Aruncus dioicus var. kamtschaticu: A Newly Identified Source of Lactucaxanthin (ε,ε-Carotene-3,3′-diol)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Reagents, and Standards
2.2. Extraction of Lipophilic Metabolites
2.3. LC–DAD–MS Analysis of CAs and α-TL
2.4. GC–MS Analysis of PHs
2.5. GC-FID and GC–MS Analysis of FAs
3. Results
3.1. CAs and α-TL in Lettuce and Goat’s Beard
3.2. PHs in Goat’s Beard
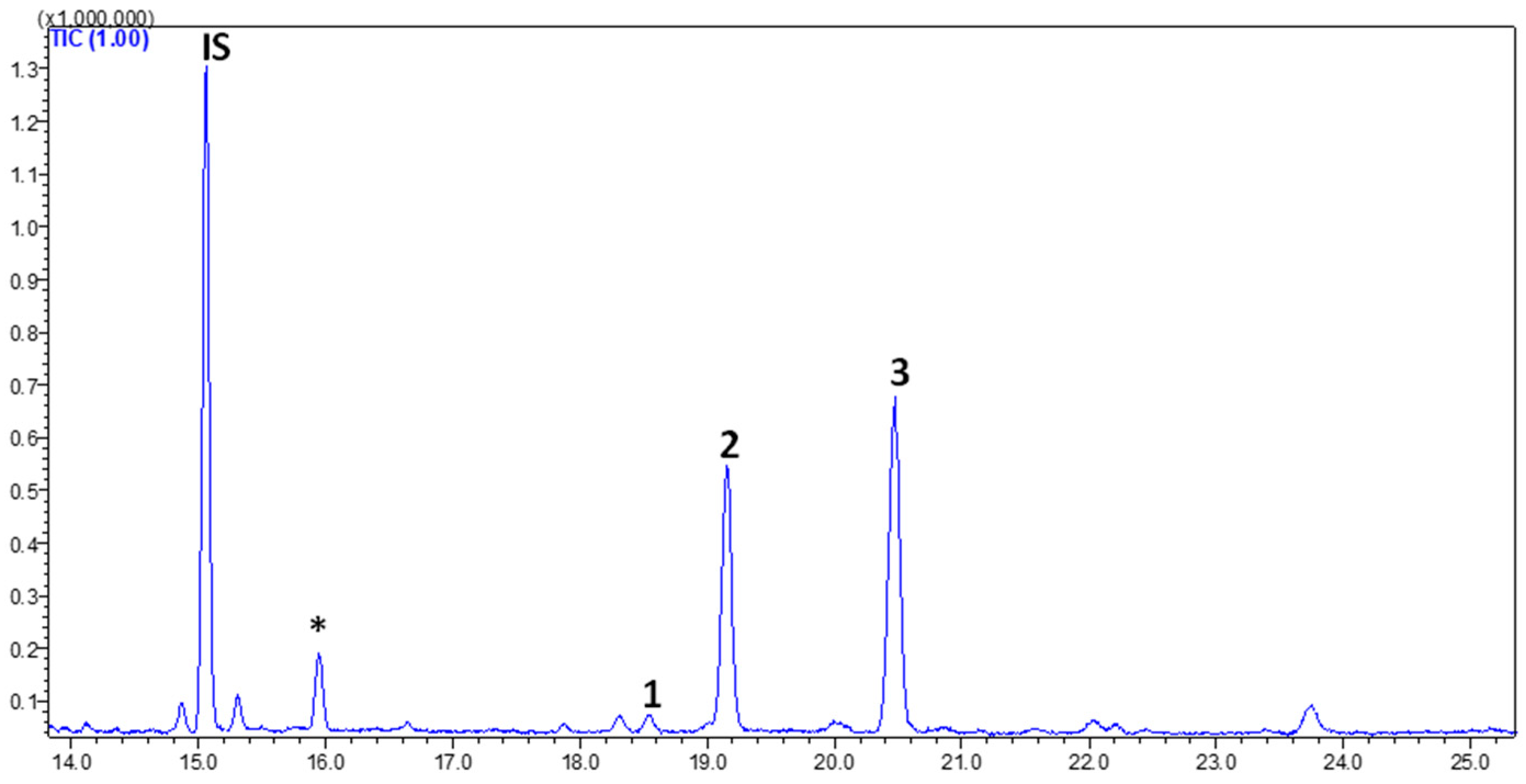
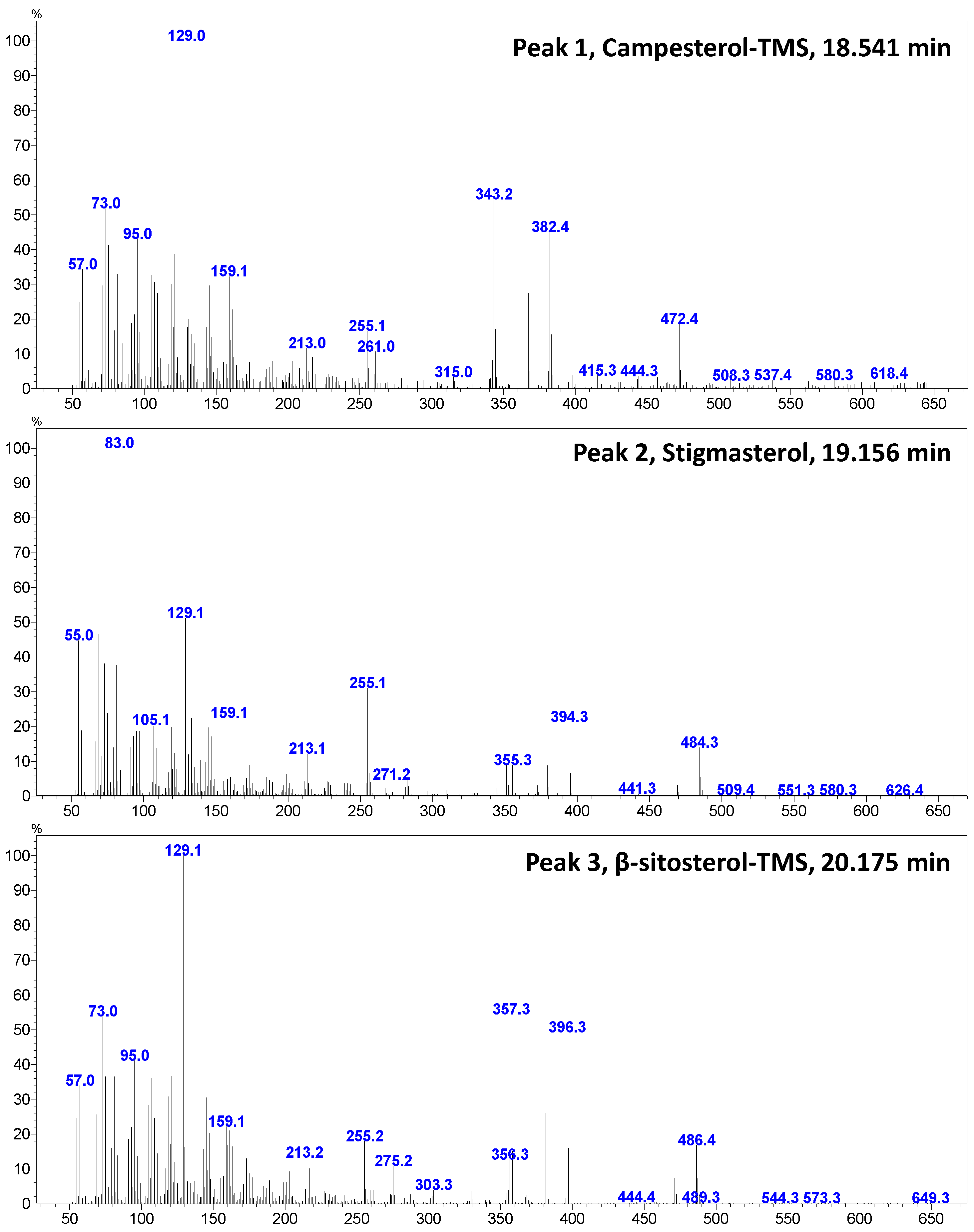
3.3. FAs in Goat’s Beard
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| S/N | Compound Class | Compound | Ret. Time | LOQ (µg/g) | LOD (µg/g) |
|---|---|---|---|---|---|
| 1 | Carotenoids | (all-E)-violaxanthin | 14.809 | 0.81 | 0.27 |
| 2 | 9-Z-neoxanthin | 15.889 | 3.27 | 1.09 | |
| 3 | (all-E)-lactucaxanthin | 18.609 | 0.37 | 0.12 | |
| 4 | (all-E)-lutein | 19.738 | 0.35 | 0.12 | |
| 5 | (all-E)-β-carotene | 32.179 | 0.52 | 0.17 | |
| 6 | Tocols | α-tocopherol | 16.969 | 1.32 | 0.44 |
References
- Oak, M.K.; Song, J.H.; Hong, S.P. Sexual dimorphism in a gynodioecious species, Aruncus aethusifolius (Rosaceae). Plant Syst. Evol. 2018, 304, 473–484. [Google Scholar] [CrossRef]
- Kim, D.H.; Moon, Y.S.; An, B.J.; Son, J.H. Potent anti-aging activity of Aruncus dioicus, a native plant of Ulleung-do, South Korea, in CCD-986sk fibroblasts via suppression of matrix metalloproteinases. J. Nat. Med. 2012, 66, 631–636. [Google Scholar] [CrossRef]
- Shin, K.-O.; Hwang, H.-J.; Han, K.-S.; Lee, Y.-J. Quality Characteristics of Substitute Meat Patties Developed Using Aruncus dioicus var. kamtschaticus Hara. Foods 2022, 11, 1341. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, J.-Y.; Seo, H.T.; Seong, H.-A.; Ji, Y.-J.; Lee, S.E.; Seo, K.H.; Kim, H.D. Samnamul (Shoots of Aruncus dioicus) Inhibit Adipogenesis by Downregulating Adipocyte-Specific Transcription Factors in 3T3-L1 Adipocytes. Processes 2020, 8, 1576. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, K.H.; Jo, J.E.; Choi, J.J.; Kim, Y.J.; Kim, J.H.; Jang, S.A.; Yook, H.S. Antioxidative and antimicrobial activities of Aruncus dioicus var. kamtschaticus Hara Extracts. J. Korean Soc. Food Sci. Nutr. 2011, 40, 47–55. [Google Scholar] [CrossRef]
- Shin, J.W. Effect of ethanol extracts of goat’s beard on streptozotocin induced diabetic symptoms and oxidative stress in rats. J. East Asian Soc. Diet. Life 2008, 18, 939–948. [Google Scholar]
