Genome-Wide Identification of the Class III Peroxidase Gene Family in Ginger and Expression Analysis under High Temperature and Intense Light Stress
Abstract
:1. Introduction
2. Materials and Methods
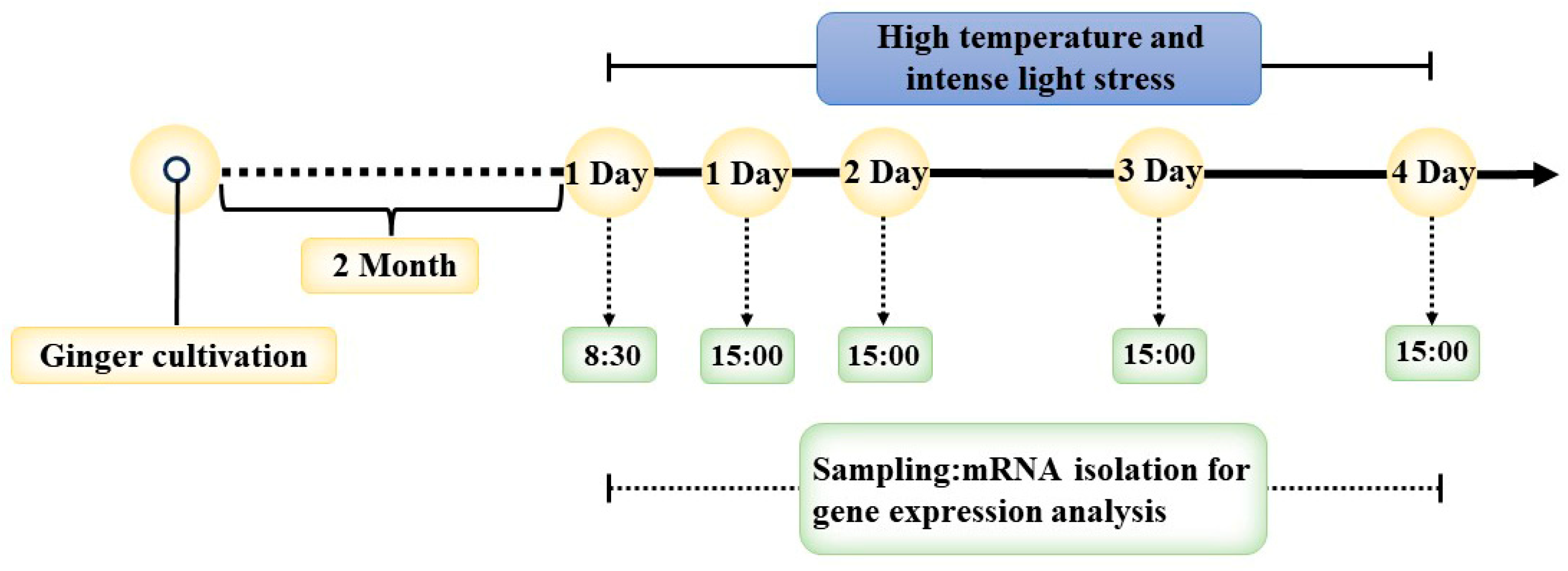
2.1. Plant Materials
2.2. Identification and Physicochemical Properties Analysis
2.3. Phylogenetic Analysis
2.4. Genetic Structure, Motif Composition, Gene Duplication and Cis-Acting Elements
2.5. Gene Expression and qRT-PCR Analysis
3. Results
3.1. Genome-Wide Identification of the PRX Family in Ginger
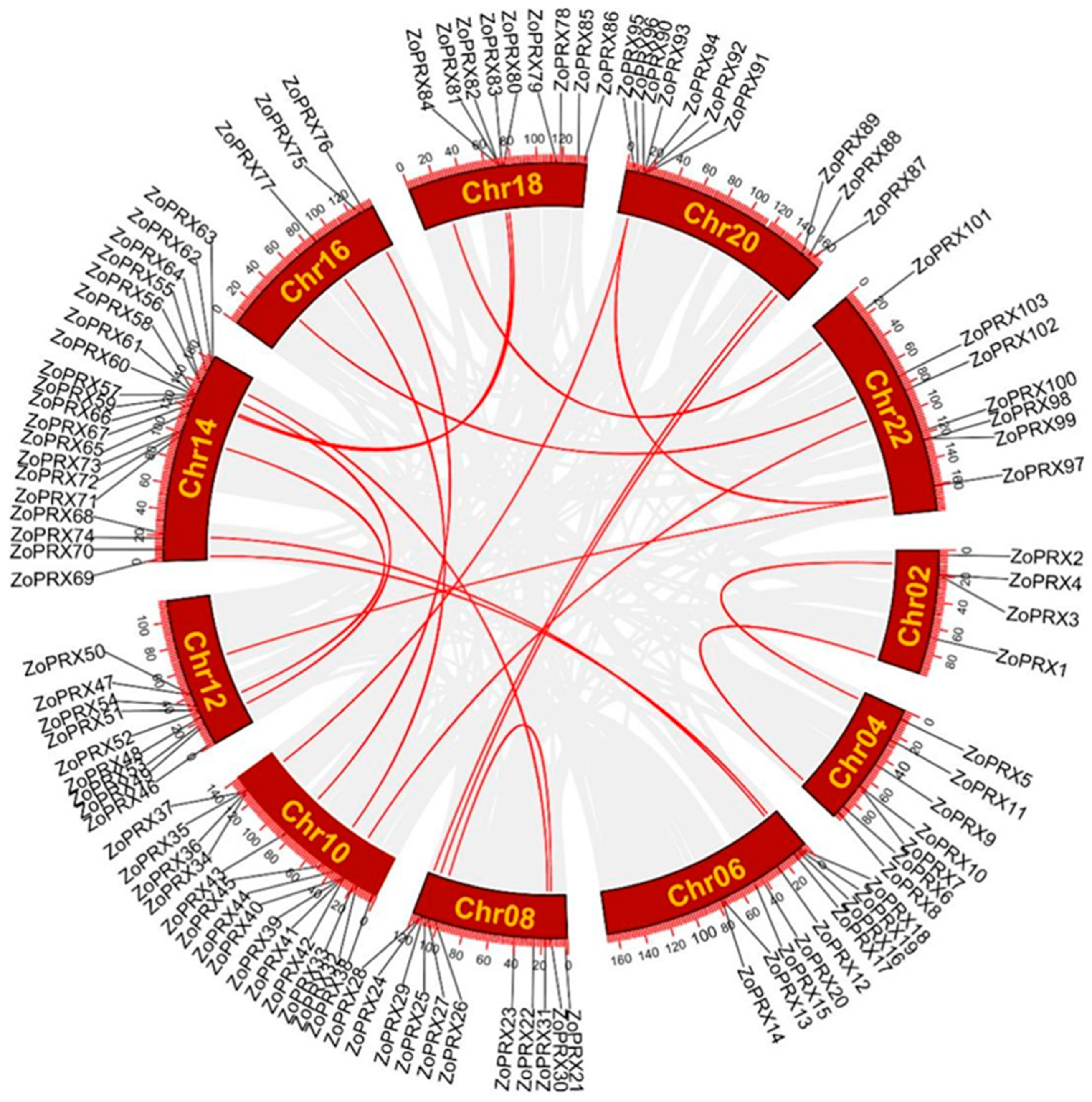
3.2. Chromosomal Distribution of the ZoPRX Genes
3.3. Conserved Motif Analysis of ZoPRXs
3.4. Cis-Element Analysis of ZoPRXs
3.5. Evolutionary Relationships of the ZoPRXs
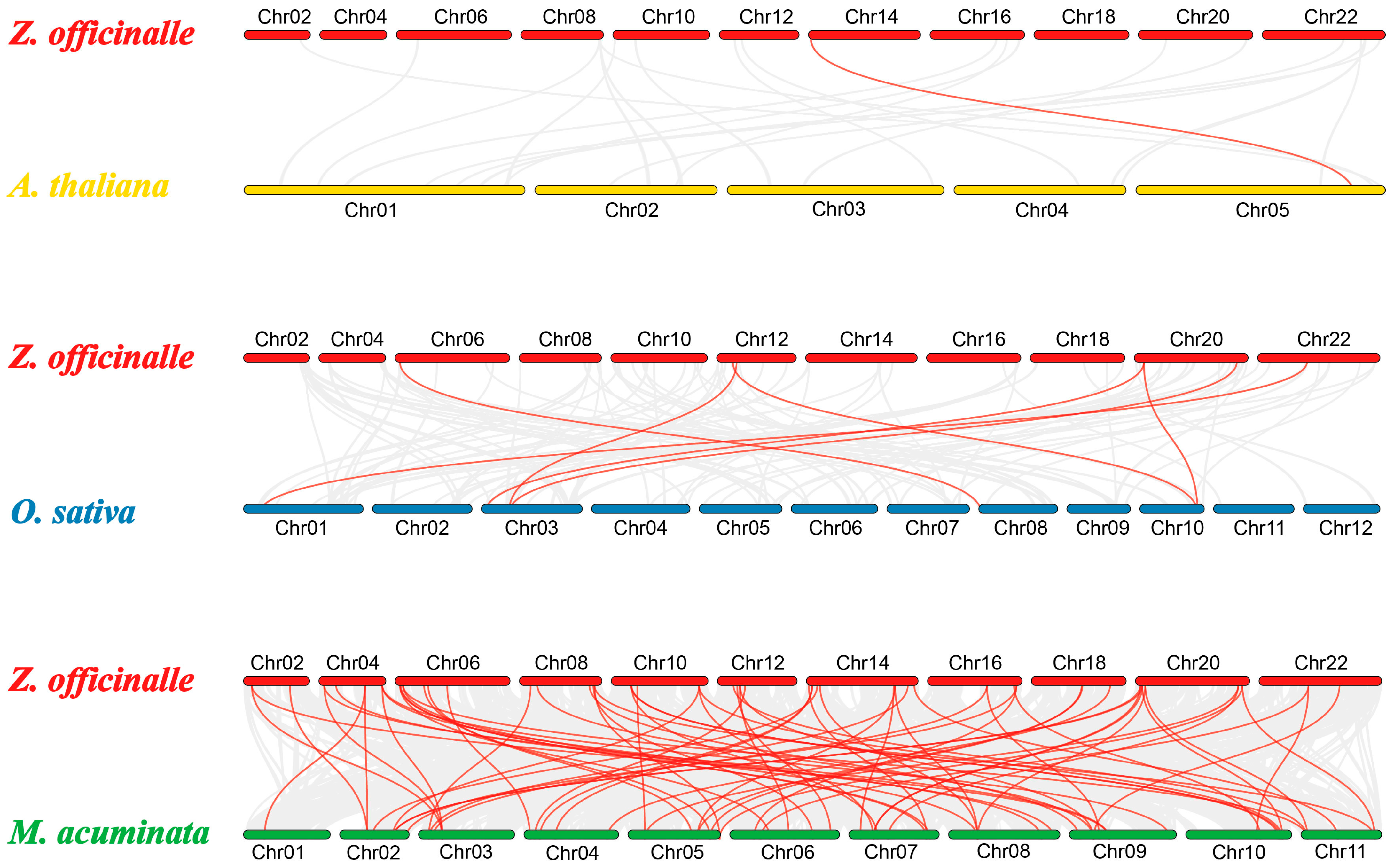
3.6. Genome-Wide Duplication Events and Synteny Analysis of ZoPRXs
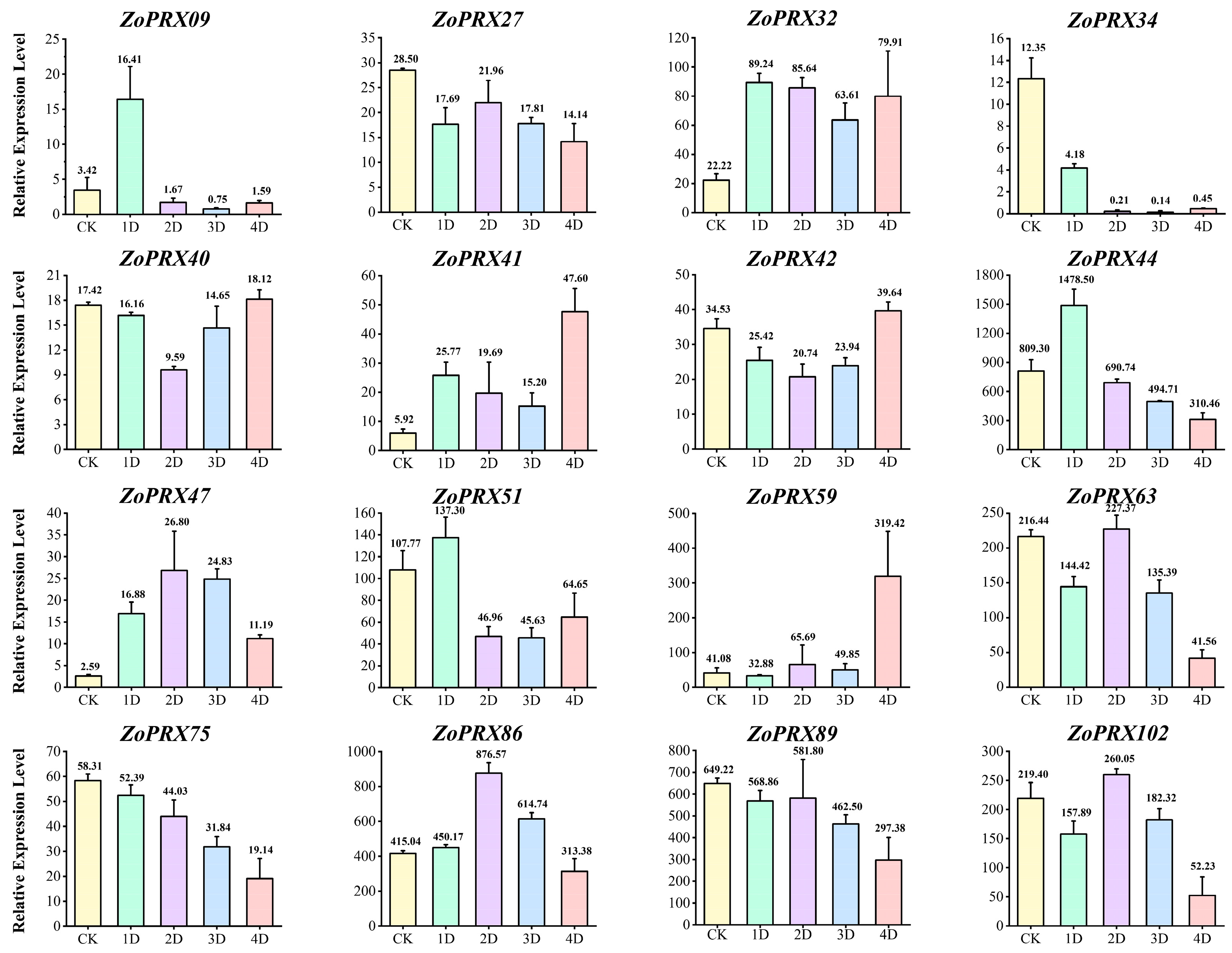
3.7. Expression Patterns of ZoPRX Genes in High Temperature and Intense Light Stresses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomez-Zavaglia, A.; Mejuto, J.C.; Simal-Gandara, J. Mitigation of emerging implications of climate change on food production systems. Food Res. Int. 2020, 134, 109256. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.A.; Bañón, S.; Vicente, M.M.J.; Miralles, J.; Martínez-Sánchez, J.J. Review Article: Root development in horticultural plants grown under abiotic stress conditions—A review. J. Hortic. Sci. Biotechnol. 2011, 86, 543–556. [Google Scholar] [CrossRef]
- Castoria, R.; Caputo, L.; De Curtis, F.; De Cicco, V. Resistance of postharvest biocontrol yeasts to oxidative stress: A possible new mechanism of action. Phytopathology 2003, 93, 564–572. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing climate-resilient crops: Improving plant tolerance to stress combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef]
