Alterations in the Growth Responses of Pelargonium × hortorum Irrigated with Microalgae Production Wastewater
Abstract
:1. Introduction
2. Materials and Methods
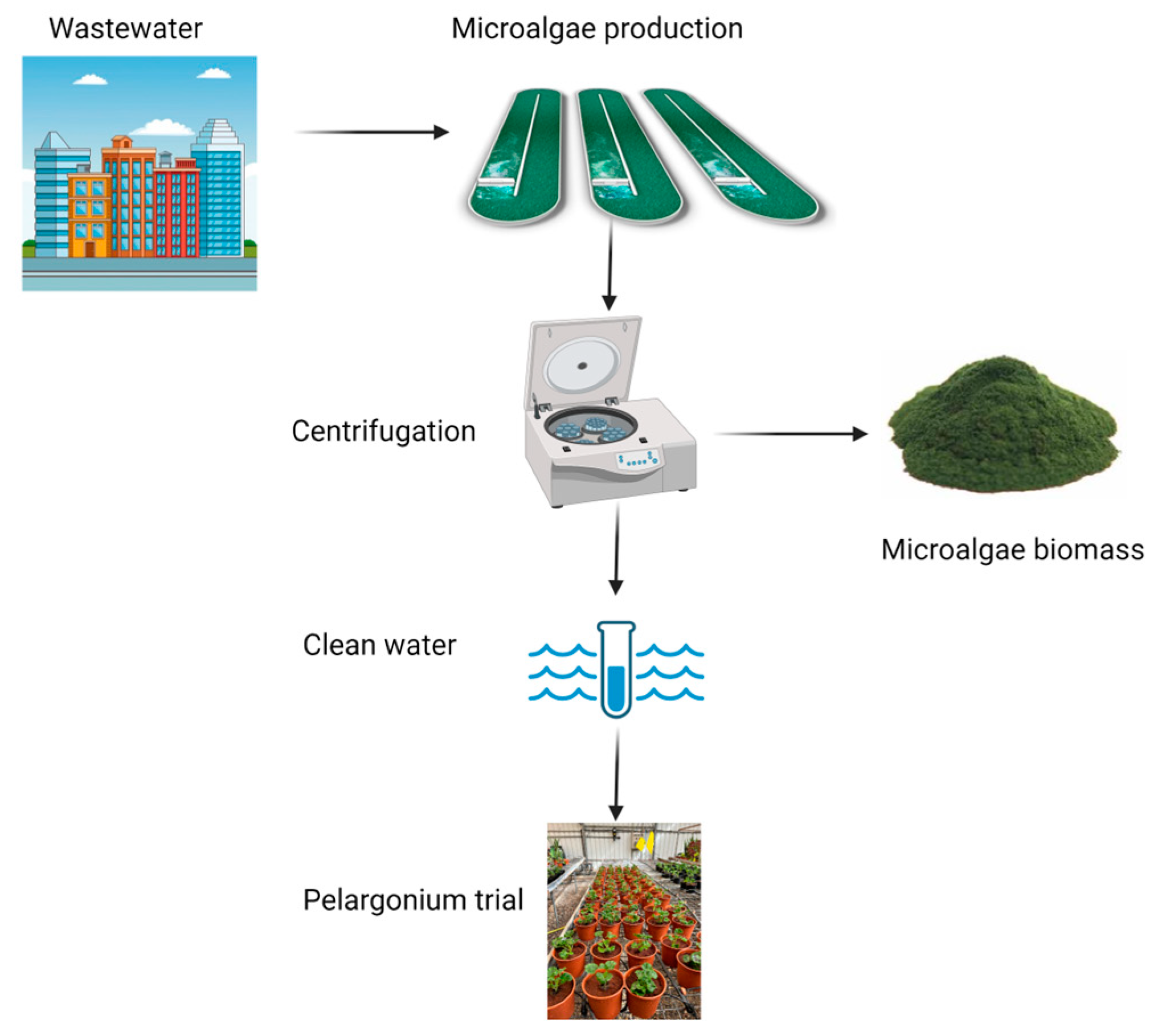
2.1. Facilities
2.2. Plant Material
2.3. Treatments
2.4. Inlet Wastewater
2.5. Outlet Wastewater
2.6. Sampling and Analysis
2.6.1. Water Analysis
2.6.2. Biometric Parameters
2.7. Experimental Design and Statistical Analysis
3. Results
3.1. Irrigation Water Quality
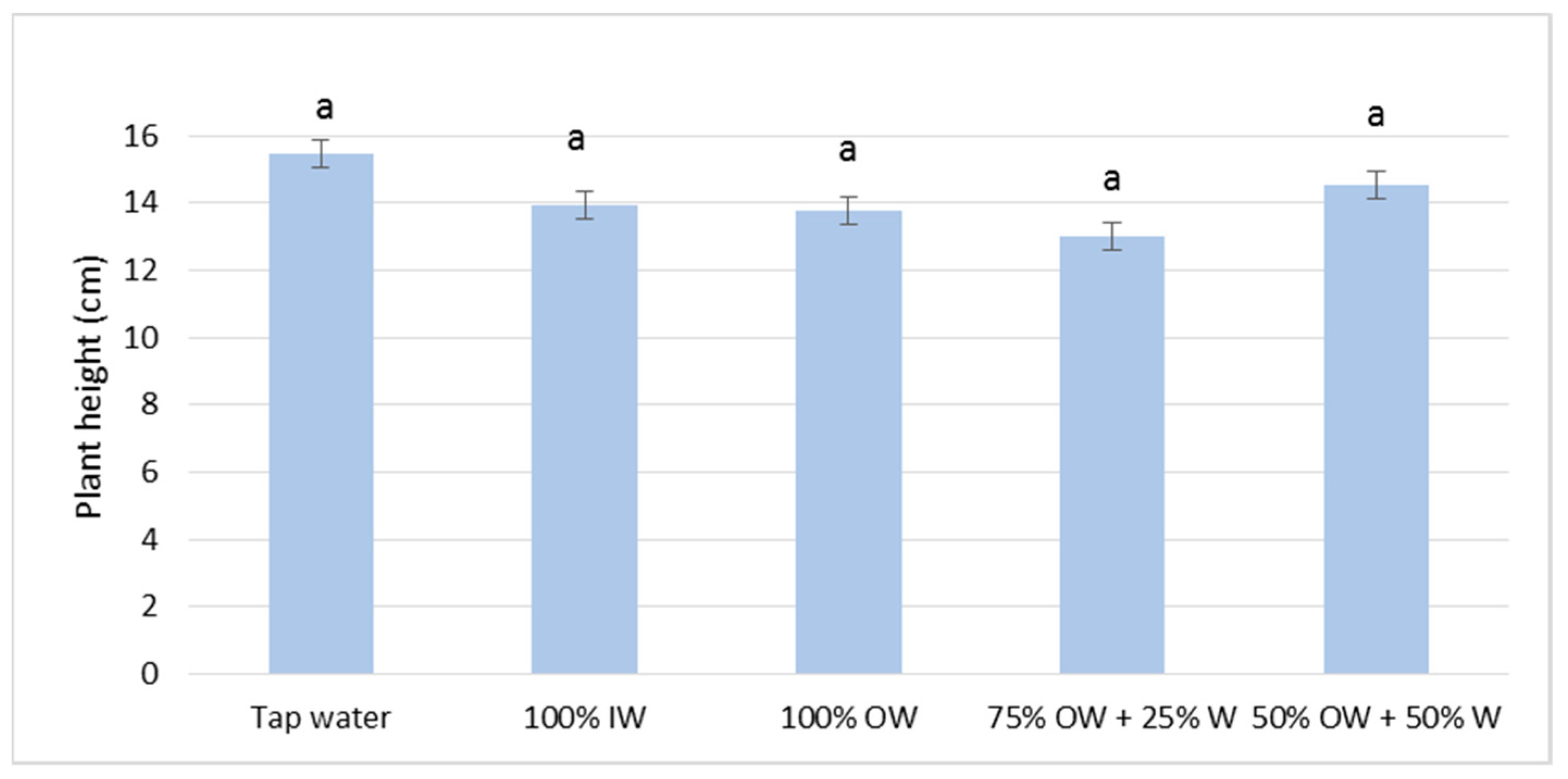
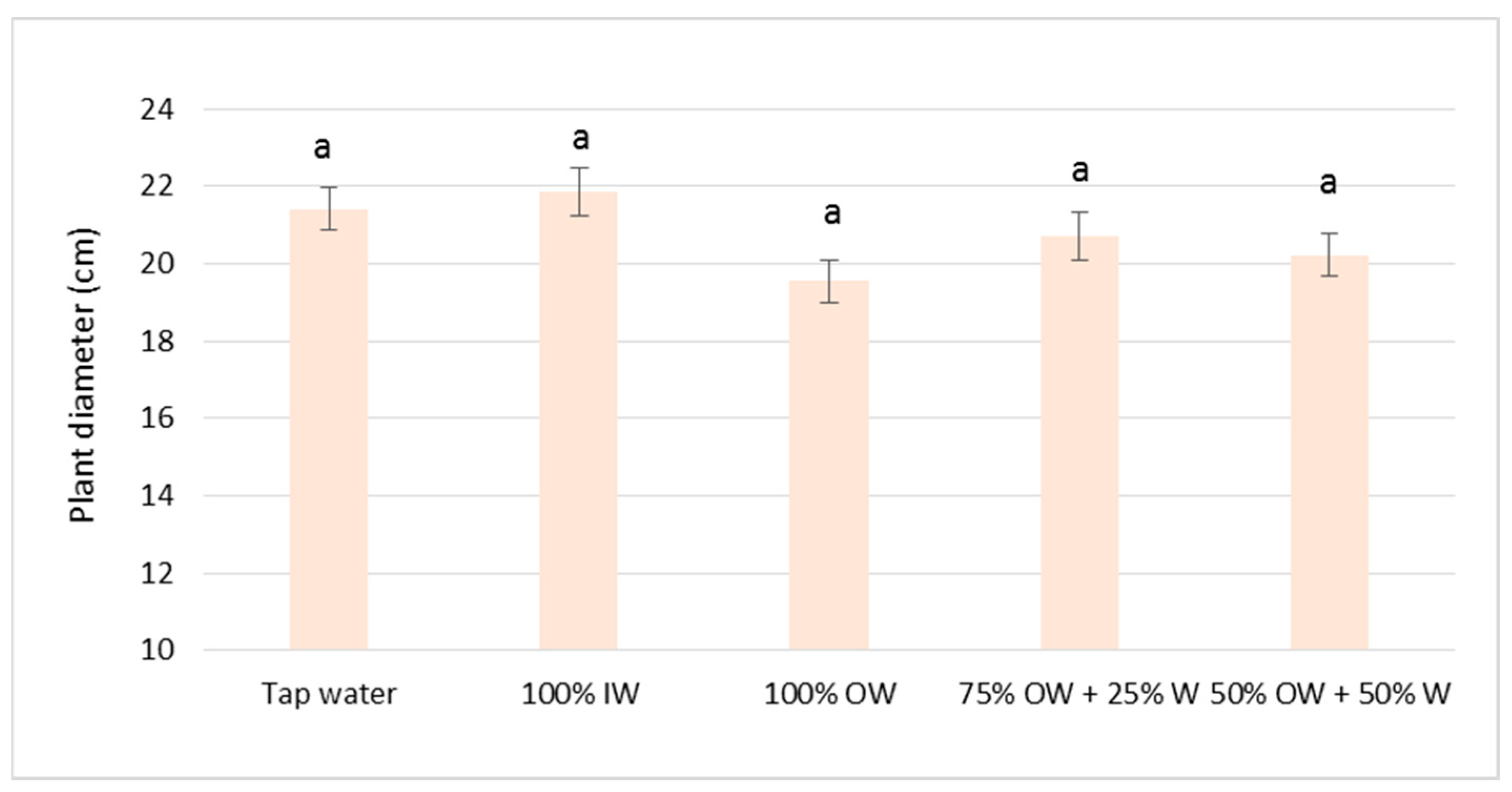
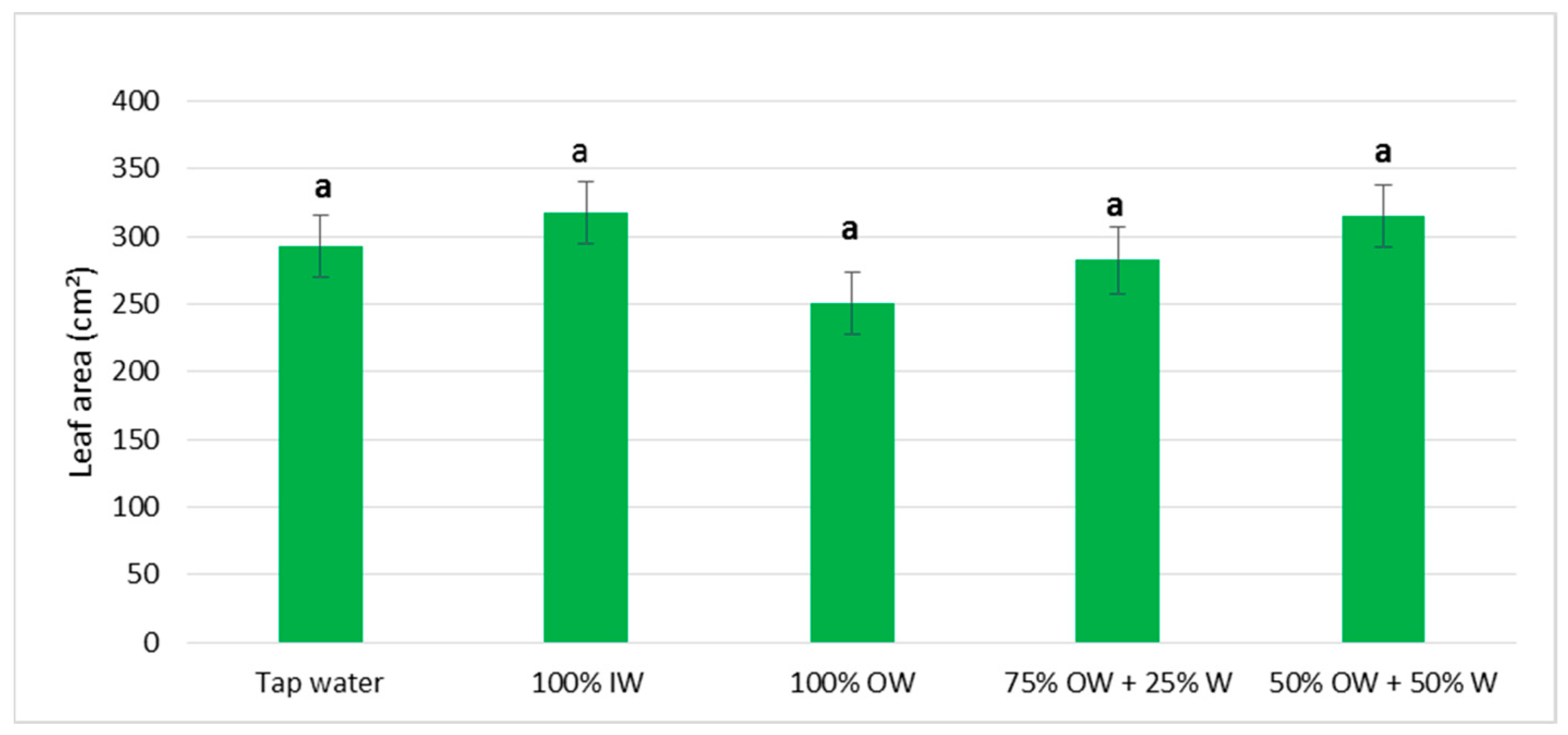
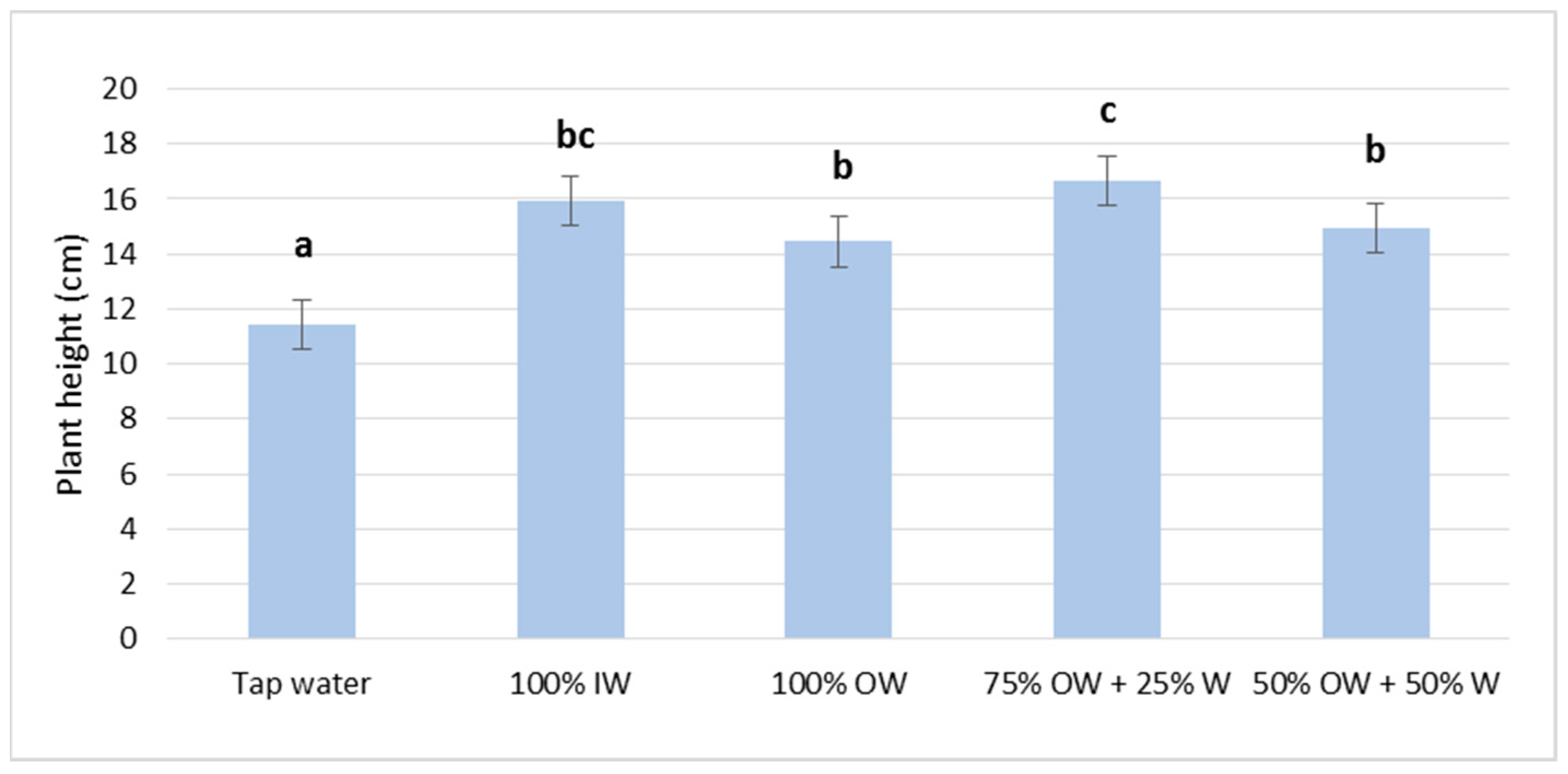
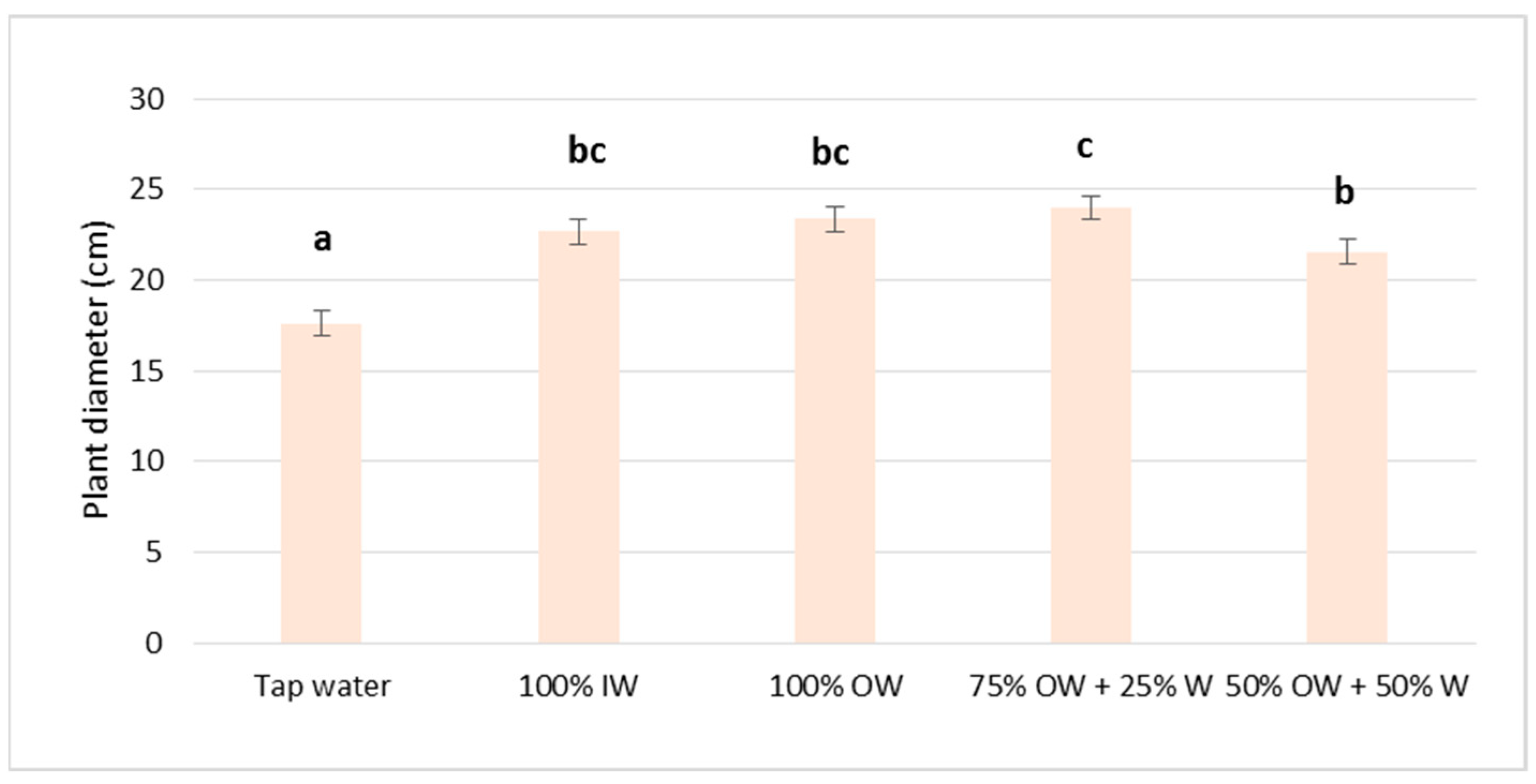
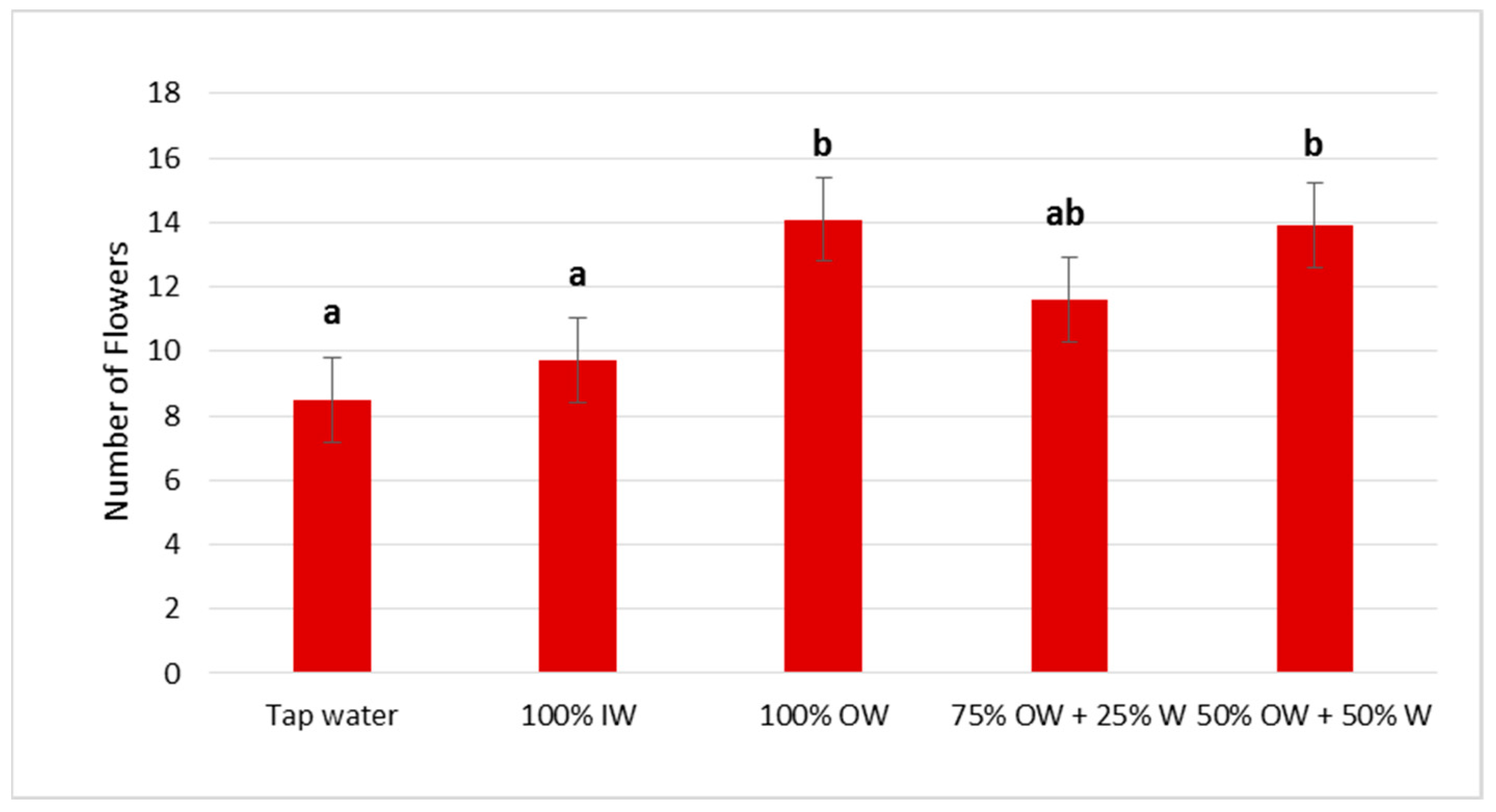
3.2. Plant Biometric Parameters
3.2.1. Autumn Growing Season
3.2.2. Spring Growing Season
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Melo, M.R.d.S.; Dias, N.d.S.; de Medeiros, I.J.N.; Travassos, K.D.; Miranda, N.d.O.; Gurgel, M.T.; Neto, H.d.S.L.; Fernandes, C.d.S. Strategies for applying gray water effluent on ornamental sunflower crops. Environ. Sci. Pollut. Res. 2020, 27, 38537–38544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Guo, H.; Zhang, Y.; Li, Z.; Liu, Y.; Wang, S.; Fu, Z. A coupling simulation and optimization method developed for environmental-economic management of Lake watershed. J. Environ. Manag. 2022, 318, 115546. [Google Scholar] [CrossRef]
- Kolekar, S.; Sankapal, P.; Khare, K.; Chinnasamy, P. Assessment of recycled treated wastewater for sustainable tomato crop production: A comprehensive review. Environ. Chall. 2023, 13, 100791. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J.; Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESA: Paris, France, 2012. [Google Scholar] [CrossRef]
