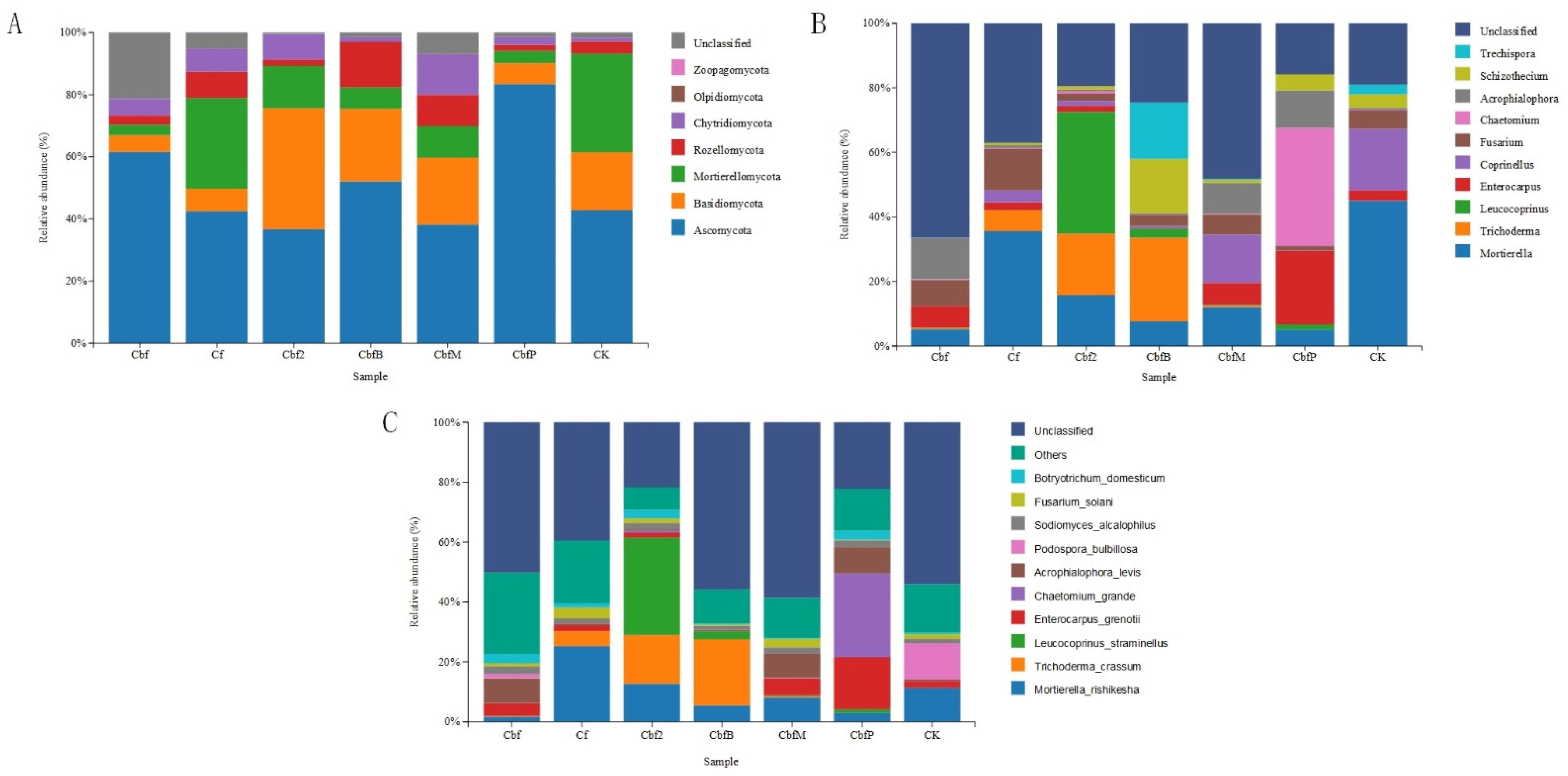
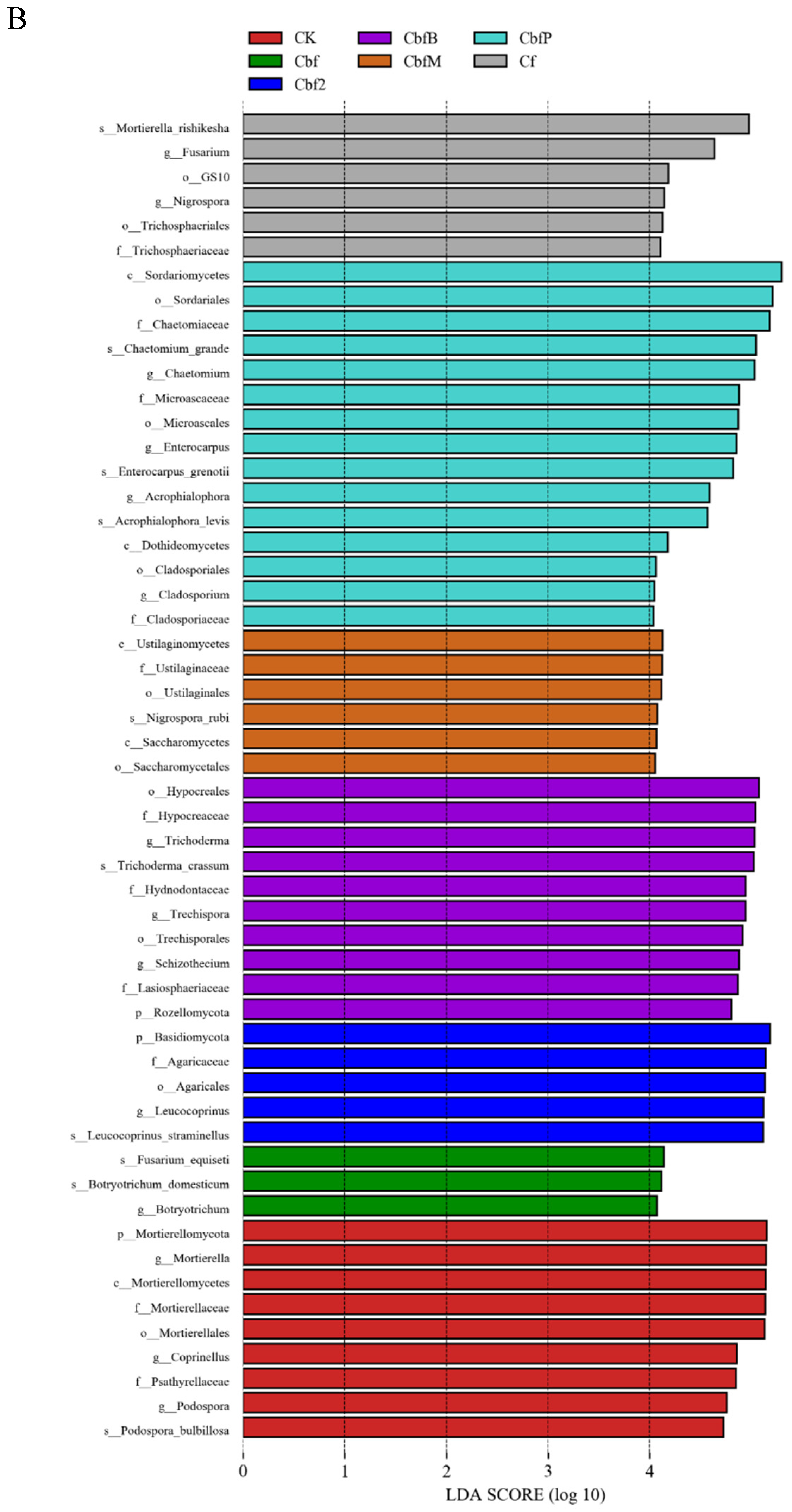
3.3. Differences in Soil Fungal Community Fractions in Vineyards with Different Fertilization Practices
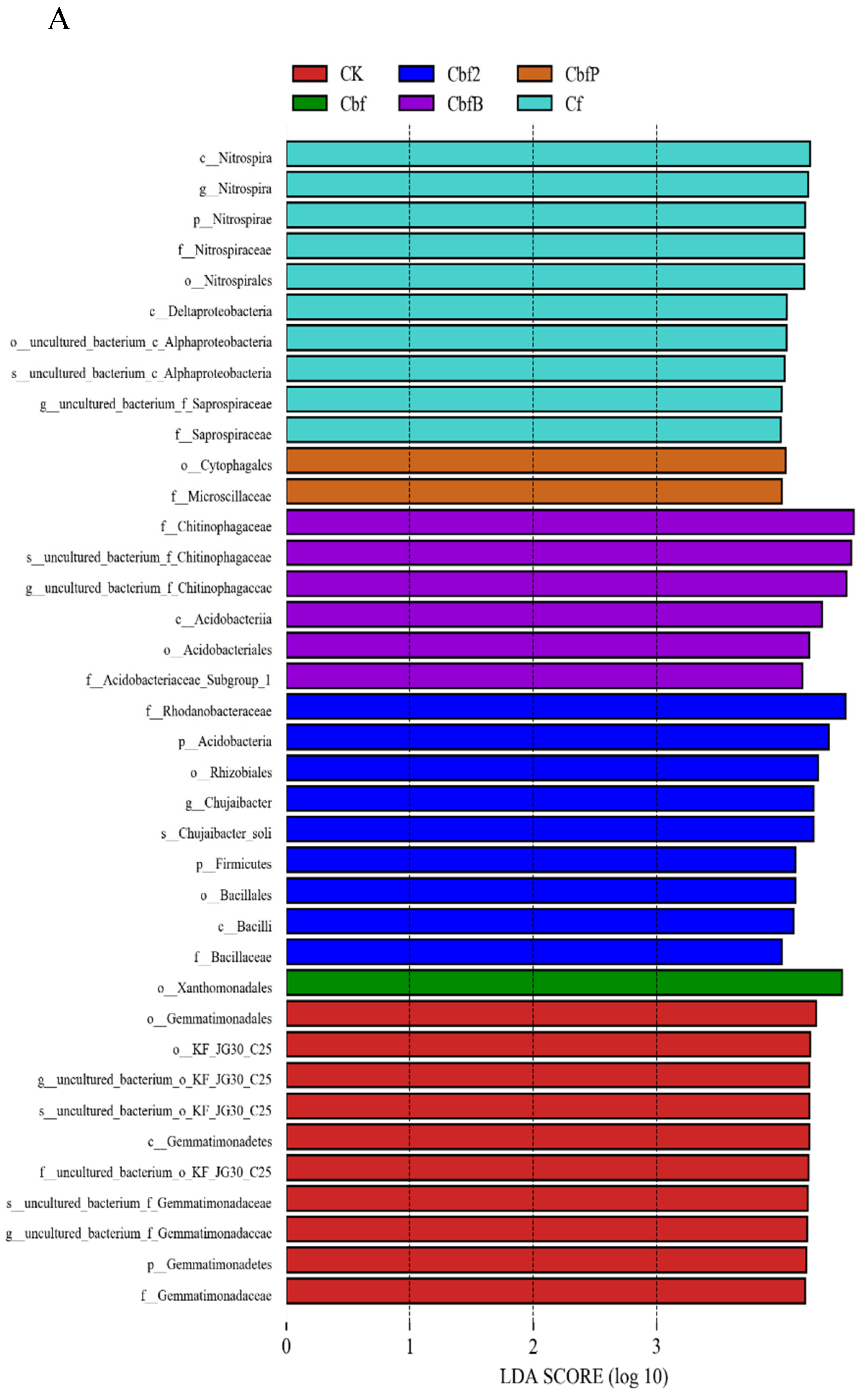
3.4. Analysis of Soil Microbial Variability in Vineyards with Different Fertilization Methods
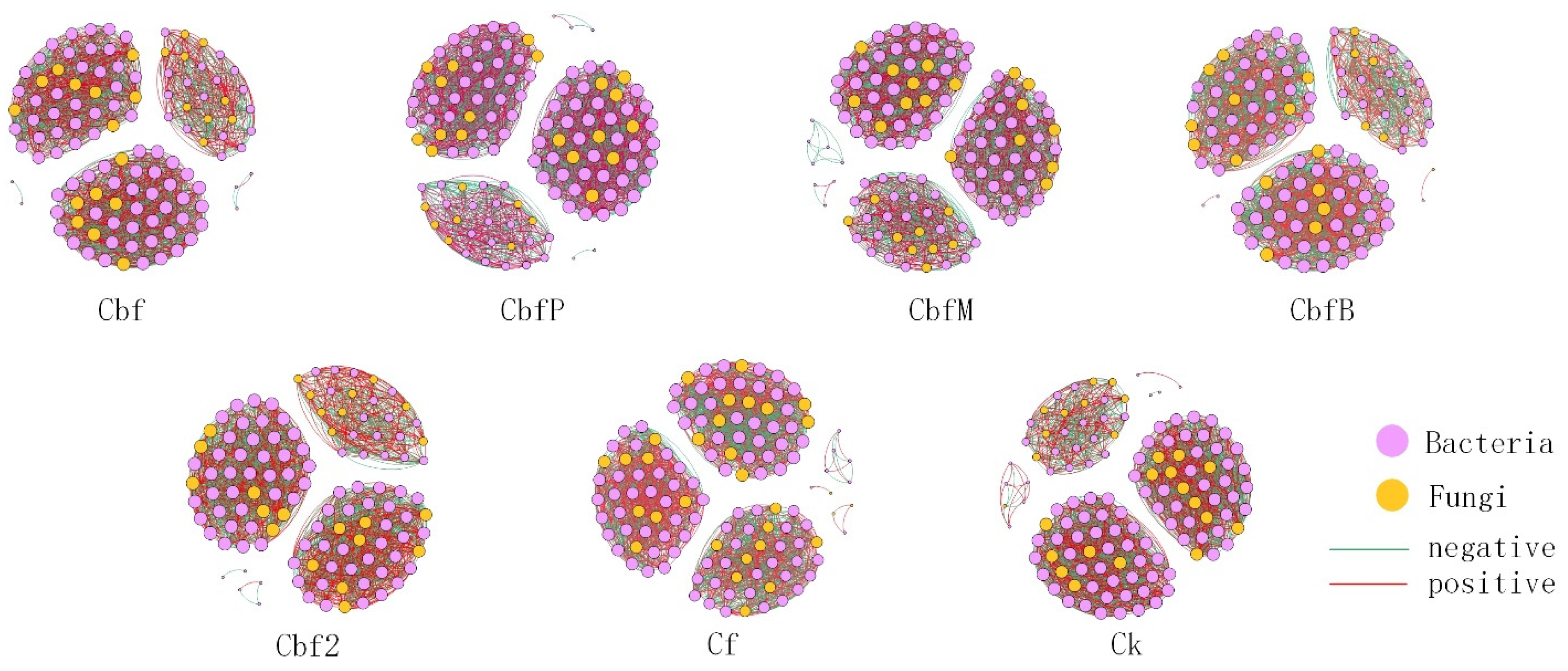
3.5. Co-Occurrence Network of Vineyard Soil Microorganisms
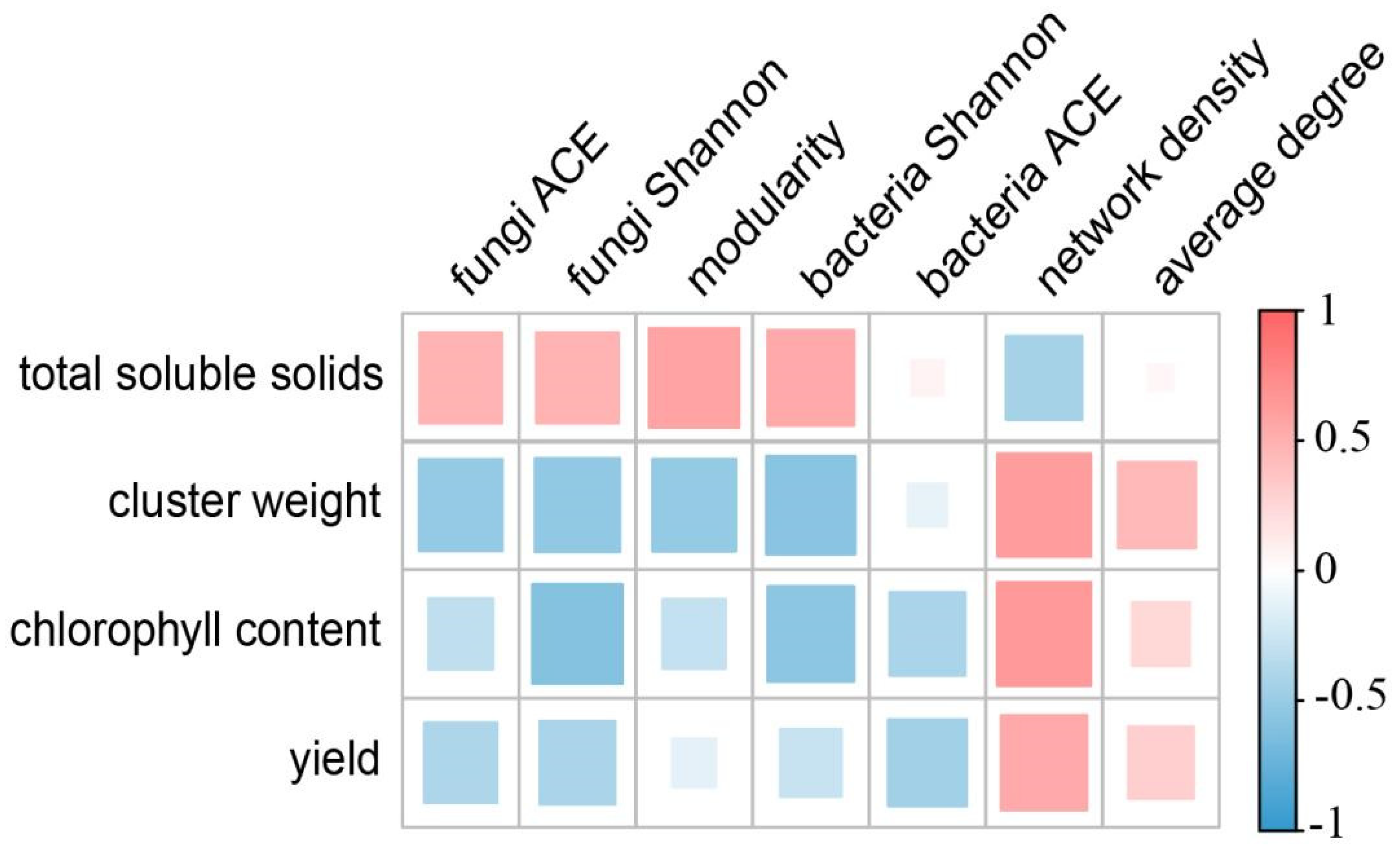
3.6. Grape Agronomic Traits and Their Relationship with Microbial Network Topological Attribute Parameters
4. Discussion
4.1. Effects of Different Fertilization Methods on Soil Microbial Community Diversity
4.2. Effects of Different Fertilizer Application Methods on Soil Microbial Composition
4.3. Effect of Different Fertilization Methods on Agronomic Traits of Grapes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, R.P.; Mo, Y.L.; Liu, C.M.; Wang, Y.Q.; Ma, J.X.; Zhang, Y.; Li, H.; Zhang, X. The Effects of Cattle Manure and Garlic Rotation on Soil under Continuous Cropping of Watermelon (Citrullus lanatus L.). PLoS ONE 2016, 11, e0156515. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, L.; Zhang, J.B.; Yin, J.; Huang, S.M. Bacterial Community Structure after Long-term Organic and Inorganic Fertilization Reveals Important Associations between Soil Nutrients and Specific Taxa Involved in Nutrient Transformations. Front. Microbiol. 2017, 8, 187. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, Z.Y.; Li, D.; Ni, H.W.; Sun, B.; Liang, Y.T. Soil pH Filters the Association Patterns of Aluminum-Tolerant Microorganisms in Rice Paddies. mSystems 2022, 7, e01022-21. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical Fertilizers and Their Impact on Soil Health. In Microbiota and Biofertilizers; Springer: Cham, Switzerland, 2021; Volume 2, pp. 1–20. [Google Scholar] [CrossRef]