- Ippolito, A.; Nigro, F. Impact of preharvest application of biological control agents on postharvest diseases of fresh fruits and vegetables. Crop Prot. 2000, 19, 715–723. [Google Scholar] [CrossRef]
- Kong, F.; Ran, Z.; Zhang, J.X.; Zhang, M.G.; Wu, K.B.; Zhang, R.T.; Liao, K.; Cao, J.Y.; Zhang, L.; Xu, J.L.; et al. Synergistic effects of temperature and light intensity on growth and physiological performance in Chaetoceros calcitrans. Aquac. Rep. 2021, 21, 100805. [Google Scholar] [CrossRef]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef]
- Roxas, V.P.; Lodhi, S.A.; Garrett, D.K.; Mahan, J.R.; Allen, R.D. Stress Tolerance in Transgenic Tobacco Seedlings that Overexpress Glutathione S-Transferase/Glutathione Peroxidase. Plant Cell Physiol. 2000, 41, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Chao-Zeng, Z.; Lei, Z.; Li-Juan, Y.; Ming, C.; Qing-Yu, W.; Lian-Cheng, L.; Zhao-Shi, X.; You-Zhi, M.; Malcolm, B. Two Wheat Glutathione Peroxidase Genes Whose Products Are Located in Chloroplasts Improve Salt and H2O2 Tolerances in Arabidopsis. PLoS ONE 2013, 8, e73989. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Wood, Z.A.; Schr?Der, E.; Harris, J.R.; Poole, L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. 2003, 28, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Almagro, L.; Gómez Ros, L.; Belchi-Navarro, S.; Bru, R.; Ros Barceló, A.; Pedreño, M. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef]
- Piontek, K.; Smith, A.T.; Blodig, W. Lignin peroxidase structure and function. Biochem. Soc. Trans. 2001, 29, 111–116. [Google Scholar] [CrossRef]
