Effects of Different Shade Treatments on the Epidermal Wax Deposition of Hosta Genotypes with Different Glaucousness of Leaf Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Shading Treatments
2.2. Micromorphology of Epicuticular Wax Crystals by SEM
2.3. Total Epicuticular Wax Determination and Composition Analysis
3. Results
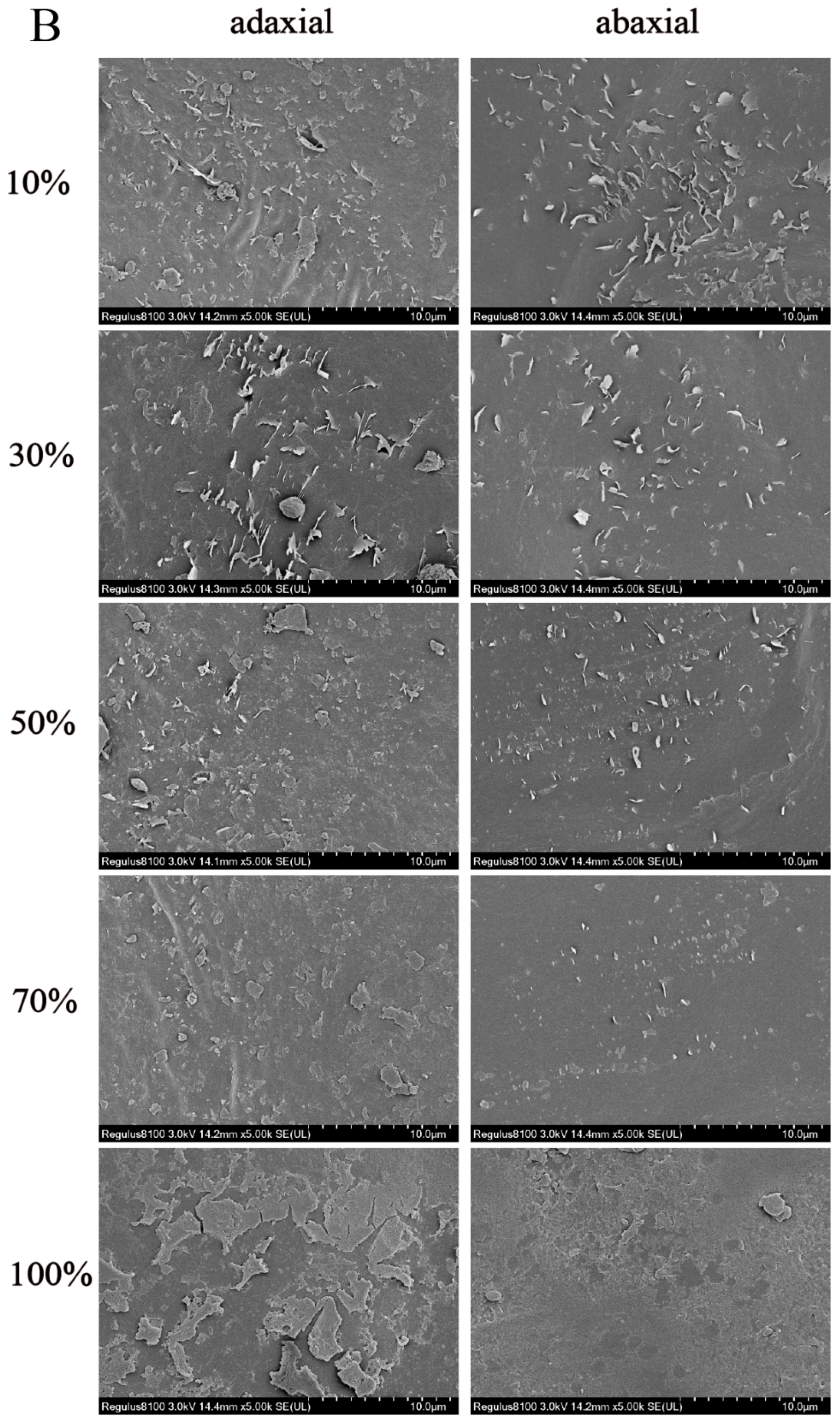
3.1. The Micromorphology of Epicuticular Wax Crystals in Different Shade Treatments
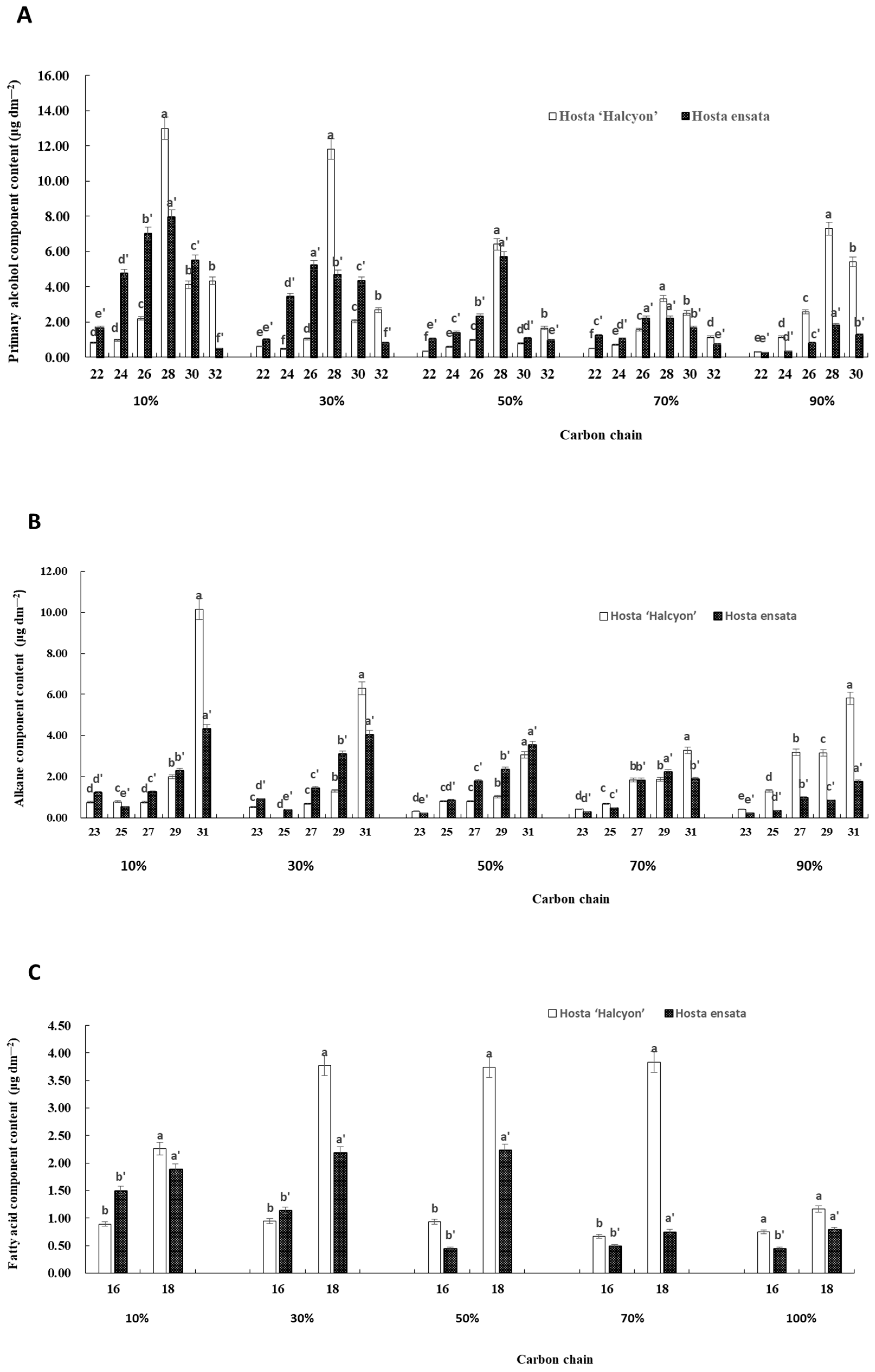
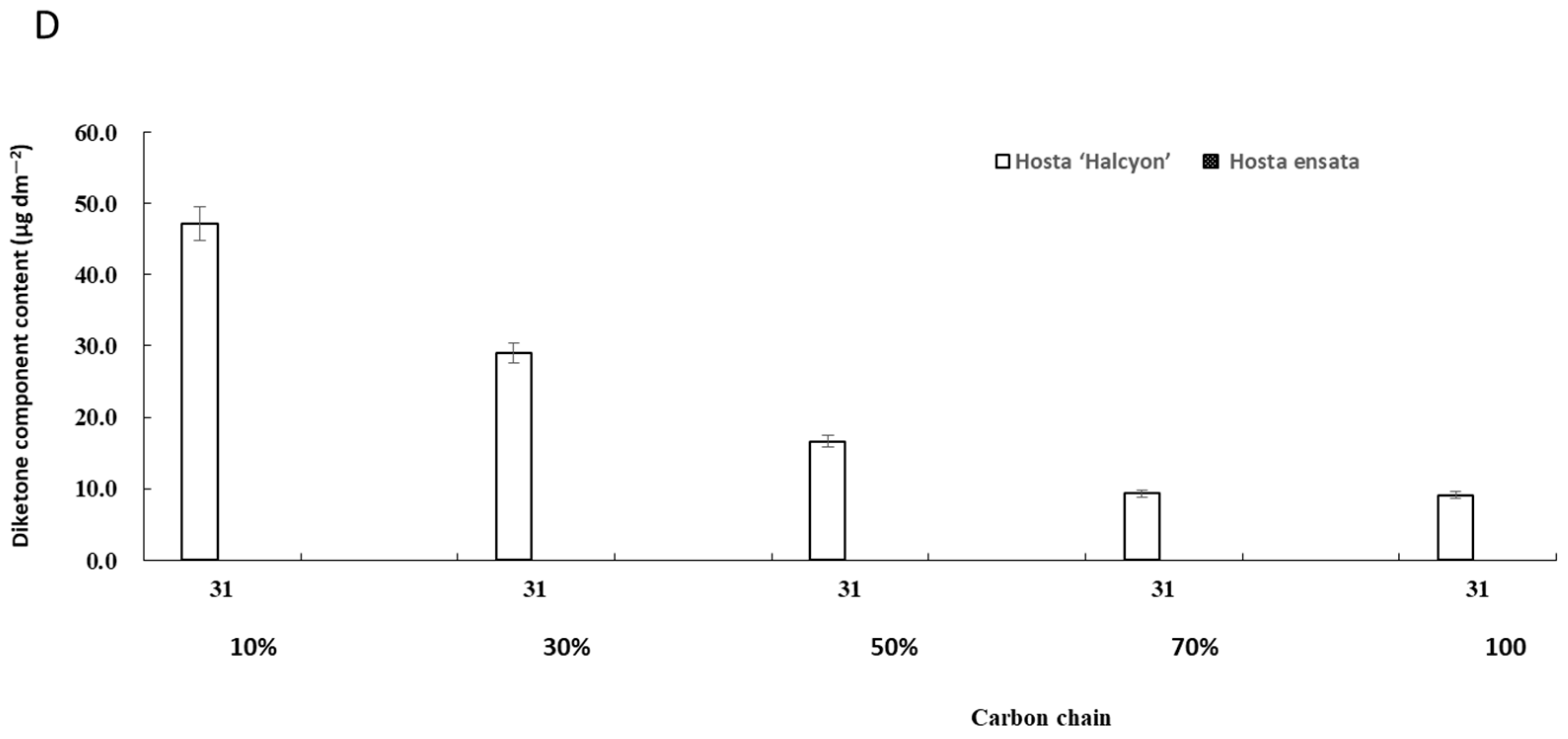
3.2. Effect of Different Shade Treatments on the Chemical Composition and the Content of Epidermal Wax
3.3. The Carbon Chain Length of Epicuticular Wax under Different Light Intensities
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yeats, T.H.; Rose, J.K. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, J.C.; Wei, J.; Niu, C.D.; Yang, D.G.; Jiang, B.W. Response of photosynthesis, the xanthophyll cycle, and wax in Japanese yew (Taxus cuspidata L.) seedlings and saplings under high light conditions. PeerJ 2023, 11, e14757. [Google Scholar] [CrossRef] [PubMed]
- Grünhofer, P.; Herzig, L.; Sent, S.; Viktoria, V.Z.D.; Schreiber, L. Increased cuticular wax deposition does not change residual foliar transpiration. Plant Cell Environ. 2022, 45, 1157–1171. [Google Scholar] [CrossRef]
- Nyadanu, D.; Lowor, S.; Akrofi, A.; Adomako, B.; Dzahini-Obiatey, H.; Akromah, R.; Awuah, R.; Kwoseh, C.; Adu-Amoah, R.; Kwarteng, A. Mode of inheritance and combining ability studies on epicuticular wax production in resistance to black pod disease in cacao (Theobroma cacao L.). Sci. Hortic. 2019, 243, 34–40. [Google Scholar] [CrossRef]
- Guo, Y.-J.; NI, Y.; Guo, Y.-J.; Han, L.; Tang, H.; Yu, Y.-X. Effect of soil water deficit and high temperature on leaf cuticular waxes and physiological indices in Alfalfa (Medicago sativa) Leaf. Acta Agron. Sin. 2011, 37, 911–917. [Google Scholar] [CrossRef]
- Szafranek, B.M.; Synak, E.E. Cuticular waxes from potato (Solanum tuberosum) leaves. Phytochemistry 2006, 67, 80–90. [Google Scholar] [CrossRef]
