Impact of CO2 Enrichment on Growth, Yield and Fruit Quality of F1 Hybrid Strawberry Grown under Controlled Greenhouse Condition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Greenhouse Setup
2.2. Carbon Dioxide Injection System
2.3. Vegetative Growth
2.4. Yield and Its Component
2.5. Vitamin C
2.6. Titratable Acidity (TA)
2.7. Total Soluble Solids (TSS)
2.8. Nutrient Analysis
2.9. Leaf Gas Exchange Properties
2.10. Chlorophyll Traits
2.11. Statistical Analysis
3. Results and Discussion
3.1. Vegetative Growth Traits
3.2. Photosynthesis and Chlorophyll Traits
3.3. Fruit Yield
3.4. Fruit Quality
3.5. Macronutrient Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vågsholm, I.; Arzoomand, N.S.; Boqvist, S. Food security, safety, and sustainability—Getting the trade-offs right. Front. Sustain. Food Syst. 2020, 4, 16. [Google Scholar] [CrossRef]
- World Food and Agriculture. Statistical Yearbook. 2021; Available online: https://openknowledge.fao.org/handle/20.500.14283/cb4477en (accessed on 1 December 2021).
- Goddek, S.; Körner, O.; Keesman, K.J.; Tester, M.A.; Lefers, R.; Fleskens, L.; Joyce, A.; van Os, E.; Gross, A.; Leemans, R. How greenhouse horticulture in arid regions can contribute to climate-resilient and sustainable food security. Glob. Food Secur. 2023, 38, 100701. [Google Scholar] [CrossRef]
- Ghoulem, M.; El Moueddeb, K.; Nehdi, E.; Boukhanouf, R.; Calautit, J.K. Greenhouse design and cooling technologies for sustainable food cultivation in hot climates: Review of current practice and future status. Biosyst. Eng. 2019, 183, 121–150. [Google Scholar] [CrossRef]
- Ministry of Environment, Water and Agriculture. Census Book; Ministry of Environment, Water and Agriculture: Riyadh, Saudi Arabia. Available online: https://www.mewa.gov.sa/en/Pages/default.aspx (accessed on 19 February 2020).
- Ouammi, A.; Achour, Y.; Dagdougui, H.; Zejli, D. Optimal operation scheduling for a smart greenhouse integrated microgrid. Energy Sustain. Dev. 2020, 58, 129–137. [Google Scholar] [CrossRef]
- Cuce, P.M.; Riffat, S. A state of the art review of evaporative cooling systems for building applications. Renew. Sustain. Energy Rev. 2016, 54, 1240–1249. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Physiological Ecology; Springer: Berlin/Heidelberg, Germany, 2008; Volume 2. [Google Scholar]
- Achour, Y.; Ouammi, A.; Zejli, D. Technological progresses in modern sustainable greenhouses cultivation as the path towards precision agriculture. Renew. Sustain. Energy Rev. 2021, 147, 111251. [Google Scholar] [CrossRef]
- Poudel, M.; Dunn, B. Greenhouse Carbon Dioxide Supplementation; Oklahoma Cooperative Extension Service: Oklahoma City, OK, USA, 2017. [Google Scholar]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M.; Miller, H.L.; IPCC [Intergovernmental Panel on Climate Change]. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK, 2007; Volume 499–587, pp. 434–497. [Google Scholar]
- Rangaswamy, T.C.; Sridhara, S.; Ramesh, N.; Gopakkali, P.; El-Ansary, D.O.; Mahmoud, E.A.; Abdelmohsen, S.A.; Abdelbacki, A.M.; Elansary, H.O.; Abdel-Hamid, A.M.J.P. Assessing the impact of higher levels of CO2 and temperature and their interactions on tomato (Solanum lycopersicum L.). Plants 2021, 10, 256. [Google Scholar] [CrossRef]
- Balasooriya, H.N.; Dassanayake, K.B.; Seneweera, S.; Ajlouni, S. Interaction of elevated carbon dioxide and temperature on strawberry (Fragaria× ananassa) growth and fruit yield. Int. J. Agric. Biosyst. Eng. 2018, 12, 279–287. [Google Scholar]
- Kawashima, H.; Takaichi, M.; BaBa, M.; Yasui, K.; Nakano, Y. Effects of energy saving and the reduction of carbon dioxide emissions with a hybrid-heating system using an air-to-air heat pump for greenhouse heating. Bull. Natl. Inst. Veg. Tea Sci. 2008, 7, 27–36. [Google Scholar]
- Gruda, N.; Tanny, J. Protected crops. In Horticulture: Plants for People and Places; Springer: Berlin/Heidelberg, Germany, 2014; Volume 1, pp. 327–405. [Google Scholar]
- Dong, J.; Gruda, N.; Lam, S.K.; Li, X.; Duan, Z. Effects of Elevated CO2 on Nutritional Quality of Vegetables: A Review. Front. Plant Sci. 2018, 9, 924. [Google Scholar] [CrossRef]
- Hidaka, K.; Nakahara, S.; Yasutake, D.; Zhang, Y.; Okayasu, T.; Dan, K.; Kitano, M.; Sone, K. Crop-local CO2 enrichment improves strawberry yield and fuel use efficiency in protected cultivations. Sci. Hortic. 2022, 301, 111104. [Google Scholar] [CrossRef]