- Bhandari, M.; Kumar, P.; Bhatt, P.; Simsek, H.; Kumar, R.; Chaudhary, A.; Malik, A.; Prajapati, S.K. An integration of algae-mediated wastewater treatment and resource recovery through anaerobic digestion. J. Environ. Manag. 2023, 342, 118159. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.-H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M.; et al. Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances, and future prospects. Environ. Sci. Ecotechnol. 2023, 13, 100205. [Google Scholar] [CrossRef] [PubMed]
- Procházková, M.; Touš, M.; Horňák, D.; Miklas, V.; Vondra, M.; Máša, V. Industrial wastewater in the context of European Union water reuse legislation and goals. J. Clean. Prod. 2023, 426, 139037. [Google Scholar] [CrossRef]
- Bonaguro, J.E.; Coletto, L.; Sambo, P.; Nicoletto, C.; Zanin, G. Environmental Analysis of Sustainable Production Practices Applied to Cyclamen and Zonal Geranium. Horticulturae 2021, 7, 8. [Google Scholar] [CrossRef]
- Navarro, T. Water reuse and desalination in Spain–challenges and opportunities. J. Water Reuse Desalination 2018, 8, 153–168. [Google Scholar] [CrossRef]
- Cao, X.; Bosch, D.; Pease, J. Recycling Irrigation Water on Ornamental Nursery Operations: Will Consumer Premiums Compensate for Growers’ Costs? In Proceedings of the Agricultural and Applied Economics Association Annual Meeting, Chicago, IL, USA, 30 July–1 August 2017; p. 259126. [Google Scholar] [CrossRef]