- Zhang, Q.; Kim, H. DNA damage protection and anti-inflammatory activity of different solvent fractions from Aruncus dioicus var. kamtschaticus. Korean J. Plant Res. 2014, 27, 714–719. [Google Scholar] [CrossRef]
- Park, S.B.; Kang, J.Y.; Kim, J.M.; Park, S.K.; Yoo, S.K.; Lee, U.; Kim, D.-O.; Heo, H.J. Effect of Aruncus dioicus var. kamtschaticus Extract on Neurodegeneration Improvement: Ameliorating Role in Cognitive Disorder Caused by High-Fat Diet Induced Obesity. Nutrients 2019, 11, 1319. [Google Scholar] [CrossRef]
- Zhao, B.T.; Jeong, S.Y.; Vu, V.D.; Min, B.S.; Kim, Y.H.; Woo, M.H. Cytotoxic and antioxidant constituents from the aerial parts of Aruncus dioicus var. kamtschaticus. Nat. Prod. Sci. 2013, 19, 66–70. [Google Scholar]
- Jeong, S.Y.; Jun, D.Y.; Kim, Y.H.; Min, B.S.; Min, B.K.; Woo, M.H. Monoterpenoids from the aerial parts of Aruncus dioicus var. kamtschaticus and their antioxidant and cytotoxic activities. Bioorg. Med. Chem. Lett. 2011, 21, 3252–3256. [Google Scholar] [CrossRef]
- Kim, M.S.; Sohn, H.Y. Anti-thrombosis activity of the aerial parts of Aruncus dioicus var kamtschaticus. J. Life Sci. 2014, 24, 515–521. [Google Scholar] [CrossRef]
- Byung Sun, M.; Bae, K.; Young Ho, K.; Shimotohno, K.; Miyashiro, H.; Hattori, M. Inhibitory activities of Korean plants on HIV-1 protease. Nat. Prod. Sci. 1998, 4, 241–244. [Google Scholar]
- Youn, J.-S.; Shin, S.-Y.; Wu, Y.; Hwang, J.-Y.; Cho, J.-H.; Ha, Y.-G.; Kim, J.-K.; Park, M.-J.; Lee, S.-H.; Kim, T.-H. Antioxidant and anti-wrinkling effects of Aruncus dioicus var. Kamtschaticus extract. Korean J. Food Preserv. 2012, 19, 393–399. [Google Scholar] [CrossRef]
- Han, C.R.; Jun, D.Y.; Woo, H.J.; Jeong, S.Y.; Woo, M.H.; Kim, Y.H. Induction of microtubule-damage, mitotic arrest, Bcl-2 phosphorylation, Bak activation, and mitochondria-dependent caspase cascade is involved in human Jurkat T-cell apoptosis by aruncin B from Aruncus dioicus var. kamtschaticus. Bioorg. Med. Chem. Lett. 2012, 22, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, U.; Kang, J.; Kim, J.; Park, S.; Park, S.; Choi, S.; Heo, H. Protective effects of Aruncus dioicus var. kamtschaticus extract against hyperglycemic-induced neurotoxicity. Korean J. Food Sci. Technol. 2017, 49, 668–675. [Google Scholar]
