Effect of a Directional Electromagnetic Field on the Early Stages of Plant (Raphanus sativus and Saccharum officinarum) Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
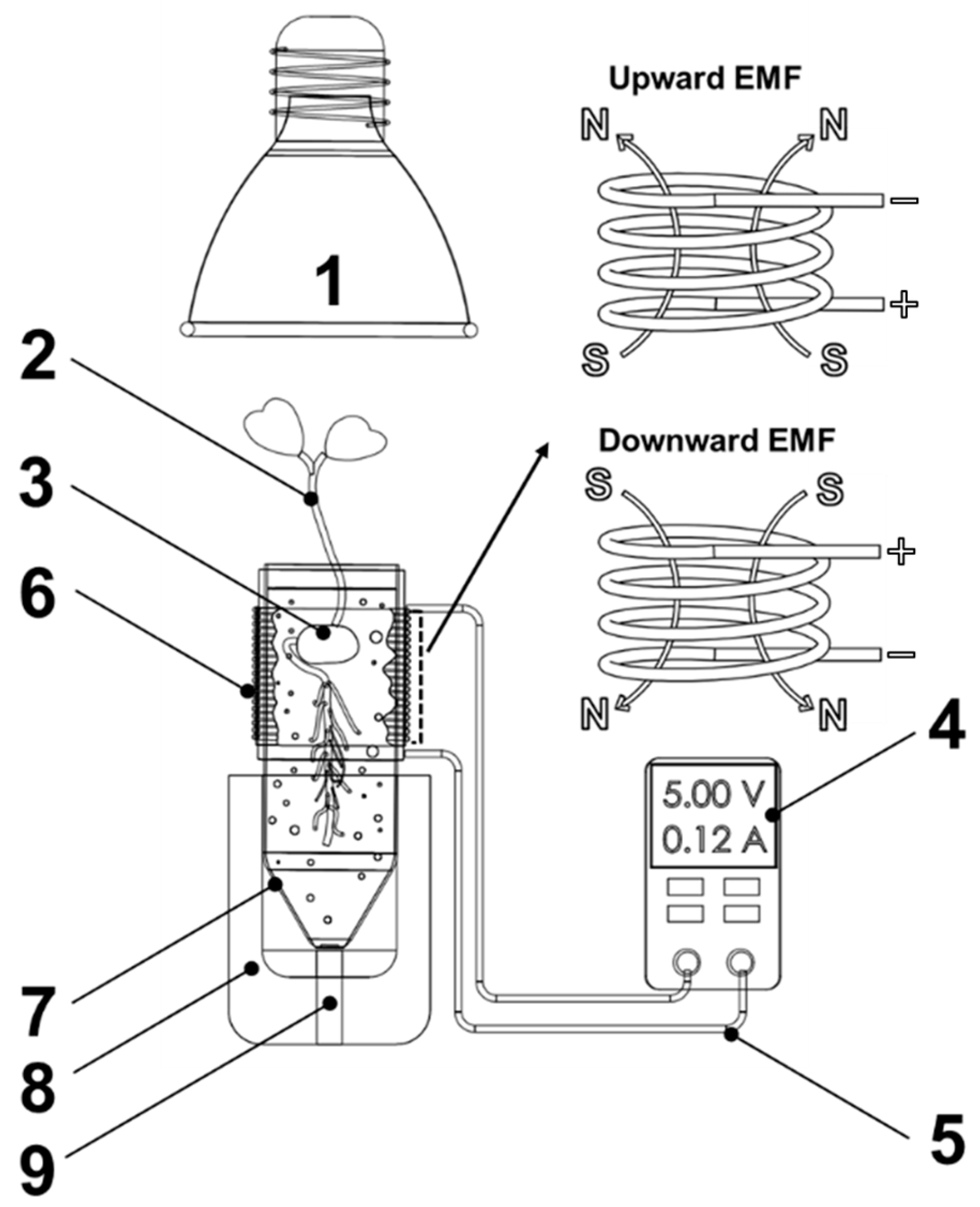
2.2. Solenoid-Type EMF Generator
2.3. Measurement of Plant Growth
2.4. IAA Analysis
2.5. Statistical Analysis
3. Results and Discussion
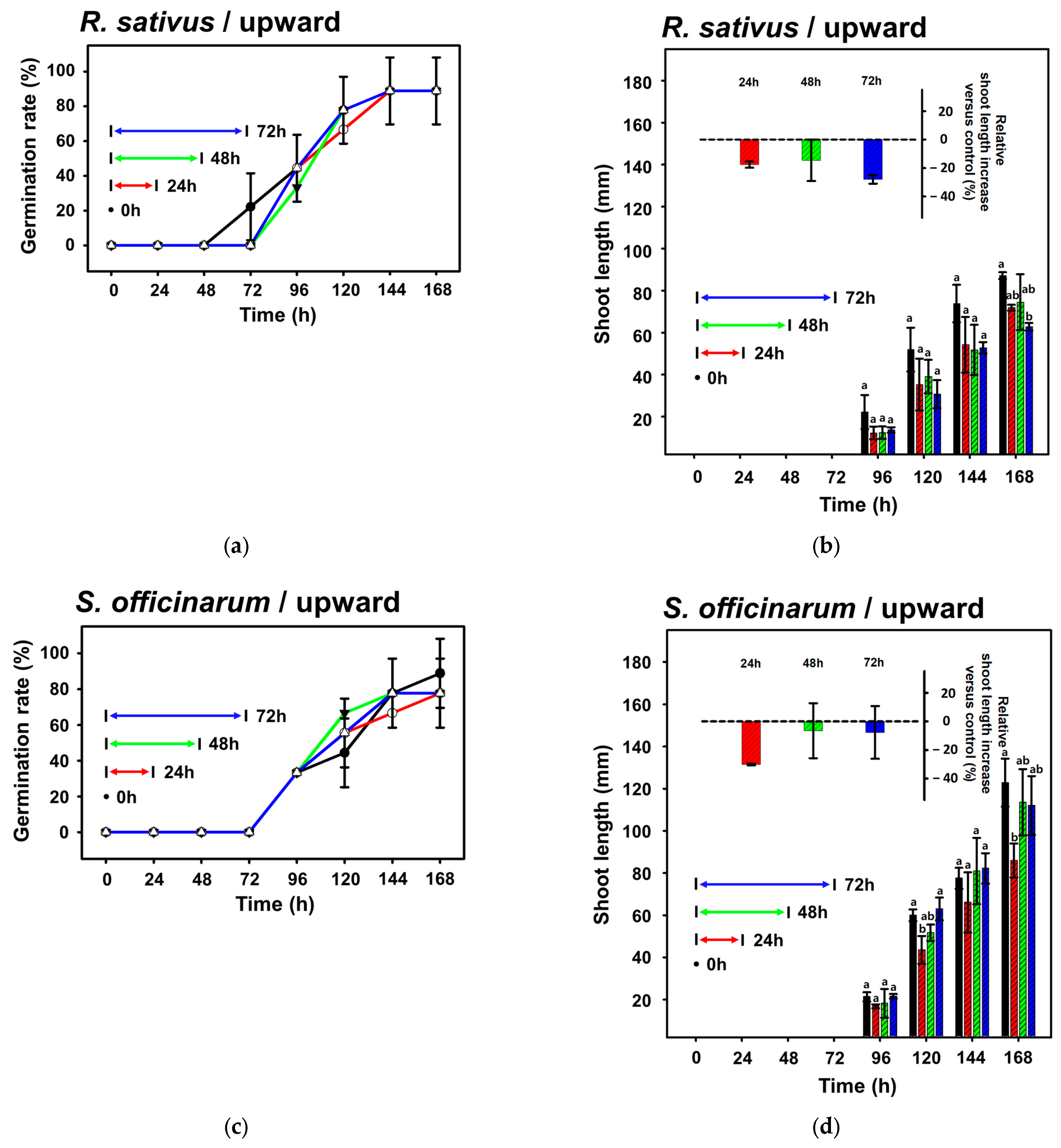
3.1. Effect of an Applied EMF on Seed Germination
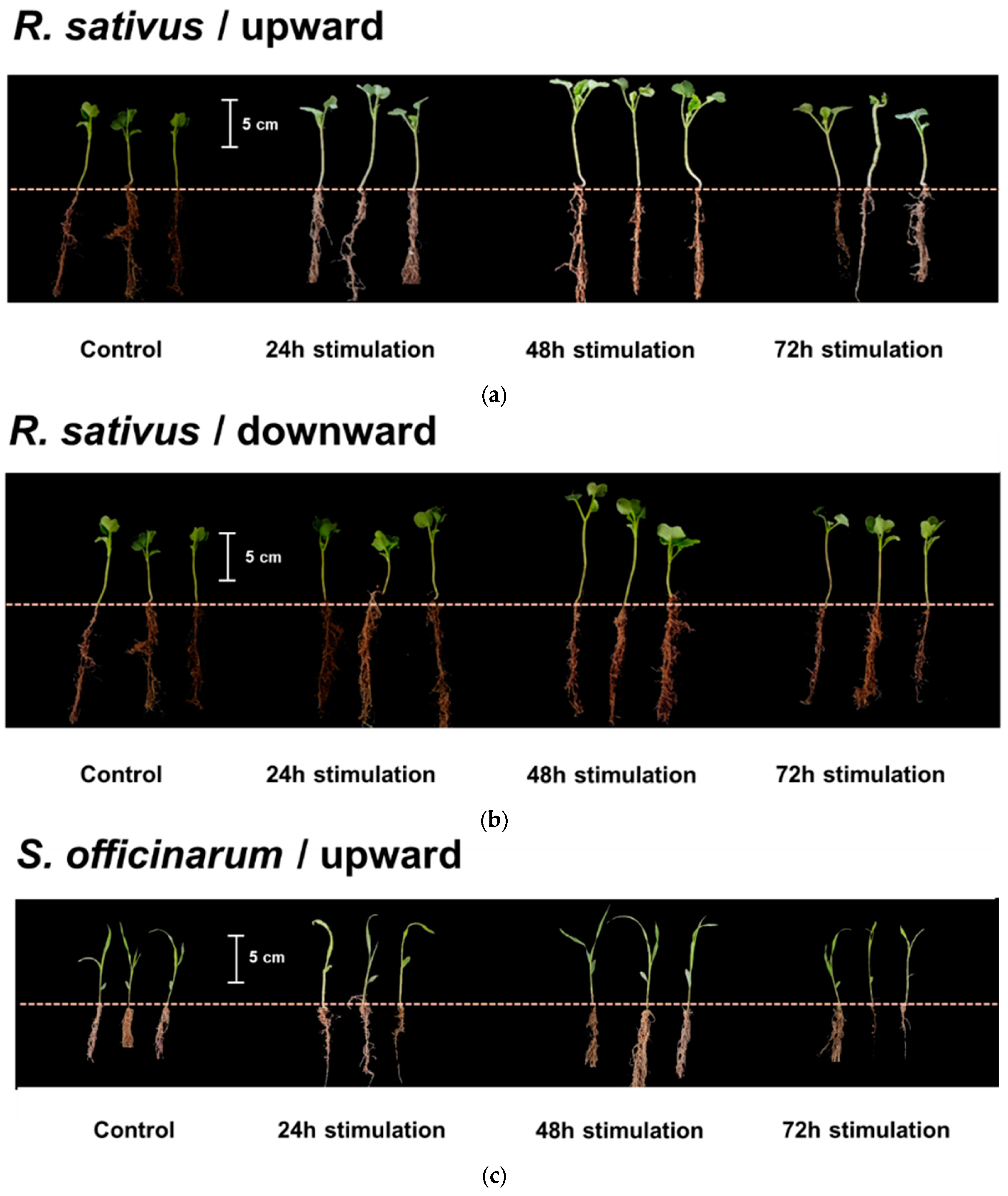
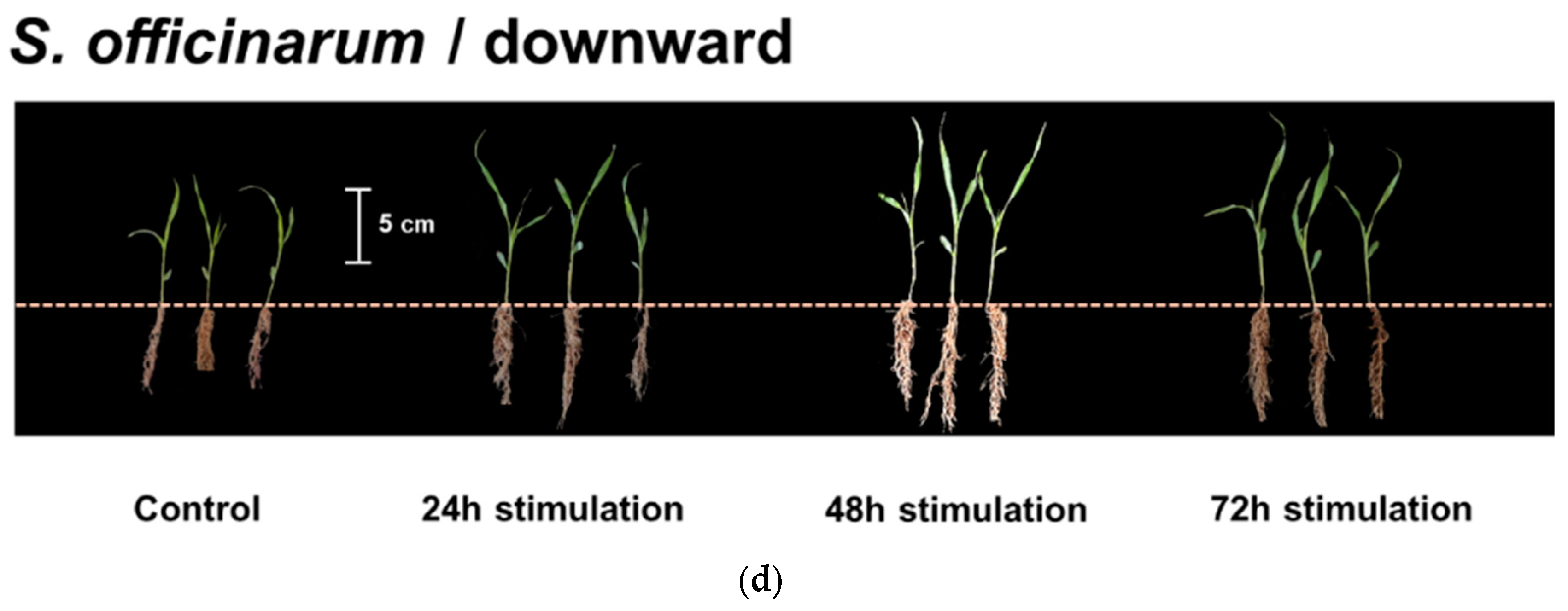
3.2. Effect of Different Directional EMFs on Plant Shoot Growth
3.3. Effect of Different Directional EMFs on Endogenous IAA Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maffei, M.E. Magnetic field effects on plant growth, development, and evolution. Front. Plant Sci. 2014, 5, 445. [Google Scholar] [CrossRef] [PubMed]
- Shabrangy, A.; Ghatak, A.; Zhang, S.; Priller, A.; Chaturvedi, P.; Weckwerth, W. Magnetic field induced changes in the shoot and root proteome of barley (Hordeum vulgare L.). Front. Plant Sci. 2021, 12, 622795. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Ranjitha Kumari, B.D.R. Pulsed magnetic field: A contemporary approach offers to enhance plant growth and yield of soybean. Plant Physiol. Biochem. 2012, 51, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Çelik, O.; Atak, Ç.; Rzakulieva, A. Stimulation of rapid regeneration by a magnetic field in Paulownia node cultures. J. Cent. Eur. Agric. 2008, 9, 297–304. [Google Scholar]
- Shabrangi, A.; Majad, A. Effect of magnetic fields on growth and antioxidant systems in agricultural plants. In Proceedings of the PIERS Proceedings, Beijing, China, 23–27 March 2009. [Google Scholar]