- Zámocký, M.; Furtmüller, P.G.; Obinger, C. Evolution of structure and function of Class I peroxidases. Arch. Biochem. Biophys. 2010, 500, 45–57. [Google Scholar] [CrossRef]
- Shigeto, J.; Tsutsumi, Y. Diverse functions and reactions of class III peroxidases. New Phytol. 2016, 209, 1395–1402. [Google Scholar] [CrossRef]
- Susumu, H.; Katsutomo, S.; Hiroyuki, I.; Yuko, O.; Hirokazu, M. A Large Family of Class III Plant Peroxidases. Plant Cell Physiol. 2001, 462, 462–468. [Google Scholar]
- Passardi, F.; Penel, C.; Dunand, C. Performing the paradoxical: How plant peroxidases modify the cell wall. Trends Plant Sci. 2004, 9, 534–540. [Google Scholar] [CrossRef]
- Passardi, F.; Theiler, G.; Zamocky, M.; Cosio, C.; Rouhier, N.; Teixera, F.; Margis-Pinheiro, M.; Ioannidis, V.; Penel, C.; Falquet, L. PeroxiBase: The peroxidase database. Phytochemistry 2007, 68, 1605–1611. [Google Scholar] [CrossRef]
- Kidwai, M.; Ahmad, I.Z.; Chakrabarty, D. Class III peroxidase: An indispensable enzyme for biotic/abiotic stress tolerance and a potent candidate for crop improvement. Plant Cell Rep. 2020, 39, 1381–1393. [Google Scholar] [CrossRef]
- Shang, H.; Fang, L.; Qin, L.; Jiang, H.; Duan, Z.; Zhang, H.; Yang, Z.; Cheng, G.; Bao, Y.; Xu, J. Genome-wide identification of the class III peroxidase gene family of sugarcane and its expression profiles under stresses. Front. Plant Sci. 2023, 14, 1101665. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Z.; How, J.; Xu, H.; Chen, L.; Li, K. Overexpression of a peroxidase gene (AtPrx64) of Arabidopsis thaliana in tobacco improves plant’s tolerance to aluminum stress. Plant Mol. Biol. 2017, 95, 157–168. [Google Scholar] [CrossRef]