- Yang, W.; Li, C.; Wang, S.; Zhou, B.; Mao, Y.; Rensing, C.; Xing, S. Influence of biochar and biochar-based fertilizer on yield, quality of tea and microbial community in an acid tea orchard soil. Appl. Soil Ecol. 2021, 166, 104005. [Google Scholar] [CrossRef]
- Ren, T.; Fan, P.; Zuo, W.; Liao, Z.; Wang, F.; Wei, Y.; Cai, X.; Liu, G. Biochar-based fertilizer under drip irrigation: More conducive to improving soil carbon pool and promoting nitrogen utilization. Ecol. Indic. 2023, 154, 110583. [Google Scholar] [CrossRef]
- Liu, Q.; Meki, K.; Zheng, H.; Yuan, Y.; Shao, M.; Luo, X.; Li, X.; Jiang, Z.; Li, F.; Xing, B. Biochar application in remediating salt-affected soil to achieve carbon neutrality and abate climate change. Biochar 2023, 5, 45. [Google Scholar] [CrossRef]
- Moreno-Lora, A.; Velasco-Sánchez, Á.; Delgado, A. Effects of Microbial Inoculants and Organic Amendments on Wheat Nutrition and Development in a Variety of Soils. J. Soil Sci. Plant Nutr. 2023, 23, 3329–3342. [Google Scholar] [CrossRef]
- Glogoza, B.; Aldrich-Wolfe, L.; Prasifka, J.R.; Prischmann-Voldseth, D.A. Impact of multiple soil microbial inoculants on biomass and biomass allocation of the legume crop field pea (Fabaceae: Pisum sativum L.). J. Sustain. Agric. Environ. 2023, 2, 314–327. [Google Scholar] [CrossRef]
- Ahsan, T.; Tian, P.-C.; Gao, J.; Wang, C.; Liu, C.; Huang, Y.-Q. Effects of microbial agent and microbial fertilizer input on soil microbial community structure and diversity in a peanut continuous cropping system. J. Adv. Res. 2023, S2090-1232. [Google Scholar] [CrossRef]
- Liu, J.; Xie, W.; Yang, J.; Yao, R.; Wang, X.; Li, W. Effect of Different Fertilization Measures on Soil Salinity and Nutrients in Salt-Affected Soils. Water 2023, 15, 3274. [Google Scholar] [CrossRef]
- Matchado, M.S.; Lauber, M.; Reitmeier, S.; Kacprowski, T.; List, M. Network analysis methods for studying microbial communities: A mini review. Comput. Struct. Biotechnol. J. 2021, 19, 2687–2698. [Google Scholar] [CrossRef]
