Mutagenesis and Flowering Promotion through Sodium Azide In Vitro Culture of Cymbidium faberi Rolfe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Explant Preparation
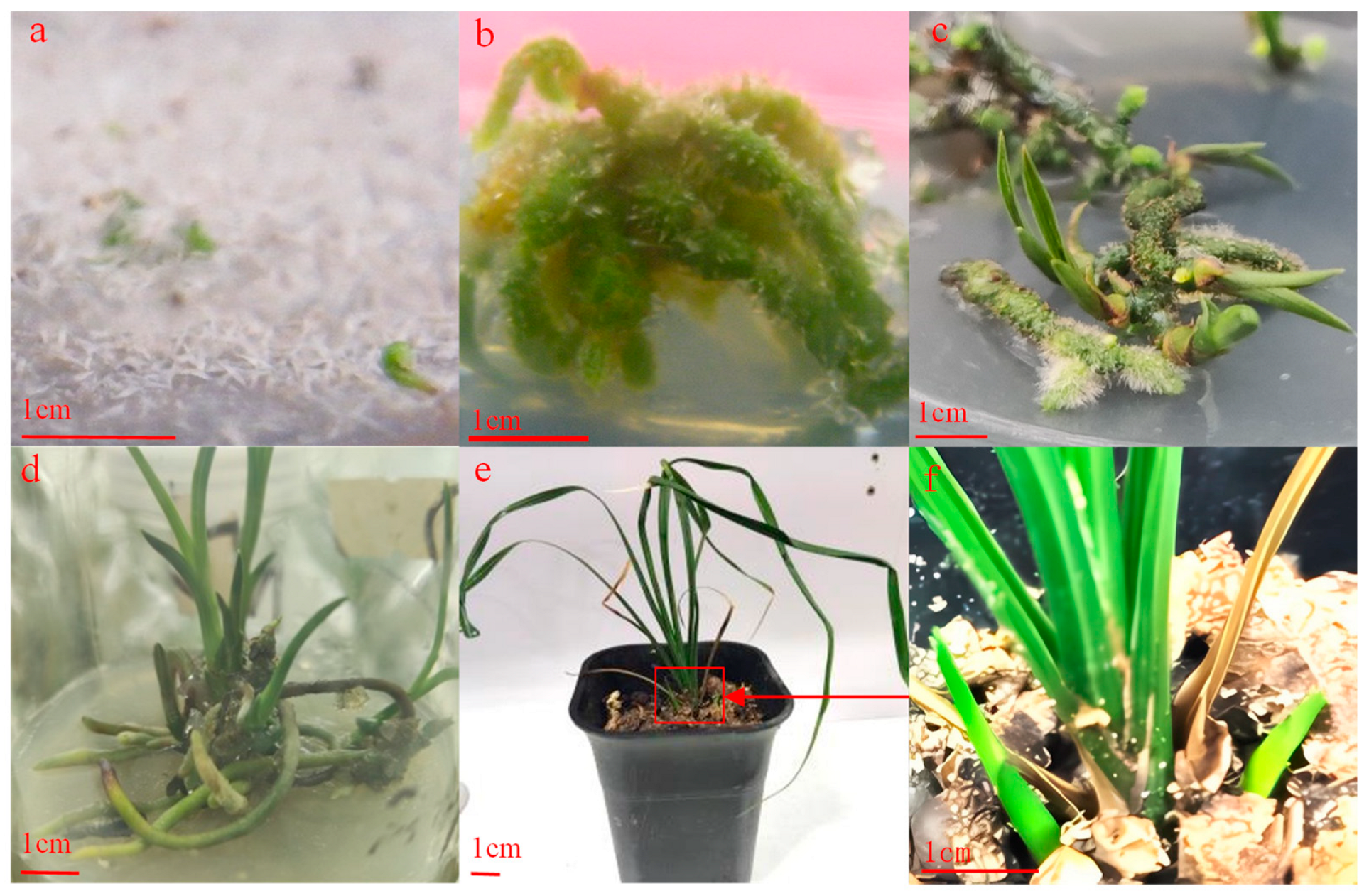
2.2. Building a Cymbidium faberi Rolfe Regeneration System
2.3. NaN3 Mutagenesis
2.4. Data Analysis
3. Results
3.1. Effect of PGRs on Proliferation of PLBs
3.2. Effect of PGRs on Induced Rooting of Cymbidium faberi Rolfe Seedlings
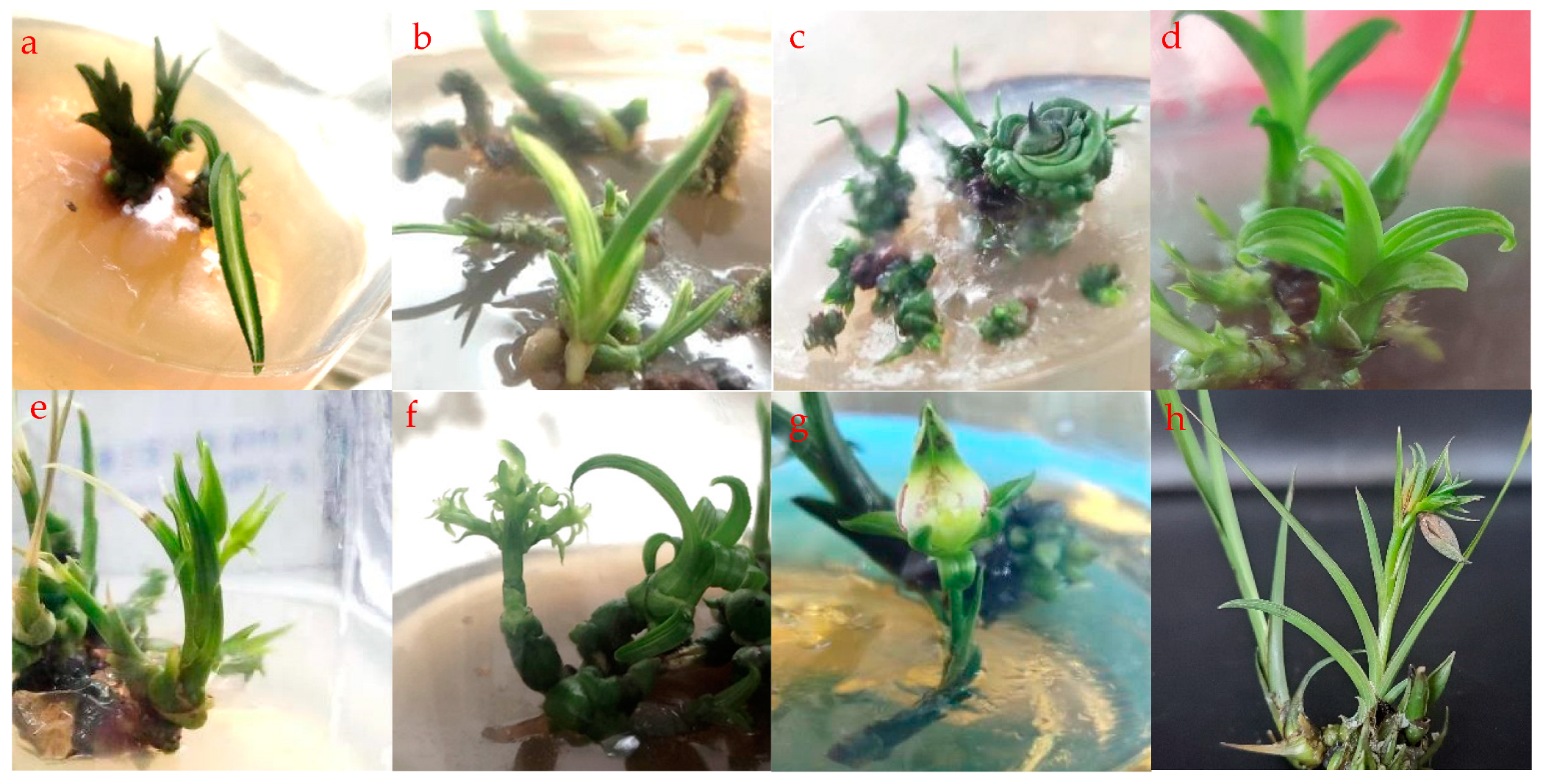
3.3. Mutagenesis of PLBs through Low Concentration of NaN3
3.4. Mutagenesis of Shoots by High Concentration of NaN3
3.5. NaN3 Promotes Flowering in Cymbidium faberi Rolfe
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.C. Chinese Orchid Species and Their Distribution. Flowers 2018, 23, 25–27. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Liu, X.; Liu, Y.Q. In vitro plant regeneration from the immature seeds of Cymbidium faberi. Plant Cell Tissue Organ Cult. 2005, 81, 247–251. [Google Scholar] [CrossRef]
- Zeng, R.Z.; Zhu, J.; Xu, S.Y.; Du, G.H.; Guo, H.R.; Chen, J.J.; Zhang, Z.S.; Xie, L. Unreduced Male Gamete Formation in Cymbidium and Its Use for Developing Sexual Polyploid Cultivars. Front. Plant Sci. 2020, 11, 558. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.G.; Tian, Y.F. Cloning and bioinformatics analysis of a PEBP family gene from Cymbidium faberi. Acta Agric. Zhejiangensis 2022, 34, 1679–1691. [Google Scholar]
- Liu, Y.; Zhang, H.L.; Guo, H.R.; Xie, L.; Zeng, R.Z.; Zhang, X.Q.; Zhang, Z.S. Transcriptomic and Hormonal Analyses Reveal that YUC-Mediated Auxin Biogenesis Is Involved in Shoot Regeneration from Rhizome in Cymbidium. Front. Plant Sci. 2017, 8, 1866. [Google Scholar] [CrossRef]
- Yang, J.Q. Observations on Pollination in Wild Cymbidium faberi Rolfe. South China Agric. 2017, 11, 38–39. [Google Scholar] [CrossRef]
- Pradhan, S.; Tiruwa, B.; Subedee, B.R.; Pant, B. In vitro germination and propagation of a threatened medicinal orchid, Cymbidium aloifolium (L.) Sw. through artificial seed. Asian Pac. J. Trop. Biomed. 2014, 4, 971–976. [Google Scholar] [CrossRef]
