Optimizing Sweet Cherry Attributes through Magnesium and Potassium Fertilization
Abstract
:1. Introduction
2. Materials and Methods
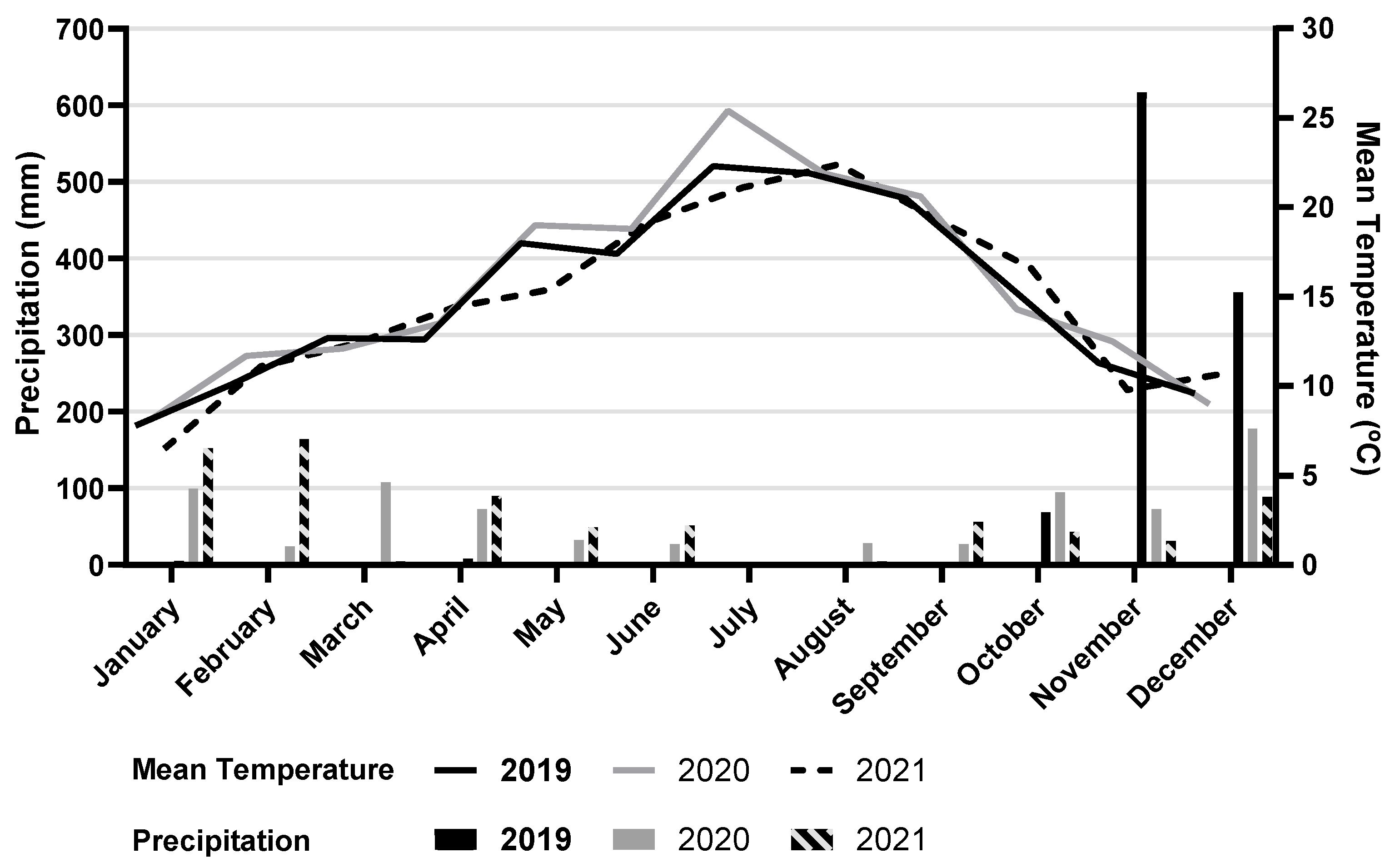
2.1. Experimental Trial
2.2. Mineral Composition
2.3. Sweet Cherry Quality Parameters
2.3.1. Biometric Parameters
2.3.2. Chromatic Parameters
2.3.3. Texture Parameters
2.3.4. Total Soluble Solids, pH, Titratable Acidity, and Maturity Index
2.4. Sensorial Analysis
2.5. Statistical Analysis
3. Results
3.1. Mineral Composition
3.2. Effect of Magnesium and Potassium on Sweet Cherry Quality Parameters
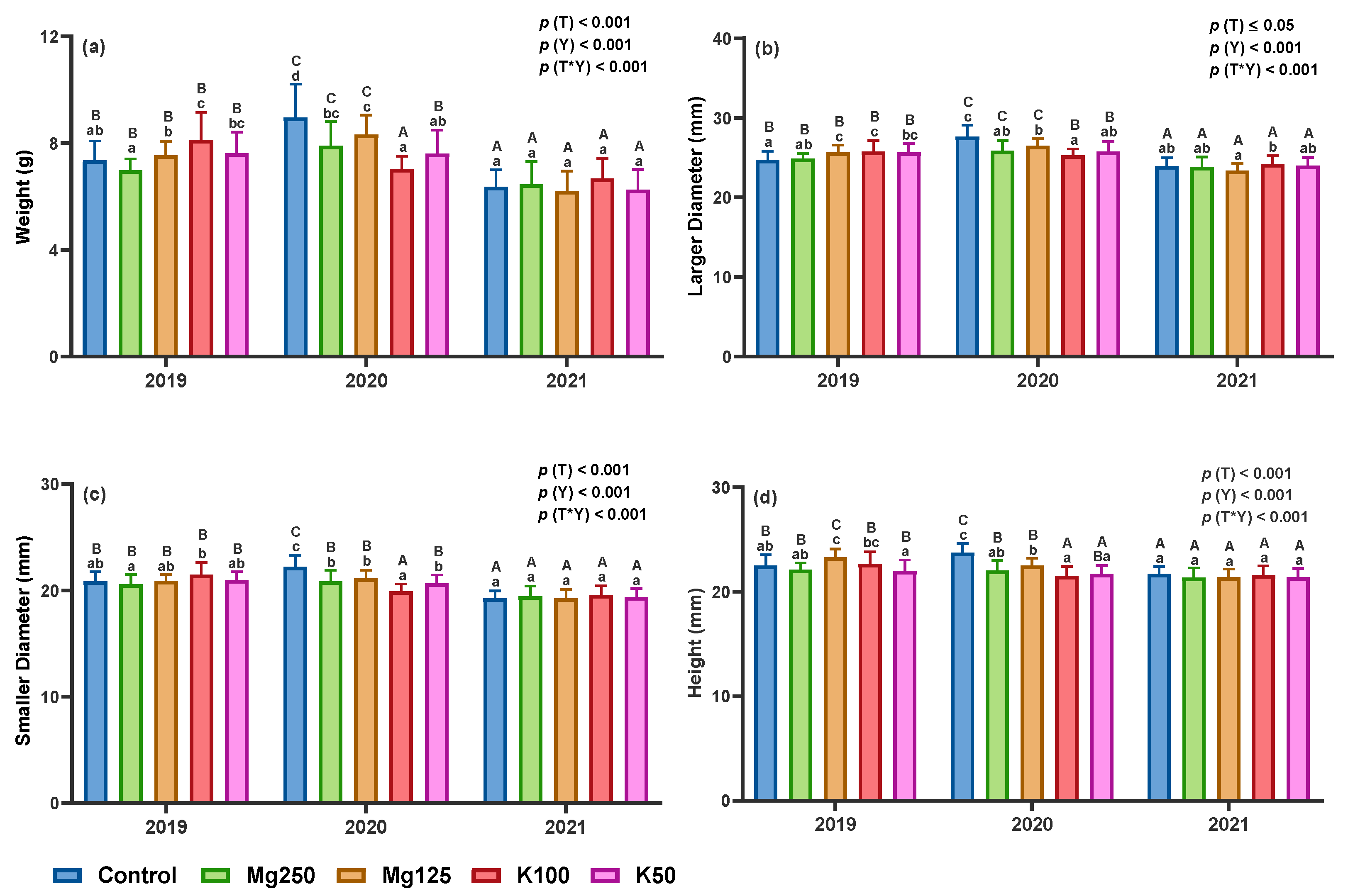
3.2.1. Biometric Parameters
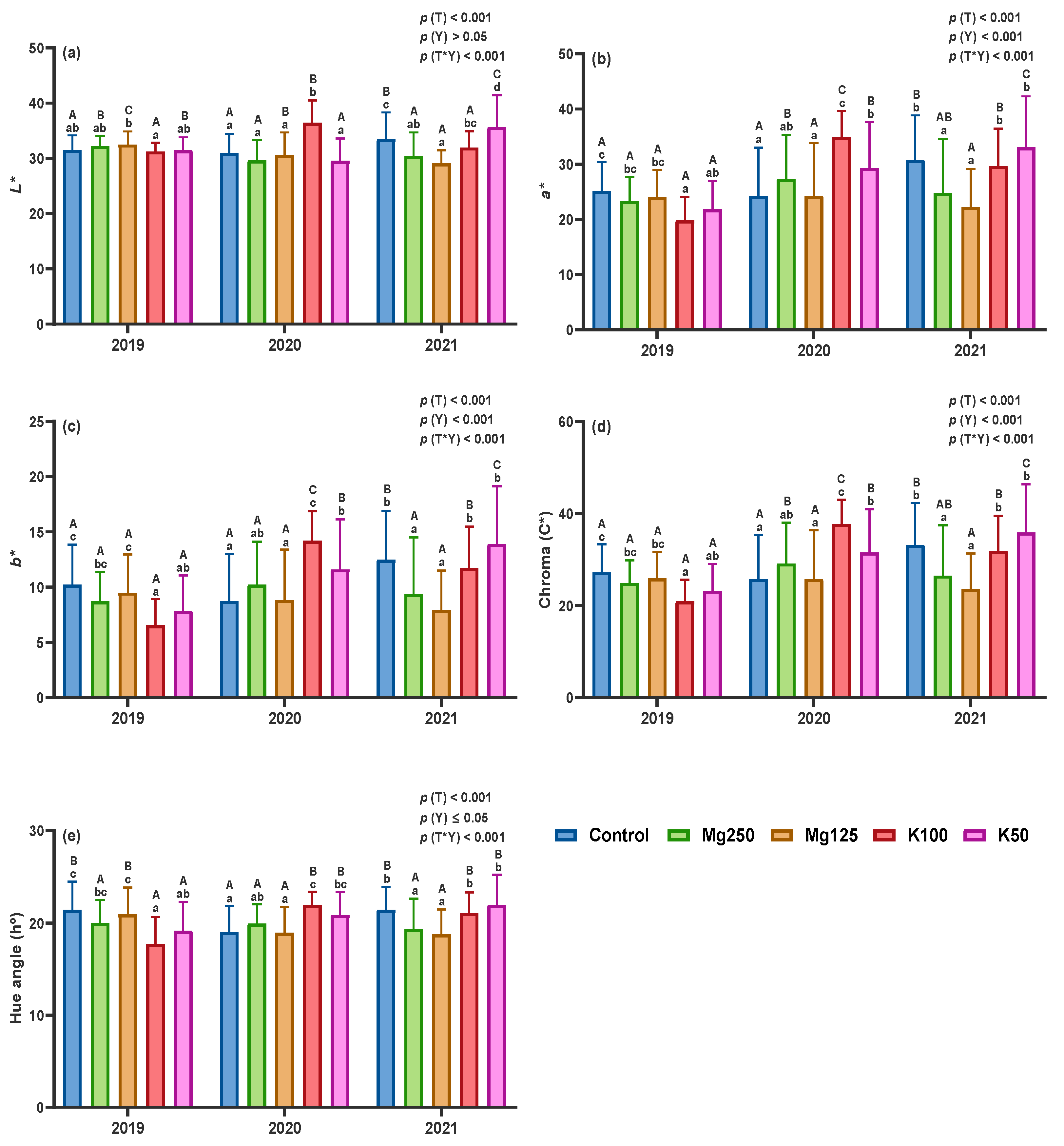