- Jenks, M.A.; Rich, P.J.; Rhodes, D.; Ashworth, E.N.; Axtell, J.D.; Ding, C.K. Leaf sheath cuticular waxes on bloomless and sparse-bloom mutants of Sorghum bicolor. Phytochemistry 2000, 54, 577–584. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C.; Cutler, D. Classification and terminology of plant epicuticular waxes. Bot. J. Linn. Soc. 1998, 126, 237–260. [Google Scholar]
- Sarkar, S.; Arya, G.C.; Negin, B.; Manasherova, E.; Levy, M.; Aharoni, A.; Cohen, H. Compositional variances in cuticular lipids of wild and domesticated barley leaves and their impact on plant-environment interactions. Environ. Exp. Bot. 2023, 206, 105140. [Google Scholar] [CrossRef]
- Cheng, G.; Huang, H.; Zhou, L.; He, S.; Zhang, Y.; Cheng, X. Chemical composition and water permeability of the cuticular wax barrier in rose leaf and petal: A comparative investigation. Plant Physiol. Biochem. 2019, 135, 404–410. [Google Scholar] [CrossRef]
- Jetter, R.; Schäffer, S.; Riederer, M. Leaf cuticular waxes are arranged in chemically and mechanically distinct layers: Evidence from Prunus laurocerasus L. Plant Cell Environ. 2001, 23, 619–628. [Google Scholar] [CrossRef]
- Koch, K.; Ensikat, H.-J. The hydrophobic coatings of plant surfaces: Epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron 2008, 39, 759–772. [Google Scholar] [CrossRef]
- Jenks, M.A.; Rich, P.J.; Ashworth, E.N. Involvement of cork cells in the secretion of epicuticular wax filaments on Sorghum bicolor (L.) Moench. Int. J. Plant Sci. 1994, 155, 506–518. [Google Scholar]
- Negin, B.; Shelly, H.A.; Efrat, A.S.; Shachar, L.; Jander, G.; Aharoni, A. Tree tobacco (Nicotiana glauca) cuticular wax composition is essential for leaf retention during drought, facilitating a speedy recovery following rewatering. New Phytol. 2022, 237, 1574–1589. [Google Scholar] [CrossRef] [PubMed]
- Thippeswamy, H.; Krishna, H.; Sinha, N.; Gajghate, R.; Jain, N.; Singh, P.K.; Singh, G.P. Assessing the role of glaucousness in imparting tolerance to moisture and heat stress in wheat. Biologia 2022, 77, 3279–3289. [Google Scholar] [CrossRef]
- Dodd, R.S.; Poveda, M.M. Environmental gradients and population divergence contribute to variation in cuticular wax composition in Juniperus communis. Biochem. Syst. Ecol. 2003, 31, 1257–1270. [Google Scholar] [CrossRef]
