Abstract
Green leafy vegetables are rich in lipophilic metabolites such as carotenoids, phytosterols, tocopherols, and fatty acids, known for their significant health benefits. Goat’s beard (Aruncus dioicus var. kamtschaticus), a wild leafy vegetable native to Ulleungdo Island, South Korea, is reported to possess various biological activities and bioactive compounds. However, the content and profiles of lipophilic metabolites, especially carotenoids, have not been reported. This study investigated the composition of lipophilic metabolites in the foliage of goat’s beard using liquid chromatography–diode-array detection–mass spectrometry, gas chromatography–mass spectrometry, and gas chromatography–flame ionization detection. Five carotenoids (violaxanthin, neoxanthin, lactucaxanthin, lutein, β-carotene) and α-tocopherol were identified and quantified using liquid chromatography–diode-array detection–mass spectrometry. Lactucaxanthin, previously discovered in lettuce, was identified for the first time in goat’s beard foliage, with a higher concentration (45.42 ± 0.80 µg/g FW) compared to red lettuce (19.05 ± 0.67 µg/g FW). Furthermore, total carotenoids and α-tocopherol contents were higher in goat’s beard than in red lettuce. Gas chromatography–mass spectrometry analysis showed the presence of three phytosterols, namely, campesterol (3.53 ± 0.20 µg/g FW), stigmasterol (65.30 ± 4.87 µg/g FW), and β-sitosterol (89.54 ± 2.46 µg/g FW). Gas chromatography–flame ionization detection analysis revealed the presence of five essential fatty acids, with α-linolenic acid (57.03 ± 0.47%) being the most abundant, contributing to a favorable polyunsaturated FA/saturated FA ratio. These findings underscore the nutritional potential of a goat’s beard, suggesting its promising use in dietary supplements and the commercial extraction of valuable lipophilic antioxidants, particularly lactucaxanthin, for nutraceuticals and functional foods.
1. Introduction
The genus Aruncus, belonging to the family Rosaceae, comprises three species of herbaceous perennials (Aruncus dioicus, Aruncus gombalanus, and Aruncus aethusifolius) that are commonly distributed in the Northern Hemisphere [1]. Aruncus species can be used as vegetables and have high medicinal and ornamental values. Aruncus dioicus var. kamtschaticus (goat’s beard) is a wild perennial leafy vegetable native to Ulleungdo Island, South Korea [2], distributed in China, Japan, and Korea. Goat’s beard has been cultivated for food, ornamental, and medicinal purposes due to its refreshing taste, aroma, nutrients, attractive flowers, and saponin content [3]. It is traditionally used for blood-stopping, detoxification, skin care, and tonsillitis [2]. Goat’s beard extracts have a variety of health benefits. These include anti-adipogenesis [4], anti-aging [2], anti-bacterial [5], anti-diabetic [6], and anti-inflammatory [7] properties, as well as anti-obesity [8], antioxidant [5,9,10], anti-thrombosis [11], and anti-viral [12] effects. Additionally, they have anti-wrinkling [13], apoptogenic [14], and cytotoxic [9,10] activities and inhibit enzymes such as acetylcholinesterase [15], α-glucosidase, and pancreatic lipase [16]. They also exhibit hypoglycemic and hypolipidemic [17] effects and are known for their whitening [18] properties. Furthermore, goat’s beard extracts have been found to alleviate psoriatic symptoms [19] and reduce brain [20] and renal [21] injuries.
Goat’s beard is reported to contain various groups of metabolites, such as cyanogenic glycosides (prunasin and sambunigrin) [10,22], flavonol glycosides (hyperoside) [9], phenolic glycosides (1-O-caffeoyl-β-D-glucopyranose, β-sitosterol-3-O-β-D-glucopyranoside) [9,22], fatty alcohols (Pentacosan-1-ol) [9], fatty acids (palmitic acid) [9], monoterpenoids (aruncin A–E, and aruncide A–E) [10,22,23], phenolics (caffeic acid, chlorogenic acid, 1-O-caffeoylglucose, 4-O-caffeoylglucose, 5-O-p-coumaroylquinic acid, cinnamic acid, caffeic acid derivative I–V, ferulic acid, isoquercetin, kaempferol O-mallonylhexoside, kaempferol 3-O-glucoside, 2,4-dihydroxycinnamic acid, quercetin 3-O-rhamnoside, hyperoside, shanzhiside methyl ester, 3,5-O-dicaffeoylquinic acid, sergeolide, and veratric acid) [4,9,22,24,25], sterol-triterpenes (10-nonacosanol, β-sitosterol) [9], and tocopherols [26]. These phytochemicals exhibit various biological actions, including antioxidant and anticancer activities.
Green leafy vegetables are also a rich source of lipophilic compounds, including carotenoids, phytosterols, and tocopherols, which are reported to possess anti-cancer [27], anti-inflammatory [28,29], antioxidant [28,30], anti-obesity [28,31], and neuroprotective [32] activities. Of the lipophilic compounds, carotenoids (colored pigments) play important roles, like harvesting light (photosynthesis) and protecting cells against oxidative damage in algae, photosynthetic bacteria, and plants. However, the content and composition of carotenoids are not disclosed in goat’s beard. Thus, in this study, we investigated the profile of lipophilic metabolites such as carotenoids (CAs), phytosterols (PHs), tocopherols (TLs), and fatty acids (FAs) in goat’s beard. Five carotenoids (CAs), three phytosterols (PHs), α-tocopherol (α-TL), and five fatty acids (FAs) were identified and quantified using liquid chromatography–diode-array detection–mass spectrometry (LC–DAD–MS), gas chromatography–mass spectrometry (GC–MS), and gas chromatography–flame ionization detection (GC-FID). We identified (all-E)-lactucaxanthin (CA–Lac), a carotenoid uncommonly present in plants but found in lettuce, for the first time in the foliage of goat’s beard. The level of CA–Lac was also higher in goat’s beard foliage than in lettuce foliage. The results suggest that goat’s beard foliage could be a potential source for commercially extracting CA–Lac.
2. Materials and Methods
2.1. Plant Materials, Reagents, and Standards
The seeds of red lettuce (cv. Super caesar red; Lactuca sativa L.) were purchased from Asia Seeds Corporations, limited (Seoul, Republic of Korea), and cultivated between April and May 2023, as shown in our recent study [33]. Aruncus dioicus var. kamtschaticus (Maxim.) H. Hara (goat’s beard) was obtained from the Hanaro market, Seoul, Republic of Korea, in April 2023 and identified by Dr. Eun Young Ko and Dr. Iyyakkannu Sivanesan. Authentic standards of (all-E)-β-carotene (CA–βCar), FA standard mix (CRM47885), 5-β-cholestan-3α-ol (PH–βCho), stigmasterol (PH–Sti), β-sitosterol (PH–βSit) (24α-ethyl cholesterol), and campesterol (PH-Cam) (24α-methyl cholesterol) were obtained from Merck Ltd., Seoul, Republic of Korea. α-TL was purchased from ChromaDex, Inc., Irvine, CA, USA. (all-E)-Violaxanthin (CA–Vio), 9-Z-neoxanthin (CA–Neo), all-(E)-lactucaxanthin (CA–Lac), and (all-E)-lutein (CA–Lut) used in the present study were purified from lettuce [34].
2.2. Extraction of Lipophilic Metabolites
The lipophilic metabolites, including CAs, α-TL, PHs, and FAs, were extracted from goat’s beard and lettuce foliage according to Saini et al. [35], with slight modifications. Fresh foliage (2 g) was placed in a 50 mL Falcon tube and homogenized with 25 mL extraction solvent [acetone(1)/ethanol(1)/cyclohexane(2)], containing 0.1% (w/v) synthetic antioxidant (butylated hydroxytoluene, BHT). The mixture was sonicated (JAC-2010; 300 w and 60 Hz) for 10 min, followed by ultra-shaking for 2 min. After vacuum filtration, the pellets were subjected to a second extraction with 20 mL of extraction solvent. The pooled filtrates were vacuum-dried using a rotary evaporator at 35 °C, dissolved in 4 mL of acetone containing 0.1% BHT, filtered (PTFE syringe filter, 0.45 µm, Whatman, Maidstone, UK) into a 5 mL glass vial, and used for further analyses.
2.3. LC–DAD–MS Analysis of CAs and α-TL
The identification and quantification of CAs and α-TL in goat’s beard and lettuce extracts were achieved using a Shimadzu LCMS (9030) system coupled with a Shimadzu PDA detector (SPD-M40) and a Shimadzu autosampler (SIL40C x3) in Tokyo, Japan. For CAs and α-TL separation, a 3 μm, 150 mm × 4.6 mm C-30 carotenoid YMC column (Wilmington, NC, USA) was utilized, with the column oven temperature set at 20 °C, an injection volume of 20 μL, and a flow rate of 0.5 mL/min. The mobile phases (A: methanol (95)/water(5); B: methyl tertiary butyl ether (90)/methanol(7)/water(3)) contained 5 mM of ammonium formate with a flow rate of 5 mL/min, following a gradient of 0% B to 100% B over 45 min, with a 5 min post-run at 0% B. MS parameters included an MS program (0 min–diverter valve to drain and 8 min–diverter value to MS), ionization method (atmospheric pressure chemical ionization positive mode, APCI+), interface temperature (400 °C), corona needle voltage (4.0 kV), DL temperature (300 °C), heat block temperature (300 °C), drying gas flow (10 L/min), nebulizing gas flow (3 L/min), data acquisition (1.86 Hz), and Q1 resolution (±20 ppm). CAs and α-TL were identified by co-chromatography with authentic standards, UV–VIS spectral features, and mass spectra characteristics and quantified using five-point external curves of standards [33]. The limits of detection (LOD) and limits of quantitation (LOQ) were established based on signal-to-noise (S/N) ratios greater than 3 and 10, respectively, for quantitative analysis using LC-MS.