3.2.2. Chromatic Parameters
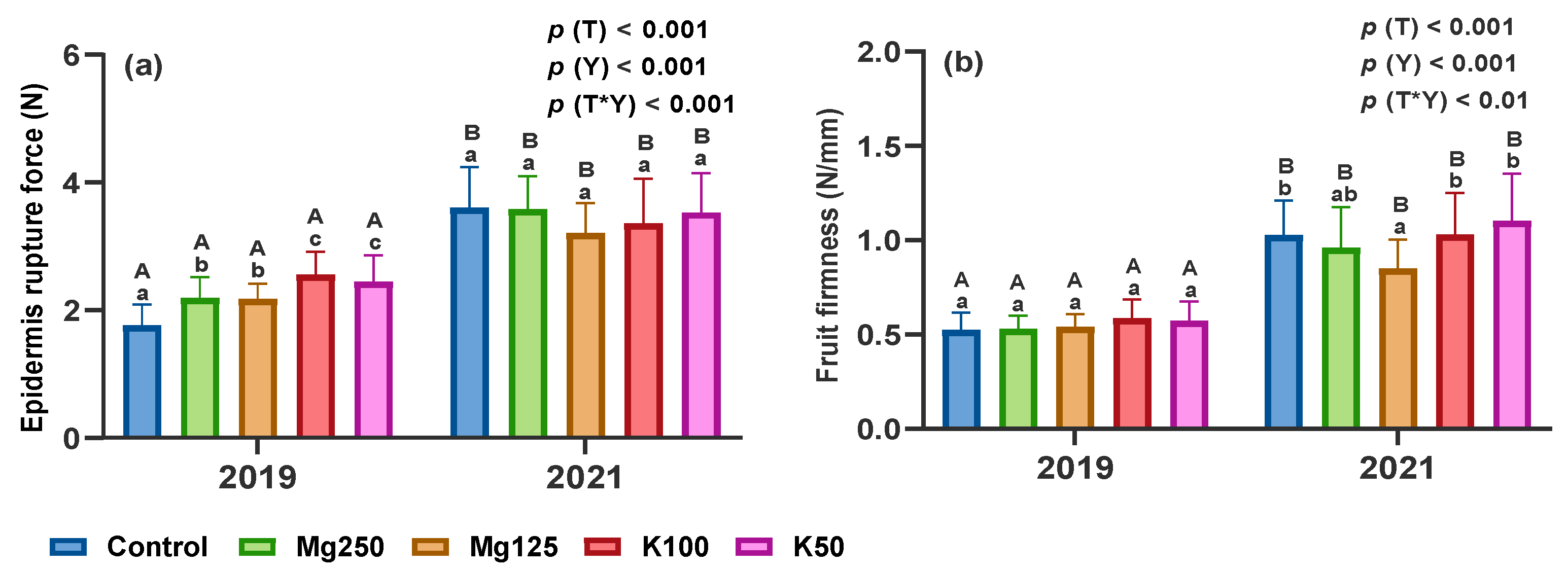
3.2.3. Texture Parameters
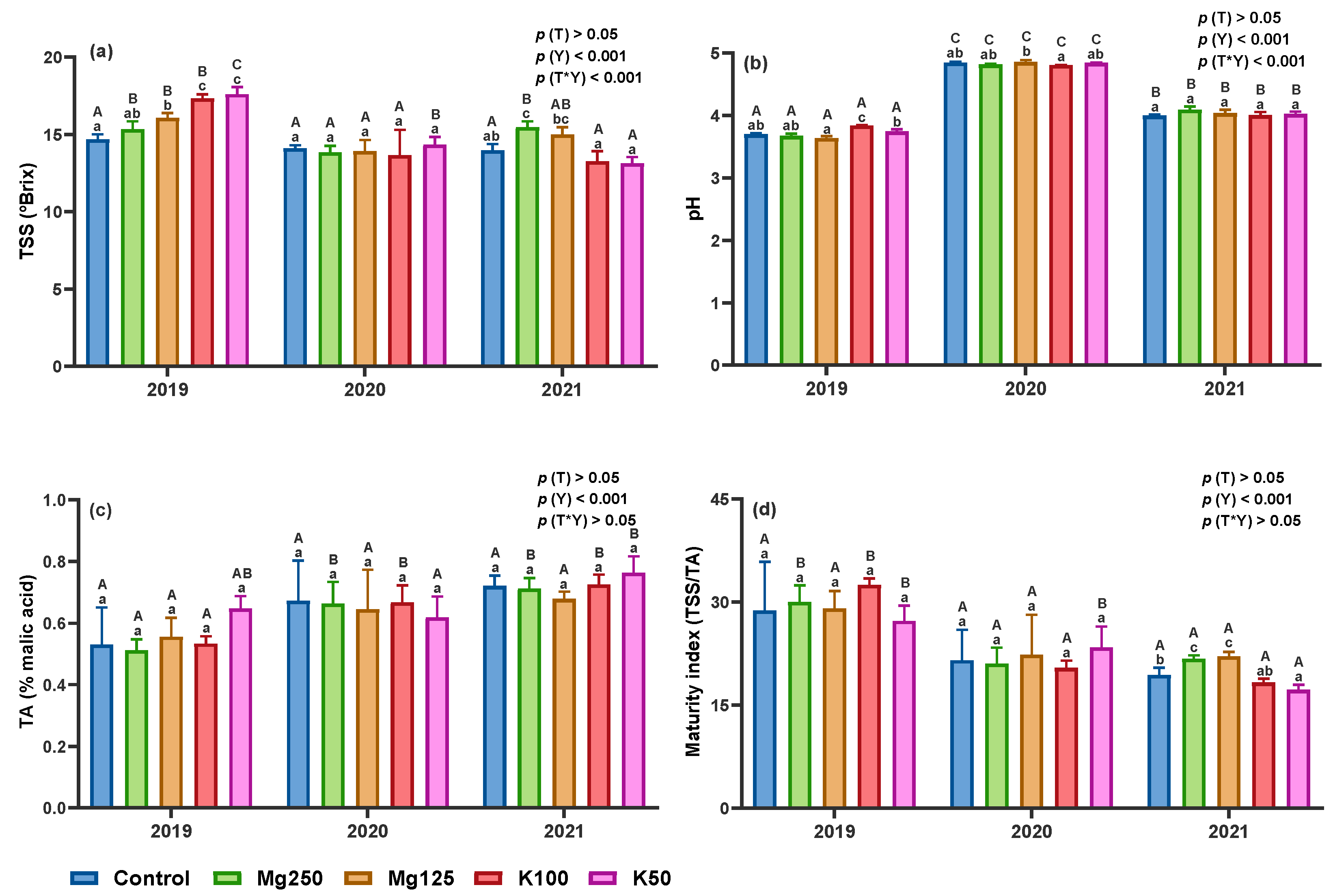
3.2.4. Total Soluble Solids, pH, Titratable Acidity, and Maturity Index
3.2.5. Correlations (between Cherry Quality Parameters)
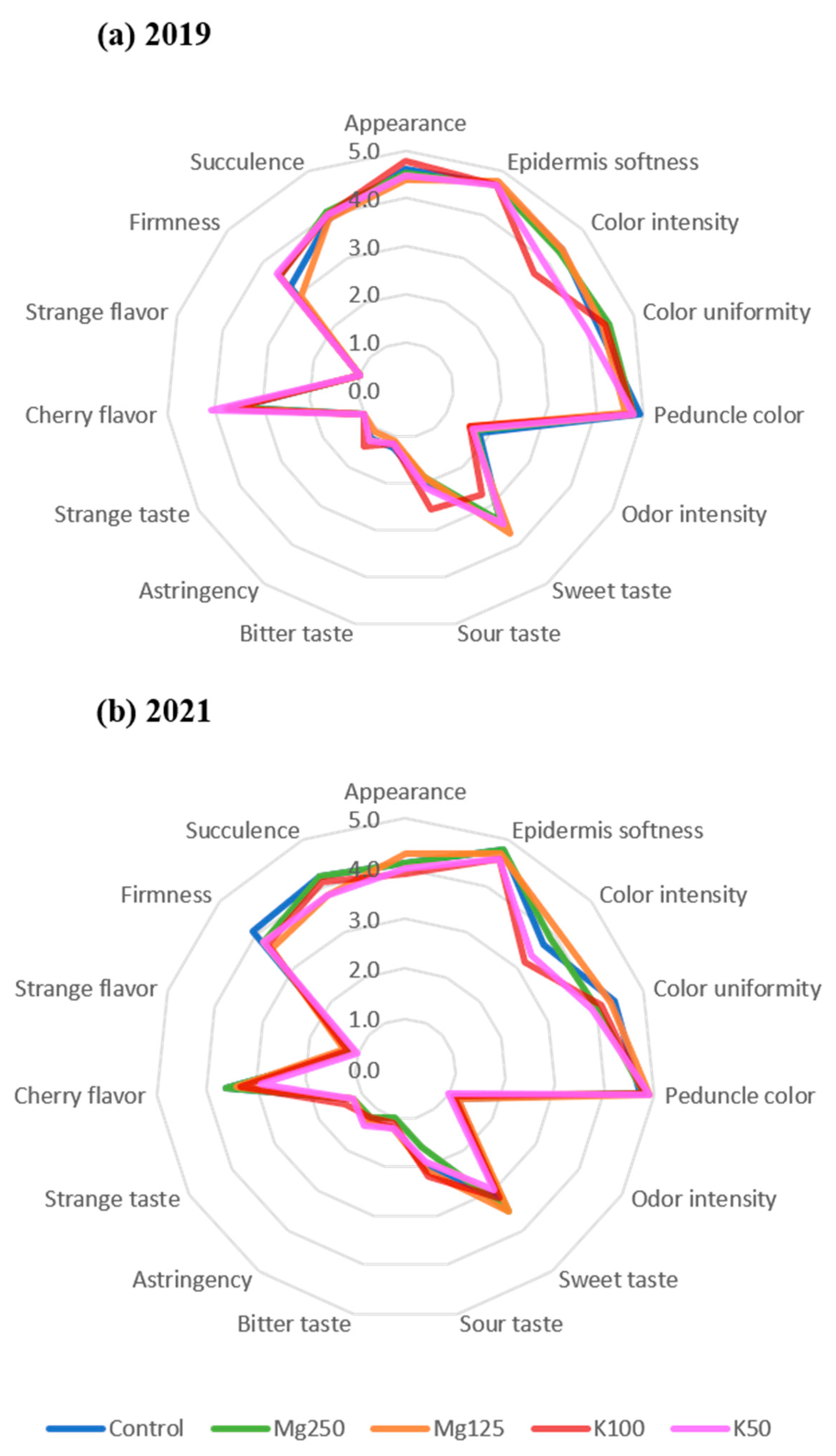
3.3. Sensorial Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraga, H.; Santos, J.A. Assessment of climate change impacts on chilling and forcing for the main fresh fruit regions in Portugal. Front. Plant Sci. 2021, 12, 689121. [Google Scholar] [CrossRef]
- Roussos, P.A. Climate change impacts on fruit trees and mitigation strategies of adverse effects. AgroLife Sci. J. 2020, 9, 269–276. [Google Scholar]