- Shepherd, T.; Robertson, G.W.; Griffiths, D.W.; Birch, A.N.E.; Duncan, G. Effects of environment on the composition of epicuticular wax from kale and Swede. Phytochemistry 1997, 40, 407–417. [Google Scholar] [CrossRef]
- Steinmüller, D.; Tevini, M. Action of ultraviolet radiation (UV-B) upon cuticular waxes in some crop plants. Planta 1985, 164, 557–564. [Google Scholar] [CrossRef]
- Hatterman, V.H.; Pitty, A.; Owen, M. Environmental effects on velvetleaf (Abutilon theophrasti) epicuticular wax deposition and herbicide absorption. Weed Sci. 2011, 59, 14–21. [Google Scholar] [CrossRef]
- Sánchez, F.J.; Manzanares, M.; de Andrés, E.F.; Tenorio, J.L.; Ayerbe, L. Residual transpiration rate, epicuticular wax load and leaf colour of pea plants in drought conditions. Influence on harvest index and canopy temperature. Eur. J. Agron. 2001, 15, 57–70. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, S.; Zhao, S.; Song, J.; Sun, J.; Cui, N.; Chen, X.; Qu, B. Effects of light intensity on the photosynthetic characteristics of Hosta genotypes differing in the glaucousness of leaf surface. Sci. Hortic. 2024, 327, 112834. [Google Scholar] [CrossRef]
- Shao, M.N.; Hao, X.; Cui, N.; Qu, B.; Guan, P.; Jia, W.K.; Xu, Y.F. Effects of epicuticular waxes on the physiological characteristics of blue-leaf Hosta. Acta Hortic. Sin. 2020, 47, 1401–1411. [Google Scholar] [CrossRef]
- Shi, A.P.; Zhang, J.Z.; Zhang, Q.X.; Shi, L. Growth characteristic analyse of shading levels on four Hosta cultivars. Bull. Bot. Res. 2004, 24, 486–490. Available online: https://bbr.nefu.edu.cn/EN/Y2004/V24/I4/486 (accessed on 8 May 2024).
- Cajuste, J.F.; González-Candelas, L.; Veyrat, A.; García-Breijo, F.J.; Reig-Armiñana, J.; Lafuente, M.T. Epicuticular wax content and morphology as related to ethylene and storage performance of ‘Navelate’ orange fruit. Postharvest Biol. Technol. 2010, 55, 29–35. [Google Scholar] [CrossRef]