- Wang, S.; Zhang, X.; Fan, Y.; Yang, R.; Wu, J.; Xu, J.; Tu, K. Effect of magnetic field pretreatment on germination characteristics, phenolic biosynthesis, and antioxidant capacity of quinoa. Plant Physiol. Biochem. 2024, 212, 108734. [Google Scholar] [CrossRef]
- Dhiman, S.K.; Wu, F.; Galland, P. Effects of weak static magnetic fields on the development of seedlings of Arabidopsis thaliana. Protoplasma 2023, 260, 767–786. [Google Scholar] [CrossRef]
- Chang, M.J.; Kim, C.J.; Choi, Y.K.; Song, H.J.; Shim, S.Y.; Yang, Y.; Kim, K.J.; Kim, H.J. Effect of electrical ground connection on plant growth. Bull. Korean Chem. Soc. 2017, 38, 1491–1494. [Google Scholar] [CrossRef]
- Lee, S.; Oh, M.M. Electric field: A new environmental factor for controlling plant growth and development in agriculture. Hortic. Environ. Biotechnol. 2023, 64, 955–961. [Google Scholar] [CrossRef]
- Dannehl, D. Effects of electricity on plant responses. Sci. Hortic. 2018, 234, 382–392. [Google Scholar] [CrossRef]
- Nyakane, N.E. The effects of magnetic fields on plants growth: A comprehensive review. Int. J. Food Eng. 2019, 5, 79–87. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, W.; Su, Z.; Guo, S.; Du, Y.; Song, X.; Shi, X.; Li, X.; Liu, Y.; Liu, Z. Effect of pulsed electric field treatment on seed germination and seedling growth of Scutellaria baicalensis. Agriculture 2024, 14, 158. [Google Scholar] [CrossRef]
- Wechsler, D.J. Electro-Horticulture; Leanpub: Victoria, BC, Canada, 2015. [Google Scholar]