- Haokip, S.W.; Shankar, K.; Lalrinngheta, J. Climate change and its impact on fruit crops. J. Pharmacogn. Phytochem. 2020, 9, 435–438. [Google Scholar]
- Roy, A.; Viswanath, M.; Chatarjee, S.; Biswas, A. Climate change crash on fruit crops and management approaches. Pharma Innov. J. 2021, 10, 315–319. [Google Scholar]
- Hirpo, F.H.; Gebeyehu, M.N. Review on the effects of climate change variability on horticultural productivity. Int. J. Environ. Sci. Nat. Resour. 2019, 17, 555969. [Google Scholar] [CrossRef]
- Blando, F.; Oomah, B.D. Sweet and sour cherries: Origin, distribution, nutritional composition and health benefits. Trends Food Sci. Technol. 2019, 86, 517–529. [Google Scholar] [CrossRef]
- Di Matteo, A.; Russo, R.; Graziani, G.; Ritieni, A.; Di Vaio, C. Characterization of autochthonous sweet cherry cultivars (Prunus avium L.) of southern Italy for fruit quality, bioactive compounds and antioxidant activity. J. Sci. Food Agric. 2017, 97, 2782–2794. [Google Scholar] [CrossRef]
- Mirto, A.; Iannuzzi, F.; Carillo, P.; Ciarmiello, L.F.; Woodrow, P.; Fuggi, A. Metabolic characterization and antioxidant activity in sweet cherry (Prunus avium L.) Campania accessions: Metabolic characterization of sweet cherry accessions. Food Chem. 2018, 240, 559–566. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize2023 (accessed on 20 September 2023).