- Huber, P.; Metz, S.; Unrein, F.; Mayora, G.; Devercelli, M. Environmental heterogeneity determines the ecological processes that govern bacterial metacommunity assembly in a floodplain river system. ISME J. 2020, 14, 2951–2966. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.E.; Wu, S.; Bhattacharjee, A.S.; Hamilton, J.J.; Mcmahon, K.D.; Goel, R.; Noguera, D.R. Metabolic network analysis reveals microbial community interactions in anammox granules. Nat. Commun. 2017, 8, 15416. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Köljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Nave Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, T.J.; Coll, R.; Calsyn, E.; Loose, M.D.; Eijk, M.J.T.V.; Roldán-Ruiz, I. Estimating genetic conformity between related ryegrass (Lolium) varieties. 1. Morphology and biochemical characterisation. Mol. Breed. 2000, 6, 569–580. [Google Scholar] [CrossRef]
- Quagliariello, A.; Del Chierico, F.; Russo, A.; Reddel, S.; Conte, G.; Lopetuso, L.R.; Ianiro, G.; Dallapiccola, B.; Cardona, F.; Gasbarrini, A.; et al. Gut Microbiota Profiling and Gut-Brain Crosstalk in Children Affected by Pediatric Acute-Onset Neuropsychiatric Syndrome and Pediatric Autoimmune Neuropsychiatric Disorders Associated With Streptococcal Infections. Front. Microbiol. 2018, 9, 675. [Google Scholar] [CrossRef]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. In Proceedings of the Third International Conference on Weblogs and Social Media, ICWSM 2009, San Jose, CA, USA, 17–20 May 2009; pp. 361–362. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Chen, J.H.; Liu, X.Y.; Zheng, J.W.; Zhang, B.; Lu, H.F.; Chi, Z.Z.; Pan, G.X.; Li, L.Q.; Zheng, J.F.; Zhang, X.H.; et al. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl. Soil Ecol. 2013, 71, 33–44. [Google Scholar] [CrossRef]