- Song, H.J.; Yang, S.W.; Jo, J.W.; Choi, Y.K.; Lee, I.S.; Lee, B.U.; Lee, S.H.; Kim, H.H.; Kim, K.J.; Kim, H.J. Submerged leaves of live indoor foliage plants adsorb H1N1 influenza virus from suspension. Plant Signal. Behav. 2023, 18, 2163869. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lee, E.B.; Kim, J.E.; Song, H.J.; Choi, Y.K.; Kim, K.J.; Kumaran, R.S.; Lee, S.H.; Yang, Y.H.; Kim, H.H. Monitoring plant moisture content using an induction coil sensor. Bull. Korean Chem. Soc. 2019, 40, 1138–1141. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, J.E.; Song, H.J.; Lee, E.B.; Choi, Y.K.; Jo, J.W.; Jeon, H.J.; Kim, H.H.; Kim, K.J.; Kim, H.J. Methodology: Non-invasive monitoring system based on standing wave ratio for detecting water content variations in plants. Plant Methods 2021, 17, 56. [Google Scholar] [CrossRef]
- He, F.; Zheng, Y.; Wang, Q.; Li, H. Electromagnetic force control system of solenoidal coil. In Proceedings of the 37th Chinese Control Conference, Wuhan, China, 25–27 July 2018; pp. 8525–8530. [Google Scholar] [CrossRef]
- Hafeez, M.B.; Zahra, N.; Ahmad, N.; Raza, A.; Wang, X.; Li, J. Growth, physical, biochemical and molecular changes in plants induced by magnetic fields: A review. Plant Biol. 2022, 25, 8–23. [Google Scholar] [CrossRef]
- Manzoor, A.; Bashir, M.A.; Naveed, M.S.; Cheema, K.L.; Cardarelli, M. Role of different abiotic factors in inducing preharvest physiological disorders in radish (Rhaphanus sativus). Plants 2021, 10, 2003. [Google Scholar] [CrossRef]
- Humbert, R.P. The Growing of Sugar Cane; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Cecchetti, D.; Pawełek, A.; Wyszkowska, J.; Antoszewski, M.; Szmidt-Jaworska, A. Treatment of winter wheat (Triticum aestivum L.) seeds with electromagnetic field influences germination and phytohormone balance depending on seed size. Agronomy 2022, 12, 1423. [Google Scholar] [CrossRef]