- Merrill, B.F.; Lu, N.; Yamaguchi, T.; Takagaki, M.; Maruo, T.; Kozai, T.; Yamori, W. Next evolution of agriculture: A review of innovations in plant factories. In Handbook of Photosynthesis; CRC Press: Boca Raton, FL, USA, 2018; pp. 723–740. [Google Scholar]
- Noctor, G.; Mhamdi, A. Climate change, CO2, and defense: The metabolic, redox, and signaling perspectives. Trends Plant Sci. 2017, 22, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, L. Effects of elevated CO2 concentrations on growth and yield of eight vegetable species in a cool climate. Sci. Hortic. 1994, 58, 177–185. [Google Scholar] [CrossRef]
- Pérez-López, U.; Miranda-Apodaca, J.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. Growth and nutritional quality improvement in two differently pigmented lettuce cultivars grown under elevated CO2 and/or salinity. Sci. Hortic. 2015, 195, 56–66. [Google Scholar] [CrossRef]
- Sánchez-Guerrero, M.; Lorenzo, P.; Medrano, E.; Castilla, N.; Soriano, T.; Baille, A. Effect of variable CO2 enrichment on greenhouse production in mild winter climates. Agric. For. Meteorol. 2005, 132, 244–252. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Zhang, M.; Zhang, Y.; Wang, Q. Effect of carbon dioxide enrichment on health-promoting compounds and organoleptic properties of tomato fruits grown in greenhouse. Food Chem. 2014, 153, 157–163. [Google Scholar] [CrossRef]
- Fan, X.; Cao, X.; Zhou, H.; Hao, L.; Dong, W.; He, C.; Xu, M.; Wu, H.; Wang, L.; Chang, Z.; et al. Carbon dioxide fertilization effect on plant growth under soil water stress associates with changes in stomatal traits, leaf photosynthesis, and foliar nitrogen of bell pepper (Capsicum annuum L.). Environ. Exp. Bot. 2020, 179, 104203. [Google Scholar] [CrossRef]
- Holley, J.; Mattson, N.; Ashenafi, E.; Nyman, M. The Impact of CO2 Enrichment on Biomass, Carotenoids, Xanthophyll, and Mineral Content of Lettuce (Lactuca sativa L.). Horticulturae 2022, 8, 820. [Google Scholar] [CrossRef]
- Tagawa, A.; Ehara, M.; Ito, Y.; Araki, T.; Ozaki, Y.; Shishido, Y. Effects of CO2 Enrichment on Yield, Photosynthetic Rate, Translocation and Distribution of Photoassimilates in Strawberry ‘Sagahonoka’. Agronomy 2022, 12, 473. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Murakami, S.; Kobayashi, T.; Worarad, K.; Yonezu, Y.; Umeda, H.; Okayama, T.; Inoue, E. Local CO2 Application within Strawberry Plant Canopy Increased Dry Matter Production and Fruit Yield in Summer and Autumn Culture. Int. J. Fruit Sci. 2022, 22, 675–685. [Google Scholar] [CrossRef]
- Dong, J.; Gruda, N.; Li, X.; Tang, Y.; Zhang, P.; Duan, Z. Sustainable vegetable production under changing climate: The impact of elevated CO2 on yield of vegetables and the interactions with environments—A review. J. Clean. Prod. 2019, 253, 119920. [Google Scholar] [CrossRef]
- Allen, L.; Kimball, B.; Bunce, J.; Yoshimoto, M.; Harazono, Y.; Baker, J.; Boote, K.; White, J.J.A.; Meteorology, F. Fluctuations of CO2 in Free-Air CO2 Enrichment (FACE) depress plant photosynthesis, growth, and yield. Agric. For. Meteorol. 2020, 284, 107899. [Google Scholar] [CrossRef]
- Vermeulen, P. Alternative sources of CO2 for the greenhouse horticulture. In Proceedings of the 2nd International Symposium Energy Challenges Mechanics (ISECM2), Aberdeen, UK, 18–20 August 2014; pp. 19–21. [Google Scholar]
- Kuroyanagi, T. History of carbon dioxide application in greenhouses and engineering approach for efficient application. Agric. Hortic. 2014, 89, 143. [Google Scholar]
- Karim, M.F.; Hao, P.; Nordin, N.H.B.; Qiu, C.; Zeeshan, M.; Khan, A.A.; Shamsi, I.H. Effects of CO2 enrichment by fermentation of CRAM on growth, yield and physiological traits of cherry tomato. Saudi J. Biol. Sci. 2020, 27, 1041. [Google Scholar]
- Henz, G.P. Desafios enfrentados por agricultores familiares na produção de morango no Distrito Federal. Hortic. Bras. 2010, 28, 260–265. [Google Scholar]
- Al-Khayri, J.M.; Islam, R. Genetic Improvement of Strawberry (Fragaria× ananassa Duchesne). Adv. Plant Breed. Strateg. Fruits 2018, 3, 217–275. [Google Scholar]
- World Food and Agriculture. Statistical Yearbook. 2019. Available online: https://openknowledge.fao.org/handle/20.500.14283/ca6463en (accessed on 1 December 2020).
