Abstract
Frankliniella occidentalis, commonly known as the western flower thrips (WFT), is one of the world’s most significant cosmopolitan pests. This pest infests many ornamental species, including Alstroemeria, leading to substantial economic losses. F. occidentalis damages flowers, transmits viruses, and causes the rejection of shipments for exports. Farmers have observed variations in the occurrence of F. occidentalis among different Alstroemeria cultivars. It is hypothesized that differences in flower color and odor among cultivars may influence the host-choice behavior of this insect, potentially explaining the observed variations in incidence and damage in the field. To test this hypothesis, we analyzed one year’s worth of sampling data for the occurrence of the WFT complex in a commercial Alstroemeria greenhouse crop. This analysis identified cultivars with high and low thrips incidences. The ‘Himalaya’ and ‘Whistler’ cultivars exhibited the highest incidence, while the ‘Shakira’ and ‘Nora’ cultivars showed the lowest incidence values. To investigate the potential role of host odors in these field incidence differences, we conducted behavioral tests (choice, non-choice, and repellency) using glass boxes where visual stimuli were blocked. These tests confirmed a differential attraction response of thrips to the flowers of Alstroemeria cultivars, although all cultivars were viable options for WFT choice. Preferences under laboratory conditions differed from the incidence observed in the greenhouse, ruling out a repellency effect of some cultivars on insects. This study provides evidence that differential emissions from Alstroemeria flowers can influence host selection by WFT, a phenomenon that warrants further in-depth exploration in future studies.
1. Introduction
Alstroemeria, commonly known as the Lily of the Incas, is native to the Andes and is widely cultivated as an ornamental plant due to its striking colors, long vase life, and numerous cultivars [1]. The western flower thrips (WFT) complex causes significant damage to Alstroemeria plants, particularly to the flowers [2]. This damage can be direct, resulting from insect behavior including feeding and oviposition, or indirect, through the transmission of viruses such as the tomato spotted wilt virus (TSWV) [3,4]. The severity of thrips damage increases as the flowers open [5].
Thrips species are mostly polyphagous cosmopolitan and possess evolutionary traits that enable successful adaptation. Their cryptic morphology allows them to hide in various parts of the plant and evade predators and handling techniques. Frankliniella occidentalis, in particular, has developed resistance to 30 active ingredients and insecticides, and is listed among the world’s most critical and invasive pests [6]. Thus, developing new management tactics is a priority research area.
Although thrips are considered generalist insects, they exhibit preferences for different plant varieties and cultivars within the same species [7]. This behavior suggests that physical and chemical stimuli may influence their incidence on and/or preference for certain plants. The spatial distribution of WFT can be affected by environmental factors such as climate [8,9] and wind direction [10], which can vary even under greenhouse conditions. In this regard, the range and population size of thrips are influenced by temperature and humidity. McDonald et al. [11] found that the minimum thermal threshold for this species is 7.9 °C. At 10 °C, it took approximately 118.6 days for an individual to complete development from an egg to an adult. This time was drastically reduced at a temperature of 30 °C, for which only 11.8 days were required to complete development into adulthood. The above highlights the notable effect that temperature has on the population dynamics of the insect and its ability to colonize areas with the presence of its hosts. According to Wang et al. [12], the current and future distribution of F. occidentalis, as predicted by the MaxEnt model, is mainly influenced by the annual mean temperature, temperature seasonality, and the mean temperature of the coldest month [12]. High humidity conditions (relative humidity (RH) ≈ 90%) are ideal for F. occidentalis pupation [13], as the larvae have high mortality rates at RH < 80%, and in general, the population decreases drastically with RH values < 50% [14]. Plant characteristics, such as the state of flower development [15], petal color, flower shape, and volatile compounds secreted by the plant [16,17], also influence the thrips distribution. Previous studies have reported differences in WFT incidence and laboratory preferences for rose cultivars, partially attributed to differential chemotactic responses associated with the volatile organic compound (VOC) profiles of different cultivars [18]. In Alstroemeria, a differential greenhouse incidence may be related to both varietal characteristics and environmental factors, while laboratory preferences, excluding visual stimuli, are likely associated with differences in VOC emissions from various cultivars.
Under this context, the aims of this study were to determine changes in the incidence of the WFT complex among Alstroemeria cultivars in commercial greenhouse crops and to evaluate the potential association between these distribution patterns and the differential preference of thrips for Alstroemeria cultivars. We assessed the occurrence of the WFT complex under commercial Alstroemeria production conditions using exploratory field analyses and compared these results with preferences observed in choice, non-choice, and repellency tests conducted under controlled conditions. This research aimed to provide a deeper understanding of thrips’ host selection behavior, which can inform more effective pest management strategies for Alstroemeria and other ornamental crops.