2.4. GC–MS Analysis of PHs
For hydrolysis, 20 µg internal standard (PH–βCho) was added to 0.5 mL goat’s beard lipophilic extract in a 5 mL glass vial and evaporated to dryness under a gentle stream of nitrogen; then, 1.5 mL of 0.5 M methanolic potassium hydroxide was added, and the mixture was heated to 85 °C in a water bath for 30 min, cooled immediately, and partitioned with 1 mL (1 M) sodium chloride and 1.5 mL of hexane. The upper organic phase (1 mL) was transferred into a glass vial (5 mL) and the mixture was evaporated to dryness under a gentle stream of nitrogen; then, silylating mixture (50 µL of N, O-bis(trimethylsilyl)trifluoroacetamide containing 1% trimethylchlorosilane and 1 mL pyridine) was added, and the mixture was kept at 60 °C for 60 min, cooled immediately, and filtered (PTFE syringe filter, 0.45 µm, Whatman) into a 5 mL glass vial for GC–MS analysis.
The analysis of PHs was conducted using a Shimadzu GC–MS (QP2010 SE) system (Tokyo, Japan), equipped with a DB-5 ms (30 m length, 0.25 μm film thickness, 0.25 mm inner diameter) column (Agilent Technologies Canada, Inc., Mississauga, ON, Canada). The GC operated under the following conditions: the column oven temperature was initially set at 150 °C and held for 1 min, then increased to 20 °C/min until reaching 300 °C and held for 30 min. The injection temperature was maintained at 260 °C, utilizing a split injection mode. Helium served as the carrier gas, with flow control set to liner velocity at 36.7 cm/s and a pressure of 86.5 kPa. Column flow was 0.93 mL/min, purge flow 3.0 mL/min, and total flow 8.6 mL/min. The total program time was 38.5 min. MS conditions included an ion source temperature of 260 °C, an interface temperature of 280 °C, a solvent cut time of 3 min, a start time of 6 min, and an end time of 38 min. Data acquisition was conducted in scan mode with a scan speed of 2500, an event time of 30 s, and a mass range from start m/z 50.00 to end m/z 650.00. Identification of PHs was confirmed by comparing their mass fragmentation patterns with authentic standards and reference databases (Wiley9, NIST08, and NIST08S). The individual PHs were quantified using five-point (5–50 µg/mL) external curves of standards.
2.5. GC-FID and GC–MS Analysis of FAs
To prepare fatty acid methyl esters (FAMEs), 0.5 mL of hydrolyzed goat’s beard lipophilic extract was partitioned with 1 mL of sodium chloride (1 M) and 1.5 mL of diethyl ether. The upper clear organic phase was then transferred into a 5 mL glass vial and evaporated to dryness under a gentle stream of nitrogen. Subsequently, 1.5 mL of methanolic-HCl solution (5% HCl in methanol) was added, and the mixture was kept at 60 °C in a heat block for 60 min. After cooling, 1.5 mL of hexane and 1 mL of sodium chloride (1 M) were added. The upper organic phase (1 mL) was filtered through a 0.45 µm PTFE syringe filter (Whatman, Maidstone, UK) into another 5 mL glass vial for GC-FID and GC–MS analysis.
The analysis of FAs was carried out using an Agilent GC (7890B) system equipped with FID, an autosampler, and an SP-2560 capillary column [length (100 m), film thickness (0.20 μm), inner diameter (0.25 mm)] from Merck KgaA in Darmstadt, Germany. The GC operated under the following conditions: the column oven temperature was set at 140 °C and held for 1 min, then increased to 5 °C/min until reaching 240 °C, where it was held for 30 min. The injection temperature was 250 °C, and the injection mode was split (5:1). An injection volume of 1 μL was used, with a 10 mL/min split flow. The inlet pressure was set at 54.901 psi, with an inlet total flow of 15 mL/min and a septum purge flow of 3 mL/min. Nitrogen was the carrier gas with a 2 mL/min flow rate. The detector settings were Hz flow at 30 mL/min, airflow at 400 mL/min, and a detector temperature of 260 °C. The total run time was 45 min, with a 5 min post-run time. To accurately identify FAMEs, mass spectra were recorded using the Shimadzu GS-MS (QP2010 SE) system following the GC-FID thermal program. The mass fragmentation pattern was compared with authentic standards and reference databases (NIST08, NIST08S, and Wiley9) to confirm the identity of FAMEs.
3. Results
3.1. CAs and α-TL in Lettuce and Goat’s Beard
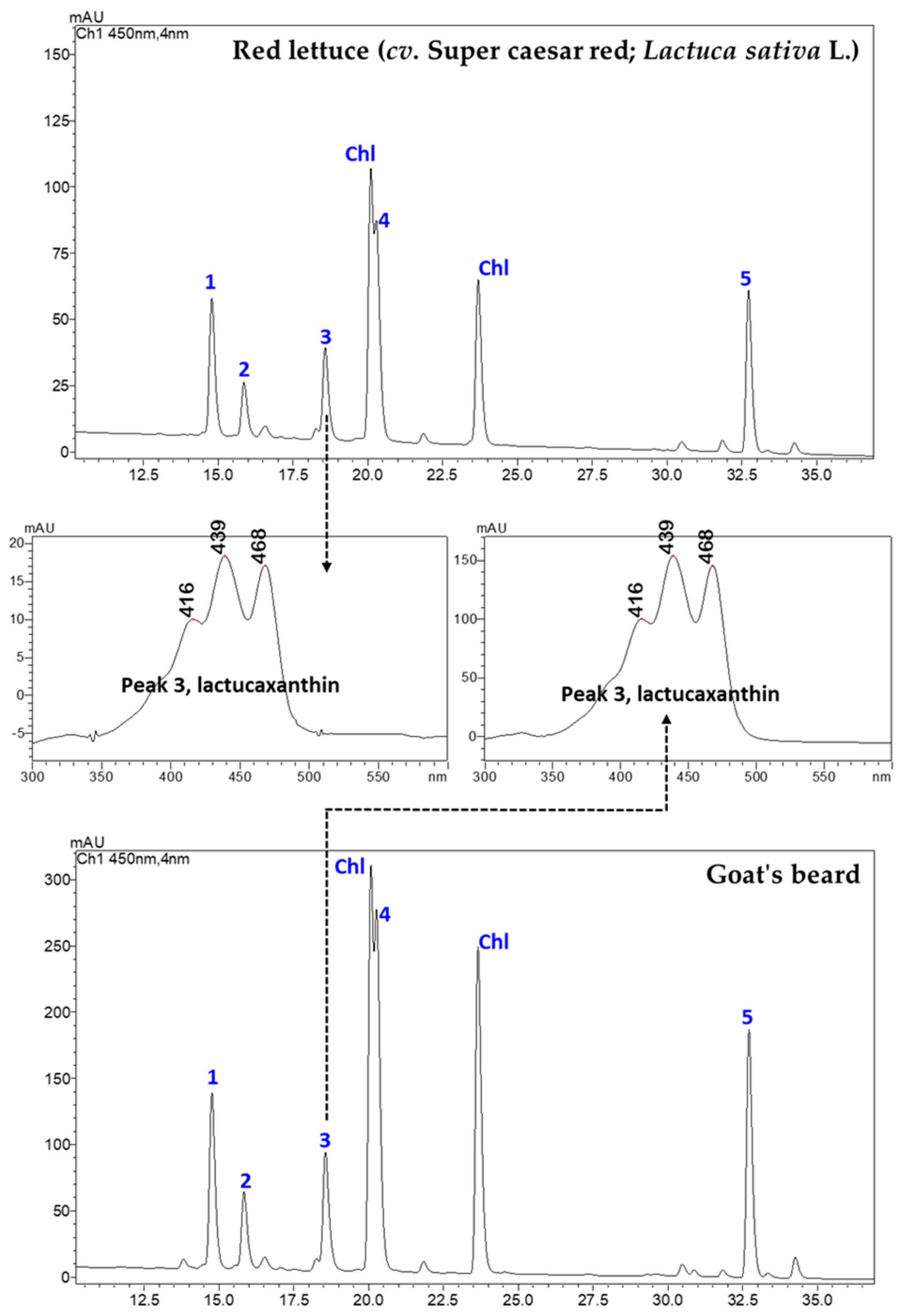
Figure 1 shows an LC-PDA chromatogram of CAs in red lettuce and goat’s beard. Five CAs, namely, CA–Vio (1), CA–Neo (2), CA–Lac (3), CA–Lut (4), and CA–βCar (5), were identified by their retention times (RTs), authentic standards, UV–VIS spectral features, and mass spectra characteristics (Figure 2 and Figure 3). These CAs were quantified using five-point external calibration curves of standards. The obtained concentrations of all carotenoids were well above the LOQ (Table A1 in Appendix A).
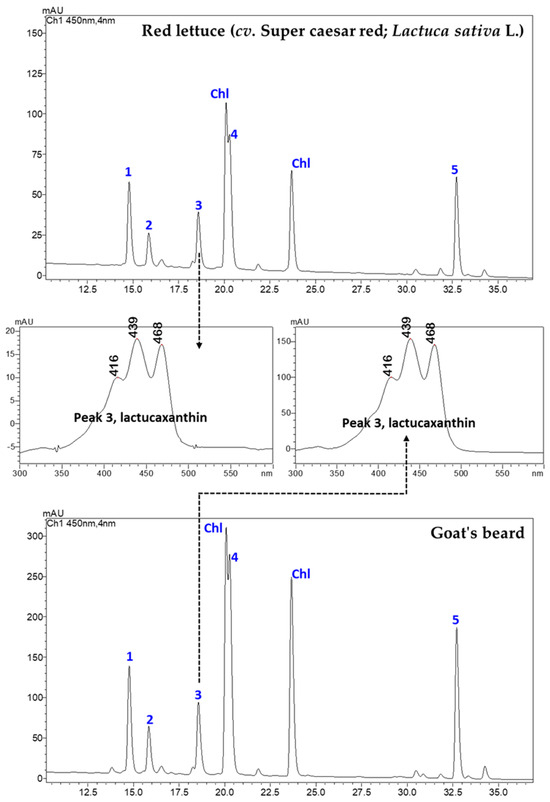
Figure 1.