- Liu, H.; Dong, S.; Li, M.; Gu, F.; Yang, G.; Guo, T.; Chen, Z.; Wang, J. The Class III peroxidase gene OsPrx30, transcriptionally modulated by the AT-hook protein OsATH1, mediates rice bacterial blight-induced ROS accumulation. J. Integr. Plant Biol. 2021, 63, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Coego, A.; Ramirez, V.; Ellul, P.; Mayda, E.; Vera, P. The H2O2-regulated Ep5C gene encodes a peroxidase required for bacterial speck susceptibility in tomato. Plant J. 2010, 42, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Tognolli, M.; Penel, C.; Greppin, H.; Simon, P. Analysis and expression of the class III peroxidase large gene family in Arabidopsis thaliana. Gene 2002, 288, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Valério, L.; De Meyer, M.; Penel, C.; Dunand, C. Expression analysis of the Arabidopsis peroxidase multigenic family. Phytochemistry 2004, 65, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Duroux, L.; Welinder, K.G. The Peroxidase Gene Family in Plants: A Phylogenetic Overview. J. Mol. Evol. 2003, 57, 397–407. [Google Scholar] [CrossRef]
- Passardi, F.; Longet, D.; Penel, C.; Dunand, C. The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry 2004, 65, 1879–1893. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhao, Y.; Han, G.; Zhu, S. Systematic analysis of maize class III peroxidase gene family reveals a conserved subfamily involved in abiotic stress response. Gene 2015, 566, 95–108. [Google Scholar] [CrossRef]
- Yunpeng, C.; Yahui, H.; Dandan, M.; Dahui, L.; Qing, J.; Yi, L.; Yongping, C. Structural, Evolutionary, and Functional Analysis of the Class III Peroxidase Gene Family in Chinese Pear (Pyrus bretschneideri). Front. Plant Sci. 2016, 7, 1874. [Google Scholar]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, H.; Gao, Y.; Xiong, R.; Xiang, Y. The TCP transcription factor PeTCP10 modulates salt tolerance in transgenic Arabidopsis. Plant Cell Rep. 2021, 40, 1971–1987. [Google Scholar] [CrossRef] [PubMed]