- Ahn, H.; Kim, J.; Kim, J.; Auh, J.; Choe, E. In vitro α-glucosidase and pancreatic lipase inhibitory activities and antioxidants of Samnamul (Aruncus dioicus) during rehydration and cooking. Food Sci. Biotechnol. 2014, 23, 1287–1293. [Google Scholar] [CrossRef]
- Kim, J.I.; Yun, J.A.; Jeong, Y.K.; Baek, H.J. Hypoglycemic and hypolipidemic effects of samnamul (shoot of Aruncus dioicus var. kamtschaticus Hara) in mice fed a high-fat/high-sucrose diet. Food Sci. Biotechnol. 2018, 27, 1467–1473. [Google Scholar] [CrossRef]
- Kim, D.H.; Moon, Y.S.; Park, T.S.; Hwang, J.Y.; Son, J.H. Potent whitening activity of Aruncus dioicus extract in B16F10 melanoma cell by suppression of melanin biosynthesis. Hortic. Sci. Technol. 2013, 31, 813–820. [Google Scholar]
- Dorjsembe, B.; Joo, H.; Nho, C.; Ham, J.; Kim, J.-C. Aruncus dioicus var. kamtschaticus Extract Ameliorates Psoriasis-like Skin Inflammation via Akt/mTOR and JAK2/STAT3 Signaling Pathways in a Murine Model. Nutrients 2022, 14, 5094. [Google Scholar] [CrossRef]
- Han, H.-S.; Lee, J.-W. Attenuation of Brain Injury by Water Extract of Goat’s-beard (Aruncus dioicus) and Its Ethyl Acetate Fraction in a Rat Model of Ischemia-Reperfusion. Prev. Nutr. Food Sci. 2011, 16, 217–223. [Google Scholar] [CrossRef]
- Baek, H.S.; Lim, S.H.; Ahn, K.S.; Lee, J. Methanol extract of goat’s-beard (Aruncus dioicus) reduces renal injury by inhibiting apoptosis in a rat model of ischemia-reperfusion. Prev. Nutr. Food Sci. 2012, 17, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Vo, Q.H.; Nguyen, P.H.; Zhao, B.T.; Thi, Y.N.; Nguyen, D.H.; Kim, W.I.; Seo, U.M.; Min, B.S.; Woo, M.H. Bioactive constituents from the n-butanolic fraction of Aruncus dioicus var. kamtschaticus. Nat. Prod. Sci. 2014, 20, 274–280. [Google Scholar]
- Granica, S.; Fusani, P.; Stanisławska, I.; Piwowarski, J.P.; Melck, D.; Motta, A.; Zidorn, C. Monoterpenoids from the traditional North Italian vegetable Aruncus dioicus (Walter) Fernald var. vulgaris (Maxim.) H.Hara (Rosaceae). Food Chem. 2017, 221, 1851–1859. [Google Scholar] [CrossRef]
- Kim, A.-N.; Lee, K.Y.; Rahman, M.S.; Kim, H.-J.; Chun, J.; Heo, H.J.; Kerr, W.L.; Choi, S.-G. Effect of water blanching on phenolic compounds, antioxidant activities, enzyme inactivation, microbial reduction, and surface structure of samnamul (Aruncus dioicus var kamtschaticus). Int. J. Food Sci. Technol. 2020, 55, 1754–1762. [Google Scholar] [CrossRef]