The representative LC-PDA chromatograms (450 nm) of CA extracts from red lettuce (cv. Super caesar red; Lactuca sativa L.) and goat’s beard foliage. The absorbance spectrum of peak 3 (CA–lactucaxanthin) is also shown from both the foliage. The peak numbers 1–5 correspond to Table 1. Chl: chlorophylls.
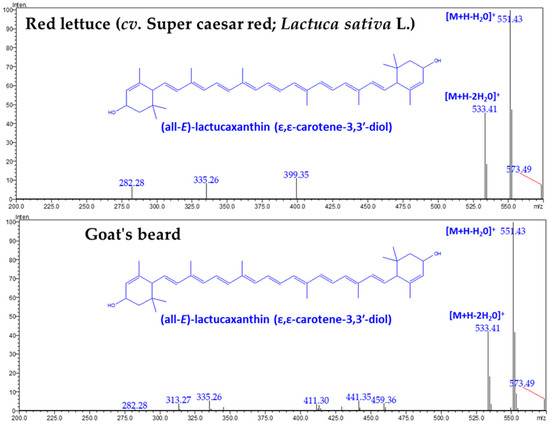
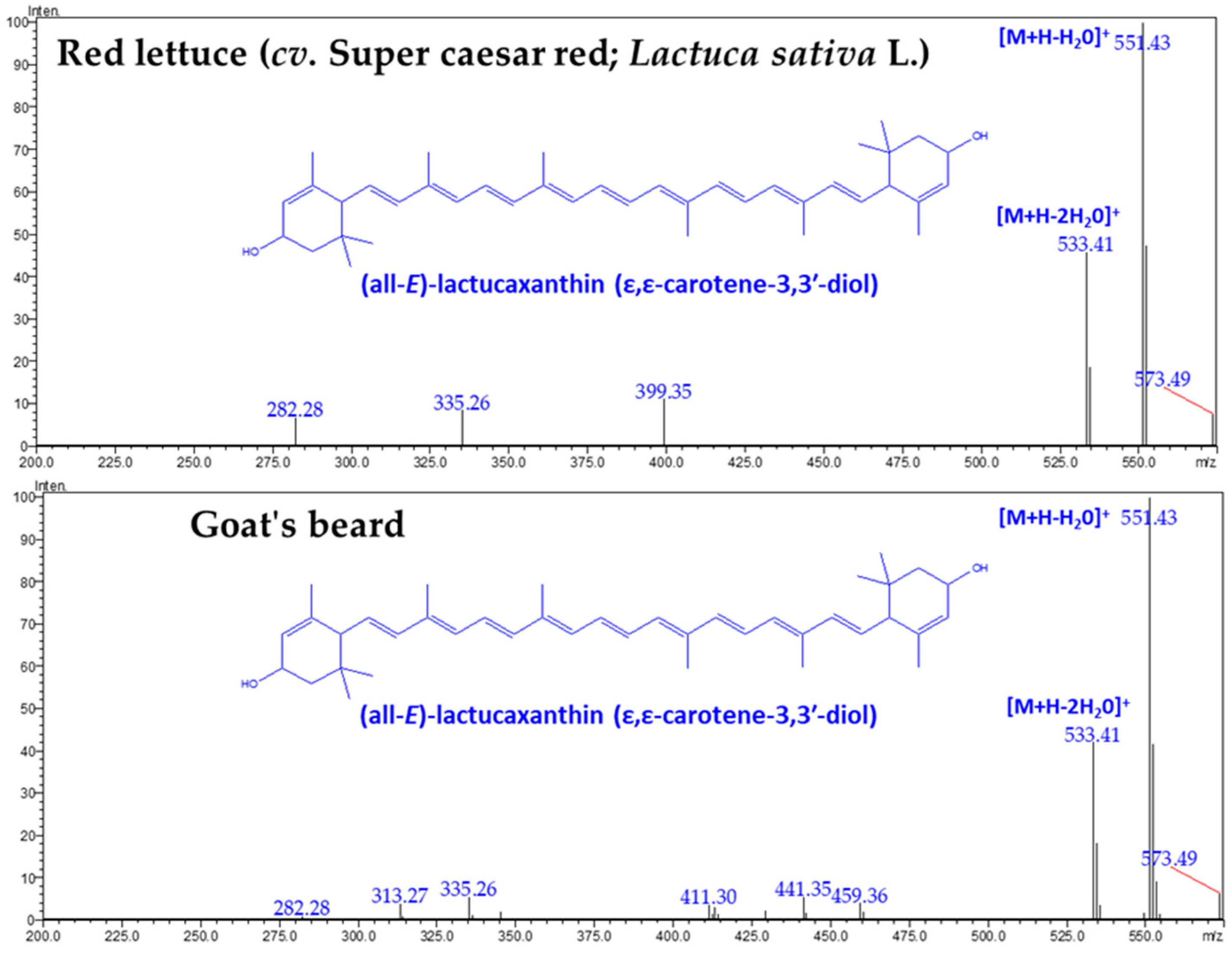
Figure 2.
The LC-Q1 mass spectrum of CA–lactucaxanthin was obtained from red lettuce (cv. Super caesar red; Lactuca sativa L.) and goat’s beard foliage.
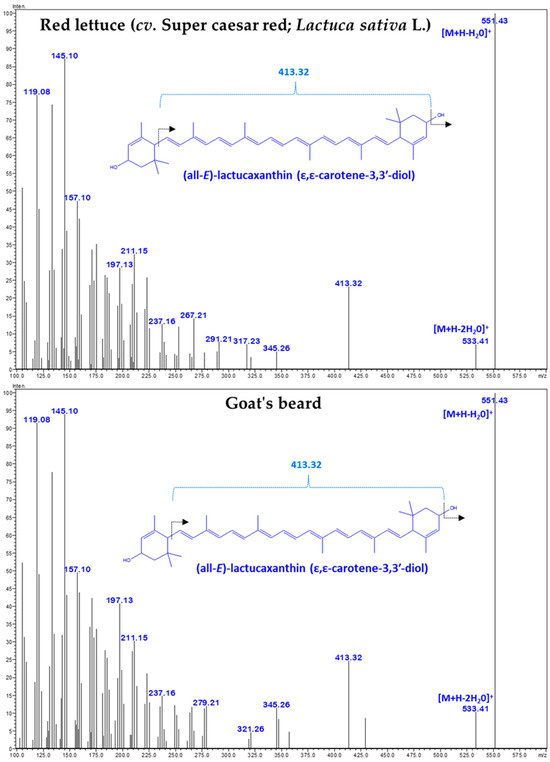
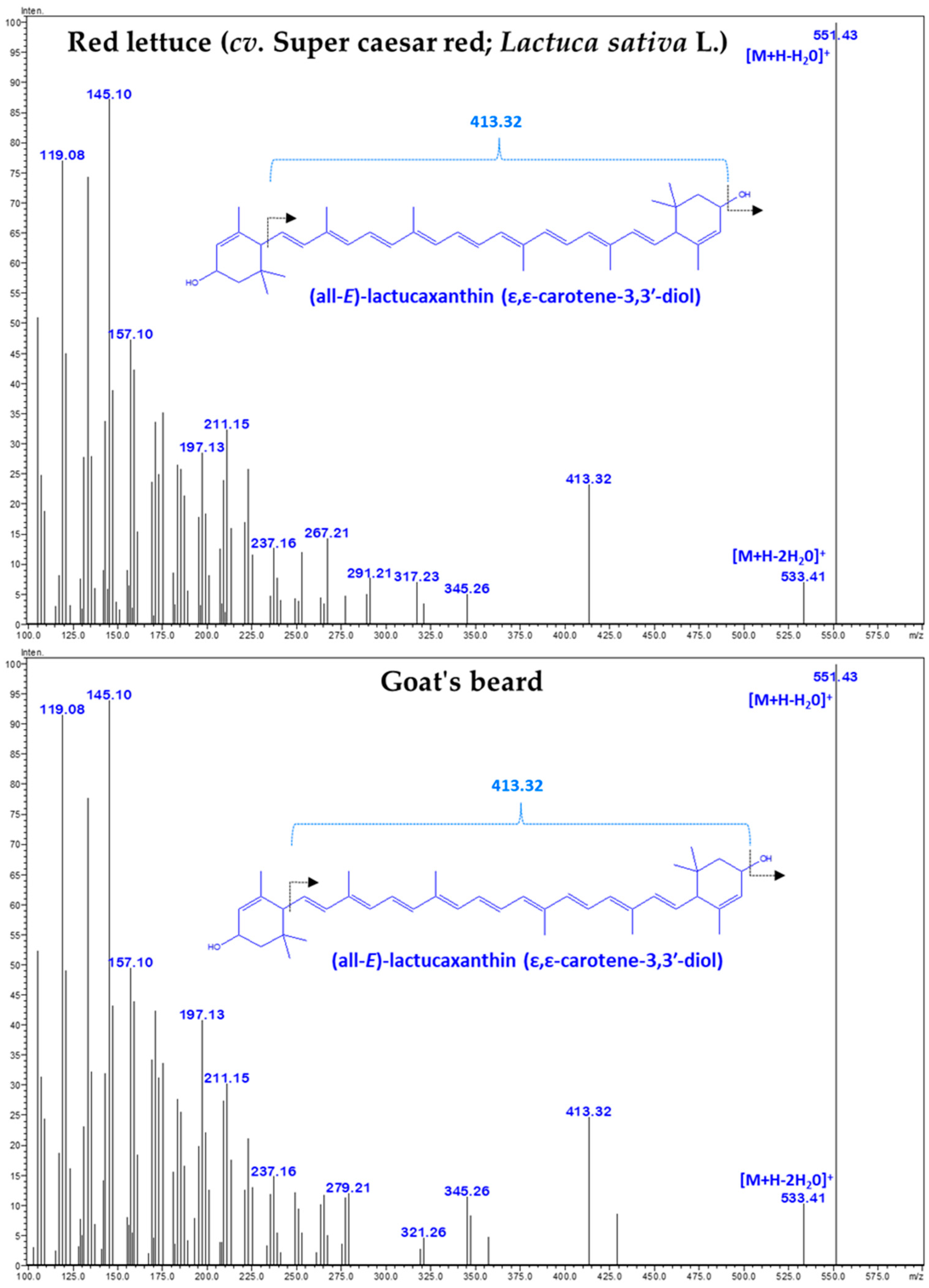
Figure 3.