- Soares, J.S.; Rosa, Y.B.C.J.; Tatara, M.B.; Sorgato, J.C.; Lemes, C.S.R. Identificação da viabilidade de sementes de orquídeas pelo teste de tetrazólio. Semin. Ciênc. Agrárias 2014, 35, 2275–2284. [Google Scholar] [CrossRef]
- Custódio, C.C.; Marks, T.R.; Pritchard, W.; Hosomi, S.T.; Machado-Neto, N.B. Improved tetrazolium viability testing in orchid seeds with a thick carapace (Dactylorhiza fuchsii) or dark seed coat (Vanda curvifolia). Seed Sci. Technol. 2016, 44, 177–188. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Senthilkumar, R.; Vijayakumar, S. In vitro micropropagation of orchid, Oncidium sp. (Dancing Dolls). Afr. J. Biotechnol. 2007, 6, 1171–1174. [Google Scholar]
- Sun, C.B.; Liu, M.; Shi, J.S.; Guo, F.Q.; Li, X. Aseptic germination of Cymbidium faberi seeds and in vitro plant regeneration. Acta Agric. Zhejiangensis 2008, 20, 231–235. [Google Scholar]
- Yu, Y.C. Study on Tissue Culture of Cymbidium faberi Da Yipin’ and Cold Resistance of Chinese Cymbidium. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2014. [Google Scholar]
- Türkoğlu, A.; Haliloğlu, K.; Tosun, M.; Szulc, P.; Demirel, F.; Eren, B.; Bujak, H.; Karagöz, H.; Selwet, M.; Özkan, G.; et al. Sodium Azide as a Chemical Mutagen in Wheat (Triticum aestivum L.): Patterns of the Genetic and Epigenetic Effects with iPBS and CRED-iPBS Techniques. Agriculture 2023, 13, 1242. [Google Scholar] [CrossRef]
- Wannajindaporn, A.; Poolsawat, O.; Chaowiset, W.; Tantasawat, P.A. Evaluation of genetic variability in in vitro sodium azide-induced Dendrobium ‘Earsakul’ mutants. Genet. Mol. Res. GMR 2014, 13, 5333–5342. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.h.; Zhao, Y.; Zhang, Q.; Sun, Y.J.; Zhou, Z.Y. Effect of azide sodium in mutagenesis on physiological traits of Phalaenosis protocorm-like body in vitro. J. Nucl. Agric. Sci. 2010, 24, 411–414. [Google Scholar]
- Cui, G.R.; Zhang, Z.X.; Zhang, C.Y.; Hu, N.B.; Sui, Y.H.; Li, J.Q. Study on NaN3 chemical induction for Phalaenopsis Tsuei Foa Lady in in vitro culture and RAPD screening of regenerated plants. Chin. J. Trop. Crops 2010, 31, 592–599. [Google Scholar]