- Fusani, P.; Piwowarski, J.P.; Zidorn, C.; Kiss, A.K.; Scartezzini, F.; Granica, S. Seasonal variation in secondary metabolites of edible shoots of Buck’s beard [Aruncus dioicus (Walter) Fernald (Rosaceae)]. Food Chem. 2016, 202, 23–30. [Google Scholar] [CrossRef] [PubMed]
- An, H.C.; Choe, E.O. Content changes of pigments and antioxidants of dried samnamul (Aruncus dioicus) and daraesoon (Actinidia arguta) during rehydration and high temperature cooking. Korean J. Food Cook. Sci. 2016, 32, 383–389. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Zhang, Z.; Liu, J.; Hong, L. β-Sitosterol as a Promising Anticancer Agent for Chemoprevention and Chemotherapy: Mechanisms of Action and Future Prospects. Adv. Nutr. 2023, 14, 1085–1110. [Google Scholar] [CrossRef]
- Martínez-García, M.; Garduño-Solórzano, G.; Lopes, G.; Sanchez, B.A.; Urbatzka, R.; Hentschke, G.S.; Campos, J.E.; Vasconcelos, V.M.O. Antioxidant, Anti-Inflammatory and Anti-Obesity Potential of Extracts Containing Phenols, Chlorophyll and Carotenoids from Mexican Wild Populations of Bacopa monnieri (L.) Wettst. Biology 2023, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Kopańska, M.; Batoryna, M.; Banaś-Ząbczyk, A.; Błajda, J.; Lis, M.W. The Effect of α-Tocopherol on the Reduction of Inflammatory Processes and the Negative Effect of Acrylamide. Molecules 2022, 27, 965. [Google Scholar] [CrossRef]
- Tian, M.; Bai, Y.; Tian, H.; Zhao, X. The Chemical Composition and Health-Promoting Benefits of Vegetable Oils—A Review. Molecules 2023, 28, 6393. [Google Scholar] [CrossRef]
- Vezza, T.; Canet, F.; de Marañón, A.M.; Bañuls, C.; Rocha, M.; Víctor, V.M. Phytosterols: Nutritional Health Players in the Management of Obesity and Its Related Disorders. Antioxidants 2020, 9, 1266. [Google Scholar] [CrossRef]
- La Torre, M.E.; Cianciulli, A.; Monda, V.; Monda, M.; Filannino, F.M.; Antonucci, L.; Valenzano, A.; Cibelli, G.; Porro, C.; Messina, G.; et al. α-Tocopherol Protects Lipopolysaccharide-Activated BV2 Microglia. Molecules 2023, 28, 3340. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Song, M.-H.; Yu, J.-W.; Lee, J.-H.; Ahn, H.-Y.; Keum, Y.-S.; Lee, J.