The tandem mass spectrum (MS/MS) of CA–lactucaxanthin (m/z 551.43) was obtained from red lettuce (cv. Super caesar red; Lactuca sativa L.) and goat’s beard.
There was no difference in the retention time (18.609 min) and specific absorbance maxima (λmax) for CA–Lac in both plant samples (Figure 1). The Q1 mass spectra displayed identical fragmentation patterns, with prominent peaks at m/z values of 145.10, 119.08, 157.10, 187.13, 211.15, 237.16, 413.32, 551.43 ([M+H-H2O]+), and 533.41 ([M+H-2H2O]+) (Figure 3).
In goat’s beard foliage, the contents of various CAs were notably higher than in red lettuce (Table 1). For instance, CA–Vio content was 55.92 ± 1.92 µg/g FW in goat’s beard, while it was 26.79 ± 0.83 µg/g FW in red lettuce. Similarly, CA–Lut was found at a concentration of 101.36 ± 1.20 µg/g FW in goat’s beard foliage, whereas in red lettuce, it measured 28.66 ± 0.62 µg/g FW. This pattern is consistent across other CAs such as CA–Neo, CA–Lac, and CA–βCar, where goat’s foliage consistently displayed higher concentrations than red lettuce. Notably, CA–Lac, a carotenoid rarely found in plants but discovered in lettuce, was identified for the first time in goat’s beard foliage. Its content was 2.4-fold higher than red lettuce, marking a unique discovery in our research. Moreover, the total CA content in goat’s beard foliage was 2.9-fold higher compared to red lettuce. Additionally, α-TL, a form of vitamin E, was also present at higher levels in goat’s beard foliage, with a concentration of 49.17 ± 3.74 µg/g FW, in contrast to 23.34 ± 2.52 µg/g FW in red lettuce.

Table 1.
The contents of carotenoids and α-tocopherol in goat’s beard and red lettuce (cv. Super caesar red; Lactuca sativa L.) foliage.
3.2. PHs in Goat’s Beard
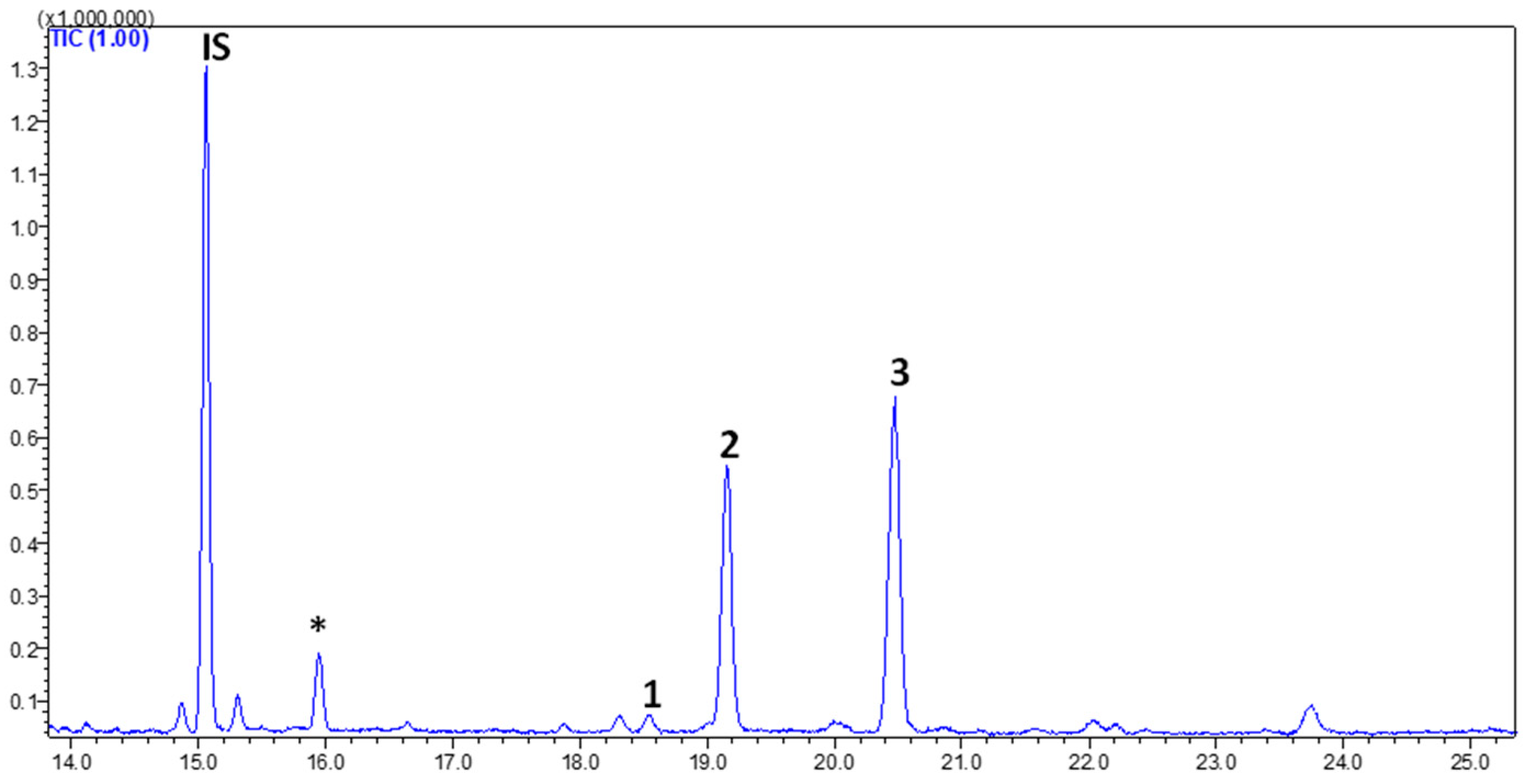
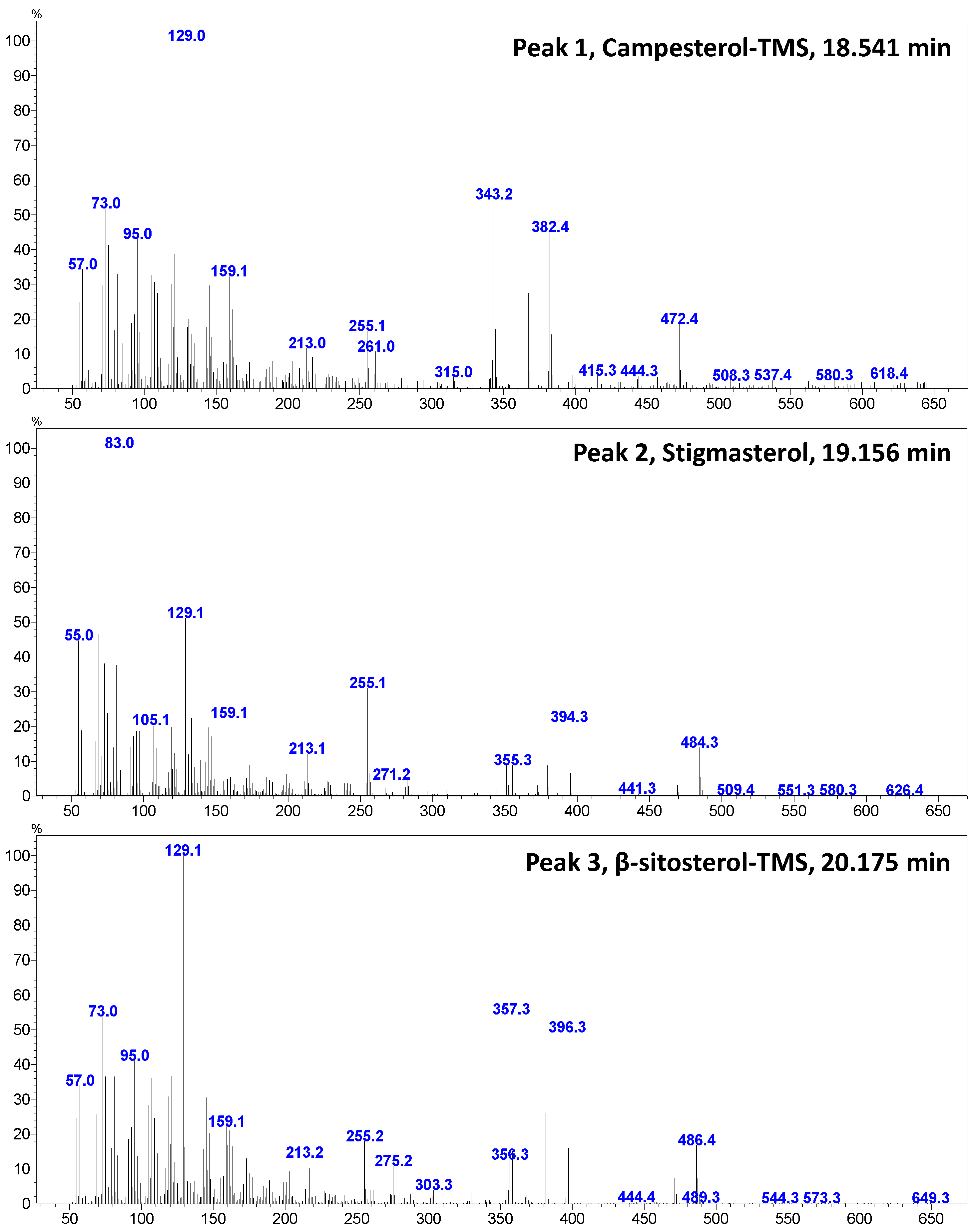
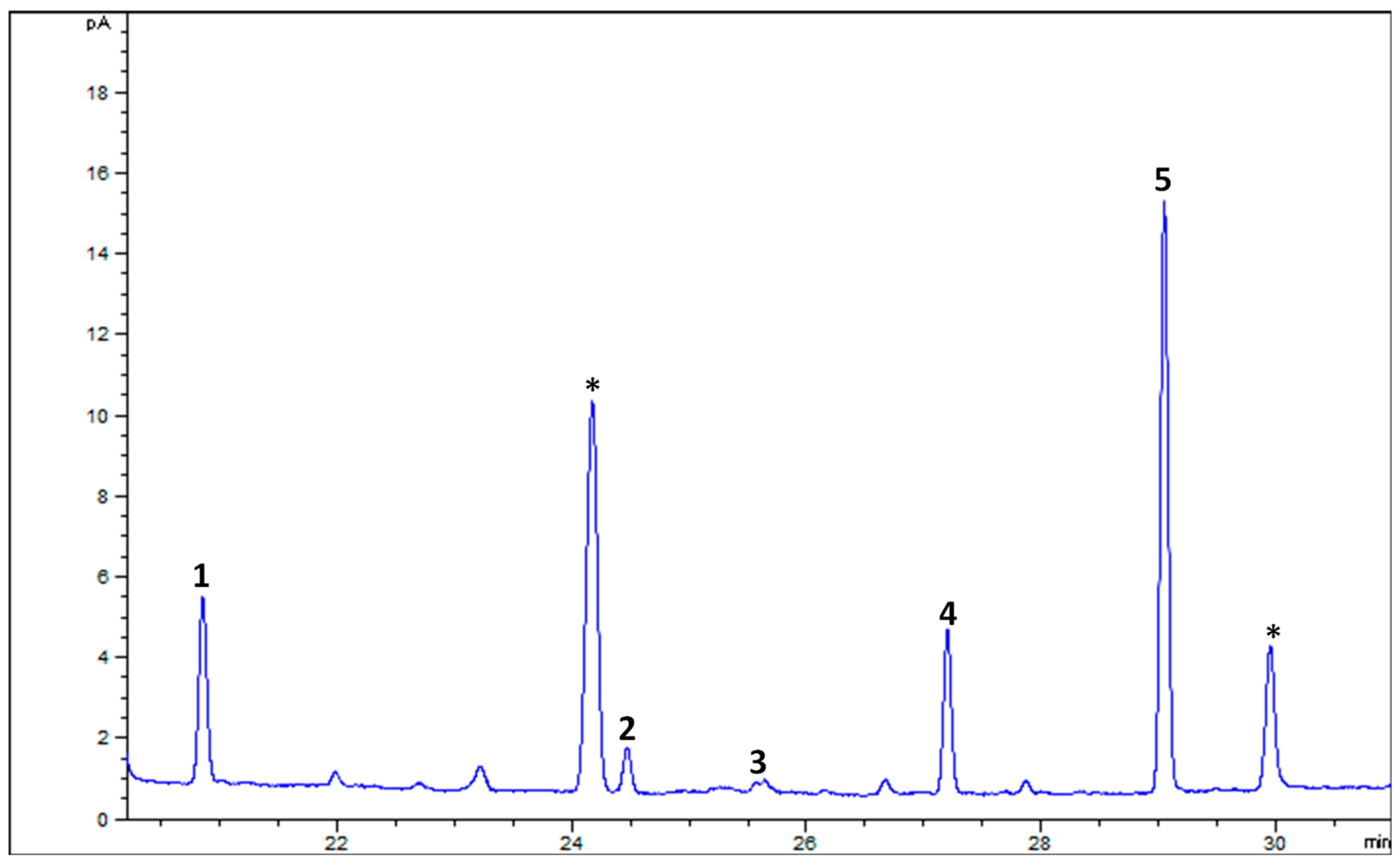