- Cervantes, L.; Ariza, M.T.; Miranda, L.; Lozano, D.; Medina, J.J.; Soria, C.; Martínez-Ferri, E. Stability of Fruit Quality Traits of Different Strawberry Varieties under Variable Environmental Conditions. Agronomy 2020, 10, 1242. [Google Scholar] [CrossRef]
- Bentvelsen, G.; Van Der Vange, A. Advances in F1 hybrid day-neutral strawberry breeding at ABZ Seeds, Andijk, The Netherlands. In Proceedings of the IX International Strawberry Symposium, Rimini, Italy, 1–5 May 2021; Volume 1309, pp. 241–246. [Google Scholar]
- Sonneveld, C.; Voogt, W. Plant Nutrition of Greenhouse Crops; Springer: Dordrecht, The Netherlands, 2009; pp. 393–403. [Google Scholar]
- Baur, F.J.; Ensminger, L. Association of Official Analytical Chemists (AOAC) Methods. J. Am. Oil Chem. Soc. 1977, 54, 171–172. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C. Nitrogen total. Methods Soil Anal. 1982, 9, 595–624. [Google Scholar]
- Jones, J.B. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Smith, J.H.; Benitez, A. Chlorophylls: Analysis in plant materials. Mod. Methods Plant Anal. 1955, 4, 142–196. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics; McGraw-Hill Book, Co.: New York, NY, USA, 1960; p. 481. [Google Scholar]
- Hong, T.; Cai, Z.; Li, R.; Liu, J.; Li, J.; Wang, Z.; Zhang, Z. Effects of water and nitrogen coupling on watermelon growth, photosynthesis and yield under CO2 enrichment. Agric. Water Manag. 2021, 259, 107229. [Google Scholar] [CrossRef]
- Chen, D.; Mei, Y.; Liu, Q.; Wu, Y.; Yang, Z.J.A.J. Carbon dioxide enrichment promoted the growth, yield, and light-use efficiency of lettuce in a plant factory with artificial lighting. Agron. J. 2021, 113, 5196–5206. [Google Scholar] [CrossRef]
- Jones, H. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology; Cambridge University Press: Cambridge, UK, 2013; pp. 1–407. [Google Scholar] [CrossRef]
- Reddy, A.R.; Rasineni, G.K.; Raghavendra, A.S. The impact of global elevated CO2 concentration on photosynthesis and plant productivity. Curr. Sci. 2010, 99, 46–57. [Google Scholar]
- Jiang, Q.; Zhang, J.; Xu, X.; Liu, G.; Zhu, J. Effects of free-air CO2 enrichment (FACE) and nitrogen (N) supply on N uptake and utilization of indica and japonica cultivars (Oryza sativa L.). Ecol. Process. 2020, 9, 1–12. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Kumar, S.; Mishra, J.S.; Prakash, V.; Rao, K.K.; Bhatt, B.P.; Srivastava, A.K. Interactive effect of elevated [CO2] and temperature on the photosynthetic process, anti-oxidative properties, and grain yield of rice. J. Agron. Crop Sci. 2022, 208, 384–393. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhong, Y.; Blennow, A.; Liang, K.; Liu, F. Effects of elevated CO2 on grain yield and quality in five wheat cultivars. J. Agron. Crop. Sci. 2022, 208, 733–745. [Google Scholar] [CrossRef]