- Hualsawat, S.; Khairum, A.; Chueakhunthod, W.; Tharapreuksapong, A.; Tantasawat, P.A. Profiling of black rot resistant Dendrobium ‘Earsakul’ induced by in vitro sodium azide mutagenesis. Eur. J. Hortic. Sci. 2022, 87, 13. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.R.; Zhang, Z.X.; Zhang, C.Y.; Hu, N.B.; Sui, Y.H.; Li, J.Q. Study on NaN3 chemical induction for Oncidium in in vitro culture and RAPD screening. Guihaia 2011, 31, 836–843. [Google Scholar]
- AL-qurainy, F.; AL-hemaid, F.M.; Khan, S.; Ali, M.A.; Tarroum, M.; Ashraf, M. Detection of sodium azide-induced mutagenicity in the regenerated shoots of Artemisia annua L., using internal transcribed spacer (ITS) sequences of nrDNA. Pak. J. Bot. 2011, 43, 2183–2186. [Google Scholar]
- Qian, Y.Y.; Han, X.; Liu, Y.; Li, J.L.; Cui, S.F.; Zhang, H.N.; Wang, G.E.; Jin, W.P. Application of Sodium Azide (NaN3) Mutation in Crop Character Improvement. J. Anhui Agric. Sci. 2017, 45, 136–138+141. [Google Scholar] [CrossRef]
- Zhang, R.C.; Li, W.; Pan, S.J.; Dai, L.Y.; Liu, S.M. Application of Chemical Mutagenesis in Improving Germplasm Resource. Mol. Plant Breed. 2017, 15, 5189–5196. [Google Scholar] [CrossRef]
- Salim, K.; Fahad, A.-Q.; Firoz, A. Sodium Azide: A Chemical Mutagen for Enhancement of Agronomic Traits of Crop Plants. Environ. We Int. J. Sci. Tech. 2009, 4, 1–21. [Google Scholar]
- Jenks, M.A.; Hasegawa, P.M.; Jain, S.M. Advances in Molecular Breeding Toward Drought and Salt Tolerant Crops; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Skoog, F.K.; Miller, C.O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 1957, 11, 118–130. [Google Scholar]
- Novak, S.D.; Luna, L.J.; Gamage, R.N. Role of Auxin in Orchid Development. Plant Signal. Behav. 2014, 9, e972277. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Yu, L.; Kong, F.; Zhao, D. Effects of plant growth regulators on in vitro propagation of Cymbidium. Afr. J. Biotechnol. 2012, 10, 15639–15646. [Google Scholar]
- Fu, X.; Zhou, H.; Fang, Z.; Weiting, H. Study on Tissue Culture and Rapid Propagation System of Cymbidium faberi Rolfe. North. Hortic. 2018, 24, 104–109. [Google Scholar]
- Chang, C.; Chang, W.-C. Effect of thidiazuron on bud development of Cymbidium sinense Willd in vitro. Plant Growth Regul. 2000, 30, 171–175. [Google Scholar] [CrossRef]