Figure 4 shows a GC–MS chromatogram of PHs identified and quantified from goat’s beard foliage. The peaks 1–3 were identified as PH–Cam (RT:18.541 min), PH–Sti (RT:19.156 min), and PH–βSit (RT:20.175 min), and their respective mass spectra are shown in Figure 5. The PH composition of goat’s beard foliage includes three main compounds: PH–Cam, PH–Sti, and PH–βSit. Of the PHs, PH–βSit was found to be the most abundant PH (89.54 ± 2.46 µg/g FW), followed by PH–Sti (65.30 ± 4.87 µg/g FW) and PH–Cam (3.53 ± 0.20 µg/g FW). The total PH content in goat’s beard foliage was 158.37 ± 7.32 µg/g FW (Table 2).
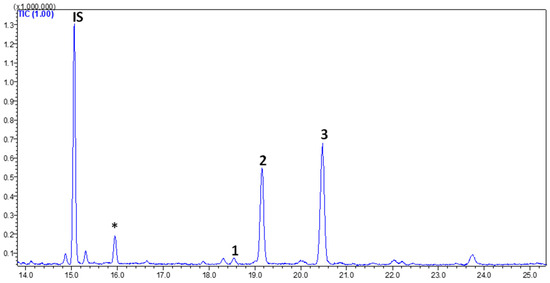
Figure 4.
The GC–MS chromatogram of PH extracts from goat’s beard foliage. IS, internal standard; (1) campesterol (RT—18.541 min); (2) stigmasterol (RT—19.156 min); and (3) β-Sitosterol (RT—20.175 min). * Not a PH.
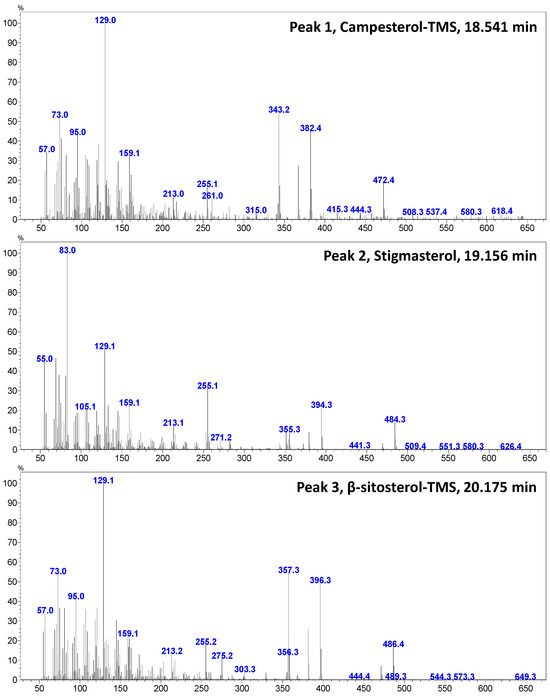
Figure 5.
The GC–mass spectrum of major sterols identified and quantified from goat’s beard foliage.

Table 2.
The contents of phytosterols in goat’s beard foliage.
3.3. FAs in Goat’s Beard
The GC-FID analysis of FAMEs obtained from goat’s beard foliage revealed the presence of various FAs (Figure 6). Palmitic acid (C16:0) was detected at 19.35 ± 0.19%. Stearic acid (C18:0) was found in lower amounts, measuring 4.21 ± 0.42%. Oleic acid (C18:1n9c) was present at 2.28 ± 0.58%, while linoleic acid (C18:2n6c) was detected at 17.12 ± 0.50%. The most abundant fatty acid identified was α-linolenic acid (C18:3n3), constituting a significant portion of the fatty acid profile at 57.03 ± 0.47% (Table 3). The sum of saturated fatty acids (∑SFAs) in goat’s beard foliage was 23.57 ± 0.61%, while the sum of polyunsaturated fatty acids (∑PUFAs) was notably higher (74.15 ± 0.03%). The ratio of ∑PUFAs to ∑SFAs was 3.15 ± 0.08, indicating a relatively high proportion of PUFAs compared to SFAs in the fatty acid profile of goat’s beard foliage.
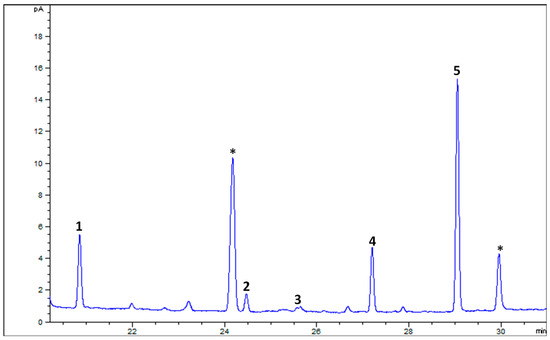
Figure 6.
The representative GC-FID chromatogram of FAMEs of goat’s beard foliage. The peak numbers (1–5) correspond to Table 3. * Not a FAME.

Table 3.
Fatty acid composition of goat’s beard foliage.