- Ballester, P.; Cerdá, B.; Arcusa, R.; Marhuenda, J.; Yamedjeu, K.; Zafrilla, P. Effect of ginger on inflammatory diseases. Molecules 2022, 27, 7223. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Wu, L.; Dong, Z.; Jiang, Y.; Jiang, S.; Xing, H.; Li, Q.; Liu, G.; Tian, S.; Wu, Z. Haplotype-resolved genome of diploid ginger (Zingiber officinale) and its unique gingerol biosynthetic pathway. Hortic. Res. 2021, 8, 189. [Google Scholar] [CrossRef]
- Gholizadeh, F.; Mirmazloum, I.; Janda, T. Genome-wide identification of HKT gene family in wheat (Triticum aestivum L.): Insights from the expression of multiple genes (HKT, SOS, TVP and NHX) under salt stress. Plant Stress 2024, 13, 100539. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Llorente, F.; López-Cobollo, R.M.; Catalá, R.; Martínez-Zapater, J.M.; Salinas, J. A novel cold-inducible gene from Arabidopsis, RCI3, encodes a peroxidase that constitutes a component for stress tolerance. Plant J. 2010, 32, 13–24. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, J.; Luo, W.; Qin, M.; Yang, J.; Wu, W.; Xie, X. Genome-wide identification and expression analysis of the class III peroxidase gene family in potato (Solanum tuberosum L.). Front. Genet. 2020, 11, 593577. [Google Scholar] [CrossRef]
- Han, Y.; Ding, T.; Su, B.; Jiang, H. Genome-Wide Identification, Characterization and Expression Analysis of the Chalcone Synthase Family in Maize. Int. J. Mol. Sci. 2016, 17, 161. [Google Scholar] [CrossRef]