- Jenks, M.A.; Gaston, C.H.; Goodwin, M.S.; Keith, J.A.; Teusink, R.S. Seasonal variation in cuticular waxes on Hosta genotypes differing in leaf surface glaucousness. Hortscience 2002, 37, 673–677. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Wang, J. Effects of Light Intensity and Temperature on the Photosynthesis Characteristics and Yield of Lettuce. Horticulturae 2022, 8, 178. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Hang, T.; Li, P. Photosynthetic characteristics and growth performance of lettuce (Lactuca sativa L.) under different light/dark cycles in mini plant factories. Photosynthetica 2020, 58, 740–747. [Google Scholar] [CrossRef]
- Benítez, J.J.; Moreno, A.G.; Puyol, S.G.; Guerrero, J.A.H.; Heredia, A.; Domínguez, E. The response of tomato fruit cuticle membranes against heat and light. Front. Plant Sci. 2022, 12, 807723. [Google Scholar] [CrossRef]
- Shaheenuzzamn, M.; Shi, S.; Sohail, K.; Wu, H.; Liu, T.; An, P.; Wang, Z.; Hasanuzzaman, M. Regulation of cuticular wax biosynthesis in plants under abiotic stress. Plant Biotechnol. Rep. 2021, 15, 1–12. [Google Scholar] [CrossRef]
- Huang, L.; Xiao, Q.L.; Zhao, X.; Wang, D.K.; Wei, L.L.; Li, X.T.; Liu, Y.T.; He, Z.B.; Kang, L.; Guo, Y.J. Responses of cuticular waxes of faba bean to light wavelengths and selection of candidate genes for cuticular wax biosynthesis. Plant Genome 2020, 13, e20058. [Google Scholar] [CrossRef]
- Upadhyaya, M.K.; Furness, N.H. Influence of light intensity and water stress on leaf surface characteristics of Cynoglossum officinale, Centaurea spp., and Tragopogon spp. Can. J. Bot. 1994, 72, 1379–1386. [Google Scholar]
- Lu, W.J.; Zheng, W.W.; Wu, Y.N.; Zang, Y.X. Research review on features and molecular mechanism of wax formation in Brassicaceae. J. Zhejiang AF Univ. 2021, 38, 205–213. [Google Scholar] [CrossRef]
- Lee, S.B.; Suh, M.C. Regulatory mechanisms underlying cuticular wax biosynthesis. J. Exp. Bot. 2022, 73, 2799–2816. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Li, W. Genetic interactions underlying the biosynthesis and inhibition of β-Diketones in wheat and their impact on glaucousness and cuticle permeability. PLoS ONE 2013, 8, e54129. [Google Scholar] [CrossRef]