- Ullah, I.; Demirsoy, H.; Soysal, D.; Lizalo, A.; Doğan, D.E.; Demirsoy, L. Evaluation of Strawberry Cultivars Based on Growth-Related Attributes. Appl. Fruit Sci. 2024, 66, 431–439. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Hidaka, K.; Okayasu, T.; Yasutake, D.; Kitano, M. Effects of Local CO2 Enrichment on Strawberry Cultivation during the Winter Season. Environ. Control. Biol. 2017, 55, 165–170. [Google Scholar] [CrossRef]
- Zhang, N.; Berman, S.R.; Joubert, D.; Vialet-Chabrand, S.; Marcelis, L.F.M.; Kaiser, E. Variation of Photosynthetic Induction in Major Horticultural Crops Is Mostly Driven by Differences in Stomatal Traits. Front. Plant Sci. 2022, 13, 860229. [Google Scholar] [CrossRef]
- Acevedo-Siaca, L.G.; Coe, R.; Wang, Y.; Kromdijk, J.; Quick, W.P.; Long, S.P. Variation in photosynthetic induction between rice accessions and its potential for improving productivity. New Phytol. 2020, 227, 1097–1108. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, J.; Zhang, Z.; Lu, Y.; Zhang, H.; Hu, J. A high efficiency CO2 concentration interval optimization method for lettuce growth. Sci. Total Environ. 2023, 879, 162731. [Google Scholar] [CrossRef]
- Sun, P.; Mantri, N.; Lou, H.; Hu, Y.; Sun, D.; Zhu, Y.; Dong, T.; Lu, H. Effects of Elevated CO2 and Temperature on Yield and Fruit Quality of Strawberry (Fragaria × ananassa Duch.) at Two Levels of Nitrogen Application. PLoS ONE 2012, 7, e41000. [Google Scholar] [CrossRef] [PubMed]
- Miri, H.R.; Rastegar, A.; Bagheri, A.R. The impact of elevated CO2 on growth and competitiveness of C3 and C4 crops and weeds. Eur. J. Exp. Biol. 2012, 2, 1144–1150. [Google Scholar]
- Nilsen, S.; Hovland, K.; Dons, C.; Sletten, S.P. Effect of CO2 enrichment on photosynthesis, growth and yield of tomato. Sci. Hortic. 1983, 20, 1–14. [Google Scholar] [CrossRef]
- Dabu, X.; Li, S.; Cai, Z.; Ge, T.; Hai, M. The effect of potassium on photosynthetic acclimation in cucumber during CO2 enrichment. Photosynthetica 2019, 57, 640–645. [Google Scholar] [CrossRef]
- Giri, A.; Armstrong, B.; Rajashekar, C.B. Elevated Carbon Dioxide Level Suppresses Nutritional Quality of Lettuce and Spinach. Am. J. Plant Sci. 2016, 7, 246–258. [Google Scholar] [CrossRef]
- Keutgen, N.; Chen, K.; Lenz, F. Responses of strawberry leaf photosynthesis, chlorophyll fluorescence and macronutrient contents to elevated CO2. J. Plant Physiol. 1997, 150, 395–400. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, F.; Hao, L.; Yu, J.; Guo, L.; Zhou, H.; Ma, C.; Zhang, X.; Xu, M. Elevated CO2 concentration induces photosynthetic down-regulation with changes in leaf structure, non-structural carbohydrates and nitrogen content of soybean. BMC Plant Biol. 2019, 19, 255. [Google Scholar] [CrossRef] [PubMed]
- Kimball, B.A. Crop responses to elevated CO2 and interactions with H2O, N, and temperature. Curr. Opin. Plant Biol. 2016, 31, 36–43. [Google Scholar] [CrossRef]
