Physiological Responses and Quality Alterations of Pea Sprouts under Salt Stress: Implications for Salt-Tolerant Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Salt Stress Effect on Pea Sprouts’ Seed Germination
2.2. Determination of Germination Index for Pea Sprouts Seeds
2.3. Effects of Salt Stress on the Growth and Development of Pea Seedlings
2.4. Determination of Morphological and Physiological Indicators of Pea Sprouts
2.5. Statistical Analysis
3. Results
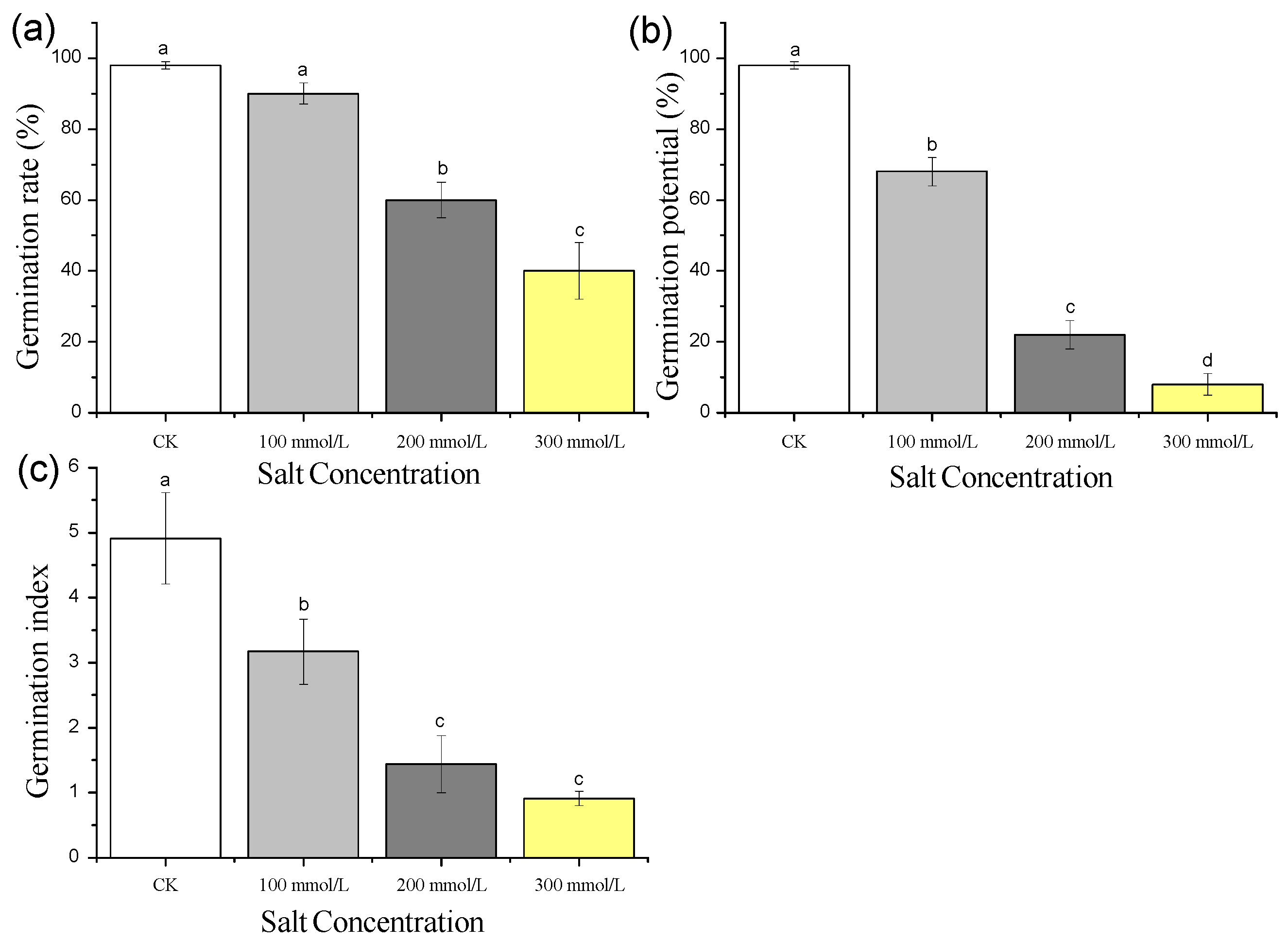
3.1. Effects of Different Concentrations of Salt Treatment on Pea Sprouts’ Seed Germination
3.2. Effects of Different Concentrations of Salt Treatment on Morphological and Physiological Indicators of Pea Sprouts
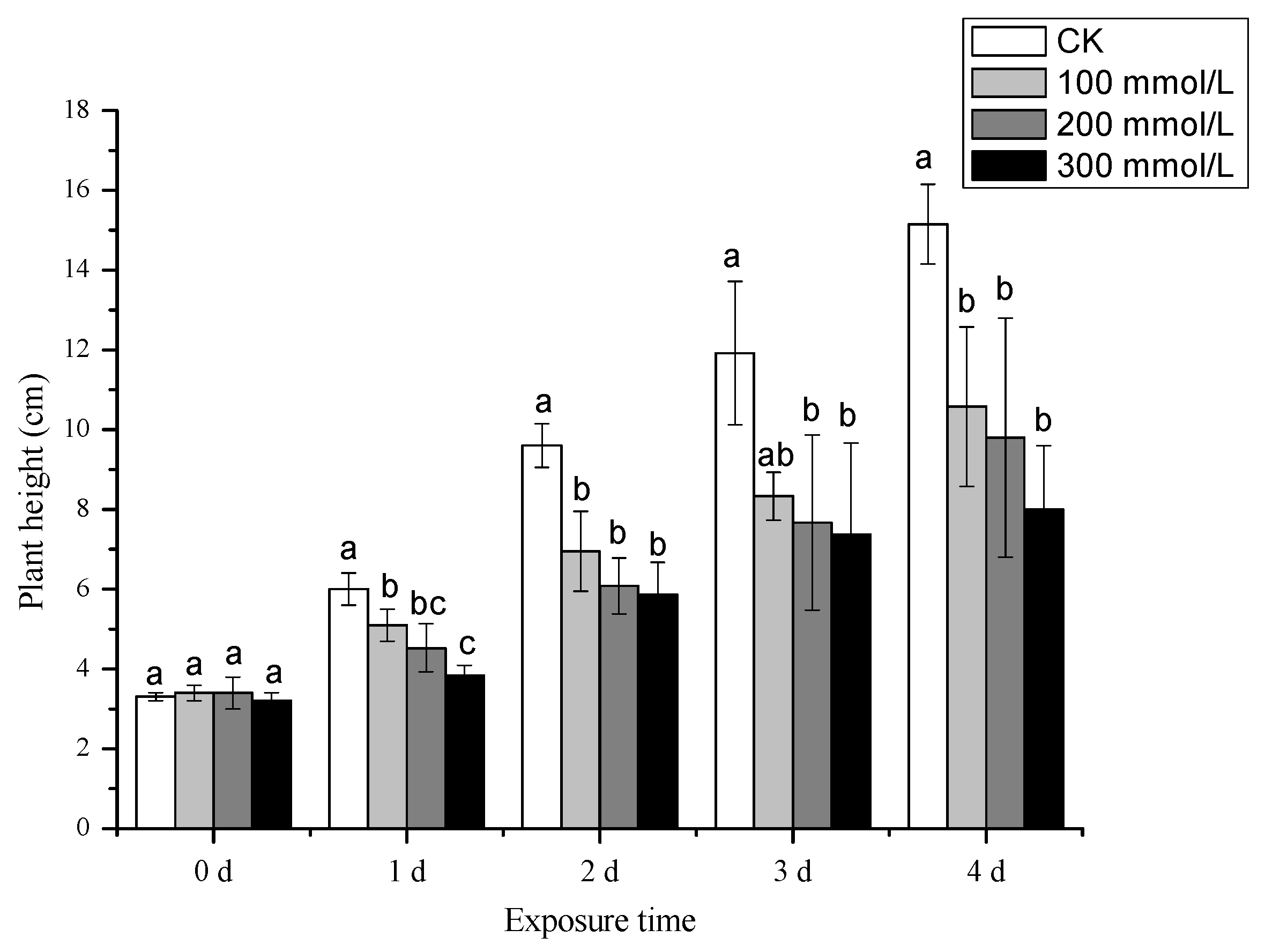
3.2.1. Effects of Salt Treatment Concentrations on Plant Height and Yield of Pea Sprouts
3.2.2. Effects of Salt Treatment Concentrations on Chlorophyll Content of Pea Sprouts
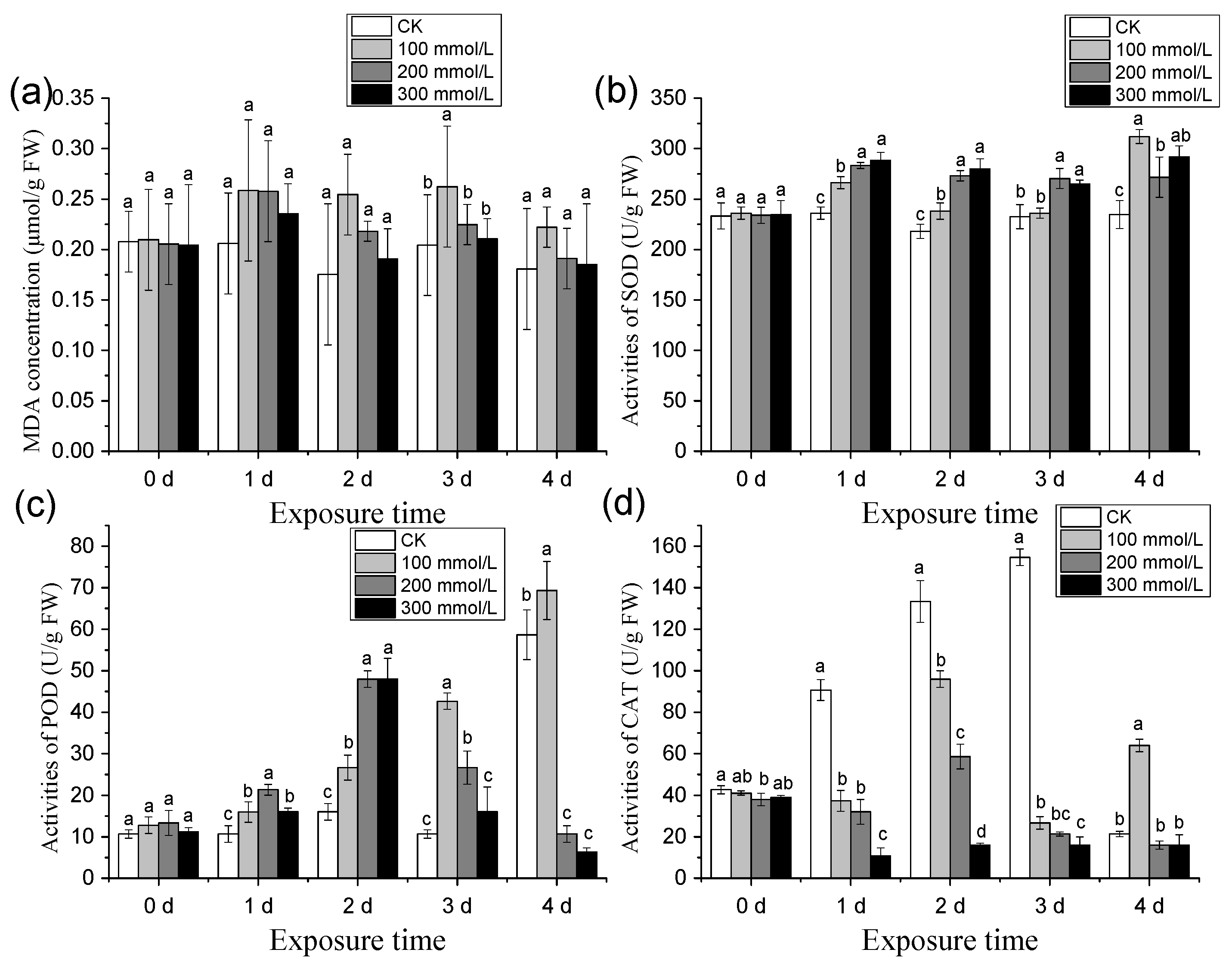
3.2.3. Effects of Different Salt Treatment Concentrations on the Relative Conductivity and MDA Content of Pea Sprouts
3.2.4. Effects of Different Salt Treatment Concentrations on SOD, POD, and CAT Activity of Pea Sprouts
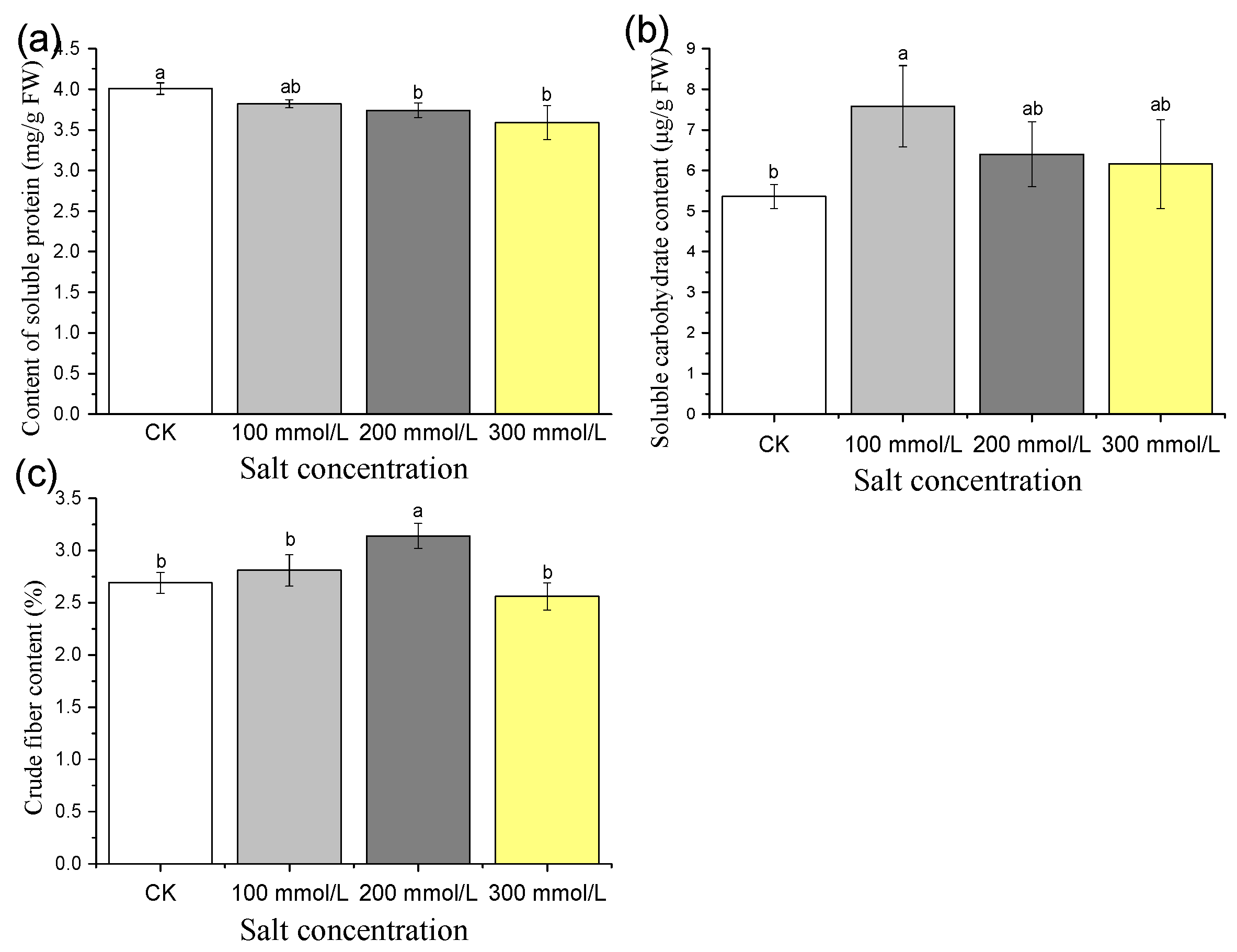
3.2.5. Short-Term Effects of Different Salt Treatment Concentrations on Soluble Protein, Soluble Sugar, and Crude Fiber Content of Pea Sprouts
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebert, A.W. Sprouts and microgreens—Novel food sources for healthy diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, Y.; Zhang, Z.; Fan, S.; Qi, Y.; Wang, F.; Wang, M.; Feng, M.; Liu, X.; Ren, H. Advance research on the pre-harvest sprouting trait in vegetable crop seeds. Int. J. Mol. Sci. 2023, 24, 17171. [Google Scholar] [CrossRef]