- Feng, H.L.; Xu, C.S.; He, H.H.; Zeng, Q.; Liu, G.S. Effect of Biochar on Soil Enzyme Activity & the Bacterial Community and Its Mechanism. Environ. Sci. 2021, 42, 422–432. [Google Scholar] [CrossRef]
- Yan, T.; Xue, J.; Zhou, Z.; Wu, Y. Biochar-based fertilizer amendments improve the soil microbial community structure in a karst mountainous area. Sci. Total Environ. 2021, 794, 148757. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, B.; Gu, W.; Li, J. Effects of Carbon-Based Fertilizer on Soil Physical and Chemical Properties, Soil Enzyme Activity and Soil Microorganism of Maize in Northeast China. Agronomy 2023, 13, 877. [Google Scholar] [CrossRef]
- Lu, X.; Yin, Y.; Feng, J.X.; Ma, H.; Gao, R. Effects of Chinese fir litter and its biochar amendment on soil microbial community structure. Acta Sci. Circumstantiae 2019, 39, 3090–3098. [Google Scholar] [CrossRef]
- Xu, X.N.; Lei, X.H.; Liao, S.Q.; Li, Y.M.; Sun, Y.X. Foliar application of potassium silicate, potassium fulvate and betaine improve summer-time tomato yield by promoting plant nitrogen and potassium uptake. Folia Hortic. 2022, 34, 125–138. [Google Scholar] [CrossRef]
- Zhang, M.M.; Li, X.Y.; Wang, X.L.; Feng, J.P.; Zhu, S.P. Potassium fulvic acid alleviates salt stress of citrus by regulating rhizosphere microbial community, osmotic substances and enzyme activities. Front. Plant Sci. 2023, 14, 1161469. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Z.; Guo, S. Effect of microbial agent A73 on yield and quality of tomato in protected area. Shandong Agricul Sci 2006, 2006, 54–55. [Google Scholar]
- Huang, Y.; Zheng, L.; Huang, Y.; Jia, Z.; Song, S.; Li, Z. Effects of different application methods of Bacillus subtilis agent on soil microbial diversity and growth of muskmelon. Chin. J. Biotechnol. 2020, 36, 2644–2656. [Google Scholar] [CrossRef]