- Lavanya, C.; Ashoka, J.; Sreenivasa, A.; Nadagoud, S.; Beladhadi, B. Effect of Elevated Carbon Dioxide and Temperature on Growth, Yield and Quality Parameters of Mulberry. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3351–3356. [Google Scholar] [CrossRef]
- Doddrell, N.H.; Lawson, T.; A Raines, C.; Wagstaff, C.; Simkin, A.J. Feeding the world: Impacts of elevated [CO2] on nutrient content of greenhouse grown fruit crops and options for future yield gains. Hortic. Res. 2023, 10, uhad026. [Google Scholar] [CrossRef]
- Takahashi, Y.; Satoh, T.; Ibe, A.; Shibasaki, N.; Nomizu, K.; Ito, M. Effect of CO2 gas application to the strawberry for green house forcing culture in Niigata region. Bull. Fac. Agric. 2006, 58, 97–102. [Google Scholar]
- Jan, S.; Baba, J.; Sharma, M.; Wani, T.; Nisar, S.; Angmo, T.; Din, S.; Kossar, S. Evaluation of Different Strawberry Cultivars for Growth, Yield and Quality Characters under Temperate Conditions of North Western Himalayas. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 837–844. [Google Scholar]
- Antunes, L.E.C.; Ristow, N.C.; Krolow, A.C.R.; Carpenedo, S.; Júnior, C.R. Yield and quality of strawberry cultivars. Hortic. Bras. 2010, 28, 222–226. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. RISING ATMOSPHERIC CARBON DIOXIDE: Plants FACE the Future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Sun, B.; Zhang, S.; Zhang, Y.; Liao, Y.; Zhou, Y.; Xia, X.; Shi, K.; Yu, J. Stimulated Leaf Dark Respiration in Tomato in an Elevated Carbon Dioxide Atmosphere. Sci. Rep. 2013, 3, 3433. [Google Scholar] [CrossRef]
- Idso, S.B.; Kimball, B.A.; Shaw, P.E.; Widmer, W.; Vanderslice, J.T.; Higgs, D.J.; Montanari, A.; Clark, W. The effect of elevated atmospheric CO2 on the vitamin C concentration of (sour) orange juice. Agric. Ecosyst. Environ. 2002, 90, 1–7. [Google Scholar] [CrossRef]
- Wang, S.; Bunce, J. Elevated carbon dioxide affects fruit flavor in field-grown strawberries (Fragaria × ananassa Duch). J. Sci. Food Agric. 2004, 84, 1464–1468. [Google Scholar] [CrossRef]
- Robinson, J.M.; Sicher, R.C. Antioxidant levels decrease in primary leaves of barley during growth at ambient and elevated carbon dioxide levels. Int. J. Plant Sci. 2004, 165, 965–972. [Google Scholar] [CrossRef]
- Högy, P.; Fangmeier, A. Atmospheric CO2 enrichment affects potatoes: 2. Tuber quality traits. Eur. J. Agron. 2008, 30, 85–94. [Google Scholar] [CrossRef]
- Khan, I.; Azam, A.; Mahmood, A. The impact of enhanced atmospheric carbon dioxide on yield, proximate composition, elemental concentration, fatty acid and vitamin C contents of tomato (Lycopersicon esculentum). Environ. Monit. Assess. 2012, 185, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin C Content in Fruits: Biosynthesis and Regulation. Front. Plant Sci. 2019, 9, 2006. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, B.; Gilna, B.; Zhang, Y.; Zhu, C.; Ma, H.; Pang, J.; Chen, G.; Zhu, J. Elevated CO 2 effects on nutrient competition between a C 3 crop (Oryza sativa L.) and a C 4 weed (Echinochloa crusgalli L.). Nutr. Cycl. Agroecosyst. 2011, 89, 93–104. [Google Scholar] [CrossRef]