-H. Profiling of Nutritionally Vital Bioactive Compounds in Emerging Green Leafy Vegetables: A Comparative Study. Foods 2022, 11, 3867. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Moon, S.H.; Gansukh, E.; Keum, Y.S. An efficient one-step scheme for the purification of major xanthophyll carotenoids from lettuce, and assessment of their comparative anticancer potential. Food Chem. 2018, 266, 56–65. [Google Scholar] [CrossRef]
- Saini, R.K.; Yu, J.-W.; Song, M.-H.; Ahn, H.-Y.; Lee, J.-H.; Keum, Y.-S.; Lee, J.-H. Profiling of Redox-Active Lipophilic Constituents in Leaf Mustard (Brassica juncea (L.) Czern.) Cultivars Using LC-MS and GC-MS. Antioxidants 2022, 11, 2464. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids: Distribution, Function in Nature, and Analysis Using LC-Photodiode Array Detector (DAD)-MS and MS/MS System. Mass Spectrom. 2023, 12, A0133. [Google Scholar] [CrossRef] [PubMed]
- Siefermann-Harms, D.; Hertzberg, S.; Borch, G.; Liaaen-Jensen, S. Lactucaxanthin, an ε,ε-carotene-3,3′-diol from Lactuca sativa. Phytochemistry 1981, 20, 85–88. [Google Scholar] [CrossRef]
- Rivera, S.M.; Christou, P.; Canela-Garayoa, R. Identification of carotenoids using mass spectrometry. Mass Spectrom. Rev. 2014, 33, 353–372. [Google Scholar] [CrossRef]
- Phillip, D.; Young, A.J. Occurrence of the carotenoid lactucaxanthin in higher plant LHC II. Photosynth. Res. 1995, 43, 273–282. [Google Scholar] [CrossRef]
- Britton, G.; Khachik, F. Carotenoids in Food. In Carotenoids: Nutrition and Health, 4th ed.; Britton, G., Pfander, H., Liaaen-Jensen, S., Eds.; Birkhäuser Verlag: Basel, Switzerland, 2009; pp. 45–66. [Google Scholar]
- Muntean, E. Quantification of carotenoids from pumpkin juice by HPLC-DAD. Sci. Researches. Agroaliment. Process. Technol. 2005, 11, 123–128. [Google Scholar]
- Muntean, E.; Bele, C.; Socaciu, C. HPLC analysis of carotenoids from fruits of Cucurbita pepo L. var. melopepo Alef. Acta Agron. Hung. 2003, 51, 455–459. [Google Scholar] [CrossRef]
- Kimura, M.; Rodriguez-Amaya, D.B. Carotenoid composition of hydroponic leafy vegetables. J. Agric. Food Chem. 2003, 51, 2603–2607. [Google Scholar] [CrossRef] [PubMed]
- Niizu, P.Y.; Rodriguez-Amaya, D.B. New data on the carotenoid composition of raw salad vegetables. J. Food Compos. Anal. 2005, 18, 739–749. [Google Scholar] [CrossRef]