- Gao, R.; Wu, S.-q.; Piao, X.C.; Park, S.-Y.; Lian, M.L. Micropropagation of Cymbidium sinense using continuous and temporary airlift bioreactor systems. Acta Physiol. Plant. 2013, 36, 117–124. [Google Scholar] [CrossRef]
- Sato, M.; Gaul, H. Effect of ethyl methanesulfonate on the fertility of barley. Radiat. Bot. 1967, 7, 7–15. [Google Scholar] [CrossRef]
- Maherchandani, N. Effects of gamma radiation on the dormant seed of Avena fatua L. Radiat. Bot. 1975, 15, 439–443. [Google Scholar] [CrossRef]
- Chen, J.T.; Nargar, K. Editorial: Orchid Genomics and Developmental Biology. Front. Plant Sci. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Supriya, R.; Amal, K.B. Isolation of a white flowered mutant through seed culture in Spathoglottis plicata Blume. Jpn. Mendel Soc. 2005, 70, 1–6. [Google Scholar]
- El-Mokadem, H.E.; Mostafa, G.G. Induction of mutations in Browallia speciosa using sodium azide and identification of the genetic variation by peroxidase isozyme. Afr. J. Biotechnol. 2014, 13, 106–111. [Google Scholar] [CrossRef]
- Chen, D.J.; Yan, W.H.; Fu, L.Y.; Kaufmann, K. Architecture of gene regulatory networks controlling flower development in Arabidopsis thaliana. Nat. Commun. 2018, 9, 4534. [Google Scholar] [CrossRef]
- Kleinhofs, A.; Owais, W.M.; Nilan, R.A. Azide. Mutat. Res. Genet. Toxicol. 1978, 55, 165–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, L.; Ban, L.; An, W.G.; Liu, S.D.; Li, X.; Xue, B.; Xu, Y. Effect of sodium azide on mitochondrial membrane potential in SH-SY5Y human neuroblastoma cells. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. Acta Acad. Med. Sin. 2000, 22, 436–439. [Google Scholar]
- Vercellino, I.; Sazanov, L.A. The assembly, regulation and function of the mitochondrial respiratory chain. Nat. Rev. Mol. Cell Biol. 2022, 23, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Naaz, N.; Choudhary, S.; Sharma, N.; Hasan, N.; Al Shaye, N.A.; Abd El-Moneim, D. Frequency and spectrum of M2 mutants and genetic variability in cyto-agronomic characteristics of fenugreek induced by caffeine and sodium azide. Front. Plant Sci. 2023, 13, 1030772. [Google Scholar] [CrossRef]