- Gojon, A.; Cassan, O.; Bach, L.; Lejay, L.; Martin, A. The decline of plant mineral nutrition under rising CO2: Physiological and molecular aspects of a bad deal. Trends Plant Sci. 2023, 28, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Jenny, H. The Soil Resource: Original and Behavior (Ecological Studies) 37; Springer-Verlag: Berlin/Heidelberg, Germany, 1980. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Taub, D.R.; Wang, X. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J. Integr. Plant Biol. 2008, 50, 1365–1374. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Rubio-Asensio, J.S.; Cousins, A.B. Carbon Dioxide Enrichment Inhibits Nitrate Assimilation in Wheat and Arabidopsis. Science 2010, 328, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Houlton, B.Z.; Marklein, A.R.; Liu, J.; Zhou, G. Plant stoichiometric responses to elevated CO2 vary with nitrogen and phosphorus inputs: Evidence from a global-scale meta-analysis. Sci. Rep. 2015, 5, 18225. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, H. Alteration in biochemical constituents and nutrients partitioning of Asparagus racemosus in response to elevated atmospheric CO2 concentration. Environ. Sci. Pollut. Res. 2021, 29, 6812–6821. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, R.; Mackowiak, C.; Stutte, G.; Yorio, N.; Berry, W. Effect of elevated carbon dioxide on nutritional quality of tomato. Adv. Space Res. 1997, 20, 1975–1978. [Google Scholar] [CrossRef]
- Loladze, I. Rising atmospheric CO2 and human nutrition: Toward globally imbalanced plant stoichiometry? Trends Ecol. Evol. 2002, 17, 457–461. [Google Scholar] [CrossRef]
- Finzi, A.C.; Norby, R.J.; Calfapietra, C.; Gallet-Budynek, A.; Gielen, B.; Holmes, W.E.; Hoosbeek, M.R.; Iversen, C.M.; Jackson, R.B.; Kubiske, M.E.; et al. Increases in nitrogen uptake rather than nitrogen-use efficiency support higher rates of temperate forest productivity under elevated CO2. Proc. Natl. Acad. Sci. USA 2007, 104, 14014–14019. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Li, P.; Han, X.; Norton, R.; Lam, S.; Zong, Y.; Sun, M.; Lin, E.; Gao, Z. Effects of free-air CO2 enrichment (FACE) on N, P and K uptake of soybean in northern China. Agric. For. Meteorol. 2016, 218-219, 261–266. [Google Scholar] [CrossRef]
- Li, P.; Han, X.; Zong, Y.; Li, H.; Lin, E.; Han, Y.; Hao, X. Effects of free-air CO2 enrichment (FACE) on the uptake and utilization of N, P and K in Vigna radiata. Agric. Ecosyst. Environ. 2015, 202, 120–125. [Google Scholar] [CrossRef]
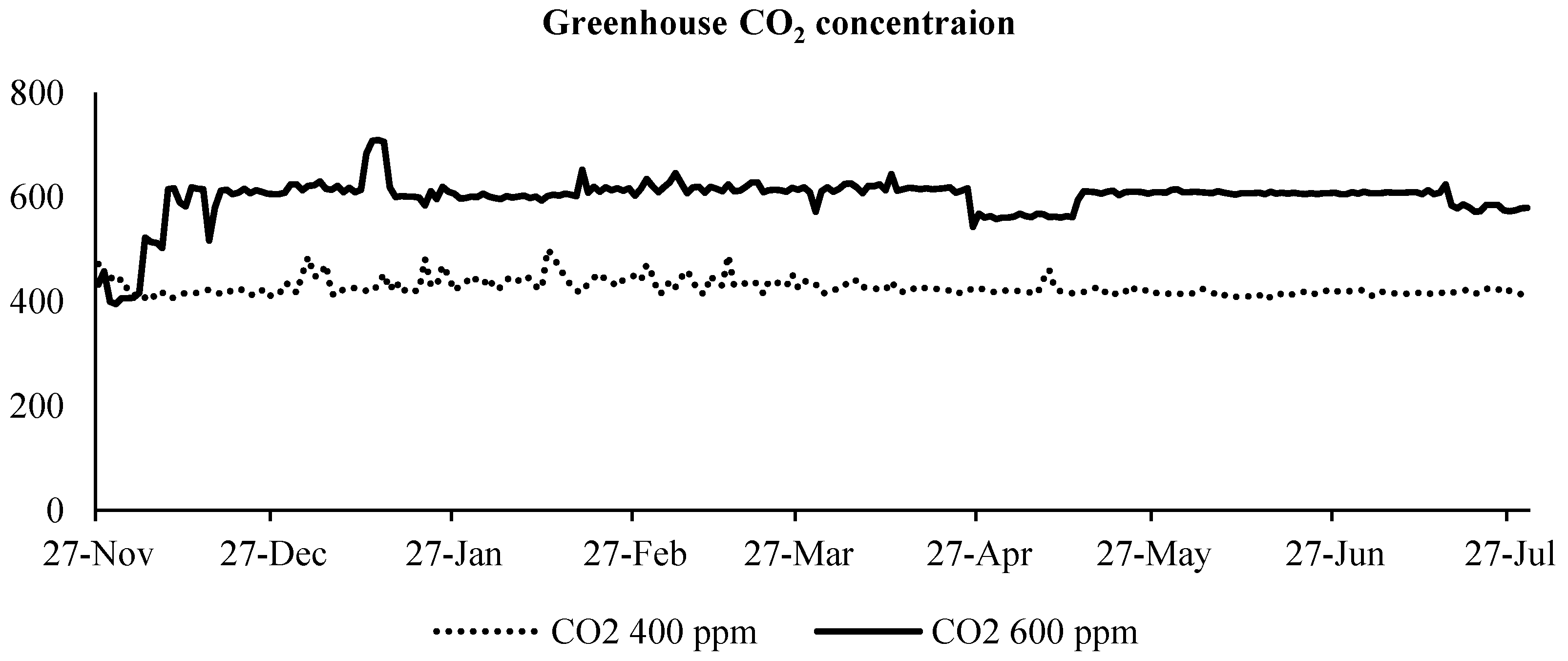