- Aloo, S.O.; Ofosu, F.K.; Kilonzi, S.M.; Shabbir, U.; Oh, D.H. Edible plant sprouts: Health benefits, trends, and opportunities for novel exploration. Nutrients 2021, 13, 2882. [Google Scholar] [CrossRef]
- Francis, H.; Debs, E.; Koubaa, M.; Alrayess, Z.; Maroun, R.G.; Louka, N. Sprouts use as functional foods. Optimization of germination of wheat (Triticum aestivum L.), alfalfa (Medicago sativa L.), and radish (Raphanus sativus L.) seeds based on their nutritional content evolution. Foods 2022, 11, 1460. [Google Scholar] [CrossRef]
- Vale, A.P.; Santos, J.; Brito, N.V.; Fernandes, D.; Rosa, E.; Beatriz, M.; Oliveira, P.P. Evaluating the impact of sprouting conditions on the glucosinolate content of Brassica oleracea sprouts. Phytochemistry 2015, 115, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Novidiyanto; Asrullah, M.; Arsanti Lestari, L.; Helmyati, S.; Farmawati, A. Effect supplementation of mung bean sprouts (Phaseolus radiatus L.) and vitamin e in rats fed high fat diet. KnE Life Sci. 2019, 4, 36. [Google Scholar]
- Khan, M.A.H.; Baset Mia, M.A.; Quddus, M.A.; Sarker, K.K.; Rahman, M.; Skalicky, M.; Brestic, M.; Gaber, A.; Alsuhaibani, A.M.; Hossain, A. Salinity-induced physiological changes in pea (Pisum sativum L.): Germination rate, biomass accumulation, relative water content, seedling vigor and salt tolerance index. Plants 2022, 11, 3493. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.B.; Alencar, N.L.M.; Enéas, G.