2. Materials and Methods
2.1. Selection of Cultivars with High and Low WFT Occurrence from Greenhouse Monitoring
Contrasting cultivars with high and low occurrences of WFT were selected from commercial Alstroemeria crops grown under greenhouse conditions, located in Sopó, Colombia (4°49′18.71″ N, 73°58′01.19″ W), at an elevation of 2600 m a.s.l., and with an average daily temperature of 19 °C and relative humidity (RH) between 60 and 90%. Data were analyzed from the farm’s pest monitoring plan, used for management decisions, and corresponded to a period of ca. 48 weeks. Due to the agroecological conditions of Colombia, Alstroemeria is produced in greenhouses, and so the respective monitoring was conducted in a commercial farm complex of 6.3 ha, divided into six greenhouses for Alstroemeria crops (Figure S1). Each greenhouse had 192 sowing rows (30 m long and 1.3 m wide, separated by 0.7 m), and each row was divided into nine parts of equal area (Figure S2).
Weekly monitoring was performed, as follows: nine flower stems were randomly sampled from each part of all rows and shaken upside down over a white surface; then, the flower organs were separated to count the WFT larvae and adults. The WFT larvae had pale yellow or white coloration and lacked wings. In contrast, adults possessed two pairs of fringed wings and elongated bodies, and their coloration was yellowish-brown or pale brown [19]. The monitoring data provided incidence information per row for Alstroemeria cultivars (n = 40), which were organized according to the logistics of the commercial farm. To quantify the occurrence of WFT in the different Alstroemeria cultivars, the average number of thrips found in nine stems per row (per planting bed) per week was measured for each cultivar over one year (Table S1). This analysis identified two cultivars each for the highest and lowest WFT occurrences among the forty examined Alstroemeria cultivars available in the crop.
2.2. Plants
Based on the previous selection, the cultivars for behavior experiments under laboratory conditions were ‘Himalaya’ and ‘Whistler’ (high WFT occurrence) and ‘Shakira’ and ‘Nora’ (low WFT occurrence). All cultivars except ‘Nora’ were bred by Könst (www.alstroemeria.com, accessed on 30 July 2024), with ‘Nora’ bred by HilverdaKoiij (www.hilverdaflorist.com, accessed on 30 July 2024). The plants were grown in the aforementioned commercial crop in Sopó, Colombia, under agronomic management standards established for Colombian flower production exports [20]. Floral buds at flower opening stages S3 (i.e., upper three anthers bent upwards) and S4 (i.e., lower three anthers bent upwards and anthesed) were selected according to the sequence proposed by Wagstaff et al. [21]. These stages were chosen for the experiments to ensure physiological uniformity and high volatile organic compound (VOC) emission [22,23].
2.3. Insects
Frankliniella occidentalis individuals were obtained from a colony fed on red clover plants (Trifolium pratense) and grown under greenhouse conditions at the Biological Control research group, Nueva Granada Campus, UMNG (12:12 h light photoperiod to mimic the conditions of Colombia and equatorial production zones, 17.84 ± 7.2 °C, 66.7 ± 19.6% RH), located in Cajicá, Colombia. Female thrips were collected and starved for 24 h, before being placed in hermetically sealed plastic boxes with appropriate ventilation (25 cm × 17 cm × 11 cm, length × width × height) at room temperature with a 12:12 h photoperiod. Females were distinguished from males by their robust abdomen and larger size [19,24,25]. Only females were used in these trials to avoid aggregation pheromone bias [26].
2.4. Behavioral Bioassays
2.4.1. Choice Bioassays
Choice tests were conducted in glass exclusion boxes (40 cm × 26 cm × 26 cm) based on a previously reported custom-made device [27]. The boxes were hermetically sealed with four internal divisions (20 cm × 13 cm × 26 cm) and circular side windows (6 cm diameter) covered with 0.03 mm metallic mesh to prevent insect escape (Figure 1). A release device (26 cm × 6 cm × 6 cm) with eight perforations (9.52 mm diameter) was placed in the center of the boxes to block visual stimuli from the flowers. The upper lid was made of glass.
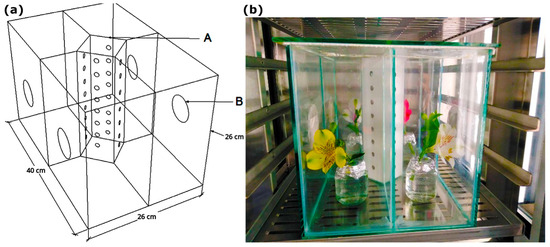
Figure 1.
(a). An exclusion box for choice, non-choice, and repellency bioassays. A. The thrips release area. B. Ventilation windows covered with metallic mesh. (b). The distribution of the flowers inside the exclusion boxes.
Flowers were kept hydrated with water for the experiments. A single flower bud of each cultivar was placed in 120 cm3 jars in the center of each internal compartment, with the petiole submerged by four centimeters, and the flask opening was closed with aluminum foil. The behavioral effects of cultivars were evaluated through all possible pairwise combinations: Nora–Whistler (NW), Himalaya–Whistler (HW), Shakira–Whistler (SW), Nora–Shakira (NS), Himalaya–Shakira (HS), and Himalaya–Nora (HN). Flower positions were changed for each repetition to eliminate bias. All told, 100 to 140 female thrips were placed into 25 mL vials and then into the release device. After 24 h, thrips in the compartment and release device were counted. Thrips found on the bottle, flower, or compartment were considered to have chosen that cultivar, while those found in the release device were scored as a non-choice.