| CO2 (ppm) | Variety | Plant Height (cm) | No. of Crowns | Crown Diameter (mm) | Leaf Area (cm2) | No. of Leaves | Leaves FW (g) | Leaves DW (g) |
|---|---|---|---|---|---|---|---|---|
| 400 | 30.6 ± 0.61 a | 2.4 ± 0.21 b | 19.7 ± 0.80 b | 847 ± 89.19 b | 31.6 ± 1.12 b | 41.6 ± 3.27 b | 8.8 ± 0.64 b | |
| 600 | 31.5 ± 0.78 a | 3.3 ± 0.44 a | 25.1 ± 1.25 a | 1378 ± 199.13 a | 53.6 ± 2.74 a | 95.5 ± 9.92 a | 13.3 ± 0.95 a | |
| D | 29.7 ± 0.54 a | 2.2 ± 0.18 c | 19.8 ± 0.14 bc | 816 ± 58.34 cd | 29.2 ± 1.01 e | 42.8 ± 1.80 d | 9.1 ± 0.46 b | |
| 400 | E | 30.4 ± 1.24 a | 2.9 ± 0.44 b | 17.0 ± 0.00 c | 1005 ± 261.29 c | 34.7 ± 2.40 d | 46.3 ± 9.82 d | 9.3 ± 2.02 b |
| S | 31.6 ± 1.36 a | 2.1 ± 0.29 c | 22.3 ± 0.80 b | 720 ± 53.61 d | 30.6 ± 0.70 de | 35.8 ± 0.57 d | 8.1 ± 0.32 b | |
| 600 | D | 29.1 ± 0.52 a | 2.9 ± 0.33 b | 23.1 ± 0.60 b | 2068 ± 144.53 a | 61.2 ± 1.4742 a | 90.2 ± 0.98 b | 15.6 ± 1.28 a |
| E | 31.5 ± 1.03 a | 3.5 ± 0.05 a | 22.0 ± 0.80 a | 745 ± 98.36 d | 44.3 ± 2.3643 c | 64.7 ± 2.02 c | 10.0 ± 0.70 b | |
| S | 33.9 0.56 a | 3.2 ± 0.44 a | 29.8 ± 0.54 a | 1321 ± 70.70 b | 55.7 ± 2.8591 b | 131.9 ± 4.87 a | 14.1 ± 0.95 a |
| CO2 | Variety | Pr (μmol. m−2. S−1) | Gs (mmol H2O.m−1. s−1) | Ci (μmol CO2.mol−1) | Tr (mmol H2O.m−1. s−1) | Chlorophyll a | Chlorophyll b | Chlorophyll (a+b) | Chlorophyll (a/b) |
|---|---|---|---|---|---|---|---|---|---|
| (ppm) | |||||||||
| 400 | 7.45 ± 0.62 b | 0.440 ± 0.03 a | 343 ± 4.61 b | 9.21 ± 0.31 a | 3.01 ± 0.01 a | 3.45 ± 0.16 a | 6.46 ± 0.15 a | 0.89 ± 0.05 a | |
| 600 | 17.11 ± 1.73 a | 0.446 ± 0.02 a | 493 ± 7.71 a | 9.82 ± 0.32 a | 2.96 ± 0.02 a | 3.85 ± 0.22 a | 6.81 ± 0.19 a | 0.78 ± 0.05 a | |
| 400 | D | 9.74 ± 0.40 b | 0.453 ± 0.09 a | 329 ± 7.43 d | 9.20 ± 0.93 b | 3.07 ± 0.33 a | 2.87 ± 0.04 b | 5.94 ± 0.04 b | 1.07 ± 0.02 a |
| E | 5.67 ± 0.16 c | 0.443 ± 0.05 a | 356 ± 2.16 c | 9.82 ± 0.31 ab | 2.99 ± 0.02 ab | 3.59 ± a 0.21 b | 6.59 ± 0.19 ab | 0.84± 0.06 ab | |
| S | 6.95 ± 0.36 c | 0.426 ± 0.02 b | 344 ± 2.29 cd | 8.63 ± 0.20 b | 2.97 ± 0.38 ab | 3.89 ± 0.09 a | 6.86 ± 0.8 a | 0.76 ±0.20 b | |
| 600 | D | 17.74 ± 1.07 a | 0.430 ± 0.37 b | 475 ±5.74 b | 9.83 ± 0.11 ab | 2.95 ± 0.03 b | 3.84 ± 0.35 ab | 6.48 ± 0.29 ab | 0.88 ± 0.08 ab |
| E | 17.21 ± 1.24 a | 0.466 ± 0.01 a | 482 ± 5.33 b | 10.71 ± 0.33 a | 2.91 ± 0.52 b | 4.26 ± 0.42 a | 7.17 ± 0.38 a | 0.70 ± 0.09 b | |
| S | 16.60 ± 0.90 a | 0.443 ± 0.26 a | 520 ± 7.81 a | 8.92 ± 0.22 b | 3.01 ± 0.04 ab | 3.47 ± 0.33 ab | 6.80 ± 0.32 ab | 0.78 ± 0.07 b |
| CO2 | Variety | Fruit Length (mm) | Fruit Diameter (mm) | Fruit FW (g) | No. Fruits (Plant) | Yield |
|---|---|---|---|---|---|---|
| (ppm) | (g/Plant) | |||||
| 400 | 37.1 ± 2.86 a | 26.3 ± 1.79 a | 19.2 ± 0.73 a | 34.9 ± 2.15 b | 678 ± 52.83 b | |
| 600 | 37.5 ± 2.51 a | 26.9 ± 1.90 a | 21.1 ± 1.53 a | 44.5 ± 2.55 a | 964 ± 114.34 a | |
| 400 | D | 41.1 ± 1.95 a | 29.8 ± 0.71 ab | 20.0 ± 0.68 bc | 37.6 ± 0.88 b | 750 ± 10.17 d |