- Camarillo-Castillo, F.; Huggins, T.D.; Mondal, S.; Reynolds, M.P.; Tilley, M.; Hays, D.B. High-resolution spectral information enables phenotyping of leaf epicuticular wax in wheat. Plant Methods 2021, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Kakani, V.G.; Reddy, K.R.; Zhao, D.; Mohammed, A.R. Effects of ultraviolet-B radiation on cotton (Gossypium hirsutum L.) morphology and anatomy. Ann. Bot. 2003, 91, 817–826. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.J.; Wang, M.K.; Bi, Q.X.; Cui, Y.F.; Wang, L.B. Transcriptome and physiological analyses provide insights into the leaf epicuticular wax accumulation mechanism in yellow horn. Hortic. Res. 2021, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Alam, B.; Singh, R.; Newaj, R. Comparative adaptive traits in green gram (Vigna radiata L.) and soybean (Glycine max L.) as influenced by varying regimes of shade. Range Manag. Agrofor. 2012, 33, 142–146. [Google Scholar]
- Martel, A.B.; Taylor, A.E.; Qaderi, M.M. Individual and interactive effects of temperature and light intensity on canola growth, physiological characteristics and methane emissions. Plant Physiol. Biochem. 2020, 157, 160–168. [Google Scholar] [CrossRef]
- Lykholat, Y.V.; Khromykh, N.O.; Pirko, Y.V.; Alexeyeva, A.A.; Pastukhova, N.L.; Blume, Y.B. Epicuticular wax composition of leaves of Tilia L. trees as a marker of adaptation to the climatic conditions of the steppe dnieper. Cytol. Genet. 2018, 52, 323–330. [Google Scholar] [CrossRef]
- Liakoura, V.; Manetas, Y.; Karabourniotis, G. Seasonal fluctuations in the concentration of U.V.-absorbing compounds in the leaves of some Mediterranean plants under field conditions. Physiol. Plant. 2001, 111, 491–500. [Google Scholar] [CrossRef] [PubMed]
- He, Z.-S.; Tang, R.; Li, M.-J.; Jin, M.-R.; Xin, C.; Liu, J.-F.; Hong, W. Response of photosynthesis and chlorophyll fluorescence parameters of Castanopsis kawakamii seedlings to forest gaps. Forests 2020, 11, 21. [Google Scholar] [CrossRef]
- Bi, J.S.; Zhai, G.Q.; Guo, S.Y.; Zhang, H.; Li, J.P.; Zhou, S.T.; Zhang, X.; Song, C.P. Trade-offs between the accumulation of cuticular wax andjasmonic acid-mediated herbivory resistance in maize. J. Integr. Plant Biol. 2023, 66, 143–159. [Google Scholar] [CrossRef]