4. Discussion
The LC–DAD–MS/MS instrument is a powerful tool for identifying and quantifying natural carotenoids [36]. Peak 3 was identified as CA–Lac using LC–DAD chromatograms and mass spectra (Figure 1, Figure 2 and Figure 3). This CA is not commonly found in plants but is present in lettuce. The presence of CA–Lac in lettuce was first revealed by Siefermann-Harms et al. [37]. In this study, we compared the carotenoid profiles of red lettuce and goat’s beard to confirm the presence of CA–Lac in goat’s beard. In the tandem mass spectrum of CA–Lac, the fragment at 413.32 m/z likely resulted from consecutive losses of a hydroxyl group and the ε-ring moiety from the molecule [34]. Additionally, the loss of water molecules is a common phenomenon in the mass spectrometry of hydroxylated xanthophylls in positive ion mode, producing the [M+H-18]+ and [M+H-36]+ ions [38]. Coupled with the identical absorbance maxima observed in the LC-PDA chromatograms, the mass spectrometry data strongly support the presence of CA–Lac in both lettuce and goat’s beard. Thus, both PDA and MS analyses confirm that CA–Lac is present in goat’s beard. CA–Lac is also reported in a few members of Compositae and Cucurbitaceae [38,39,40,41,42]. The content of CA–Lac in the foliage of goat’s beard was higher (45.42 µg/g FW) than in pumpkin fruit (0.15 µg/g FW) [41], crown gourd immature fruit (traces) [42], Boston lettuce (11.8 µg/g FW) [43], and (7.5 µg/g FW) [44], curly lettuce (8.2 µg/g FW) [43], and (6.7 µg/g FW) [44], freelice lettuce (6.9 µg/g FW), French lettuce (11.9 µg/g FW) [43], romaine lettuce (14.8 µg/g FW) [45], red lettuce (19.05 ± 0.67 µg/g FW, Table 2), and lettuce (3.6 µg/g DW) [46]. CA–Lac is gaining importance due to its biological activities, including anti-angiogenic [47], anti-diabetic [46], anti-cancer [34], antioxidant [47,48], and anti-tumor [49] properties, as well as its cytotoxicity [34,48]. These studies show lettuce serves as a source of CA–Lac. The content of CA–Lac in goat’s beard foliage is about 2.4-fold higher than in lettuce; thus, it could be a potential source for the large-scale extraction of CA–Lac.
In goat’s beard foliage, concentrations of key carotenoids (CA–Lut, CA–βCar, CA–Vio, CA–Neo) were also notably higher than those found in red lettuce. These CAs are powerful antioxidants that exhibit anti-aging [50], anti-bacterial [51], anti-cancer [52], anti-obesity [53], anti-inflammatory [54], and chemo-preventive [55] activities. Of the key CAs, CA–Lut was the most abundant (101.36 ± 1.20 µg/g FW) CA in goat’s beard foliage, followed by CA–βCar (65.52 ± 3.02 µg/g FW), CA–Vio (55.92 ± 1.92 µg/g FW), and CA–Neo (30.21 ± 0.89 µg/g FW) (Table 1). A similar trend was observed in 26 green leafy vegetables [56]. The content (µg/g FW) of CA–Lut in goat’s beard foliage was notably higher than that in celery (2.4 ± 0.8), iceberg lettuce (5.0 ± 2.0), leeks (19.0 ± 6.0), Ceylon spinach (green-stemmed) (27.0 ± 2.0), kangkong (41.0 ± 3.0), swordleaf lettuce (43.0 ± 2.0), green amaranth (44.0 ± 15), pea shoots (51.0 ± 6.0), chayote leaves (55.0 ± 12), garland chrysanthemum (68.0 ± 11), garlic chives (68.0 ± 6.0), pegaga (77.0 ± 2.0), sweet potato leaves (77.0 ± 9.0), and turmeric leaves (82.0 ± 6.0), while lower than spinach (114.0 ± 23), ulam raja (114.0 ± 33), gongura leaves (115.0 ± 13), agathi leaves (120.0 ± 16), melinjau (120.8 ± 0.3), kale (126.0 ± 21), fenugreek leaves (127.0 ± 24), wolfberry leaves (158.0 ± 22), moringa leaves (160.0 ± 33), cassava leaves (171.0 ± 21), and sweet leaf bush (213.0 ± 8.0) [56]. In this study, α-TL, a non-enzymatic antioxidant [57] (µg/g FW) in goat’s beard foliage, was higher (49.17 ± 3.74) than that found in red lettuce (23.34 ± 2.52). The results agree with Saini et al. [33], who recorded a similar α-TL content (µg/g FW) in green lettuce (30.1 ± 3.31), green-red lettuce (25.1 ± 0.85), and red lettuce (25.18 ± 2.75). Furthermore, the α-TL content in goat’s beard foliage was also higher than that in some green leafy vegetables such as green amaranth (1.9 ± 0.5), iceberg lettuce (2.6 ± 0.2), celery (4.3 ± 0.9), garland chrysanthemum (4.0 ± 0.4), kangkong (5.0 ± 0.3), Ceylon spinach (6.0 ± 1.0), leek (6.0 ± 2.0), agathi leaves (7.0 ± 2.0), swordleaf lettuce (7.0 ± 0.6), sweet potato leaves (7.4 ± 0.1), ulam raja (7.7 ± 0.1), pea shoots (9.5 ± 0.9), chayote leaves (11.0 ± 5.0), spinach (16.0 ± 3.0), pegaga (17.0 ± 14), garlic chives (24.0 ± 3.0), ponnaganni (24.0 ± 3.0), wolfberry leaves (28.0 ± 8.0), fenugreek leaves (31.2 ± 0.9), melinjau (34.0 ± 10), and kale (46.0 ± 12) [56].
PHs are important bioactive metabolites reported to possess several biological activities [31]. PH–βSit is an abundant PH in many plants, including green leafy vegetables [33], and it has antioxidant, anti-cancer, anti-inflammatory, and anti-tumor properties [58,59]. In the present study, PH–βSit was found to be the major PH in goat’s beard foliage, and its content (µg/g FW) was higher (89.54 ± 2.46) than in green lettuce (53.3 ± 5.57), green-red lettuce (70.6 ± 7.76), and red lettuce (73.7 ± 5.72) [33]. Similarly, the PH–Sti content (µg/g FW) in goat’s beard foliage (65.30 ± 4.87) (Table 2) was also higher than in green lettuce (40.9 ± 6.43), red lettuce (40.6 ± 1.23), and moringa (20.4 ± 0.63) [33]. So far, the FA profile of the goat’s beard has not been disclosed. In this study, five FAs were identified and quantified in goat’s beard foliage. The major FAs in goat’s beard foliage were α-linolenic acid (57.03 ± 0.47%), palmitic acid (19.35 ± 0.19%), and linoleic acid (17.12 ± 0.50%). Similarly, these three FAs were higher in several leafy vegetables [60,61]. α-Linolenic acid, an essential PUFA needed for human health, has been reported to have anti-cancer, anti-inflammatory, anti-osteoporotic, anti-oxidative, cardiovascular protective, and neuroprotective effects [62,63].
5. Conclusions
This study revealed that goat’s beard foliage contains notably higher levels of CAs and α-TL than red lettuce, highlighting its potential as a superior source of these bioactive compounds. The identification of CA–Lac in goat’s beard foliage for the first time, along with the higher concentrations of other CAs, α-TL, and PHs, underscores its nutritional and therapeutic value. The FA profile, dominated by α-linolenic acid (PUFA), further enhances its health benefits, making the goat’s beard a promising candidate for dietary supplementation and large-scale extraction of valuable lipophilic antioxidants, mainly CA-Lac.
Author Contributions
Conceptualization, R.K.S. and I.S.; methodology, R.K.S., Y.-S.K. and J.-H.L.; validation, E.-Y.K., Y.-S.K. and J.-H.L.; formal analysis, R.K.S., Y.-S.K. and J.-H.L.; investigation, R.K.S. and I.S.; resources, E.-Y.K. and S.C.C.; writing—original draft preparation, R.K.S. and I.S.; writing—review and editing, R.K.S., Y.-S.K., J.-H.L., S.C.C. and I.S. All authors have read and agreed to the published version of the manuscript.
Funding
Konkuk University, Project number: 2024-A019-0050.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
This paper was supported by Konkuk University in 2024.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
The limits of quantitation (LOQ; lower limit of quantitation) and limits of detection (LOD; lower limit of detection) and of CAS and TLs analyzed utilizing LC-MS/MS.
Table A1.