- Morillas-España, A.; Lafarga, T.; Sánchez-Zurano, A.; Acién-Fernández, F.G.; Rodríguez-Miranda, E.; Gómez-Serrano, C.; González-López, C.V. Year-long evaluation of microalgae production in wastewater using pilot-scale raceway photobioreactors: Assessment of biomass productivity and nutrient recovery capacity. Algal Res. 2021, 60, 102500. [Google Scholar] [CrossRef]
- Zhuang, L.-L.; Yang, P.; Liang, S.; Yu, T.; Liu, C.; Zhao, T.; Tan, G.; Niu, Y.; Zhang, J. Comparison of microalgae cultivation modes for advanced wastewater purification and pollutant-resource conversion: A pilot study. J. Water Process Eng. 2023, 56, 104374. [Google Scholar] [CrossRef]
- Renuka, N.; Prasanna, R.; Sood, A.; Bansal, R.; Bidyarani, N.; Singh, R.; Shivay, Y.S.; Nain, L.; Ahluwalia, A.S. Wastewater grown microalgal biomass as inoculants for improving micronutrient availability in wheat. Rhizosphere 2017, 3, 150–159. [Google Scholar] [CrossRef]
- Lu, W.; Asraful Alam, M.; Liu, S.; Xu, J.; Parra Saldivar, R. Critical processes and variables in microalgae biomass production coupled with bioremediation of nutrients and CO2 from livestock farms: A review. Sci. Total Environ. 2020, 716, 135247. [Google Scholar] [CrossRef] [PubMed]
- Renuka, N.; Sood, A.; Prasanna, R.; Ahluwalia, A.S. Phycoremediation of wastewaters: A synergistic approach using microalgae for bioremediation and biomass generation. Int. J. Environ. Sci. Technol. 2015, 12, 1443–1460. [Google Scholar] [CrossRef]
- Pittman, J.K.; Dean, A.P.; Osundeko, O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 2011, 102, 17–25. [Google Scholar] [CrossRef]