-G. Comparison between the water and salt stress effects on plant growth and development. Responses Org. Water Stress 2013, 4, 67–94. [Google Scholar]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rathod, S.; Manohara, K.K.; Gireesh, C.; Anantha, M.S.; Sakhare, A.S.; Parmar, B.; Yadav, B.K.; Bandumula, N.; Raihan, F.; et al. Salt stress in plants and mitigation approaches. Plants 2022, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Bano, A.; Khan, N. Climate change and salinity effects on crops and chemical communication between plants and plant growth-promoting microorganisms under stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Poojan Yadav, S.; Bharadwaj, R.; Nayak, H.; Mahto, R.; Kumar Singh, R.; Kumar Prasad, S. Impact of salt stress on growth, productivity and physicochemical properties of plants: A review. Int. J. Chem. Stud. 2019, 7, 1793–1798. [Google Scholar]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Tarchoun, N.; Saadaoui, W.; Mezghani, N.; Pavli, O.I.; Falleh, H.; Petropoulos, S.A. The effects of salt stress on germination, seedling growth and biochemical responses of tunisian squash (cucurbita maxima duchesne) germplasm. Plants 2022, 11, 800. [Google Scholar] [CrossRef] [PubMed]
- Plessis, A. Abiotic stress experiments need a reality check to improve translation to the field. J. Exp. Bot. 2023, 74, 1741–1744. [Google Scholar] [CrossRef]
- Gul, Z.; Tang, Z.H.; Arif, M.; Ye, Z. An insight into abiotic stress and influx tolerance mechanisms in plants to cope in saline environments. Biology 2022, 11, 597. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Bioch. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Lu, Q.; Ge, G.T.; Sa, D.W.; Wang, Z.J.; Hou, M.L.; Jia, Y.S. Effects of salt stress levels on nutritional quality and microorganisms of alfalfa-influenced soil. PeerJ 2021, 9, e11729. [Google Scholar] [CrossRef]