Choice bioassays were conducted under greenhouse conditions at UMNG (as described above) and in a climatic chamber (12:12 h light, 70 ± 2% RH, 20 ± 1 °C) [9]. Three replicates were conducted in the greenhouse and four under controlled conditions, with exclusion boxes cleaned between replicates using 70% ethyl alcohol and hot air flow. An additional choice test exposed a number of thrips to the VOCs of all four cultivars simultaneously. This test was conducted under the same climatic chamber conditions, with flower positions modified between replicates. Female thrips (n = 120) were placed in groups of 30 in each compartment, and after 24 h, their choices were quantified. Four repetitions were performed.
2.4.2. Non-Choice Bioassay
The non-choice test assessed each cultivar’s attractiveness without the competition effect of another cultivar. A flower of the same cultivar was placed in each compartment of the exclusion boxes under the same conditions as the choice trials. Four boxes (one per cultivar) were used, with 120 female thrips deposited into the release device. After 24 h, the number of thrips in each compartment was counted. This experiment was repeated four times.
2.4.3. Repellency Bioassay
This test established the possible repellency effect of flowers on female thrips. Two flowers of the same cultivar were placed diagonally in two compartments of the exclusion boxes, alternating between two empty compartments only containing a glass container filled with water and covered with perforated aluminum foil. If a repellent effect existed, thrips would be located in empty chambers. Female thrips (n = 120) were placed in the release device, and after 24 h, their positions were counted. Flower positions were modified for each repetition to avoid bias. Four replicates were performed.
2.5. Statical Analysis
One year’s worth of thrips occurrence data were analyzed to compare the WFT incidence among Alstroemeria cultivars under greenhouse conditions. A generalized linear model analysis assuming a negative binomial distribution (GLMNB) accounted for overdispersion [28]. The cultivars were considered treatments, and the number of thrips was considered the response variable. Several models were fitted, and the intercept was varied to obtain specific comparisons between contrasting cultivars.
For paired choice and repellency tests, generalized linear models (GLM) assuming a binomial error distribution with a logit link function were used to find differences in the proportion of thrips choosing each cultivar. The response variable was the proportion of individuals choosing a cultivar relative to the number released, and the treatment was the flower cultivar. The control treatment (without flowers) was the intercept in repellency tests.
GLM analyses assuming binomial distribution with a logit link function were performed in the simultaneous choice trial with four cultivars and the non-choice trial. The response variable was the thrips’ choice proportion, with the highest-choice cultivar as the intercept. A p-value < 0.05 was considered significant. All statistical analyses were conducted using the R statistical language (Auckland, New Zealand) [29].
3. Results
3.1. Occurrence of WFT in Alstroemeria under Greenhouse Conditions
The average number of thrips measured per planting bed per week throughout one year suggested different WFT occurrences between assessed Alstroemeria cultivars (n = 40) under greenhouse conditions (Figure 2). The cultivars ‘Himalaya’ and ‘Whistler’ demonstrated the highest occurrence rates, with average thrips numbers of 6.58 ± 0.18 and 2.91 ± 0.13, respectively. Conversely, the cultivars ‘Shakira’ and ‘Nora’ exhibited significantly lower occurrences, with ‘Shakira’ averaging only 0.22 thrips per planting bed, and ‘Nora’ showing similarly low levels.
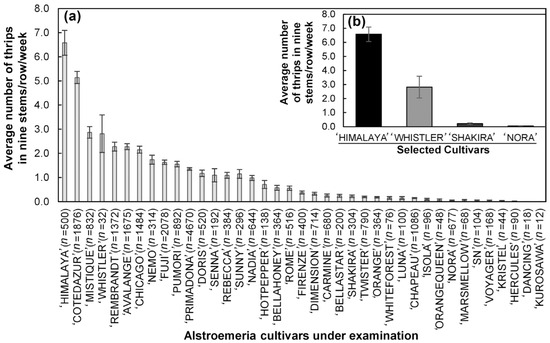
Figure 2.
The mean occurrence (average number of individuals per planting bed (nine stems/row) per week) of WFT as obtained from commercial field data. (a) The mean occurrence of the examined commercial Alstroemeria cultivars (n = 40). (b) A comparison between the selected four contrasting cultivars. The error bars correspond to the standard error of the mean (SEM).
Table 1 presents the p-values obtained from the generalized linear model with GLMNB-based comparison tests. These tests highlighted significant differences in thrips occurrence between the cultivars. For instance, ‘Himalaya’ showed highly significant differences when compared to ‘Nora’ (p = 2 × 10−16) and ‘Shakira’ (p = 2 × 10−16). Similarly, ‘Whistler’ showed significant differences compared to ‘Himalaya’ (p = 0.00195), ‘Nora’ (p = 0.0218), and ‘Shakira’ (p = 0.0366).

Table 1.
p-values obtained for GLMNB-based comparison tests. The asterisks indicate the significance levels: *** p < 0.001, ** p < 0.01, * p < 0.05.
3.2. Behavioral Bioassays
3.2.1. Choice Bioassays
The choice bioassays conducted under greenhouse conditions revealed significant preferences among thrips for certain cultivars. In the HN combination (i.e., ‘Himalaya’ vs. ‘Nora’), ‘Nora’ was significantly more attractive to thrips (p = 1.57 × 10−5). In the NW combination (i.e., ‘Nora’ vs. ‘Whistler’), ‘Whistler’ was the more attractive cultivar (p = 0.00499). Additionally, in the HW combination (i.e., ‘Himalaya’ vs. ‘Whistler’), ‘Whistler’ again showed higher attractiveness (p = 0.0297). Other combinations did not show significant differences in terms of thrips preference (Figure 3a).