- Kordatzaki, G.; Katsenios, N.; Giannoglou, M.; Andreou, V.; Chanioti, S.; Katsaros, G.; Savvas, D.; Efthimiadou, A. Effect of foliar and soil application of plant growth promoting bacteria on kale production and quality characteristics. Sci. Hortic. 2022, 301, 111094. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.T.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef]
- Carrión, V.J.; Perez-Jaramillo, J.; Cordovez, V.; Tracanna, V.; Raaijmakers, J.M. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science 2019, 366, 606–612. [Google Scholar] [CrossRef]
- Li, X.N.; Yao, S.; Bian, Y.R.; Jiang, X.; Song, Y. The combination of biochar and plant roots improves soil bacterial adaptation to PAH stress: Insights from soil enzymes, microbiome, and metabolome. J. Hazard. Mater. 2020, 400, 123227. [Google Scholar] [CrossRef]
- De Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Sun, R.H.; Li, J.A.; Zhai, L.M.; Cui, H.L.; Fan, B.Q.; Wang, H.Y.; Liu, H.B. Combined organic-inorganic fertilization builds higher stability of soil and root microbial networks than exclusive mineral or organic fertilization. Soil Ecol. Lett. 2023, 5, 220142. [Google Scholar] [CrossRef]
- Wu, Y.-M.; Yang, X.-Q.; Li, S.-Y.; Sun, L.-J.; Cen, R.-H.; Zhao, L.-X.; Yang, Y.-B.; Ding, Z.-T. Three New Azaphilone Phytotoxins from Coculture of the Phytopathogens Nigrospora oryzae and Colletotrichum gloeosporioides and Antifungal Activities Against N. oryzae. Chem. Nat. Compd. 2022, 58, 848–852. [Google Scholar] [CrossRef]
- Syahmi, Z.A.; Rita, M.; Aswad, W.; Syahirah, M.A.; Erneeza, M.H. First Report of Leaf Blight on Parthenium hysterophorus Caused by Nigrospora sphaerica in Malaysia. Plant Dis. 2021, 141, 105497. [Google Scholar] [CrossRef]
- Jia, W.Y.; Luo, M.Y.; Wei, T.P.; Zhang, H.; Zeng, Y.; Jiang, Y.L. Nigrospora musae and N. oryzae as new causal agents of broad bean leaf spot disease in China. Crop Prot. 2024, 177, 106567. [Google Scholar] [CrossRef]