- Trușcă, M.; Gâdea, Ș.; Vidican, R.; Stoian, V.; Vâtcă, A.; Balint, C.; Stoian, V.A.; Horvat, M.; Vâtcă, S. Exploring the research challenges and perspectives in ecophysiology of plants affected by salinity stress. Agriculture 2023, 13, 734. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Zeng, Y.; Xiang, L.; Lei, Z.; Huang, Q.; Li, T.; Shen, F.; Cheng, Q. A salt tolerance evaluation method for sunflower (Helianthus annuus L.) at the seed germination stage. Sci. Rep. 2020, 10, 10626. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, H.; Chen, Y.; Zhang, L.; Kudusi, K.; Song, J. Effects of drought and salt stress on seed germination of ephemeral plants in desert of northwest china. Front. Ecol. Evol. 2022, 10, 1026095. [Google Scholar] [CrossRef]
- Guo, J.; Du, M.; Tian, H.; Wang, B. Exposure to high salinity during seed development markedly enhances seedling emergence and fitness of the progeny of the extreme halophyte suaeda salsa. Front. Plant Sci. 2020, 11, 1291. [Google Scholar] [CrossRef] [PubMed]
- Muradoglu, F.; Gundogdu, M.; Ercisli, S.; Encu, T.; Balta, F.; Ze Jaafar, H.; Zia-Ul-Haq, M. Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol. Res. 2015, 48, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Peng, J.; Wang, J. Protective enzyme activity regulation in cotton (Gossypium hirsutum L.) in response to scirpus planiculmis stress. Front. Plant Sci. 2022, 13, 1068419. [Google Scholar] [CrossRef]