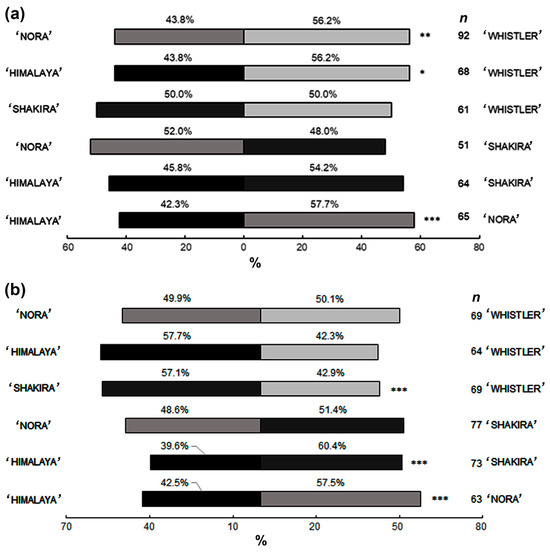
Figure 3.
The percentage preference of adult female western flower thrips (WFT) for different Alstroemeria cultivars in paired choice trials. The sample size (n) corresponds to the number of individuals making a choice. (a) Greenhouse test (n = 140). (b) The test was conducted in a climatic chamber (n = 130). Asterisks indicate the significance level based on GLM statistical tests: *** p < 0.001, ** p < 0.01, * p < 0.05.
Under climatic chamber conditions, the thrips showed a significantly higher preference for ‘Shakira’ in the SW (i.e., ‘Shakira’ vs. ‘Whistler’) (p = 0.000192) and the HS (i.e., ‘Himalaya’ vs. ‘Shakira’) (p = 5.19 × 10−7) combinations. ‘Nora’ was preferred over ‘Himalaya’ in the HN combination (p = 3.26 × 10−5). No significant differences were observed in other pairwise comparisons (Figure 3b).
In the multiple-choice tests conducted under climatic chamber conditions employing the four varieties simultaneously, the WFT complex exhibited a clear preference for the ‘Shakira’ cultivar over the other cultivars, with a highly significant difference (p = 2.00 × 10−16) (Figure 4a). The ‘Nora’ cultivar exhibited the lowest thrips selection, which coincided with results from the field examinations.
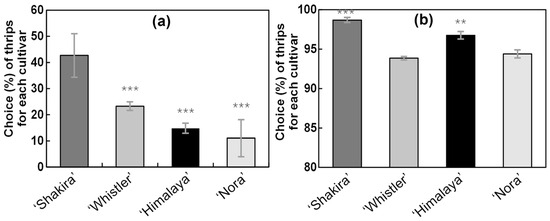
Figure 4.
Results from the multiple-choice and non-choice trials for different Alstroemeria cultivars. (a) The average percentage preference of adult female western flower thrips (WFT) in the multiple-choice test with the four varieties simultaneously. (b) The preference of adult female WFT for different Alstroemeria cultivars in non-choice trials (n = 120). Error bars represent the standard error for the proportions. Asterisks indicate the degree of significance based on GLM statistical tests relative to the cultivar with the highest preference (‘Shakira’): *** p < 0.001, ** p < 0.01.
3.2.2. Non-Choice Bioassay
The non-choice bioassay confirmed that all tested Alstroemeria cultivars were attractive to the WFT. However, ‘Shakira’ and ‘Himalaya’ cultivars showed a significantly higher average percentage of thrips compared to Whistler and Nora (p < 0.05) (Figure 4b).
3.2.3. Repellency Bioassay
The repellency bioassay outcomes indicated that there was no repellency effect by any of the cultivars towards the thrips. In all cases, the choice percentage for cultivars exceeded 73%, suggesting that thrips were generally attracted to the flowers of all tested varieties (Figure 5).
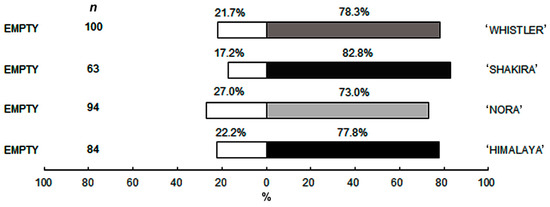
Figure 5.
The evaluation of the repellency of the WFT complex against four Alstroemeria cultivars (n = 120).
4. Discussion
This study aimed to assess the behavioral response of WFT to Alstroemeria cultivars, exploring the role of cultivar-dependent floral emission differences as the primary factor determining pest incidence under field conditions. Alstroemeria flowers were found to induce differential responses in the WFT complex, making some cultivars more frequented than others. However, the cultivars registering greater preference by thrips in the different trials did not necessarily coincide with those exhibiting higher incidence under greenhouse conditions.