| E | 26.3 ± 1.99 b | 19.4 ± 0.98 c | 16.8 ± 0.11 cd | 28.0 ± 3.79 c | 471 ± 5.25 f | |
| S | 44.0 ± 0.60 a | 30.1 ± 0.53 b | 20.7 ± 1.23 b | 39.3 ± 1.58 b | 813 ±11.84 c | |
| 600 | D | 39.9 ± 1.91 a | 29.3 ± 0.72 b | 22.8 ± 0.67 b | 46.8 ± 1.83 a | 1067 ± 24.86 b |
| E | 29.4 ± 4.18 b | 19.6 ± 0.83 c | 15.2 ± 0.63 d | 34.8 ± 2.09 b | 528 ± 4.04 e | |
| S | 43.3 ± 1.29 a | 31.9 ± 0.74 a | 25.0 ± 1.96 a | 51.9 ± 0.79 a | 1297 ± 27.13 a |
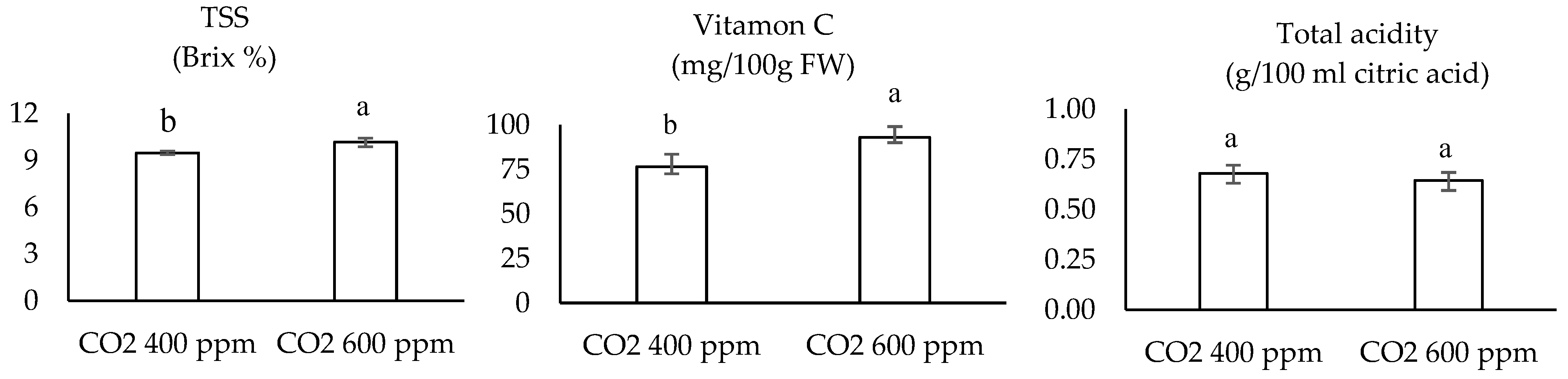
| CO2 (ppm) | Variety | TSS (Brix %) | Vitamin C (mg/100 g FW Fruit) | Total Acidity (gm/100 mL Citric Acid) |
|---|---|---|---|---|
| 400 | D | 9.533 ± 0.08 ab | 66.67 ± 1.09 d | 0.7067 ± 0.65 a |
| E | 8.733 ± 0.27 b | 82.93 ± 2.53 c | 0.71 ± 0.06 a | |
| S | 10.2 ± 0.36 a | 80.27 ± 1.18 c | 0.6233 ± 0.09 a | |
| 600 | D | 10.167 ± 0.52 a | 82.4 ± 1.28 c | 0.6267 ± 0.03 a |
| E | 10.067 ± 0.13 a | 102.8 ± 1.66 a | 0.6333 ± 0.02 a | |
| S | 10.3 ± 0.10 a | 94 ± 1.38 b | 0.6733 ± 0.02 a |
| CO2 (ppm) | Variety | N % | P % | K % | Ca % | Mg % |
|---|---|---|---|---|---|---|
| 400 | 2.79 ± 0.03 a | 0.98 ± 0.10 a | 0.60 ± 0.02 a | 1.94 ± 0.21 b | 0.55 ± 0.03 a | |
| 600 | 2.37 ± 0.02 a | 0.80 ± 0.06 a | 0.47 ± 0.03 a | 2.55 ± 0.23 a | 0.42 ± 0.02 a | |
| 400 | D | 2.81 ± 0.05 a | 0.92 ± 0.03 ab | 0.54± 0.04 b | 1.46 0.04 c | 0.51 ± 0.02 b |
| E | 2.75 ± 0.01 a | 1.03 ± 0.39 a | 0.62 ± 0.02 a | 2.36 ± 0.52 b | 0.64 ± 0.04 a | |
| S | 2.83 ± 0.04 a | 1.01± 0.03 a | 0.66 ± 0.04 a | 2.00 ± 0.09 b | 0.50 ± 0.04 b | |
| 600 | D | 2.39 ± 0.02 a | 0.65 ± 0.03 c | 0.39 ± 0.02 c | 1.86 ± 0.34 bc | 0.37 ± 0.04 c |
| E | 2.36 ± 0.10 b | 0.86 ± 0.02 b | 0.48 ± 0.06 ab | 2.94 ± 0.03 a | 0.48 ± 0.06 ab | |
| S | 2.38 ± 0.85 b | 0.91 ± 0.16 ab | 0.54 ± 0.04 b | 2.83 ± 0.07 ab | 0.43 ± 0.03 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, M.; Qaryouti, M.; Alharbi, S.; Alghamdi, B.; Al-Soqeer, A.; Alharbi, A.; Almutairi, K.; Abdelaziz, M.E. Impact of CO2 Enrichment on Growth, Yield and Fruit Quality of F1 Hybrid Strawberry Grown under Controlled Greenhouse Condition. Horticulturae 2024, 10, 941. https://doi.org/10.3390/horticulturae10090941
Osman M, Qaryouti M, Alharbi S, Alghamdi B, Al-Soqeer A, Alharbi A, Almutairi K, Abdelaziz ME. Impact of CO2 Enrichment on Growth, Yield and Fruit Quality of F1 Hybrid Strawberry Grown under Controlled Greenhouse Condition. Horticulturae. 2024; 10(9):941. https://doi.org/10.3390/horticulturae10090941
Chicago/Turabian StyleOsman, Mohamed, Muein Qaryouti, Saif Alharbi, Budour Alghamdi, Abdulrahman Al-Soqeer, Abdulaziz Alharbi, Khalid Almutairi, and Mohamed Ewis Abdelaziz. 2024. "Impact of CO2 Enrichment on Growth, Yield and Fruit Quality of F1 Hybrid Strawberry Grown under Controlled Greenhouse Condition" Horticulturae 10, no. 9: 941. https://doi.org/10.3390/horticulturae10090941