- Eigenbrode, S. The effects of plant epicuticular waxy blooms on attachment and effectiveness of predatory insects. Arthropod Struct. Dev. 2004, 33, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.F.A.; Meirelles, T.S.; Salatino, A. Epicuticular waxes from caatinga and cerrado species and their efficiency against water loss. An. Acad. Bras. Cienc. 2003, 75, 431–439. [Google Scholar] [CrossRef]
- Brennan, E.B.; Hrusa, G.F.; Weinbaum, S.A.; Leviso, W.J. Resistance of Eucalyptus species to Glycaspis brimblecombei (Homoptera: Psyllidae) in the San Francisco Bay area. Pan-Pac. Entomol. 2001, 77, 249–253. [Google Scholar]
- Stork, N.E. Role of Waxblooms in preventing attachment to brassicas by the mustard beetle, Phaedon cochleariae. Entomol. Exp. Appl. 1980, 28, 100–107. [Google Scholar]
- Wang, B.; Jeffers, S.N. Fusarium root and crown rot: A disease of container-grown Hostas. Plant Dis. 2000, 84, 980–988. [Google Scholar] [CrossRef][Green Version]
- Jagdale, G.B.; Grewal, P.S. Influence of the entomopathogenic nematode Steinernema carpocapsae infected host cadavers or their extracts on the foliar nematode Aphelenchoides fragariae on Hosta in the greenhouse and laboratory. Biol. Control 2008, 44, 13–23. [Google Scholar] [CrossRef]
- Li, H.Y.; Soares, M.A.; Torres, M.S.; Bergen, M.; White, J.F. Endophytic bacterium, Bacillus amyloliquefaciens, enhances ornamental hosta resistance to diseases and insect pests. J. Plant Interact. 2015, 10, 224–229. [Google Scholar] [CrossRef]
- Bhanot, V.; Fadanavis, S.V.; Panwar, J. Revisiting the architecture, biosynthesis and functional aspects of the plant cuticle: There is more scope. Environ. Exp. Bot. 2021, 183, 104364. [Google Scholar] [CrossRef]
- Pilon, J.J.; Lambers, H.; Baas, W.; Tosserams, M.; Rozema, J.; Atkin, O.K. Leaf waxes of slow-growing alpine and fast-growing lowland Poa species: Inherent differences and responses to UV-B radiation. Phytochemistry 1999, 50, 571–580. [Google Scholar] [CrossRef]
- Gonzalez, R.; Paul, N.D.; Percy, K.; Ambrose, M.; McLaughlin, C.K.; Barnes, J.D.; Areses, M.; Wellburn, A.R. Responses to ultraviolet-B radiation (280–315 nm) of pea (Pisum sativum) lines differing in leaf surface wax. Physiol. Plant. 1996, 98, 852–860. [Google Scholar] [CrossRef]