The limits of quantitation (LOQ; lower limit of quantitation) and limits of detection (LOD; lower limit of detection) and of CAS and TLs analyzed utilizing LC-MS/MS.
| S/N | Compound Class | Compound | Ret. Time | LOQ (µg/g) | LOD (µg/g) |
|---|---|---|---|---|---|
| 1 | Carotenoids | (all-E)-violaxanthin | 14.809 | 0.81 | 0.27 |
| 2 | 9-Z-neoxanthin | 15.889 | 3.27 | 1.09 | |
| 3 | (all-E)-lactucaxanthin | 18.609 | 0.37 | 0.12 | |
| 4 | (all-E)-lutein | 19.738 | 0.35 | 0.12 | |
| 5 | (all-E)-β-carotene | 32.179 | 0.52 | 0.17 | |
| 6 | Tocols | α-tocopherol | 16.969 | 1.32 | 0.44 |
References
- Oak, M.K.; Song, J.H.; Hong, S.P. Sexual dimorphism in a gynodioecious species, Aruncus aethusifolius (Rosaceae). Plant Syst. Evol. 2018, 304, 473–484. [Google Scholar] [CrossRef]
- Kim, D.H.; Moon, Y.S.; An, B.J.; Son, J.H. Potent anti-aging activity of Aruncus dioicus, a native plant of Ulleung-do, South Korea, in CCD-986sk fibroblasts via suppression of matrix metalloproteinases. J. Nat. Med. 2012, 66, 631–636. [Google Scholar] [CrossRef]
- Shin, K.-O.; Hwang, H.-J.; Han, K.-S.; Lee, Y.-J. Quality Characteristics of Substitute Meat Patties Developed Using Aruncus dioicus var. kamtschaticus Hara. Foods 2022, 11, 1341. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, J.-Y.; Seo, H.T.; Seong, H.-A.; Ji, Y.-J.; Lee, S.E.; Seo, K.H.; Kim, H.D. Samnamul (Shoots of Aruncus dioicus) Inhibit Adipogenesis by Downregulating Adipocyte-Specific Transcription Factors in 3T3-L1 Adipocytes. Processes 2020, 8, 1576. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, K.H.; Jo, J.E.; Choi, J.J.; Kim, Y.J.; Kim, J.H.; Jang, S.A.; Yook, H.S. Antioxidative and antimicrobial activities of Aruncus dioicus var. kamtschaticus Hara Extracts. J. Korean Soc. Food Sci. Nutr. 2011, 40, 47–55. [Google Scholar] [CrossRef]
- Shin, J.W. Effect of ethanol extracts of goat’s beard on streptozotocin induced diabetic symptoms and oxidative stress in rats. J. East Asian Soc. Diet. Life 2008, 18, 939–948. [Google Scholar]
- Zhang, Q.; Kim, H. DNA damage protection and anti-inflammatory activity of different solvent fractions from Aruncus dioicus var. kamtschaticus. Korean J. Plant Res. 2014, 27, 714–719. [Google Scholar] [CrossRef]
- Park, S.B.; Kang, J.Y.; Kim, J.M.; Park, S.K.; Yoo, S.K.; Lee, U.; Kim, D.-O.; Heo, H.J. Effect of Aruncus dioicus var. kamtschaticus Extract on Neurodegeneration Improvement: Ameliorating Role in Cognitive Disorder Caused by High-Fat Diet Induced Obesity. Nutrients 2019, 11, 1319. [Google Scholar] [CrossRef]
- Zhao, B.T.; Jeong, S.Y.; Vu, V.D.; Min, B.S.; Kim, Y.H.; Woo, M.H. Cytotoxic and antioxidant constituents from the aerial parts of Aruncus dioicus var. kamtschaticus. Nat. Prod. Sci. 2013, 19, 66–70. [Google Scholar]
- Jeong, S.Y.; Jun, D.Y.; Kim, Y.H.; Min, B.S.; Min, B.K.; Woo, M.H. Monoterpenoids from the aerial parts of Aruncus dioicus var. kamtschaticus and their antioxidant and cytotoxic activities. Bioorg. Med. Chem. Lett. 2011, 21, 3252–3256. [Google Scholar] [CrossRef]
- Kim, M.S.; Sohn, H.Y. Anti-thrombosis activity of the aerial parts of Aruncus dioicus var kamtschaticus. J. Life Sci. 2014, 24, 515–521. [Google Scholar] [CrossRef]
- Byung Sun, M.; Bae, K.; Young Ho, K.; Shimotohno, K.; Miyashiro, H.; Hattori, M. Inhibitory activities of Korean plants on HIV-1 protease. Nat. Prod. Sci. 1998, 4, 241–244. [Google Scholar]
- Youn, J.-S.; Shin, S.-Y.; Wu, Y.; Hwang, J.-Y.; Cho, J.-H.; Ha, Y.-G.; Kim, J.-K.; Park, M.-J.; Lee, S.-H.; Kim, T.-H. Antioxidant and anti-wrinkling effects of Aruncus dioicus var. Kamtschaticus extract. Korean J. Food Preserv. 2012, 19, 393–399. [Google Scholar] [CrossRef]
- Han, C.R.; Jun, D.Y.; Woo, H.J.; Jeong, S.Y.; Woo, M.H.; Kim, Y.H. Induction of microtubule-damage, mitotic arrest, Bcl-2 phosphorylation, Bak activation, and mitochondria-dependent caspase cascade is involved in human Jurkat T-cell apoptosis by aruncin B from Aruncus dioicus var. kamtschaticus. Bioorg. Med. Chem. Lett. 2012, 22, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, U.; Kang, J.; Kim, J.; Park, S.; Park, S.; Choi, S.; Heo, H. Protective effects of Aruncus dioicus var. kamtschaticus extract against hyperglycemic-induced neurotoxicity. Korean J. Food Sci. Technol. 2017, 49, 668–675. [Google Scholar]
- Ahn, H.; Kim, J.; Kim, J.; Auh, J.; Choe, E. In vitro α-glucosidase and pancreatic lipase inhibitory activities and antioxidants of Samnamul (Aruncus dioicus) during rehydration and cooking. Food Sci. Biotechnol. 2014, 23, 1287–1293. [Google Scholar] [CrossRef]
- Kim, J.I.; Yun, J.A.; Jeong, Y.K.; Baek, H.J. Hypoglycemic and hypolipidemic effects of samnamul (shoot of Aruncus dioicus var. kamtschaticus Hara) in mice fed a high-fat/high-sucrose diet. Food Sci. Biotechnol. 2018, 27, 1467–1473. [Google Scholar] [CrossRef]
- Kim, D.H.; Moon, Y.S.; Park, T.S.; Hwang, J.Y.; Son, J.H. Potent whitening activity of Aruncus dioicus extract in B16F10 melanoma cell by suppression of melanin biosynthesis. Hortic. Sci. Technol. 2013, 31, 813–820. [Google Scholar]
- Dorjsembe, B.; Joo, H.; Nho, C.; Ham, J.; Kim, J.-C. Aruncus dioicus var. kamtschaticus Extract Ameliorates Psoriasis-like Skin Inflammation via Akt/mTOR and JAK2/STAT3 Signaling Pathways in a Murine Model. Nutrients 2022, 14, 5094. [Google Scholar] [CrossRef]
- Han, H.-S.; Lee, J.-W. Attenuation of Brain Injury by Water Extract of Goat’s-beard (Aruncus dioicus) and Its Ethyl Acetate Fraction in a Rat Model of Ischemia-Reperfusion. Prev. Nutr. Food Sci. 2011, 16, 217–223. [Google Scholar] [CrossRef][Green Version]
- Baek, H.S.; Lim, S.H.; Ahn, K.S.; Lee, J. Methanol extract of goat’s-beard (Aruncus dioicus) reduces renal injury by inhibiting apoptosis in a rat model of ischemia-reperfusion. Prev. Nutr. Food Sci. 2012, 17, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Vo, Q.H.; Nguyen, P.H.; Zhao, B.T.; Thi, Y.N.; Nguyen, D.H.; Kim, W.I.; Seo, U.M.; Min, B.S.; Woo, M.H. Bioactive constituents from the n-butanolic fraction of Aruncus dioicus var. kamtschaticus. Nat. Prod. Sci. 2014, 20, 274–280. [Google Scholar]
- Granica, S.; Fusani, P.; Stanisławska, I.; Piwowarski, J.P.; Melck, D.; Motta, A.; Zidorn, C. Monoterpenoids from the traditional North Italian vegetable Aruncus dioicus (Walter) Fernald var. vulgaris (Maxim.) H.Hara (Rosaceae). Food Chem. 2017, 221, 1851–1859. [Google Scholar] [CrossRef]
- Kim, A.-N.; Lee, K.Y.; Rahman, M.S.; Kim, H.-J.; Chun, J.; Heo, H.J.; Kerr, W.L.; Choi, S.-G. Effect of water blanching on phenolic compounds, antioxidant activities, enzyme inactivation, microbial reduction, and surface structure of samnamul (Aruncus dioicus var kamtschaticus). Int. J. Food Sci. Technol. 2020, 55, 1754–1762. [Google Scholar] [CrossRef]
- Fusani, P.; Piwowarski, J.P.; Zidorn, C.; Kiss, A.K.; Scartezzini, F.; Granica, S. Seasonal variation in secondary metabolites of edible shoots of Buck’s beard [Aruncus dioicus (Walter) Fernald (Rosaceae)]. Food Chem. 2016, 202, 23–30. [Google Scholar] [CrossRef] [PubMed]
- An, H.C.; Choe, E.O. Content changes of pigments and antioxidants of dried samnamul (Aruncus dioicus) and daraesoon (Actinidia arguta) during rehydration and high temperature cooking. Korean J. Food Cook. Sci. 2016, 32, 383–389. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Zhang, Z.; Liu, J.; Hong, L. β-Sitosterol as a Promising Anticancer Agent for Chemoprevention and Chemotherapy: Mechanisms of Action and Future Prospects. Adv. Nutr. 2023, 14, 1085–1110. [Google Scholar] [CrossRef]
- Martínez-García, M.; Garduño-Solórzano, G.; Lopes, G.; Sanchez, B.A.; Urbatzka, R.; Hentschke, G.S.; Campos, J.E.; Vasconcelos, V.M.O. Antioxidant, Anti-Inflammatory and Anti-Obesity Potential of Extracts Containing Phenols, Chlorophyll and Carotenoids from Mexican Wild Populations of Bacopa monnieri (L.) Wettst. Biology 2023, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Kopańska, M.; Batoryna, M.; Banaś-Ząbczyk, A.; Błajda, J.; Lis, M.W. The Effect of α-Tocopherol on the Reduction of Inflammatory Processes and the Negative Effect of Acrylamide. Molecules 2022, 27, 965. [Google Scholar] [CrossRef]