- Salachna, P. Trends in Ornamental Plant Production. Horticulturae 2022, 8, 413. [Google Scholar] [CrossRef]
- Gabellini, S.; Scaramuzzi, S. Evolving consumption trends, marketing strategies, and governance settings in ornamental horticulture: A review of the grey literature. Horticulturae 2022, 8, 234. [Google Scholar] [CrossRef]
- Darras, A.I.; Grigoropoulou, K.; Dimiza, K.; Zulfiqar, F. Effects of brief UV-C irradiation treatments on the rooting performance of Pelargonium × hortorum stem cuttings (L.h. bailey). Horticulturae 2022, 8, 897. [Google Scholar] [CrossRef]
- Torres, A.P.; Lopez, R.G. Commercial greenhouse production: Measuring daily light integral in a greenhouse. Purdue Agr. 2010, 7, HO-238-W. [Google Scholar]
- Kesari, K.K.; Soni, R.; Jamal, Q.M.S.; Tripathi, P.; Lal, J.A.; Jha, N.K.; Siddiqui, M.H.; Kumar, P.; Tripathi, V.; Ruokolainen, J. Wastewater treatment and reuse: A review of its applications and health implications. Water Air Soil Pollut. 2021, 232, 208. [Google Scholar] [CrossRef]
- Giuffrida, F.; Rouphael, Y.; Toscano, S.; Scuderi, D.; Romano, D.; Rivera, C.M.; Colla, G.; Leonardi, C. A simple model for nondestructive leaf area estimation in bedding plants. Photosynthetica 2011, 49, 380. [Google Scholar] [CrossRef]
- Sharma, M.; Agarwal, S.; Agarwal Malik, R.; Kumar, G.; Pal, D.B.; Mandal, M.; Sarkar, A.; Bantun, F.; Haque, S.; Singh, P.; et al. Recent advances in microbial engineering approaches for wastewater treatment: A review. Bioengineered 2023, 14, 2184518. [Google Scholar] [CrossRef] [PubMed]