- Liang, M.L.; Wang, T. Calculating the magnetic field of the infinite solenoid and understanding the ampere circuital law from the magnetic field of moving charges. Eur. J. Phys. 2015, 36, 065043. [Google Scholar] [CrossRef]
- Singh, B.C.S.; Adugna, A.D.; Rani, A.R. A method a rapid in vitro proliferation and morphological characterization of the medical plant Aloe vera L. Afr. J. Biotechnol. 2017, 16, 2201–2214. [Google Scholar]
- Wang, L.; Ruan, Y.L. Regulation of cell division and expansion by sugar and auxin signaling. Front. Plant Sci. 2013, 4, 163. [Google Scholar] [CrossRef]
- Seo, D.H.; Jeong, H.; Choi, Y.D.; Jang, G. Auxin controls the division of root endodermal cells. Plant Physiol. 2021, 187, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Porfírio, S.; Gomes da Silva, M.D.R.; Peixe, A.; Cabrita, M.J.; Azadi, P. Current analytical methods for plant auxin quantification—A review. Anal. Chim. Acta 2016, 902, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.; Nikolova, M.; Dimitrova, L.; Zayova, E. Micropropagation and evaluation of flavonoid content and antioxidant activity of Saliva officinalis L. Genet. Plant Physiol. 2015, 5, 48–60. [Google Scholar]
- Wang, S.; Sun, G.; Luo, Y.; Qian, W.; Fan, K.; Ding, Z.; Hu, J. Role of Role of iaa and primary metabolites in two rounds of adventitious root formation in softwood cuttings of Carmellia sinensis (L.). Agronomy 2022, 12, 2486. [Google Scholar] [CrossRef]
- Gaid, M.; Haas, P.; Beuerle, T.; Scholl, S.; Beerhues, L. Hyperforin production in hypericum perforatum root cultures. J. Biotechnol. 2016, 222, 47–55. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, L.; Ma, C.; Liu, J.; Liu, B.; Li, H. The use of HPLC in determination of endogenous hormones in anthers of bitter melon. J. Life Sci. 2011, 5, 139–142. [Google Scholar]