- Liu, Q.W.; Wang, S.X.; Li, K.; Qiao, J.; Guo, Y.S.; Liu, Z.D.; Guo, X.W. Responses of soil bacterial and fungal communities to the long-term monoculture of grapevine. Appl. Microbiol. Biotechnol. 2021, 105, 7035–7050. [Google Scholar] [CrossRef] [PubMed]
- Zu Drewer, J.M.; Köster, M.; Abdulai, I.; Rötter, R.P.; Hagemann, N.; Schmidt, H.P. Impact of Different Methods of Root-Zone Application of Biochar-Based Fertilizers on Young Cocoa Plants: Insights from a Pot-Trial. Horticulturae 2022, 8, 328. [Google Scholar] [CrossRef]
- Liu, C.; Yang, J.; Wang, G.; Ye, B.; Wang, Y. Impact of Application of Biochar-Based Fertilizer on the Content of Phosphorus and Potassium in Soil. IOP Conf. Ser. Earth Environ. Sci. 2019, 252, 052064. [Google Scholar] [CrossRef]
- Ramos, N.C.; Abon, J.E.O.; Pascual, K.S.; Bulatao, R.M.; Monserate, J.J.; Diaz, J.M.A. Synthesis and Characterization of Rice Straw Derived Nanoscale Biochar-Based Fertilizer Infused with Nutrients. Key Eng. Mater. 2022, 913, 107–115. [Google Scholar] [CrossRef]
- Xu, L.; Mo, K.; Ran, D.; Ma, J.; Zhang, L.; Sun, Y.; Long, Q.; Jiang, G.; Zhao, X.; Zou, X. An endolysin gene from Candidatus Liberibacter asiaticus confers dual resistance to huanglongbing and citrus canker. Hortic. Res. 2023, 10, uhad159. [Google Scholar] [CrossRef]
- Jamal, A.; Farhat, H.; Urooj, F.; Rahman, A.; Irfan, M.; Ehteshamul-Haque, S. Characterization of endophytic yeast and its suppressive effect on root rotting fungi of tomato under neem cake soil amendment. Egypt. J. Biol. Pest Control 2021, 31, 152. [Google Scholar] [CrossRef]
- Banerjee, S.; Kirkby, C.A.; Schmutter, D.; Bissett, A.; Kirkegaard, J.A.; Richardson, A.E. Network analysis reveals functional redundancy and keystone taxa amongst bacterial and fungal communities during organic matter decomposition in an arable soil. Soil Biol. Biochem. 2016, 97, 188–198. [Google Scholar] [CrossRef]