- Humphries, J.M.; Khachik, F. Distribution of lutein, zeaxanthin, and related geometrical isomers in fruit, vegetables, wheat, and pasta products. J. Agric. Food Chem. 2003, 51, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.S.; Lakshmi, M.J.; Sharavana, G.; Sathaiah, G.; Sreerama, Y.N.; Baskaran, V. Lactucaxanthin—A potential anti-diabetic carotenoid from lettuce (Lactuca sativa) inhibits α-amylase and α-glucosidase activity in vitro and in diabetic rats. Food Funct. 2017, 8, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Anitha, R.E.; Janani, R.; Peethambaran, D.; Baskaran, V. Lactucaxanthin protects retinal pigment epithelium from hyperglycemia-regulated hypoxia/ER stress/VEGF pathway mediated angiogenesis in ARPE-19 cell and rat model. Eur. J. Pharmacol. 2021, 899, 174014. [Google Scholar] [CrossRef]
- Jayapala, N.; Rani Elavarasan, A.; Chaudhari, S.R.; Vallikannan, B. Cytotoxicity and 3T3-L1 cell uptake of lactucaxanthin purified and characterized by LC-MS and NMR from lettuce (Lactuca sativa). J. Liq. Chromatogr. Relat. Technol. 2020, 43, 233–246. [Google Scholar] [CrossRef]
- Tsushima, M.; Maoka, T.; Katsuyama, M.; Kozuka, M.; Matsuno, T.; Tokuda, H.; Nishino, H.; Iwashima, A. Inhibitory Effect of Natural Carotenoids on Epstein-Barr Virus Activation Activity of a Tumor Promoter in Raji Cells. A Screening Study for Anti-Tumor Promoters. Biol. Pharm. Bull. 1995, 18, 227–233. [Google Scholar] [CrossRef]
- Zheng, W.V.; Xu, W.; Li, Y.; Qin, J.; Zhou, T.; Li, D.; Xu, Y.; Cheng, X.; Xiong, Y.; Chen, Z. Anti-aging effect of beta-carotene through regulating the KAT7-P15 signaling axis, inflammation and oxidative stress process. Cell. Mol. Biol. Lett. 2022, 27, 86. [Google Scholar] [CrossRef]
- Mahavy, C.E.; Mol, A.; Andrianarisoa, B.; Duez, P.; Jaziri, M.E.; Baucher, M.; Rasamiravaka, T. The Xanthophyll Carotenoid Lutein Reduces the Invasive Potential of Pseudomonas aeruginosa and Increases Its Susceptibility to Tobramycin. Int. J. Mol. Sci. 2022, 23, 7199. [Google Scholar] [CrossRef]
- Antunes, A.; Carmo, F.; Pinto, S.; Andrade, N.; Martel, F. The Anti-Proliferative Effect of β-Carotene against a Triple-Negative Breast Cancer Cell Line Is Cancer Cell-Specific and JNK-Dependent. PharmaNutrition 2022, 22, 100320. [Google Scholar] [CrossRef]