- Paranychianakis, N.V.; Chartzoulakis, K.S. Irrigation of Mediterranean crops with saline water: From physiology to management practices. Agric. Ecosyst. Environ. 2005, 106, 171–187. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Lao, M.T. The effects of salt stress on ornamental plants and integrative cultivation practices. Sci. Hortic. 2018, 240, 430–439. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef]
- Wang, H.-J.; Wang, J.; Yu, X. Wastewater irrigation and crop yield: A meta-analysis. J. Integr. Agric. 2022, 21, 1215–1224. [Google Scholar] [CrossRef]
- Coskun, D.; White, P.J. Ion-uptake mechanisms of individual cells and roots: Short-distance transport. In Marschner’s Mineral Nutrition of Plants; Rengel, E.Z., Cakmak, I., White, P.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 11–71. [Google Scholar]
- Ortiz Tena, F.; Bickel, V.; Steinweg, C.; Posten, C. Continuous microalgae cultivation for wastewater treatment–Development of a process strategy during day and night. Sci. Total Environ. 2024, 912, 169082. [Google Scholar] [CrossRef]
- Ahsan, M.; Nafees, M.; Amin, M.; Nawaz, F.; Tufail, A.; Sardar, H.; Shokralla, S.; Mahmoud, E.A.; El-Sabrout, A.M.; Elansary, H.O. Nutrients uptake and accumulation in plant parts of fragrant Rosa species irrigated with treated and untreated wastewater. Plants 2022, 11, 1260. [Google Scholar] [CrossRef]
- Hashem, M.S.; Qi, X. Treated Wastewater irrigation—A review. Water 2021, 13, 1527. [Google Scholar] [CrossRef]