- Gonçalves, A.C.; Bento, C.; Silva, B.; Simões, M.; Silva, R.L. Nutrients, bioactive compounds and bioactivity: The health benefits of sweet cherries (Prunus avium L.). Curr. Nutr. Food Sci. 2019, 15, 208–227. [Google Scholar] [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Factors affecting quality and health promoting compounds during growth and postharvest life of sweet cherry (Prunus avium L.). Front. Plant Sci. 2017, 8, 2166. [Google Scholar] [CrossRef]
- Paunović, G.; Hajder, Đ.; Korićanac, A.; Pašalić, B.; Glišić, I.; Cvetkovic, M.; Cvetković, M. Preferences in sweet cherry fruits among consumers in Serbia and Bosnia and Herzegovina. Hortic. Sci. 2022, 49, 189–196. [Google Scholar] [CrossRef]
- Acero, N.; Gradillas, A.; Beltran, M.; García, A.; Muñoz Mingarro, D. Comparison of phenolic compounds profile and antioxidant properties of different sweet cherry (Prunus avium L.) varieties. Food Chem. 2019, 279, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Nawirska-Olszańska, A.; Kolniak-Ostek, J.; Oziembłowski, M.; Ticha, A.; Hyšpler, R.; Zadak, Z.; Židová, P.; Paprstein, F. Comparison of old cherry cultivars grown in Czech Republic by chemical composition and bioactive compounds. Food Chem. 2017, 228, 136–142. [Google Scholar] [CrossRef]
- Bujdosó, G.; Hrotko, K.; Feldmane, D.; Giovannini, D.; Demirsoy, H.; Tao, R.; Ercisli, S.; Ertek, N.; Malchev, S. What kind of sweet cherries do the final consumers prefer? S. West. J. Hortic. Biol. Environ. 2020, 11, 37–48. [Google Scholar]
- Neilsen, G.H.; Neilsen, D.; Peryea, F.J.; Fallahi, E. Tomorrow’s challenges in fruit nutrition research: Quo vadis? Acta Hortic. 2010, 868, 27–40. [Google Scholar] [CrossRef]
- Bons, H.K.; Sharma, A. Impact of foliar sprays of potassium, calcium, and boron on fruit setting behavior, yield, and quality attributes in fruit crops: A review. J. Plant Nutr. 2023, 46, 3232–3246. [Google Scholar] [CrossRef]
- Toor, M.D.; Adnan, M.; Ur Rehman, F.; Tahir, R.; Saeed, M.; Khan, A.; Pareek, V. Nutrients and their importance in agriculture crop production: A review. Indian J. Pure Appl. Biosci. 2021, 9, 1–6. [Google Scholar] [CrossRef]
- Yener, H.; Altuntaş, Ö. Effects of potassium fertilization on leaf nutrient content and quality attributes of sweet cherry fruits (Prunus Avium L.). J. Plant Nutr. 2021, 44, 946–957. [Google Scholar] [CrossRef]
- Uçgun, K.; Altindal, M. Effects of increasing doses of nitrogen, phosphorus, and potassium on the uptake of other nutrients in sweet cherry trees. Commun. Soil Sci. Plant Anal. 2021, 52, 1249–1256. [Google Scholar] [CrossRef]
- Ateş, Ö.; Alveroğlu, V.; Turhan, E.; Yalçin, G.; Taşpinar, K.; Kizilaslan, F. Effects of potassium fertilization on sweet cherry fruit (Prunus avium L.) quality and mineral content. Commun. Soil Sci. Plant Anal. 2022, 53, 1777–1782. [Google Scholar] [CrossRef]
- Csihon, A.; Gonda, I.; Orosz-Tóth, M.; Kincses, S.n.; Holb, I. Effect of N, P, K and Mg fertilizers on some vegetative and generative parameters of a sweet cherry cultivar. Int. J. Hortic. Sci. 2020, 26, 27–30. [Google Scholar] [CrossRef]
- Xie, K.; Cakmak, I.; Wang, S.; Zhang, F.; Guo, S. Synergistic and antagonistic interactions between potassium and magnesium in higher plants. Crop J. 2021, 9, 249–256. [Google Scholar] [CrossRef]
- Wang, Z.; Hassan, M.U.; Nadeem, F.; Wu, L.; Zhang, F.; Li, X. Magnesium fertilization improves crop yield in most production systems: A meta-analysis. Front. Plant Sci. 2020, 10, 1727. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Hou, Y. Effect of soil magnesium on plants: A review. IOP Conf. Ser. Earth Environ. Sci. 2018, 170, 022168. [Google Scholar] [CrossRef]
- Chaudhry, A.H.; Nayab, S.; Hussain, S.B.; Ali, M.; Pan, Z. Current understandings on magnesium deficiency and future outlooks for sustainable agriculture. Int. J. Mol. Sci. 2021, 22, 1819. [Google Scholar] [CrossRef]
- Cakmak, I.; White, P.J. Magnesium in crop production and food quality. Plant Soil 2020, 457, 1–3. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, N.; Kavino, M. Role of potassium in fruit crops—A review. Agric. Rev. 2006, 27, 284–291. [Google Scholar]