In commercial greenhouses, the ‘Himalaya’ and ‘Whistler’ cultivars showed the highest incidence of thrips. Yet, in paired choice trials, ‘Himalaya’ did not show greater preference by WFT in any combination evaluated in the greenhouse or climate chamber, nor was this seen in the multiple-choice trial. ‘Whistler’ was the preferred cultivar in some combinations, although this preference was not consistently observed between the greenhouse and the climate chamber. On the other hand, ‘Shakira’, one of the cultivars with the lowest incidences in the greenhouse, presented the highest percentages of individuals in the multiple-choice and non-choice trials. These results highlight the fact that field incidence occurs from a combination of interacting factors, with VOC-related floral emissions being one of many relevant elements. Even in the presence of highly attractive flowers in choice tests, thrips could still be present in significant numbers on less preferred hosts [30].
The high mobility of thrips can result in a wide distribution across all available flowers despite their differential host preferences. Factors such as microclimatic variation, influenced by the crops’ location, impact WFT host selection [31]. The presence of phytophagous competitors or predators can also significantly reduce the WFT incidence in plants that might otherwise be preferred under field conditions [32]. Additionally, adult thrips might select hosts they have previously encountered for feeding and oviposition to ensure a suitable resource for their progeny, which could influence the WFT incidence in Alstroemeria cultivars in commercial crops. Furthermore, plant factors such as nutritional status, health, and the presence of neighboring host plants affect pest distribution. Since the monitoring of thrips in commercial crops did not characterize the pest’s stages of development, there could be changes in plant choice driven by resource demands at different insect development stages [33,34]. All of these factors may explain the differences found in this study between the incidence of thrips in Alstroemeria cultivars under field conditions and their preferences under greenhouse and climatic chamber conditions [35].
In addition, thrips can respond to chromatic stimuli, which may explain such differences. All of the tests in this study were conducted in exclusion boxes, where the effect of color was blocked, suggesting a primarily chemotactic response by the insects. However, in greenhouse conditions, visual stimuli can be highly relevant. Elimem and Chermiti [36] and Park et al. [5] reported a higher attraction of F. occidentalis towards creamy-white and yellow flower colors. In the greenhouse, three of the four cultivars chosen for the behavioral studies exhibited these colors: ‘Himalaya’ and ‘Whistler’ are white with purple and red hues, respectively, while ‘Shakira’ is yellow with lime green hues. Since the field monitoring data were collected at flower opening stages S1–S2 [21], where color differences between cultivars are visually distinct, this factor could significantly influence the thrips’ choices. Blumthal et al. [37] suggested that the wavelengths of yellow and green colors (500–600 nm) can be attractive to F. occidentalis, and Stukenberg et al. [38] proposed the existence of specific photoreceptors in F. occidentalis which are sensitive to blue and green wavelengths. Although the ‘Shakira’ cultivar exhibits yellow with lime green undertones, which could be associated with a high visual attraction by thrips, this option must be discarded since the behavioral tests blocked color effects through the architecture of the exclusion boxes. Thus, color cannot explain the superior attraction response that ‘Shakira’ exhibited when compared to other cultivars in the trials. Furthermore, under field conditions, ‘Shakira’ does not show a high occurrence of WFT. Therefore, the higher preference of thrips for ‘Shakira’ is likely a result of the emission of attractive VOCs by its flowers.
Our results demonstrated the thrips’ responses to floral emissions generated by Alstroemeria flowers, consistent with studies on other plants such as rose, meadow-sweet, bay laurel, and sage [18,39,40]. These previous studies found behavioral responses of WFT to VOCs from host plants. Therefore, VOCs emitted by Alstroemeria flowers likely play a significant role in host selection, along with biotic and abiotic environmental factors and plant characteristics. The genetic background of each cultivar can influence the production of particular VOCs, varying among Alstroemeria hybrids [41]. Similar results were presented by Avellaneda et al. [40], who found differences in VOC emission in commercial rose cultivars and attractant activity of compounds identified in flowers, such as (+/-)-theaspirane and β-caryophyllene. In this regard, qualitative and quantitative differences between VOC profiles from flowers of different Alstroemeria cultivars are possible.
No repellency effect was found for any cultivars, suggesting that all cultivars could provide food, oviposition sites, and shelter for WFT. The behavior of thrips might have evolved to respond to attractive visual or olfactory stimuli from flowers. The floral opening stages during field monitoring coincide with intervals when VOCs from the pollen or its precursors are present, and are potentially responsible for the attractiveness of all cultivars and the differential attraction between cultivars. Pollen boosts the fecundity of several thrips species [42,43], including F. occidentalis [44], and can produce compounds attractive to thrips, such as (S)-(-)-verbenone [45]. Since the thrips’ olfactory preference is correlated with field performance in terms of developmental and survival rates [46], differences in VOC production in the pollen of Alstroemeria cultivars could partially determine the thrips’ selection behavior. The flower buds used in this study could have continued their opening process during the experiment, varying in pollen availability as they reached opening stages S3–S4 within 24 h after the start of tests, since vase life differed.