- Leonowicz, G.; Trzebuniak, K.F.; Zimak-Piekarczyk, P.; Ślesak, I.; Mysliwa-Kurdziel, B. The activity of superoxide dismutases (sods) at the early stages of wheat deetiolation. PLoS ONE 2018, 13, e0194678. [Google Scholar] [CrossRef]
- Talaat, N.B.; Nesiem, M.R.A.; Gadalla, E.G.; Ali, S.F. Gibberellic acid and salicylic acid dual application improves date palm fruit growth by regulating the nutrient acquisition, amino acid profile, and phytohormone performance. J. Soil. Sci. Plant Nutr. 2023, 23, 6216–6231. [Google Scholar] [CrossRef]
- Jacomassi, L.M.; Pacola, M.; Momesso, L.; Viveiros, J.; Júnior, O.A.; Siqueira, G.F.d.; Campos, M.d.; Crusciol, C.A.C. Foliar application of amino acids and nutrients as a tool to mitigate water stress and stabilize sugarcane yield and bioenergy generation. Plants 2024, 13, 461. [Google Scholar] [CrossRef]
- Hu, P.; Wang, L.; Hu, Z.; Jiang, L.; Hu, H.; Rao, Z.; Wu, L.; Tang, Z. Effects of multi-bacteria solid-state fermented diets with different crude fiber levels on growth performance, nutrient digestibility, and microbial flora of finishing pigs. Animals 2021, 11, 3079. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Plasma membrane permeability as an indicator of salt tolerance in plants. Biol. Plant. 2013, 57, 1–10. [Google Scholar] [CrossRef]
- Colin, L.; Ruhnow, F.; Zhu, J.K.; Zhao, C.; Zhao, Y.; Persson, S. The cell biology of primary cell walls during salt stress. Plant Cell 2023, 35, 201–217. [Google Scholar] [CrossRef]