- Imtiaz, H.; Mir, A.R.; Corpas, F.J.; Hayat, S. Impact of potassium starvation on the uptake, transportation, photosynthesis, and abiotic stress tolerance. Plant Growth Regul. 2023, 99, 429–448. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.M.; Ghosh, T.K.; Kabir, A.H.; Abdelrahman, M.; Rahman Khan, M.A.; Mochida, K.; Tran, L.-S.P. Potassium in plant physiological adaptation to abiotic stresses. Plant Physiol. Biochem. 2022, 186, 279–289. [Google Scholar] [CrossRef]
- Kumari, V.V.; Banerjee, P.; Verma, V.C.; Sukumaran, S.; Chandran, M.A.; Gopinath, K.A.; Venkatesh, G.; Yadav, S.K.; Singh, V.K.; Awasthi, N.K. Plant nutrition: An effective way to alleviate abiotic stress in agricultural crops. Int. J. Mol. Sci. 2022, 23, 8519. [Google Scholar] [CrossRef]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Predieri, S.; Dris, R.; Sekse, L.; Rapparini, F. Influence of environmental factors and orchard management on yield and quality of sweet cherry. Food Agric. Environ. 2003, 1, 263–266. [Google Scholar]
- Wang, Y.-H.; Li, Y.-J.; He, X.-H.; Karim, R.; Shi, X.-J.; Xie, D.-T.; Zhang, Y.-Q. Response of crop yields to magnesium fertilization: A meta-analysis in China. Int. J. Agric. Sci. 2016, 6, 1011–1015. [Google Scholar]
- Ishfaq, M.; Kiran, A.; ur Rehman, H.; Farooq, M.; Ijaz, N.H.; Nadeem, F.; Azeem, I.; Li, X.; Wakeel, A. Foliar nutrition: Potential and challenges under multifaceted agriculture. Environ. Exp. Bot. 2022, 200, 104909. [Google Scholar] [CrossRef]
- Micheli, E.; Schád, P.; Spaargaren, O.; Dent, D.; Nachtergaele, F.; Wrb, I. World Reference Base for Soil Resources 2006: A Framework for International Classification, Correlation and Communication: FAO; World Soil Resources Reports No. 103; FAO: Rome, Italy, 2006. [Google Scholar]
- Melo, L.C.A.; Silva, C.A. Influência de métodos de digestão e massa de amostra na recuperação de nutrientes em resíduos orgânicos. Química Nova 2008, 31, 556–561. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Ricardo-Rodrigues, S.; Laranjo, M.; Agulheiro-Santos, A.C. Methods for quality evaluation of sweet cherry. J. Sci. Food Agric. 2023, 103, 463–478. [Google Scholar] [CrossRef]
- Chauvin, M.A.; Whiting, M.; Ross, C.F. The influence of harvest time on sensory properties and consumer acceptance of sweet cherries. HortTechnology 2009, 19, 748–754. [Google Scholar] [CrossRef]
- Ucgun, K. Effects of nitrogen and potassium fertilization on nutrient content and quality attributes of sweet cherry fruits. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 47, 114–118. [Google Scholar] [CrossRef]
- Pinto, T.; Vilela, A.; Cosme, F. Fruit juices and fruit fermented beverages: Consumer acceptance, chemical and sensory characteristics. In Prime Archives in Applied Sciences, 2nd ed.; Seongwoo, W.F., Ed.; Vide Leaf: Hyderabad, India, 2023. [Google Scholar]
- Cosme, F.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Gonçalves, B. Red fruits composition and their health benefits—A review. Foods 2022, 11, 644. [Google Scholar] [CrossRef]
- Erdal, A.; Saraçoğlu, O. Role of the foliar fertilization treatments on quality attributes of sweet cherry fruits (Prunus avium). Akad. Ziraat Derg. 2018, 7, 131–136. [Google Scholar] [CrossRef]
- Woldemariam, S.; Lal, S.; Zelelew, D.; Solomon, M. Effect of potassium levels on productivity and fruit quality of tomato (Lycopersicon esculentum L.). J. Agric. Stud. 2018, 6, 104. [Google Scholar] [CrossRef]
- Afzal, I.; Hussain, B.; Maqsood, S.; Basra, A.; Ullah, S.; Shakeel, Q.; Kamran, M. Foliar application of potassium improves fruit quality and yield of tomato plants. Acta Sci. Pol. Hortorum Cultus 2015, 14, 3–13. [Google Scholar]
- Quddus, M.A.; Siddiky, M.A.; Hussain, M.J.; Rahman, M.A.; Ali, M.R.; Masud, M.A.T. Magnesium influences growth, yield, nutrient uptake, and fruit quality of tomato. Int. J. Veg. Sci. 2022, 28, 441–464. [Google Scholar] [CrossRef]
- Liu, X.; Hu, C.; Liu, X.; Riaz, M.; Liu, Y.; Dong, Z.; Tan, Q.; Sun, X.; Wu, S.; Tan, Z. Effect of magnesium application on the fruit coloration and sugar accumulation of navel orange (Citrus sinensis Osb.). Sci. Hortic. 2022, 304, 111282. [Google Scholar] [CrossRef]
- Zlamalova, T.; Elbl, J.; Baron, M.; Belikova, H.; Lampir, L.; Hlusek, J.; Losak, T. Using foliar applications of magnesium and potassium to improve yields and some qualitative parameters of vine grapes (Vitis vinifera L.). Plant Soil Environ. 2015, 61, 451–457. [Google Scholar] [CrossRef]