- Kurniawan, R.; Nurkolis, F.; Taslim, N.A.; Subali, D.; Surya, R.; Gunawan, W.B.; Alisaputra, D.; Mayulu, N.; Salindeho, N.; Kim, B. Carotenoids Composition of Green Algae Caulerpa racemosa and Their Antidiabetic, Anti-Obesity, Antioxidant, and Anti-Inflammatory Properties. Molecules 2023, 28, 3267. [Google Scholar] [CrossRef]
- Soontornchaiboon, W.; Joo, S.S.; Kim, S.M. Anti-inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoidea in RAW 264.7 macrophages. Biol. Pharm. Bull. 2012, 35, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Das, S.; Joardar, S.; Bhattacharjee, S.; Chakraborty, P. Carotenoids as Anticancer Agents. In Carotenoids: Structure and Function in the Human Body; Springer: Berlin/Heidelberg, Germany, 2021; pp. 475–512. [Google Scholar]
- Lee, H.W.; Bi, X.; Henry, C.J. Carotenoids, tocopherols and phylloquinone content of 26 green leafy vegetables commonly consumed in Southeast Asia. Food Chem. 2022, 385, 132729. [Google Scholar]
- Meulmeester, F.L.; Luo, J.; Martens, L.G.; Mills, K.; van Heemst, D.; Noordam, R. Antioxidant Supplementation in Oxidative Stress-Related Diseases: What Have We Learned from Studies on Alpha-Tocopherol? Antioxidants 2022, 11, 2322. [Google Scholar] [CrossRef]
- Bhat, A.H.; Alia, A.; Rather, G.M.; Kumar, B. Isolation & characterisation of beta-sitosterol from the rhizomes of Arisaema utile and its evaluation for antioxidant activity. Int. J. Sci. Res. Biol. Sci. 2019, 6, 111–118. [Google Scholar]
- Khan, Z.; Nath, N.; Rauf, A.; Emran, T.B.; Mitra, S.; Islam, F.; Chandran, D.; Barua, J.; Khandaker, M.U.; Idris, A.M.; et al. Multifunctional roles and pharmacological potential of β-sitosterol: Emerging evidence toward clinical applications. Chem. Biol. Interact. 2022, 365, 110117. [Google Scholar] [CrossRef]
- Almazan, A.M.; Adeyeye, S.O. Fat and fatty acid concentrations in some green vegetables. J. Food Compos. Anal. 1998, 11, 375–380. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.C.; Carvalho, A.M.; Sánchez-Mata, M.C.; Cámara, M.; Tardío, J. Fatty acids profiles of some Spanish wild vegetables. Food Sci. Int. 2012, 18, 281–290. [Google Scholar] [CrossRef]
- Kim, K.B.; Nam, Y.A.; Kim, H.S.; Hayes, A.W.; Lee, B.M. α-Linolenic acid: Nutraceutical, pharmacological and toxicological evaluation. Food Chem. Toxicol. 2014, 70, 163–178. [Google Scholar] [CrossRef]