- Oliver, K.R.; Mccomb, J.A.; Greene, W.K. Transposable Elements: Powerful Contributors to Angiosperm Evolution and Diversity. Genome Biol. Evol. 2013, 5, 1886–1901. [Google Scholar] [CrossRef]
- Ren, L.L.; Liu, Y.J.; Liu, H.J.; Qian, T.T.; Qi, L.W.; Wang, X.R.; Zeng, Q.Y. Subcellular Relocalization and Positive Selection Play Key Roles in the Retention of Duplicate Genes of Populus Class III Peroxidase Family. Plant Cell 2014, 26, 2404–2419. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Paterson, A.H. Genome and gene duplications and gene expression divergence: A view from plants. Ann. N. Y. Acad. Sci. 2012, 1256, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, J.; Ahammed, G.J.; Li, J.; Yang, Y. Genome-wide identification and expression analysis of aquaporin gene family related to abiotic stress in watermelon. Genome 2019, 62, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Mika, A.; Boenisch, M.J.; Hopff, D.; Lüthje, S. Membrane-bound guaiacol peroxidases from maize (Zea mays L.) roots are regulated by methyl jasmonate, salicylic acid, and pathogen elicitors. J. Exp. Bot. 2010, 61, 831–841. [Google Scholar] [CrossRef]
- Hwarari, D.; Guan, Y.; Li, R.; Movahedi, A.; Chen, J.; Yang, L. Comprehensive bioinformatics and expression analysis of TCP transcription factors in Liriodendron chinense reveals putative abiotic stress regulatory roles. Forests 2022, 13, 1401. [Google Scholar] [CrossRef]




| Genes Name | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| ZoTUB2 | GAACATGATGTGTGCTGCCG | ATCTTCAGCCCTTTCGGAGG |
| ZoPRX9 | AAACATCTAAACCCAAACC | ATTCTGGCATCCTTACA |
| ZoPRX27 | AGCGTTTGGTGGGTGAC | GACTTCAAGACACTACCG |
| ZoPRX32 | TGTAAGGATGCCAGAAT | ATTCTGGCATCCTTACA |
| ZoPRX34 | GTGACGACGCTCAACGG | TGACTAATCCAAGGCAC |