- Zhang, W.; Song, P.; Li, G.; Wang, E.; Lv, Z.; Zhang, Y.; Zhang, Q.; Liang, F.; Yang, J. Exogenous application of nutrient elements effectively reduces grape cracking and improves fruit quality. Sci. Hortic. 2023, 319, 112157. [Google Scholar] [CrossRef]
- Luedeling, E.; Kunz, A.; Blanke, M.M. Identification of chilling and heat requirements of cherry trees—A statistical approach. Int. J. Biometeorol. 2013, 57, 679–689. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Warang, O.; Das, S. Impact of climate change on fruit crops—A review. Curr. World Environ. 2022, 17, 319–330. [Google Scholar] [CrossRef]
- Kaufmann, H.; Blanke, M. Chilling in cherry—principles and projection—a brief introduction. Acta Hortic. 2017, 1162, 39–44. [Google Scholar] [CrossRef]
- Drogoudi, P.; Kazantzis, K.; Kunz, A.; Blanke, M.M. Effects of climate change on cherry production in Naoussa, Greece and Bonn, Germany: Adaptation strategies. Euro-Mediterr. J. Environ. Integr. 2020, 5, 12. [Google Scholar] [CrossRef]
- Whiting, M.D.; Lang, G.; Ophardt, D. Rootstock and training system affect sweet cherry growth, yield, and fruit quality. HortScience 2005, 40, 582–586. [Google Scholar] [CrossRef]
- Whiting, M.D.; Ophardt, D.; McFerson, J.R. Chemical blossom thinners vary in their effect on sweet cherry fruit set, yield, fruit quality, and crop value. HortTechnology 2006, 16, 66–70. [Google Scholar] [CrossRef]
- Paltineanu, C.; Chitu, E. Climate change impact on phenological stages of sweet and sour cherry trees in a continental climate environment. Sci. Hortic. 2020, 261, 109011. [Google Scholar] [CrossRef]
- Kappel, F.; Fisher-Fleming, B.; Hogue, E. Fruit characteristics and sensory attributes of an ideal sweet cherry. HortScience 1996, 31, 443–446. [Google Scholar] [CrossRef]
- Zheng, X.; Yue, C.; Gallardo, K.; McCracken, V.; Luby, J.; McFerson, J. What attributes are consumers looking for in sweet cherries? evidence from choice experiments. Agric. Resour. Econ. Rev. 2016, 45, 124–142. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Campos, G.; Alves, G.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Physical and phytochemical composition of 23 Portuguese sweet cherries as conditioned by variety (or genotype). Food Chem. 2021, 335, 127637. [Google Scholar] [CrossRef]
- Romano, G.S.; Cittadini, E.D.; Pugh, B.; Schouten, R. Sweet cherry quality in the horticultural production chain. Stewart Postharvest Rev. 2006, 6, 1–9. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer acceptance of ‘Brooks’ and ‘Bing’ cherries is mainly dependent on fruit SSC and visual skin color. Postharvest Biol. Technol. 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Martins, V.; Silva, V.; Pereira, S.; Afonso, S.; Oliveira, I.; Santos, M.; Ribeiro, C.; Vilela, A.; Bacelar, E.; Silva, A.P.; et al. Rootstock affects the fruit quality of ‘Early Bigi’ sweet cherries. Foods 2021, 10, 2317. [Google Scholar] [CrossRef]
- Vilela, A.; Bacelar, E.A.; Pinto, T.; Anjos, R.; Correia, E.; Gonçalves, B.; Cosme, F. Novel applications, sensory and sensor techniques in beverage and food fragrance biotechnology. In Advances in Food Science; Hien, P.P., Ed.; Vide Leaf: Hyderabad, India, 2020. [Google Scholar]
| Treatment | N | P | K | Ca | Mg | S | |
|---|---|---|---|---|---|---|---|
| Cherry Skin | Control | 7.60 ± 0.58 a | 0.94 ± 0.15 a | 17.17 ± 4.14 a | 0.87 ± 0.16 a | 0.92 ± 0.10 a | 0.39 ± 0.04 a |
| Mg250 | 7.09 ± 0.81 a | 0.99 ± 0.14 a | 18.79 ± 0.96 a | 0.88 ± 0.14 a | 0.95 ± 0.04 a | 0.40 ± 0.05 a | |
| Mg125 | 7.46 ± 0.63 a | 1.08 ± 0.08 ab | 19.68 ± 2.62 a | 0.95 ± 0.08 a | 0.87 ± 0.06 a | 0.38 ± 0.05 a | |
| K100 | 7.50 ± 0.75 a | 1.03 ± 0.10 ab | 18.36 ± 0.82 a | 0.82 ± 0.04 a | 0.86 ± 0.05 a | 0.41 ± 0.03 a | |
| K50 | 9.85 ± 0.56 b | 1.36 ± 0.16 b | 21.93 ± 0.42 a | 0.98 ± 0.19 a | 0.91 ± 0.06 a | 0.47 ± 0.09 a | |
| p-Value (T) | 0.004 | 0.019 | 0.198 | 0.594 | 0.504 | 0.360 | |
| Cherry Pulp | Control | 9.68 ± 0.91 a | 0.96 ± 0.07 a | 17.76 ± 1.99 a | 0.36 ± 0.15 a | 0.79 ± 0.11 a | 0.49 ± 0.05 a |
| Mg250 | 8.81 ± 1.07 a | 1.02 ± 0.13 a | 17.64 ± 1.27 a | 0.30 ± 0.05 a | 0.75 ± 0.10 a | 0.46 ± 0.09 a | |
| Mg125 | 9.39 ± 0.34 a | 0.97 ± 0.08 a | 18.38 ± 1.67 ab | 0.47 ± 0.01 a | 0.82 ± 0.06 a | 0.45 ± 0.08 a | |
| K100 | 8.85 ± 0.83 a | 1.08 ± 0.19 a | 17.86 ± 0.57 ab | 0.44 ± 0.03 a | 0.76 ± 0.05 a | 0.54 ± 0.09 ab | |