- Shepherd, T.; Griffiths, D.W. The effects of stress on plant cuticular waxes. New Phytol. 2006, 171, 469–499. [Google Scholar] [CrossRef]
- Gordon, D.C.; Percy, K.E.; Riding, R.T. Effects of u.v.-B radiation on epicuticular wax production and chemical composition of four Picea species. New Phytol. 1998, 138, 441–449. [Google Scholar] [CrossRef]
- Pieniazek, F.; Dasgupta, M.; Messina, V.; Devi, M.P.; Devi, Y.I.; Mohanty, S.; Singh, S.; Sahoo, B.B.; Nongdam, P.; Acharya, G.C.; et al. Differential occurrence of cuticular wax and its role in leaf physiological mechanisms of three edible aroids of Northeast India. Agriculture 2022, 12, 724. [Google Scholar] [CrossRef]
- Jansen, B.; Nierop, K.G.; Hageman, J.A.; Cleef, A.M.; Verstraten, J.M. The straight-chain lipid biomarker composition of plant species responsible for the dominant biomass production along two altitudinal transects in the Ecuadorian Andes. Org. Geochem. 2006, 37, 1514–1536. [Google Scholar] [CrossRef]
- Otto, A.; Shunthirasingham, C.; Simpson, M.J. A comparison of plant and microbial biomarkers in grassland soils from the Prairie Ecozone of Canada. Org. Geochem. 2004, 36, 425–448. [Google Scholar] [CrossRef]
- Eglinton, G.; Hamilton, R.J. Leaf Epicuticular Waxes. Science 1967, 156, 1322–1335. [Google Scholar]
- Fukuda, S.; Satoh, A.; Kasahara, H.; Matsuyama, H.; Takeuchi, Y. Effects of ultraviolet-B irradiation on the cuticular wax of cucumber (Cucumis sativus) cotyledons. J. Plant Res. 2008, 121, 179–189. [Google Scholar]
- Mohammadian, M.A.; Watling, J.R.; Hill, R.S. The impact of epicuticular wax on gas-exchange and photoinhibition in Leucadendron lanigerum (Proteaceae). Acta Oecologica 2007, 31, 93–101. [Google Scholar] [CrossRef]

| Hosta Genotype | RLI | Alkanes | Primary Alcohols | Fatty Acids | β-Diketones | Aliphatic Esters | Unknowns | Total Content |
|---|---|---|---|---|---|---|---|---|
| 10% | 14.5 ± 0.6 b | 25.4 ± 1.0 a | 3.1 ± 0.1 b | 47.1 ± 1.8 a | 1.0 ± 0.0 b | 4.0 ± 0.2 c | 95.1 ± 3.6 a | |