- Shi, M.M.; Wang, C.L.; Wang, P.; Zhang, M.L.; Liao, W.B. Methylation in DNA, histone, and RNA during flowering under stress condition: A review. Plant Sci. 2022, 324, 111431. [Google Scholar] [CrossRef]
- Sherrard, M.E.; Maherali, H. The adaptive significance of drought escape in Avena barbata, an annual grass. Evol. Int. J. Org. Evol. 2006, 60, 2478–2489. [Google Scholar] [CrossRef]
- Kolar, J.; Senkova, J. Reduction of mineral nutrient availability accelerates flowering of Arabidopsis thaliana. J. Plant Physiol. 2008, 165, 1601–1609. [Google Scholar] [CrossRef]
- Hatayama, T.; Takeno, K. The metabolic pathway of salicylic acid rather than of chlorogenic acid is involved in the stress-induced flowering of Pharbitis nil. J. Plant Physiol. 2003, 160, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, J.; Wang, L.; Xu, M. Progress of stress-induced flowering in plants. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2016, 32, 1301–1308. [Google Scholar] [CrossRef]
- Song, Y.H.; Ito, S.; Imaizumi, T. Flowering time regulation: Photoperiod- and temperature-sensing in leaves. Trends Plant Sci. 2013, 18, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Verhage, L.; Angenent, G.C.; Immink, R.G.H. Research on floral timing by ambient temperature comes into blossom. Trends Plant Sci. 2014, 19, 583–591. [Google Scholar] [CrossRef]
| Treatments | NAA (mg·L−1) | 6-BA (mg·L−1) | 2,4-D (mg·L−1) | IBA (mg·L−1) |
|---|---|---|---|---|
| B-0 | 0 | 0 | 0 | 0 |
| B-1 | 1 | 0.5 | 0.1 | 0 |
| B-2 | 1 | 1.0 | 0.1 | 0 |
| B-3 | 1 | 0.5 | 0.2 | 0 |
| B-4 | 0 | 0.5 | 0.1 | 1 |
| Treatments | New Branches | New Growth Length (mm) | New Growth Diameter (mm) |
|---|---|---|---|
| B-0 | 0 | 0.26 ± 0.04 d | 0.04 ± 0.05 e |
| B-1 | 3.10 ± 0.23 a | 4.87 ± 0.01 b | 0.96 ± 0.04 a |
| B-2 | 2.80 ± 0.17 a | 5.31 ± 0.06 a | 0.82 ± 0.03 b |
| B-3 | 2.47 ± 0.14 b | 4.53 ± 0.04 c | 0.59 ± 0.04 d |
| B-4 | 2.31 ± 0.17 b | 4.76 ± 0.05 b | 0.68 ± 0.02 c |
| Treatments | NAA (mg·L−1) | IAA (mg·L−1) | 6-BA (mg·L−1) | Rooting Rate (%) | Transplant Survival Rate (%) | Root Growth Status |