- Marroquin, A.; Holmes, K.; Salazar, D. Soil salinization and chemically mediated plant–insect interactions in a changing climate. Curr. Opin. Insect Sci. 2020, 60, 101130. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.F.F.; He, Z.L.; Stoffella, P.J.; Melfi, A.J. Reclaimed wastewater: Effects on citrus nutrition. Agric. Water Manag. 2011, 98, 1828–1833. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, S.; Fan, J.; Zhang, F.; Xiang, Y.; Zheng, J.; Guo, J. Responses of growth, fruit yield, quality and water productivity of greenhouse tomato to deficit drip irrigation. Sci. Hortic. 2021, 275, 109710. [Google Scholar] [CrossRef]
- Kwon, O.-H.; Choi, H.-G.; Kim, S.-J.; Kim, W.-H. Assessment of Four-Seasonal Quality and Yield of Cut Flower Roses Grafted onto Rosa Rootstocks. Agriculture 2022, 12, 1848. [Google Scholar] [CrossRef]
- Ting, W.H.T.; Tan, I.A.W.; Salleh, S.F.; Wahab, N.A. Application of water hyacinth (Eichhornia crassipes) for phytoremediation of ammoniacal nitrogen: A review. J. Water Process Eng. 2018, 22, 239–249. [Google Scholar] [CrossRef]
- Tabatabaei, S.H.; Nourmahnad, N.; Kermani, S.G.; Tabatabaei, S.A.; Najafi, P.; Heidarpour, M. Urban wastewater reuse in agriculture for irrigation in aridand semi-arid regions–A review. Int. J. Recycl. Org. Waste Agric. 2020, 9, 193–220. [Google Scholar] [CrossRef]



| Tap Water | 100% IW | 100% OW | 75% OW + 25% W | 50% OW + 50% W | |
|---|---|---|---|---|---|
| pH | 8.49 ± 0.45 a | 7.99 ± 0.44 a | 7.73 ± 0.62 a | 7.93 ± 0.56 a | 7.89 ± 0.57 a |
| EC | 1.18 ± 0.41 a | 3.40 ± 0.43 b | 3.32 ± 0.17 b | 2.86 ± 0.20 ab | 2.42 ± 0.28 ab |
| NO3− | 4.03 ± 0.37 a | 0.21 ± 0.10 a | 144.08 ± 51.03 b | 118.01 ± 16.38 b | 82.10 ± 22.72 ab |
| NH4+ | 0 a | 110.49 ± 7.09 b | 4.54 ± 2.78 a | 1.86 ± 0.04 a | 0.58 ± 0.73 a |