| ZoPRX40 | CGGCTACTGGGACAACG | TGGACGCAGTAAGGTGT |
| ZoPRX41 | TATTAGGTGTTTACGGAGTG | CGCACTCCGTAAACACC |
| ZoPRX42 | CGACATTTCTGCGTTCA | ACCTCTTACGCCGCACT |
| ZoPRX44 | CTTTCTTTCGCTCTTGT | ACAAGAGCGAAAGAAAG |
| ZoPRX47 | CGGTGATTCTGACAAGG | TCAGACTCACATCCACC |
| ZoPRX51 | TGGGACGAACAGGAGGAA | TTCCTCCTGTTCGTCCC |
| ZoPRX59 | GTCCGCAGCCCATACCT | AGTCGAAGATCCACGCCAGCAGCCG |
| ZoPRX63 | AAAGAAAGGCAACAATG | TAGTCGACAGATTCCCCATGAATGA |
| ZoPRX75 | ATGTTGGCTTATGACTT | GTCATAAGCCAACATTC |
| ZoPRX86 | AGTGTTTCCGTCCTCTA | ATGAACTTGATGGGTCTA |
| ZoPRX89 | ATTGCCAAGGAACTCAG | CTTGGCAATGCCATGCACATTTTTG |
| ZoPRX102 | TATCCAGAATAATCCCAATC | ATGAGGTTTTTCTTGATTCCGGGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, M.; Jiang, Y.; Tang, S.; Xing, H.; Li, H.; Gu, J.; Mao, M.; Wang, W.; Xia, M.; Li, H.-L. Genome-Wide Identification of the Class III Peroxidase Gene Family in Ginger and Expression Analysis under High Temperature and Intense Light Stress. Horticulturae 2024, 10, 911. https://doi.org/10.3390/horticulturae10090911
Gong M, Jiang Y, Tang S, Xing H, Li H, Gu J, Mao M, Wang W, Xia M, Li H-L. Genome-Wide Identification of the Class III Peroxidase Gene Family in Ginger and Expression Analysis under High Temperature and Intense Light Stress. Horticulturae. 2024; 10(9):911. https://doi.org/10.3390/horticulturae10090911
Chicago/Turabian StyleGong, Min, Yajun Jiang, Shihao Tang, Haitao Xing, Hui Li, Jiajia Gu, Minmin Mao, Wei Wang, Maoqin Xia, and Hong-Lei Li. 2024. "Genome-Wide Identification of the Class III Peroxidase Gene Family in Ginger and Expression Analysis under High Temperature and Intense Light Stress" Horticulturae 10, no. 9: 911. https://doi.org/10.3390/horticulturae10090911