- Kim, Y.; Oh, Y.J.; Park, W.J. HPLC-based quantification of indole-3-acetic acid in the primary root tip of maize. J. Nanotechnol. 2006, 3, 40–45. [Google Scholar]
- Agarwal, A.; Nigam, V.K. Nitrilase mediated conversion of indole-3-acetonitrile to indole-3-acetic acid. Biocatal. Agric. Biotechnol. 2014, 3, 351–357. [Google Scholar] [CrossRef]
- Eşitken, A.; Turan, M. Alternating magnetic field effects on yield and plant nutrient element composition of strawberry (Fragaria x ananassa cv. Camarosa). Acta Agric. Scand. 2004, 54, 135–139. [Google Scholar] [CrossRef]
- Hasan, M.M.; Alharby, H.F.; Hajar, A.S.; Hakeem, K.R.; Alzahrani, Y. The effect of magnetized water on the growth and physiological conditions of Moringa species under drought stress. Pol. J. Environ. Stud. 2019, 28, 1145–1155. [Google Scholar] [CrossRef]
- Lin, I.J.; Yotvat, J. Exposure of irrigation and drinking water to a magnetic field with controlled power and direction. J. Magn. Magn. Mater. 1990, 83, 525–526. [Google Scholar] [CrossRef]
- Taimourya, H.; Oussible, M.; Baamal, L.; Harif, A.; Zaid, E.; Guedira, A.; Smouni, A. Magnetic treatment of culture medium enhance growth and minerals uptake of strawberry (Fragaria x ananassa Duch.) and tomato (Solanum lycopersicum) in Fe deficiency conditions. Int. J. Eng. Res. 2017, 8, 1414–1436. [Google Scholar]
- Cai, T.; Meng, X.; Liu, X.; Liu, T.; Wang, H.; Jia, Z.; Yang, D.; Ren, X. Exogenous hormonal application regulates the occurrence of wheat tillers by changing endogenous hormones. Front. Plant Sci. 2018, 9, 1886. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, E. Regulation of root growth by plant hormones—Roles for auxin and gibberellin. Crit. Rev. Plant Sci. 2005, 24, 249–265. [Google Scholar] [CrossRef]