While visual stimuli were excluded in controlled tests, they play a crucial role in the thrips’ attraction to certain cultivars under greenhouse conditions. This factor should be considered in future studies and pest management strategies. As an explanatory hypothesis, these results suggest that differences in VOC production among Alstroemeria cultivars likely contribute, among other factors, to varying attractiveness levels. Thus, confirming the chemotactic response of thrips mediated by volatile compounds produced by Alstroemeria opens avenues for developing management alternatives based on ethological control. Identifying the VOCs produced by the cultivars that showed an attraction effect in the preference tests is a starting point for seeking attractant compounds that can potentially be used in traps for monitoring and/or controlling thrips, and are based on plant biology and biochemistry.
5. Conclusions
This study investigated the behavioral responses of WFT to different Alstroemeria cultivars, with a focus on the role of floral emissions in pest incidence. Our findings highlight several key conceptual insights and aspects of plant biology performance. In this regard, Alstroemeria flowers elicit varying responses from the WFT complex, making certain cultivars more frequented than others. Floral emissions seemed to significantly influence the thrips’ behavior, consistent with findings in other plant studies. In addition, the field incidence of thrips does not always correlate with cultivar preference observed in controlled trials. While ‘Himalaya’ and ‘Whistler’ showed the highest incidence of thrips in commercial greenhouses, ‘Shakira’, with low field incidence, displayed the highest attraction in multiple-choice and non-choice trials. Such discrepancies between field and controlled conditions underscore the fact that field incidence likely results from a combination of factors, with VOCs; microclimatic variations; the presence of competitors and predators; plant nutritional status; and visual stimuli potentially being the most important. These factors have not been thoroughly tested to explain the differences between field and controlled conditions and warrant further exploration in future studies. However, none of the tested cultivars exhibited a repellency effect, suggesting that all cultivars provide suitable resources for WFT, such as food, oviposition sites, and shelter. Future research should focus on pinpointing these attractant compounds and exploring their application in integrated pest management, thereby enhancing sustainable agriculture practices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10090982/s1. Table S1: Analysis outcomes for data from the farm’s pest monitoring plan; Figure S1: Plot distribution map of the commercial farm; Figure S2: Illustration of the field map for the organization of sowing rows.
Author Contributions
Conceptualization, D.R. and E.C.-B.; methodology, software, validation, formal analysis, and investigation, L.G.C.-Q. and M.A.D.; resources, D.R.; data curation, L.G.C.-Q. and E.C.-B.; writing—original draft preparation, L.G.C.-Q.; writing—review and editing, D.R. and E.C.-B.; supervision, D.R. and E.C.-B.; project administration, D.R.; funding acquisition, D.R. and E.C.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Vicerrectoría de Investigaciones at the Universidad Militar Nueva Granada (UMNG) through the research project IMP-CIAS-2922, valid during 2019–2021.
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors mainly thank Suasuque S.A.S and Flores La Serena Ltd.a. for providing information and plant material to carry out these studies. We also thank Jose Mauricio Simoes Bento from the University of Sao Paulo—ESALQ for the guidance in conducting the behavior tests. Our gratitude is extended to the UMNG for providing financial and technical resources, facilities, and laboratories.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bridgen, M.P. Alstroemeria. In Ornamental Crops; Van Huylenbroeck, J., Ed.; Springer International Publishing: Riverhead, NY, USA, 2018; Volume 11, pp. 231–236. ISBN 978-3-319-90697-3. [Google Scholar]
- Gaum, W.G.; Giliomee, J.H.; Pringle, K.L. Resistance of Some Rose Cultivars to the Western Flower Thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). Bull. Entomol. Res. 1994, 84, 487–492. [Google Scholar] [CrossRef]
- Jones, D.R. Plant Viruses Transmitted by Thrips. Eur. J. Plant Pathol. 2005, 113, 119–157. [Google Scholar] [CrossRef]
- Macharia, I.; Backhouse, D.; Wu, S.-B.; Ateka, E.M. Weed Species in Tomato Production and Their Role as Alternate Hosts of Tomato Spotted Wilt Virus and Its Vector Frankliniella occidentalis. Ann. Appl. Biol. 2016, 169, 224–235. [Google Scholar] [CrossRef]