|---|---|---|---|---|---|---|
| D-1 | 0.2 | 0 | 2 | 8.80 ± 0.80 e | 87.41 ± 0.77 d | White slender |
| D-2 | 0.5 | 0 | 2 | 10.18 ± 0.80 de | 90.29 ± 0.44 c | White slender |
| D-3 | 1 | 0 | 2 | 18.98 ± 3.21 c | 96.64 ± 0.37 b | Green thick |
| D-4 | 0.2 | 1 | 2 | 12.96 ± 0.80 d | 90.47 ± 0.24 c | White slender |
| D-5 | 2 | 0 | 1 | 49.54 ± 3.21 b | 96.21 ± 0.77 b | White thick |
| D-6 | 2 | 1 | 1 | 73.15 ± 2.12 a | 98.31 ± 0.37 a | Yellow-green thick |
| Treatments | NaN3 (mg·L−1) | PLB Mortality (%) | Differentiation Rate (%) |
|---|---|---|---|
| E-0 | 0.0 | 8.33 ± 0.41 d | 80.56 ± 0.24 a |
| E-1 | 0.5 | 16.67 ± 0.41 c | 71.17 ± 0.28 b |
| E-2 | 1.0 | 19.44 ± 0.48 c | 58.27 ± 0.34 c |
| E-3 | 1.5 | 36.11 ± 0.63 b | 49.81 ± 0.52 d |
| E-4 | 2.0 | 41.67 ± 0.72 ab | 41.34 ± 0.14 e |
| E-5 | 2.5 | 48.61 ± 0.24 a | 26.28 ± 0.40 f |
| E-6 | 3.0 | 48.33 ± 0.41 d | 10.68 ± 0.40 g |
| Treatment | NaN3 (mg·L−1) | Leaf Growth Length (mm) | Bud Mortality Rate (%) |
|---|---|---|---|
| F-0 | 0.0 | 16.33 ± 0.88 a | 0 |
| F-1 | 4.0 | 6.04 ± 0.85 b | 48.61 ± 0.24 d |
| F-2 | 5.0 | 4.38 ± 0.25 c | 52.78 ± 0.24 d |
| F-3 | 6.0 | 3.38 ± 0.33 d | 66.67 ± 0.42 c |
| F-4 | 7.0 | 1.42 ± 0.19 e | 80.56 ± 1.04 b |
| F-5 | 8.0 | 0.96 ± 0.38 e | 84.72 ± 0.24 b |
| F-6 | 9.0 | 0 ± 0 f | 93.06 ± 0.24 a |
| F-7 | 10.0 | 0 ± 0 f | 1 ± 0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Liu, S.; An, B.; Zhang, H.; Wu, J.; Li, C.; Long, Y. Mutagenesis and Flowering Promotion through Sodium Azide In Vitro Culture of Cymbidium faberi Rolfe. Horticulturae 2024, 10, 889. https://doi.org/10.3390/horticulturae10080889
Wu Z, Liu S, An B, Zhang H, Wu J, Li C, Long Y. Mutagenesis and Flowering Promotion through Sodium Azide In Vitro Culture of Cymbidium faberi Rolfe. Horticulturae. 2024; 10(8):889. https://doi.org/10.3390/horticulturae10080889
Chicago/Turabian StyleWu, Zhengjing, Sujuan Liu, Bingjie An, Hao Zhang, Jingjing Wu, Chenfang Li, and Yuan Long. 2024. "Mutagenesis and Flowering Promotion through Sodium Azide In Vitro Culture of Cymbidium faberi Rolfe" Horticulturae 10, no. 8: 889. https://doi.org/10.3390/horticulturae10080889