- Ahmed, A.; Hasnain, S. Auxins as one of the factors of plant growth improvement by plant growth promoting bacteria. Pol. J. Microbiol. 2014, 63, 261–266. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, J.W.; Yang, S.W.; Lee, G.W.; Kim, J.H.; Kim, Y.J.; Choi, Y.-K.; Kim, K.J.; Lee, H.-S.; Bang, S.W.; Kim, H.J. Effect of a Directional Electromagnetic Field on the Early Stages of Plant (Raphanus sativus and Saccharum officinarum) Growth. Horticulturae 2024, 10, 973. https://doi.org/10.3390/horticulturae10090973
Jo JW, Yang SW, Lee GW, Kim JH, Kim YJ, Choi Y-K, Kim KJ, Lee H-S, Bang SW, Kim HJ. Effect of a Directional Electromagnetic Field on the Early Stages of Plant (Raphanus sativus and Saccharum officinarum) Growth. Horticulturae. 2024; 10(9):973. https://doi.org/10.3390/horticulturae10090973
Chicago/Turabian StyleJo, Jeong Wook, Sung Woo Yang, Gyu Won Lee, Jae Hun Kim, Ye Jin Kim, Yong-Keun Choi, Kwang Jin Kim, Hyeong-Seok Lee, Sung Won Bang, and Hyung Joo Kim. 2024. "Effect of a Directional Electromagnetic Field on the Early Stages of Plant (Raphanus sativus and Saccharum officinarum) Growth" Horticulturae 10, no. 9: 973. https://doi.org/10.3390/horticulturae10090973
APA StyleJo, J. W., Yang, S. W., Lee, G. W., Kim, J. H., Kim, Y. J., Choi, Y.-K., Kim, K. J., Lee, H.-S., Bang, S. W., & Kim, H. J. (2024). Effect of a Directional Electromagnetic Field on the Early Stages of Plant (Raphanus sativus and Saccharum officinarum) Growth. Horticulturae, 10(9), 973. https://doi.org/10.3390/horticulturae10090973