- Tian, M.; Bai, Y.; Tian, H.; Zhao, X. The Chemical Composition and Health-Promoting Benefits of Vegetable Oils—A Review. Molecules 2023, 28, 6393. [Google Scholar] [CrossRef]
- Vezza, T.; Canet, F.; de Marañón, A.M.; Bañuls, C.; Rocha, M.; Víctor, V.M. Phytosterols: Nutritional Health Players in the Management of Obesity and Its Related Disorders. Antioxidants 2020, 9, 1266. [Google Scholar] [CrossRef]
- La Torre, M.E.; Cianciulli, A.; Monda, V.; Monda, M.; Filannino, F.M.; Antonucci, L.; Valenzano, A.; Cibelli, G.; Porro, C.; Messina, G.; et al. α-Tocopherol Protects Lipopolysaccharide-Activated BV2 Microglia. Molecules 2023, 28, 3340. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Song, M.-H.; Yu, J.-W.; Lee, J.-H.; Ahn, H.-Y.; Keum, Y.-S.; Lee, J.-H. Profiling of Nutritionally Vital Bioactive Compounds in Emerging Green Leafy Vegetables: A Comparative Study. Foods 2022, 11, 3867. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Moon, S.H.; Gansukh, E.; Keum, Y.S. An efficient one-step scheme for the purification of major xanthophyll carotenoids from lettuce, and assessment of their comparative anticancer potential. Food Chem. 2018, 266, 56–65. [Google Scholar] [CrossRef]
- Saini, R.K.; Yu, J.-W.; Song, M.-H.; Ahn, H.-Y.; Lee, J.-H.; Keum, Y.-S.; Lee, J.-H. Profiling of Redox-Active Lipophilic Constituents in Leaf Mustard (Brassica juncea (L.) Czern.) Cultivars Using LC-MS and GC-MS. Antioxidants 2022, 11, 2464. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids: Distribution, Function in Nature, and Analysis Using LC-Photodiode Array Detector (DAD)-MS and MS/MS System. Mass Spectrom. 2023, 12, A0133. [Google Scholar] [CrossRef] [PubMed]
- Siefermann-Harms, D.; Hertzberg, S.; Borch, G.; Liaaen-Jensen, S. Lactucaxanthin, an ε,ε-carotene-3,3′-diol from Lactuca sativa. Phytochemistry 1981, 20, 85–88. [Google Scholar] [CrossRef]
- Rivera, S.M.; Christou, P.; Canela-Garayoa, R. Identification of carotenoids using mass spectrometry. Mass Spectrom. Rev. 2014, 33, 353–372. [Google Scholar] [CrossRef]
- Phillip, D.; Young, A.J. Occurrence of the carotenoid lactucaxanthin in higher plant LHC II. Photosynth. Res. 1995, 43, 273–282. [Google Scholar] [CrossRef]
- Britton, G.; Khachik, F. Carotenoids in Food. In Carotenoids: Nutrition and Health, 4th ed.; Britton, G., Pfander, H., Liaaen-Jensen, S., Eds.; Birkhäuser Verlag: Basel, Switzerland, 2009; pp. 45–66. [Google Scholar]
- Muntean, E. Quantification of carotenoids from pumpkin juice by HPLC-DAD. Sci. Researches. Agroaliment. Process. Technol. 2005, 11, 123–128. [Google Scholar]
- Muntean, E.; Bele, C.; Socaciu, C. HPLC analysis of carotenoids from fruits of Cucurbita pepo L. var. melopepo Alef. Acta Agron. Hung. 2003, 51, 455–459. [Google Scholar] [CrossRef]
- Kimura, M.; Rodriguez-Amaya, D.B. Carotenoid composition of hydroponic leafy vegetables. J. Agric. Food Chem. 2003, 51, 2603–2607. [Google Scholar] [CrossRef] [PubMed]
- Niizu, P.Y.; Rodriguez-Amaya, D.B. New data on the carotenoid composition of raw salad vegetables. J. Food Compos. Anal. 2005, 18, 739–749. [Google Scholar] [CrossRef]
- Humphries, J.M.; Khachik, F. Distribution of lutein, zeaxanthin, and related geometrical isomers in fruit, vegetables, wheat, and pasta products. J. Agric. Food Chem. 2003, 51, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.S.; Lakshmi, M.J.; Sharavana, G.; Sathaiah, G.; Sreerama, Y.N.; Baskaran, V. Lactucaxanthin—A potential anti-diabetic carotenoid from lettuce (Lactuca sativa) inhibits α-amylase and α-glucosidase activity in vitro and in diabetic rats. Food Funct. 2017, 8, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Anitha, R.E.; Janani, R.; Peethambaran, D.; Baskaran, V. Lactucaxanthin protects retinal pigment epithelium from hyperglycemia-regulated hypoxia/ER stress/VEGF pathway mediated angiogenesis in ARPE-19 cell and rat model. Eur. J. Pharmacol. 2021, 899, 174014. [Google Scholar] [CrossRef]
- Jayapala, N.; Rani Elavarasan, A.; Chaudhari, S.R.; Vallikannan, B. Cytotoxicity and 3T3-L1 cell uptake of lactucaxanthin purified and characterized by LC-MS and NMR from lettuce (Lactuca sativa). J. Liq. Chromatogr. Relat. Technol. 2020, 43, 233–246. [Google Scholar] [CrossRef]
- Tsushima, M.; Maoka, T.; Katsuyama, M.; Kozuka, M.; Matsuno, T.; Tokuda, H.; Nishino, H.; Iwashima, A. Inhibitory Effect of Natural Carotenoids on Epstein-Barr Virus Activation Activity of a Tumor Promoter in Raji Cells. A Screening Study for Anti-Tumor Promoters. Biol. Pharm. Bull. 1995, 18, 227–233. [Google Scholar] [CrossRef]
- Zheng, W.V.; Xu, W.; Li, Y.; Qin, J.; Zhou, T.; Li, D.; Xu, Y.; Cheng, X.; Xiong, Y.; Chen, Z. Anti-aging effect of beta-carotene through regulating the KAT7-P15 signaling axis, inflammation and oxidative stress process. Cell. Mol. Biol. Lett. 2022, 27, 86. [Google Scholar] [CrossRef]
- Mahavy, C.E.; Mol, A.; Andrianarisoa, B.; Duez, P.; Jaziri, M.E.; Baucher, M.; Rasamiravaka, T. The Xanthophyll Carotenoid Lutein Reduces the Invasive Potential of Pseudomonas aeruginosa and Increases Its Susceptibility to Tobramycin. Int. J. Mol. Sci. 2022, 23, 7199. [Google Scholar] [CrossRef]
- Antunes, A.; Carmo, F.; Pinto, S.; Andrade, N.; Martel, F. The Anti-Proliferative Effect of β-Carotene against a Triple-Negative Breast Cancer Cell Line Is Cancer Cell-Specific and JNK-Dependent. PharmaNutrition 2022, 22, 100320. [Google Scholar] [CrossRef]
- Kurniawan, R.; Nurkolis, F.; Taslim, N.A.; Subali, D.; Surya, R.; Gunawan, W.B.; Alisaputra, D.; Mayulu, N.; Salindeho, N.; Kim, B. Carotenoids Composition of Green Algae Caulerpa racemosa and Their Antidiabetic, Anti-Obesity, Antioxidant, and Anti-Inflammatory Properties. Molecules 2023, 28, 3267. [Google Scholar] [CrossRef]
- Soontornchaiboon, W.; Joo, S.S.; Kim, S.M. Anti-inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoidea in RAW 264.7 macrophages. Biol. Pharm. Bull. 2012, 35, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Das, S.; Joardar, S.; Bhattacharjee, S.; Chakraborty, P. Carotenoids as Anticancer Agents. In Carotenoids: Structure and Function in the Human Body; Springer: Berlin/Heidelberg, Germany, 2021; pp. 475–512. [Google Scholar]
- Lee, H.W.; Bi, X.; Henry, C.J. Carotenoids, tocopherols and phylloquinone content of 26 green leafy vegetables commonly consumed in Southeast Asia. Food Chem. 2022, 385, 132729. [Google Scholar]
- Meulmeester, F.L.; Luo, J.; Martens, L.G.; Mills, K.; van Heemst, D.; Noordam, R. Antioxidant Supplementation in Oxidative Stress-Related Diseases: What Have We Learned from Studies on Alpha-Tocopherol? Antioxidants 2022, 11, 2322. [Google Scholar] [CrossRef]
- Bhat, A.H.; Alia, A.; Rather, G.M.; Kumar, B. Isolation & characterisation of beta-sitosterol from the rhizomes of Arisaema utile and its evaluation for antioxidant activity. Int. J. Sci. Res. Biol. Sci. 2019, 6, 111–118. [Google Scholar]
- Khan, Z.; Nath, N.; Rauf, A.; Emran, T.B.; Mitra, S.; Islam, F.; Chandran, D.; Barua, J.; Khandaker, M.U.; Idris, A.M.; et al. Multifunctional roles and pharmacological potential of β-sitosterol: Emerging evidence toward clinical applications. Chem. Biol. Interact. 2022, 365, 110117. [Google Scholar] [CrossRef]
- Almazan, A.M.; Adeyeye, S.O. Fat and fatty acid concentrations in some green vegetables. J. Food Compos. Anal. 1998, 11, 375–380. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.C.; Carvalho, A.M.; Sánchez-Mata, M.C.; Cámara, M.; Tardío, J. Fatty acids profiles of some Spanish wild vegetables. Food Sci. Int. 2012, 18, 281–290. [Google Scholar] [CrossRef]
- Kim, K.B.; Nam, Y.A.; Kim, H.S.; Hayes, A.W.; Lee, B.M. α-Linolenic acid: Nutraceutical, pharmacological and toxicological evaluation. Food Chem. Toxicol. 2014, 70, 163–178. [Google Scholar] [CrossRef]
- Cambiaggi, L.; Chakravarty, A.; Noureddine, N.; Hersberger, M. The Role of α-Linolenic Acid and Its Oxylipins in Human Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 6110. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).