- Park, J.D.; Kim, S.G.; Kim, D.I.; Cho, K. Population Dynamics of Frankliniella occidentalis on Different Rose Cultivars and Flowering Stages. J. Asia-Pac. Entomol. 2002, 5, 97–102. [Google Scholar] [CrossRef]
- Buggs, R.; Coker, T.; Denis, C.; Walker, D.; Smith, J.; Mota-Sanchez, D.; Cock, M. Plant Health—State of Research. In State of the World’s Plants; Willis, K.J., Ed.; Royal Botanic Gardens: London, UK, 2017; pp. 64–71. [Google Scholar]
- Carrizo, P.I.; Klasman, R. Frankliniella occidentalis preference for carnation varieties. Bol. Sanid. Veg. Plagas 2003, 29, 201–210. [Google Scholar]
- Katayama, H. Effect of Temperature on Development and Oviposition of Western Flower Thrips Frankliniella occidentalis (Pergande). Jpn. J. Appl. Entomol. Zool. 1997, 41, 225–231. [Google Scholar] [CrossRef]
- Olatinwo, R.O.; Prabha, T.; Paz, J.O.; Riley, D.G.; Hoogenboom, G. The Weather Research and Forecasting (WRF) Model: Application in Prediction of TSWV-Vectors Populations. J. Appl. Entomol. 2011, 135, 81–90. [Google Scholar] [CrossRef]
- Yudin, L.S.; Tabahnik, B.E.; Mitchell, W.C.; Cho, J.J. Effects of Mechanical Barriers on Distribution of Thrips (Thysanoptera: Thripidae) in Lettuce. J. Econ. Entomol. 1991, 84, 136–139. [Google Scholar] [CrossRef]
- McDonald, J.R.; Bale, J.S.; Walters, K.F.A. Effect of Temperature on Development of the Western Flower Thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). EJE 1998, 95, 301–306. [Google Scholar]
- Wang, Z.; Xu, D.; Liao, W.; Xu, Y.; Zhuo, Z. Predicting the Current and Future Distributions of Frankliniella occidentalis (Pergande) Based on the MaxEnt Species Distribution Model. Insects 2023, 14, 458. [Google Scholar] [CrossRef]
- Steiner, M.Y.; Spohr, L.J.; Goodwin, S. Relative Humidity Controls Pupation Success and Dropping Behaviour of Western Flower Thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Aust. J. Entomol. 2011, 50, 179–186. [Google Scholar] [CrossRef]
- Shipp, J.L.; Gillespie, T.J. Influence of Temperature and Water Vapor Pressure Deficit on Survival of Frankliniella occidentalis (Thysanoptera: Thripidae). Environ. Entomol. 1993, 22, 726–732. [Google Scholar] [CrossRef]
- Arévalo, H.A.; Liburd, O.E. Horizontal and Vertical Distribution of Flower Thrips in Southern Highbush and Rabbiteye Blueberry Plantings, with Notes on a New Sampling Method for Thrips Inside Blueberry Flowers. J. Econ. Entomol. 2007, 100, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, R.A. Western Flower Thrips (Frankliniella occidentalis) Management on Ornamental Crops Grown in Greenhouses: Have We Reached an Impasse? Pest Technol. 2009, 3, 1–9. [Google Scholar]
- Cloyd, R.A. Indirect Effects of Pesticides on Natural Enemies. In Pesticides–Advances in Chemical and Botanical Pesticides; Soundararajan, R.P., Ed.; IntechOpen: Rijeka, Croati, 2012; pp. 127–150. [Google Scholar]
- Avellaneda, J.; Díaz, M.; Coy-Barrera, E.; Rodríguez, D. Incidence and Preference of Frankliniella occidentalis (Thysanoptera:Thripidae) to Diferent Rose Cultivars. Arthropod Plant Interact. 2022, 16, 205–214. [Google Scholar] [CrossRef]
- Rodríguez, D.; Coy-Barrera, E. Overview of Updated Control Tactics for Western Flower Thrips. Insects 2023, 14, 649. [Google Scholar] [CrossRef]
- Durán, D. Agronomic Current Notes: Cultivating Alstroemerias; Metroflor: Norwalk, CT, USA, 2018; pp. 71–73. [Google Scholar]
- Wagstaff, C.; Bramke, I.; Breeze, E.; Thomas, B.; Buchanan-Wollaston, V.; Harrison, L.; Rogers, H.; Stead, T. Global Changes in Gene Expression during Alstroemeria Petal Senescence. Acta Hortic. 2005, 669, 127–134. [Google Scholar] [CrossRef]
- Aros, D.; Gonzalez, V.; Allemann, R.K.; Müller, C.T.; Rosati, C.; Rogers, H.J. Volatile Emissions of Scented Alstroemeria Genotypes Are Dominated by Terpenes, and a Myrcene Synthase Gene Is Highly Expressed in Scented Alstroemeria Flowers. J. Exp. Bot. 2012, 63, 2739–2752. [Google Scholar] [CrossRef]
- Aros, D.; Rogers, H.J.; Rosati, C. Floral Scent Evaluation in Alstroemeria through Gas Chromatography-Mass Spectrometry (GC-MS) and Semiquantitative RT-PCR. Acta Hortic. 2011, 886, 19–26. [Google Scholar] [CrossRef]
- Reitz, S. Frankliniella occidentalis (Western Flower Thrips); CABI Compendium; CABI International: Wallingford, UK, 2020; p. 24426. [Google Scholar]
- De Kogel, W.J.; Bosco, D.; Van Der Hoek, M.; Mollema, C. Effect of Host Plant on Body Size of Frankliniella occidentalis (Thysanoptera: Thripidae) and Its Correlation with Reproductive Capacity. Eur. J. Entomol. 1999, 96, 365–368. [Google Scholar]