- Pehlivan, N.; Sun, L.; Jarrett, P.; Yang, X.; Mishra, N.; Chen, L.; Kadioglu, A.; Shen, G.; Zhang, H. Co-overexpressing a plasma membrane and a vacuolar membrane sodium/proton antiporter significantly improves salt tolerance in transgenic arabidopsis plants. Plant Cell Physiol. 2016, 57, 1069–1084. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. The response of salinity stress-induced a. Tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Ye, Z.H.; Zhang, H.J.; Miao, L.X. Broccoli plants over-expressing an erf transcription factor gene boerf1 facilitates both salt stress and sclerotinia stem rot resistance. J. Plant Growth Regul. 2019, 38, 1–13. [Google Scholar] [CrossRef]
- Rahnama, H.; Ebrahimzadeh, H. The effect of nacl on antioxidant enzyme activities in potato seedlings. Biol. Plant. 2005, 49, 93–97. [Google Scholar] [CrossRef]
- Akbari, M.; Katam, R.; Husain, R.; Farajpour, M.; Mazzuca, S.; Mahna, N. Sodium chloride induced stress responses of antioxidative activities in leaves and roots of pistachio rootstock. Biomolecules 2020, 10, 189. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, Y.; Ji, Y.; Jiang, Z.; Wang, L. Effects of salt stress on ion content, antioxidant enzymes and protein profile in different tissues of broussonetia papyrifera. South. Afr. J. Bot. 2013, 85, 1–9. [Google Scholar] [CrossRef]
- Taïbi, K.; Taïbi, F.; Ait Abderrahim, L.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Polesskaya, O.G.; Kashirina, E.I.; Alekhina, N.D. Effect of salt stress on antioxidant system of plants as related to nitrogen nutrition. Russ. J. Plant Physl 2006, 53, 186–192. [Google Scholar] [CrossRef]
- Alharbi, K.; Al-Osaimi, A.A.; Alghamdi, B.A. Sodium chloride (nacl)-induced physiological alteration and oxidative stress generation in Pisum sativum (L.): A toxicity assessment. ACS Omega 2022, 7, 20819–20832. [Google Scholar] [CrossRef]