| N (NO3− + NH4+) | 0.91 a | 85.98 b | 36.06 ab | 28.09 ab | 18.99 a |
| PO43− | 0.00 a | 24.01 ± 0.18 d | 14.14 ± 4.03 c | 10.55 ± 2.73 bc | 7.11 ± 1.52 b |
| K+ | 2.41 ± 0.10 a | 37.57 ± 3.72 b | 36.83 ± 8.38 b | 28.27 ± 6.21 b | 29.71 ± 10.67 b |
| Ca2+ | 39.22 ± 2.39 a | 106.90 ± 28.08 b | 119.16 ± 15.38 b | 105.03 ± 19.79 b | 90.82 ± 22.72 b |
| Mg2+ | 18.28 ± 2.01 a | 62.38 ± 21.86 b | 71.93 ± 13.59 b | 62.18 ± 16.07 b | 53.09 ± 17.57 b |
| SO42− | 20.71 ± 2.51 a | 125.13 ± 22.43 b | 178.43 ± 58.86 b | 141.64 ± 51.03 b | 108.39 ± 44.22 ab |
| Cl− | 220.83 ± 20.03 a | 564.15 ± 138.56 b | 667.56 ± 52.95 b | 585.94 ± 81.27 b | 518.17 ± 125.78 b |
| Na+ | 109.99 ± 9.50 a | 394.02 ± 117.88 b | 442.35 ± 65.73 b | 369.85 ± 66.71 b | 302.94 ± 70.80 b |
| Tap Water | 100% IW | 100% OW | 75% OW + 25% W | 50% OW + 50% W | |
|---|---|---|---|---|---|
| pH | 8.50 ± 0.00 a | 7.70 ± 0.28 a | 8.12 ± 0.42 a | 7.95 ± 0.35 a | 8.15 ± 0.07 a |
| EC | 1.26 ± 0.16 a | 2.80 ± 0.06 b | 3.15 ± 0.84 c | 2.65 ± 0.54 b | 2.4 ± 0.00 b |
| NO3− | 4.68 ± 0.38 a | 3.20 ± 2.26 a | 47.06 ± 3.62 b | 39.83 ± 3.66 b | 24.47 ± 1.77 ab |
| NH4+ | 0.00 a | 131.76 ± 8.29 c | 42.25 ± 9.74 b | 31.11 ± 7.06 b | 19.25 ± 4.07 ab |
| N (NO3− + NH4+) | 1.05 a | 103.20 b | 43.48 ab | 33.18 ab | 20.50 a |
| PO43− | 0.00 a | 34.40 ± 0.79 b | 14.66 ± 5.50 b | 11.49 ± 3.16 b | 6.77 ± 2.67 a |
| K+ | 2.73 ± 0.11 a | 39.19 ± 1.30 b | 43.57 ± 10.91 b | 34.75 ± 7.65 b | 21.79 ± 5.32 b |
| Ca2+ | 48.74 ± 3.40 a | 70.95 ± 0.47 a | 83.94 ± 4.80 a | 75.66 ± 2.54 a | 65.40 ± 1.07 a |
| Mg2+ | 24.60 ± 2.47 a | 33.61 ± 0.02 a | 47.08 ± 8.66 a | 41.32 ± 5.83 a | 35.20 ± 2.73 a |