- Kirk, W.D.J. The Aggregation Pheromones of Thrips (Thysanoptera) and Their Potential for Pest Management. Int. J. Trop. Insect Sci. 2017, 37, 41–49. [Google Scholar] [CrossRef]
- Nyasani, J.O.; Meyhöfer, R.; Subramanian, S.; Poehling, H.M. Feeding and Oviposition Preference of Frankliniella occidentalis for Crops and Weeds in Kenyan French Bean Fields. J. Appl. Entomol. 2013, 137, 204–213. [Google Scholar] [CrossRef]
- Lindén, A.; Mäntyniemi, S. Using the Negative Binomial Distribution to Model Overdispersion in Ecological Count Data. Ecology 2011, 92, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Pearsall, I.A. Flower Preference Behaviour of Western Flower Thrips in the Similkameen Valley, British Columbia, Canada. Entomol. Exp. Appl. 2000, 95, 303–313. [Google Scholar] [CrossRef]
- Elimem, M.; Chermiti, B. Population Dynamics of Frankliniella occidentalis Pergande (1895) (Thysanoptera: Thripidae) and Evaluation of Its Different Ecotypes and Their Evolution in a Rose (Rosa hybrida) Greenhouse in the Sahline Region, Tunisia. Afr. J. Plant Sci. Biotechnol. 2009, 3, 53–62. [Google Scholar]
- De Kogel, W.J. Preference and Performance of Western Flower Thrips. In Thrips and Tospoviruses: Proceedings of the 7th International Symposium on Thysanoptera; Australian National Insect Collection: Canberra, Australia, 2022; pp. 181–183. [Google Scholar]
- Kakkar, G.; Seal, D.R.; Stansly, P.A.; Liburd, O.E.; Kumar, V. Abundance of Frankliniella schultzei (Thysanoptera: Thripidae) in Flowers on Major Vegetable Crops of South Florida. Fla. Entomol. 2012, 95, 468–475. [Google Scholar] [CrossRef]
- Rahman, T.; Spafford, H.; Broughton, S. Variation in Preference and Performance of Frankliniella occidentalis (Thysanoptera: Thripidae) on Three Strawberry Cultivars. J. Econ. Entomol. 2010, 103, 1744–1753. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wu, Q.J.; Li, X.F.; Zhang, Y.J.; Xu, B.Y.; Zhu, G.R. Life History of Western Flower Thrips, Frankliniella occidentalis (Thysan., Thripae), on Five Different Vegetable Leaves. J. Appl. Entomol. 2007, 131, 347–354. [Google Scholar] [CrossRef]
- Elimem, M.; Chermiti, B. Color Preference of Frankliniella occidentalis (Pergande) (Thysanoptera; Thripidae) and Orius sp. (Hemiptera; Anthocorridae) Populations on Two Rose Varieties. Floric. Ornam. Biotechnol. 2013, 7, 94–98. [Google Scholar]
- Blumthal, M.R.; Cloyd, R.A.; Spomer, L.A.; Warnock, D.F. Flower Color Preferences of Western Flower Thrips. Horttechnology 2005, 15, 846–853. [Google Scholar] [CrossRef]
- Stukenberg, N.; Pietruska, M.; Waldherr, A.; Meyhöfer, R. Wavelength-Specific Behavior of the Western Flower Blue-Green Chromatic Mechanism. Insects 2020, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Chermenscaya, T.D.; Pow, E.M.; Woodcock, C.; Maniar, S.; Shamshev, I.V.; Selytskaya, O.G.; Burov, V.N.; Roditakis, N. Behavioural Responses of Western Flower Thrips, Frankliniella occidentalis (Pergande), to Volatiles from Three Aromatic Plants. Int. J. Trop. Insect Sci. 2001, 21, 67–72. [Google Scholar] [CrossRef]
- Avellaneda, J.; Díaz, M.; Coy-Barrera, E.; Rodríguez, D.; Osorio, C. Rose Volatile Compounds Allow the Design of New Control Strategies for the Western Flower Thrips (Frankliniella occidentalis). J. Pest Sci. 2021, 94, 129–142. [Google Scholar] [CrossRef]
- Aros, D.; Suazo, M.; Rivas, C.; Zapata, P.; Úbeda, C.; Bridgen, M. Molecular and Morphological Characterization of New Interspecific Hybrids of Alstroemeria Originated from A. caryophylleae Scented Lines. Euphytica 2019, 215, 93. [Google Scholar] [CrossRef]
- Kirk, W.D.J. Pollen-Feeding and the Host Specificity and Fecundity of Flower Thrips (Thysanoptera). Ecol. Entomol. 1985, 10, 281–289. [Google Scholar] [CrossRef]
- Teulon, D.A.J.; Penman, D.R. Effects of Temperature and Diet on Oviposition Rate and Development Time of the New Zealand Flower Thrips. Thrips Obscuratus. Entomol. Exp. Appl. 1991, 60, 143–155. [Google Scholar] [CrossRef]
- Hulshof, J.; Vanninen, I. Western Flower Thrips Feeding on Pollen, and Its Implications for Control. In Thrips and Tospoviruses: Proceedings of the 7th International Symposium on Thysanoptera; Australian National Insect Collection: Canberra, Australia, 2002; pp. 173–179. [Google Scholar]
- Abdullah, Z.S.; Ficken, K.J.; Greenfield, B.P.J.; Butt, T.M. Innate Responses to Putative Ancestral Hosts: Is the Attraction of Western Flower Thrips to Pine Pollen a Result of Relict Olfactory Receptors? J. Chem. Ecol. 2014, 40, 534–540. [Google Scholar] [CrossRef]
- Cao, Y.; Reitz, S.R.; Germinara, G.S.; Wang, C.; Wang, L.; Yang, S.; Gao, Y.; Zhang, W.; Li, C. Host Preference of Thrips Hawaiiensis for Different Ornamental Plants. J. Pest Sci. 2022, 95, 761–770. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).