| K50 | 13.75 ± 2.11 b | 1.27 ± 0.13 a | 21.73 ± 1.42 b | 0.48 ± 0.23 a | 0.88 ± 0.14 a | 0.73 ± 0.11 b | |
| p-Value (T) | 0.002 | 0.071 | 0.030 | 0.403 | 0.523 | 0.014 | |
| p-Value (T) | 0.000 | 0.001 | 0.007 | 0.272 | 0.555 | 0.002 | |
| p-Value (Tissue) | 0.000 | 0.674 | 0.472 | 0.000 | 0.003 | 0.000 | |
| p-Value (T*Tissue) | 0.228 | 0.773 | 0.912 | 0.786 | 0.451 | 0.168 |
| Treatment | B | Fe | Zn | Mn | Cu | |
|---|---|---|---|---|---|---|
| Cherry Skin | Control | 16.48 ± 0.25 c | 25.28 ± 2.27 a | 4.12 ± 0.58 a | 8.21 ± 0.77 a | 5.78 ± 0.94 a |
| Mg250 | 12.99 ± 0.23 ab | 23.75 ± 1.66 a | 5.11 ± 1.04 a | 5.90 ± 1.65 a | 6.25 ± 0.84 a | |
| Mg125 | 12.48 ± 0.61 ab | 25.36 ± 1.84 a | 4.53 ± 0.97 a | 8.02 ± 1.57 a | 7.40 ± 0.86 a | |
| K100 | 12.08 ± 1.09 a | 33.25 ± 0.33 b | 6.51 ± 2.88 a | 6.24 ± 0.12 a | 6.56 ± 0.88 a | |
| K50 | 14.74 ± 1.49 bc | 37.07 ± 5.09 b | 7.00 ± 3.85 a | 7.62 ± 1.47 a | 8.08 ± 2.07 a | |
| p-Value (T) | 0.001 | 0.000 | 0.490 | 0.153 | 0.220 | |
| Cherry Pulp | Control | 13.91 ± 1.80 c | 44.88 ± 4.14 bc | 4.93 ± 1.32 a | 3.90 ± 0.64 b | 4.78 ± 0.23 ab |
| Mg250 | 12.06 ± 0.39 bc | 22.47 ± 3.02 a | 4.01 ± 1.31 a | 2.28 ± 0.43 a | 4.18 ± 0.57 a | |
| Mg125 | 8.63 ± 1.38 a | 38.86 ± 1.44 b | 4.88 ± 0.67 a | 3.74 ± 0.37 b | 5.80 ± 0.54 ab | |
| K100 | 10.80 ± 0.40 ab | 19.86 ± 0.51 a | 4.84 ± 1.74 a | 3.07 ± 0.20 ab | 5.37 ± 0.87 ab | |
| K50 | 12.96 ± 0.25 bc | 52.16 ± 6.58 c | 5.28 ± 1.77 a | 2.20 ± 0.50 a | 6.53 ± 1.50 b | |
| p-Value (T) | 0.001 | 0.000 | 0.853 | 0.002 | 0.050 | |
| p-Value (T) | 0.000 | 0.000 | 0.471 | 0.008 | 0.011 | |
| p-Value (Tissue) | 0.000 | 0.000 | 0.344 | 0.000 | 0.001 | |
| p-Value (T*Tissue) | 0.111 | 0.000 | 0.681 | 0.338 | 0.917 |
| Weight | Larger Diameter | Smaller Diameter | Height | L* | a* | b* | C* | h○ | ERF | FF | TSS | pH | TA | MI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight | 1 | 0.918 ** | 0.886 ** | 0.762 ** | −0.174 ** | −0.158 ** | −0.170 ** | −0.161 ** | −0.150 ** | −0.416 ** | −0.455 ** | 0.071 | 0.460 ** | −0.348 * | 0.269 |
| Larger Diameter | 1 | 0.811 ** | 0.667 ** | −0.126 ** | −0.111 * | −0.128 ** | −0.115 * | −0.128 ** | −0.354 ** | −0.398 ** | 0.070 | 0.519 ** | −0.217 | 0.151 | |
| Smaller Diameter | 1 | 0.716 ** | −0.200 ** | −0.229 ** | −0.225 ** | −0.229 ** | −0.174 ** | −0.467 ** | −0.507 ** | 0.205 | 0.382 ** | −0.362 * | 0.331 * | ||
| Height | 1 | −0.108 * | −0.201 ** | −0.192 ** | −0.200 ** | −0.129 ** | −0.333 ** | −0.317 ** | 0.054 | 0.094 | −0.203 | 0.179 | |||
| L* | 1 | 0.811 ** | 0.824 ** | 0.816 ** | 0.715 ** | 0.199 ** | 0.256 ** | −0.154 | 0.060 | 0.255 | −0.274 | ||||
| a* | 1 | 0.980 ** | 0.999 ** | 0.858 ** | 0.335 ** | 0.404 ** | −0.433 ** | 0.287 | 0.317 * | −0.420 ** | |||||
| b* | 1 | 0.987 ** | 0.923 ** | 0.294 ** | 0.368 ** | −0.382 ** | 0.183 | 0.263 | −0.349 * | ||||||
| C* | 1 | 0.872 ** | 0.329 ** | 0.399 ** | −0.425 ** | 0.271 | 0.308 * | −0.409 ** | |||||||
| h○ | 1 | 0.120 * | 0.190 ** | −0.257 | −0.079 | 0.084 | −0.140 | ||||||||
| ERF | 1 | 0.877 ** | −0.372 * | 0.793 ** | 0.705 ** | −0.667 ** | |||||||||
| FF | 1 | −0.601 ** | 0.736 ** | 0.774 ** | −0.793 ** | ||||||||||
| TSS | 1 | −0.509 ** | −0.380 * | 0.696 ** | |||||||||||
| pH | 1 | 0.257 | −0.440 ** | ||||||||||||
| TA | 1 | −0.914 ** | |||||||||||||
| MI | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, M.; Pereira, S.; Ferreira, H.; Sousa, J.R.; Vilela, A.; Ribeiro, C.; Raimundo, F.; Egea-Cortines, M.; Matos, M.; Gonçalves, B. Optimizing Sweet Cherry Attributes through Magnesium and Potassium Fertilization. Horticulturae 2024, 10, 881. https://doi.org/10.3390/horticulturae10080881
Santos M, Pereira S, Ferreira H, Sousa JR, Vilela A, Ribeiro C, Raimundo F, Egea-Cortines M, Matos M, Gonçalves B. Optimizing Sweet Cherry Attributes through Magnesium and Potassium Fertilization. Horticulturae. 2024; 10(8):881. https://doi.org/10.3390/horticulturae10080881
Chicago/Turabian StyleSantos, Marlene, Sandra Pereira, Helena Ferreira, João Ricardo Sousa, Alice Vilela, Carlos Ribeiro, Fernando Raimundo, Marcos Egea-Cortines, Manuela Matos, and Berta Gonçalves. 2024. "Optimizing Sweet Cherry Attributes through Magnesium and Potassium Fertilization" Horticulturae 10, no. 8: 881. https://doi.org/10.3390/horticulturae10080881