| H. ‘Halcyon’ | 30% | 9.1 ± 0.7 d | 19.6 ± 1.6 c | 4.7 ± 0.4 a | 29.0 ± 2.3 b | 1.5 ± 0.01 a | 3.8 ± 0.3 c | 67.8 ± 5.4 b |
| 50% | 5.6 ± 0.6 e | 10.3 ± 1.2 d | 4.6 ± 0.5 a | 16.5 ± 1.9 c | 0.1 ± 0.00 c | 1.3 ± 0.2 d | 38.3 ± 4.7 c | |
| 70% | 10.3 ± 0.1 c | 11.2 ± 0.1 d | 1.9 ± 0.0 c | 9.3 ± 0.1 d | 0.9 ± 0.0 b | 10.6 ± 0.1 b | 44.2 ± 0.4 c | |
| 100% | 16.2 ± 0.1 a | 22.5 ± 0.2 b | 4.3 ± 0.0 a | 9.1 ± 0.1 d | 1.4 ± 0.0 a | 16.3 ± 0.1 a | 70.3 ± 0.6 b | |
| 10% | 10.1 ± 0.0 a | 30.5 ± 0.1 a | 3.4 ± 0.0 a | nd | 0.9 ± 0.0 b | 5.2 ± 0.0 b | 50.1 ± 0.1 a | |
| H. ensata | 30% | 10.2 ± 0.4 a | 21.3 ± 0.7 b | 3.3 ± 0.1 a | nd | 01.1 ± 0.0 a | 3.1 ± 0.1 c | 39.0 ± 1.4 b |
| 50% | 10.4 ± 01.8 a | 14.1 ± 2.4 c | 2.7 ± 0.5 b | nd | 0.8 ± 0.1 c | 10.5 ± 1.7 a | 38.4 ± 6.5 bc | |
| 70% | 6.3 ± 1.2 b | 14.1 ± 1.6 c | 1.2 ± 0.1 c | nd | 0.6 ± 0.1 d | 9.7 ± 0.9 a | 32.0 ± 3.9 c | |
| 100% | 4.8 ± 0.1 b | 8.9 ± 0.3 d | 1.2 ± 0.0 c | nd | 0.2 ± 0.0 e | 5.7 ± 0.1 b | 20.8 ± 3.4 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, P.; Chen, S.; Sun, J.; Zhao, S.; Fan, R.; Xu, Y.; Qu, B. Effects of Different Shade Treatments on the Epidermal Wax Deposition of Hosta Genotypes with Different Glaucousness of Leaf Surface. Horticulturae 2024, 10, 981. https://doi.org/10.3390/horticulturae10090981
Guan P, Chen S, Sun J, Zhao S, Fan R, Xu Y, Qu B. Effects of Different Shade Treatments on the Epidermal Wax Deposition of Hosta Genotypes with Different Glaucousness of Leaf Surface. Horticulturae. 2024; 10(9):981. https://doi.org/10.3390/horticulturae10090981
Chicago/Turabian StyleGuan, Ping, Siyu Chen, Jiaying Sun, Shuyi Zhao, Ren Fan, Yufeng Xu, and Bo Qu. 2024. "Effects of Different Shade Treatments on the Epidermal Wax Deposition of Hosta Genotypes with Different Glaucousness of Leaf Surface" Horticulturae 10, no. 9: 981. https://doi.org/10.3390/horticulturae10090981
APA StyleGuan, P., Chen, S., Sun, J., Zhao, S., Fan, R., Xu, Y., & Qu, B. (2024). Effects of Different Shade Treatments on the Epidermal Wax Deposition of Hosta Genotypes with Different Glaucousness of Leaf Surface. Horticulturae, 10(9), 981. https://doi.org/10.3390/horticulturae10090981