- Imam, N.; Belda, I.; García-Jiménez, B.; Duehl, A.J.; Doroghazi, J.R.; Almonacid, D.E.; Thomas, V.P.; Acedo, A. Local Network Properties of Soil and Rhizosphere Microbial Communities in Potato Plantations Treated with a Biological Product Are Important Predictors of Crop Yield. mSphere 2021, 6, e0013021. [Google Scholar] [CrossRef]
- Zhang, Z.M.; He, P.; Hao, X.X.; Li, L.J. Long-term mineral combined with organic fertilizer supports crop production by increasing microbial community complexity. Appl. Soil Ecol. 2023, 188, 104930. [Google Scholar] [CrossRef]
| Treatment | Base Fertilizer | Fruit-Expanding Fertilizer |
|---|---|---|
| CK | / | / |
| Cbf | 400 g/plant charcoal-based fertilizer | / |
| CbfP | 400 g/plant charcoal-based fertilizer | 40 g/plant potassium fulvic acid |
| CbfM | 400 g/plant charcoal-based fertilizer | 10 g/plant composite microbial fungus |
| CbfB | 400 g/plant charcoal-based fertilizer | 10 g/plant Bacillus subtilis |
| Cbf2 | / | two applications of 200 g/plant charcoal-based fertilizer |
| Cf | / | two applications of 100 g/plant composite fertilizer |
| Treatment | Nodes | Edges | Positive Edges (%) | Negative Edges (%) | Average Degree | Network Density | Modularity |
|---|---|---|---|---|---|---|---|
| Cbf | 140 | 3034 | 58.47% | 41.53% | 43.343 | 0.312 | 0.616 |
| CbfP | 122 | 2366 | 51.94% | 48.06% | 38.787 | 0.321 | 0.602 |
| CbfM | 124 | 2244 | 49.29% | 50.71% | 36.194 | 0.294 | 0.646 |
| CbfB | 115 | 2097 | 50.79% | 49.21% | 36.470 | 0.320 | 0.615 |
| Cbf2 | 117 | 2139 | 51.75% | 48.25% | 36.564 | 0.315 | 0.616 |
| Cf | 132 | 2446 | 50.65% | 49.35% | 37.061 | 0.283 | 0.664 |
| CK | 126 | 2342 | 50.43% | 49.57% | 37.175 | 0.297 | 0.610 |
| Treatment | Chlorophyll Content (SPAD) | Cluster Weight (g) | Total Soluble Solids (%) | Yield (kg/m2) |
|---|---|---|---|---|