- López-Cristoffanini, C.; Bundó, M.; Serrat, X.; San Segundo, B.; López-Carbonell, M.; Nogués, S. A comprehensive study of the proteins involved in salinity stress response in roots and shoots of the fl478 genotype of rice (Oryza sativa L. Ssp. Indica). Crop J. 2021, 9, 1154–1168. [Google Scholar] [CrossRef]
- Ghezal, N.; Rinez, I.; Sbai, H.; Saad, I.; Farooq, M.; Rinez, A.; Zribi, I.; Haouala, R. Improvement of Pisum sativum salt stress tolerance by bio-priming their seeds using Typha angustifolia leaves aqueous extract. South. Afr. J. Bot. 2016, 105, 240–250. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional ecology of the ruminant. Nutr. Ecol. Rumin. 2019, 44, 2552–2561. [Google Scholar]
- Anderson, J.W.; Baird, P.; Davis, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Zhan, L.; Su, X.; Wang, T. Physiological Responses and Quality Alterations of Pea Sprouts under Salt Stress: Implications for Salt-Tolerant Mechanism. Horticulturae 2024, 10, 966. https://doi.org/10.3390/horticulturae10090966
Guo J, Zhan L, Su X, Wang T. Physiological Responses and Quality Alterations of Pea Sprouts under Salt Stress: Implications for Salt-Tolerant Mechanism. Horticulturae. 2024; 10(9):966. https://doi.org/10.3390/horticulturae10090966
Chicago/Turabian StyleGuo, Juxian, Liqing Zhan, Xiuxiu Su, and Tingqin Wang. 2024. "Physiological Responses and Quality Alterations of Pea Sprouts under Salt Stress: Implications for Salt-Tolerant Mechanism" Horticulturae 10, no. 9: 966. https://doi.org/10.3390/horticulturae10090966