- Cambiaggi, L.; Chakravarty, A.; Noureddine, N.; Hersberger, M. The Role of α-Linolenic Acid and Its Oxylipins in Human Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 6110. [Google Scholar] [CrossRef] [PubMed]
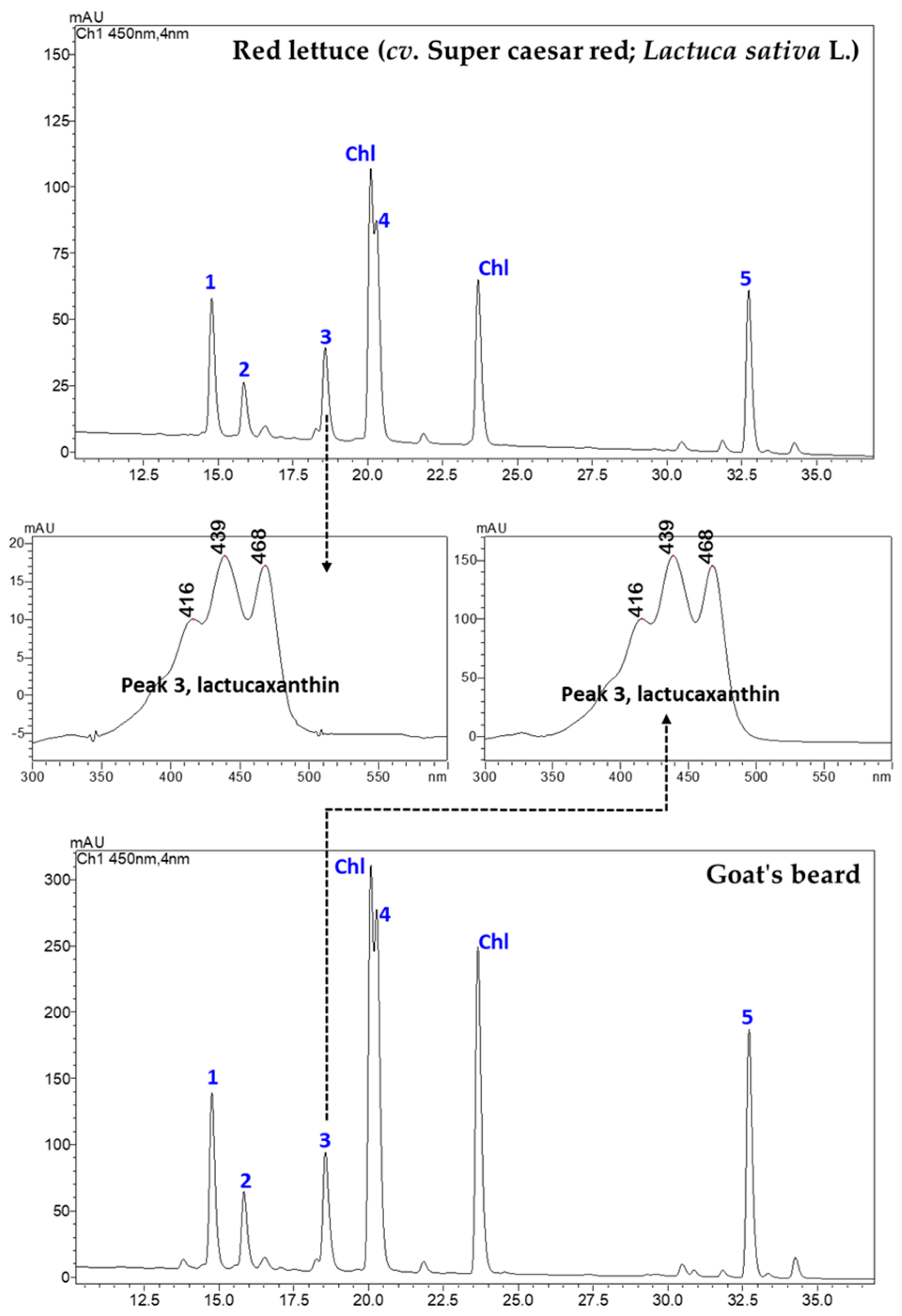
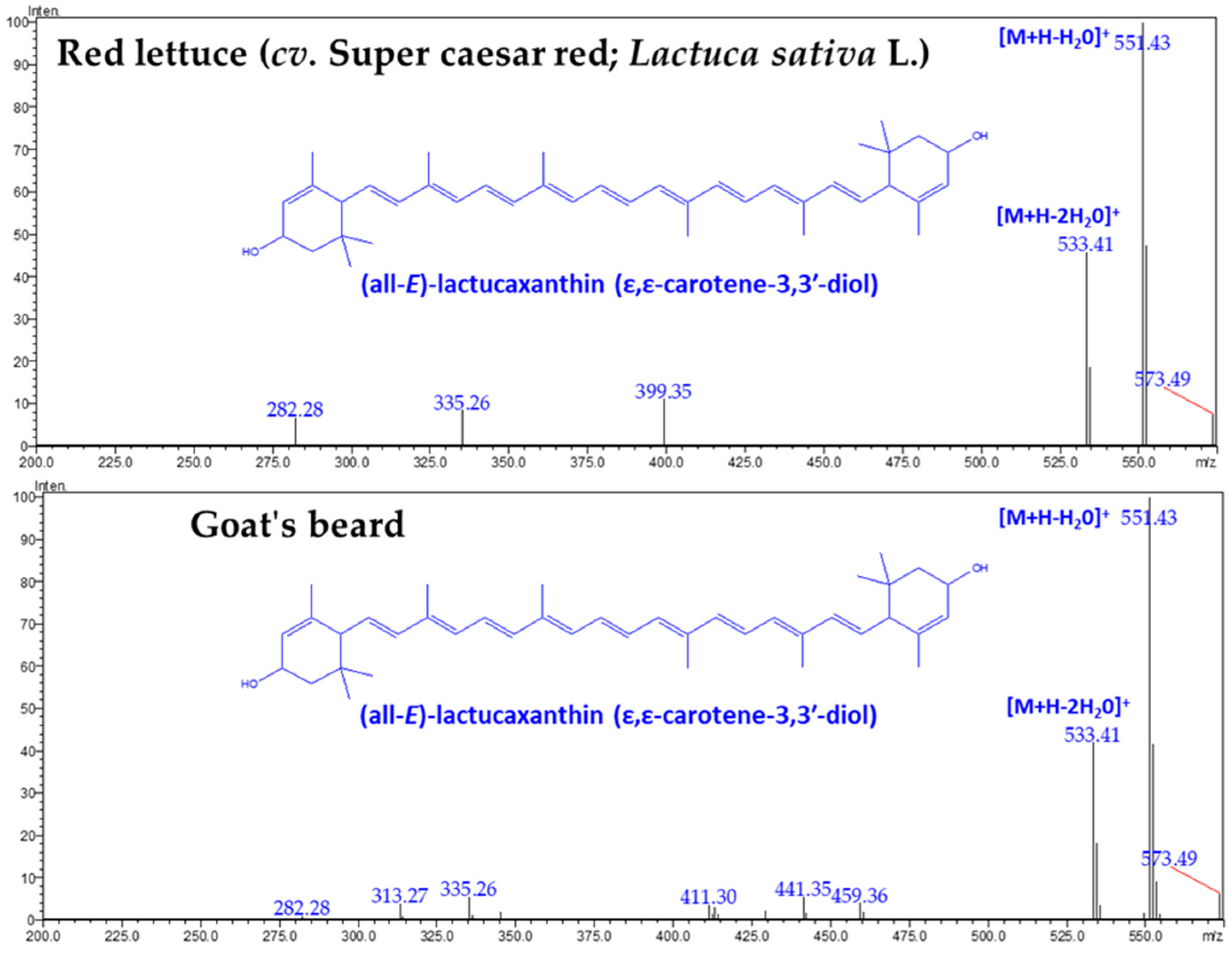

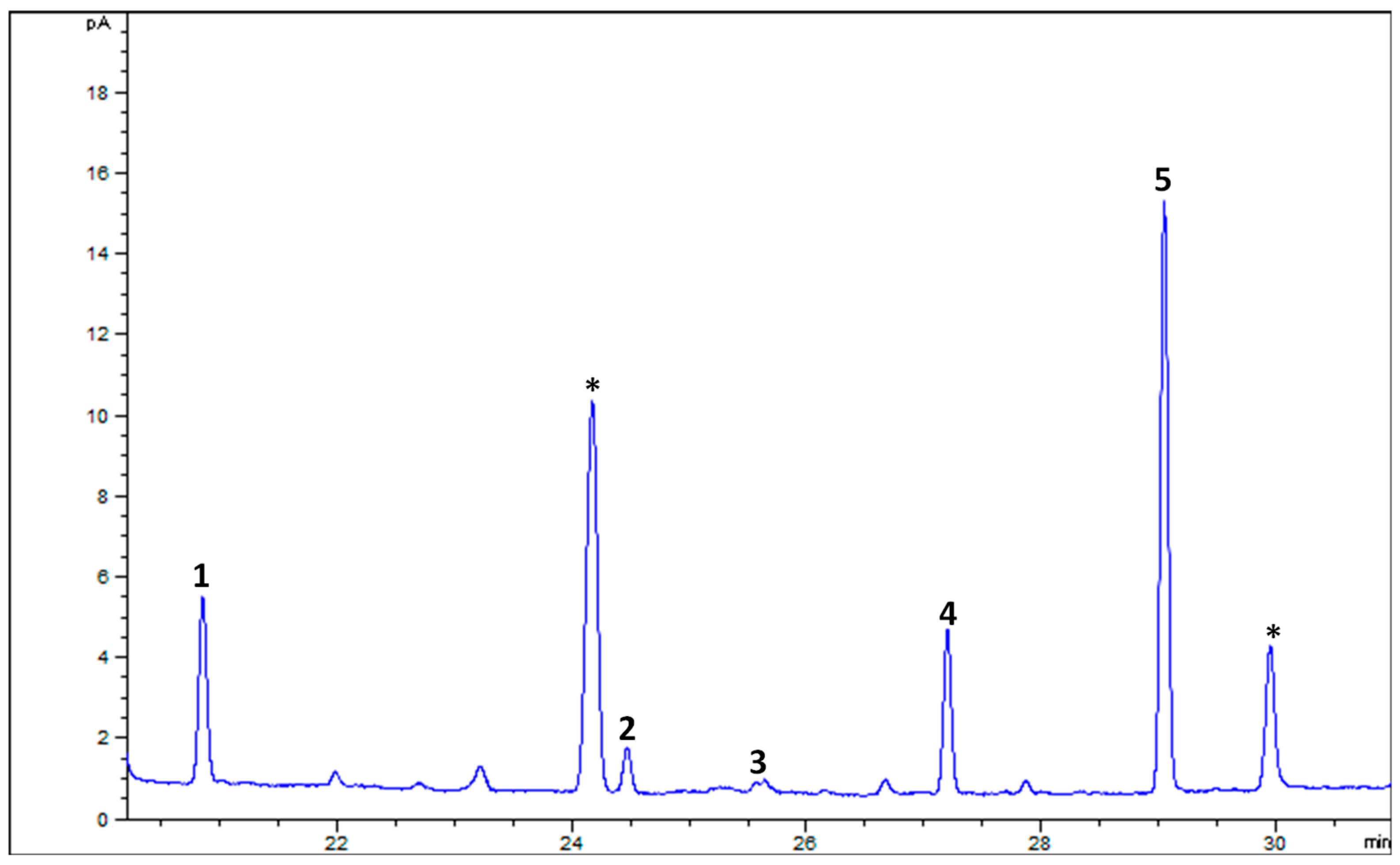
| Compounds (CA) | Retention Time (min) | Goat’s Beard | Red Lettuce |
|---|---|---|---|
| (all-E)-Violaxanthin | 14.809 | 55.92 ± 1.92 | 26.79 ± 0.83 |
| 9-Z-Neoxanthin | 15.889 | 30.21 ± 0.89 | 9.04 ± 0.12 |
| (all-E)-Lactucaxanthin | 18.609 | 45.42 ± 0.80 | 19.05 ± 0.67 |
| (all-E)-Lutein | 20.341 | 101.36 ± 1.20 | 28.66 ± 0.62 |
| (all-E)-β-Carotene | 32.759 | 65.52 ± 3.02 | 18.86 ± 1.55 |
| Total carotenoids | 298.42 ± 2.04 | 102.39 ± 0.88 | |
| α-Tocopherol | 16.969 | 49.17 ± 3.74 | 23.34 ± 2.52 |
| Peak No. | Phytosterol | RT (min) | Contents (µg/g FW) |
|---|---|---|---|
| 1 | Campesterol | 18.560 | 3.53 ± 0.20 |
| 2 | Stigmasterol | 19.171 | 65.30 ± 4.87 |
| 3 | β-Sitosterol | 20.497 | 89.54 ± 2.46 |
| Total phytosterol | 158.37 ± 7.32 |
| Peak No. | FAME | RT (min) | Content (%) |
|---|---|---|---|
| 1 | C16:0 (Palmitic) | 20.844 | 19.35 ± 0.19 |
| 2 | C18:0 (Stearic) | 24.468 | 4.21 ± 0.42 |
| 3 | C18:1n9c (Oleic) | 25.56 | 2.28 ± 0.58 |
| 4 | C18:2n6c (Linoleic) | 27.195 | 17.12 ± 0.50 |
| 5 | C18:3n3 (α-Linolenic) | 29.045 | 57.03 ± 0.47 |
| ∑SFAs | 23.57 ± 0.61 | ||
| ∑PUFAs | 74.15 ± 0.03 | ||
| ∑PUFAs/∑SFAs | 3.15 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saini, R.K.; Ko, E.-Y.; Keum, Y.-S.; Lee, J.-H.; Chun, S.C.; Sivanesan, I. Aruncus dioicus var. kamtschaticu: A Newly Identified Source of Lactucaxanthin (ε,ε-Carotene-3,3′-diol). Horticulturae 2024, 10, 891. https://doi.org/10.3390/horticulturae10080891
Saini RK, Ko E-Y, Keum Y-S, Lee J-H, Chun SC, Sivanesan I. Aruncus dioicus var. kamtschaticu: A Newly Identified Source of Lactucaxanthin (ε,ε-Carotene-3,3′-diol). Horticulturae. 2024; 10(8):891. https://doi.org/10.3390/horticulturae10080891
Chicago/Turabian StyleSaini, Ramesh Kumar, Eun-Young Ko, Young-Soo Keum, Ji-Ho Lee, Se Chul Chun, and Iyyakkannu Sivanesan. 2024. "Aruncus dioicus var. kamtschaticu: A Newly Identified Source of Lactucaxanthin (ε,ε-Carotene-3,3′-diol)" Horticulturae 10, no. 8: 891. https://doi.org/10.3390/horticulturae10080891