| Cbf | 39.61 ± 2.60 bc | 1057.77 ± 99.72 b | 18.12 ± 1.05 d | 3.57 ± 0.02 c |
| CbfP | 38.07 ± 1.21 a | 890.73 ± 54.49 a | 17.5 ± 0.7 bc | 3.55 ± 0.06 bc |
| CbfM | 38.28 ± 2.03 ab | 857.17 ± 76.6 a | 19.1 ± 1.55 e | 3.5 ± 0.06 bc |
| CbfB | 39.45 ± 1.80 bc | 899.61 ± 107.29 a | 17.88 ± 1.13 cd | 3.54 ± 0.06 bc |
| Cbf2 | 40.16 ± 1.35 d | 1101.66 ± 36.57 b | 17.21 ± 1.17 ab | 3.58 ± 0.08 c |
| Cf | 37.58 ± 2.05 a | 795.9 ± 100.02 a | 17.99 ± 1.42 d | 3.47 ± 0.06 b |
| CK | 37.14 ± 1.98 a | 884 ± 116.19 a | 16.83 ± 1.79 a | 3.33 ± 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, P.; Wu, J.; Lin, X.; Chen, C.; Zhu, J.; Wang, Y.; Zhou, J.; Wang, H.; Shen, J.; Jia, H. The Application of Carbon-Based Fertilizer Changed the Microbial Composition and Co-Occurrence Network Topological Properties of Vineyard Soil. Horticulturae 2024, 10, 871. https://doi.org/10.3390/horticulturae10080871
Sun P, Wu J, Lin X, Chen C, Zhu J, Wang Y, Zhou J, Wang H, Shen J, Jia H. The Application of Carbon-Based Fertilizer Changed the Microbial Composition and Co-Occurrence Network Topological Properties of Vineyard Soil. Horticulturae. 2024; 10(8):871. https://doi.org/10.3390/horticulturae10080871
Chicago/Turabian StyleSun, Ping, Jiaqi Wu, Xianrui Lin, Chenfei Chen, Jianxi Zhu, Yi Wang, Jian Zhou, Huaxin Wang, Jiansheng Shen, and Huijuan Jia. 2024. "The Application of Carbon-Based Fertilizer Changed the Microbial Composition and Co-Occurrence Network Topological Properties of Vineyard Soil" Horticulturae 10, no. 8: 871. https://doi.org/10.3390/horticulturae10080871
APA StyleSun, P., Wu, J., Lin, X., Chen, C., Zhu, J., Wang, Y., Zhou, J., Wang, H., Shen, J., & Jia, H. (2024). The Application of Carbon-Based Fertilizer Changed the Microbial Composition and Co-Occurrence Network Topological Properties of Vineyard Soil. Horticulturae, 10(8), 871. https://doi.org/10.3390/horticulturae10080871