| SO42− | 27.55 ± 3.00 a | 59.74 ± 3.15 ab | 108.50 ± 10.55 b | 88.67 ± 6.31 b | 65.63 ± 3.80 ab |
| Cl− | 274.52 ± 24.15 a | 402.38 ± 1.96 ab | 632.93 ± 206.62 b | 541.30 ± 148.50 b | 443.12 ± 85.54 ab |
| Na+ | 131.33 ± 10.50 a | 242.63 ± 0.91 a | 396.65 ± 141.01 b | 328.71 ± 102.63 b | 256.24 ± 62.32 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rápalo-Cruz, A.; Gómez-Serrano, C.; González-López, C.V.; Hassanpouraghdam, M.B.; Jiménez-Becker, S. Alterations in the Growth Responses of Pelargonium × hortorum Irrigated with Microalgae Production Wastewater. Horticulturae 2024, 10, 921. https://doi.org/10.3390/horticulturae10090921
Rápalo-Cruz A, Gómez-Serrano C, González-López CV, Hassanpouraghdam MB, Jiménez-Becker S. Alterations in the Growth Responses of Pelargonium × hortorum Irrigated with Microalgae Production Wastewater. Horticulturae. 2024; 10(9):921. https://doi.org/10.3390/horticulturae10090921
Chicago/Turabian StyleRápalo-Cruz, Alejandro, Cintia Gómez-Serrano, Cynthia Victoria González-López, Mohammad Bagher Hassanpouraghdam, and Silvia Jiménez-Becker. 2024. "Alterations in the Growth Responses of Pelargonium × hortorum Irrigated with Microalgae Production Wastewater" Horticulturae 10, no. 9: 921. https://doi.org/10.3390/horticulturae10090921
APA StyleRápalo-Cruz, A., Gómez-Serrano, C., González-López, C. V., Hassanpouraghdam, M. B., & Jiménez-Becker, S. (2024). Alterations in the Growth Responses of Pelargonium × hortorum Irrigated with Microalgae Production Wastewater. Horticulturae, 10(9), 921. https://doi.org/10.3390/horticulturae10090921







