Abstract
The MYB gene family, widely distributed across a variety of plants, plays a crucial role in the phenylpropane metabolic pathway. In this study, we identified 146 R2R3-MYB genes in the ‘NanGuo’ pear genome by screening its gene sequences. Phylogenetic analysis divided these genes into seven subfamilies, and we examined each for stability through analyses of conserved structural domains and motifs. In addition, differences in the expression levels between two varieties, the ‘NanGuo’ pear and its red bud sport variant ‘NanHong’ pear, were investigated using quantitative real-time PCR (qRT-PCR). The results revealed that the expression levels of 12 R2R3-MYB transcription factors (TFs) corresponded with the trends in anthocyanin content. Specifically, the expression trends of eight R2R3-MYB TFs positively correlated with anthocyanin accumulation, whereas four exhibited opposite trends, suggesting their negatively regulatory role in anthocyanin accumulation. This study not only enhances our understanding of the MYB gene family in the ‘NanGuo’ pear genome but also lays a solid foundation for future research into the functional roles of PuMYBs.
1. Introduction
MYB transcription factors (TFs), one of the largest TF families in plants [1], possess a highly conserved N-terminal MYB structural domain [2,3]. This domain comprises 1–4 tandem and incomplete repeat sequences (R) [4], each containing 50–55 conserved amino acid residues [5]. Based on the number of R structures in the MYB gene, MYB TFs are categorized into four types [6]: 1R-MYB (R1/2, R3-MYB), 2R-MYB (R2R3-MYB), 3R-MYB (R1R2R3-MYB), and 4R-MYB (R1R2R2R1/2-MYB). 1R-MYB (R1/2, R3-MYB) is mainly involved in plant morphogenesis [7,8]. 2R-MYB (R2R3-MYB) is the most common and widely distributed, containing two R structures, and it is involved in various life processes, including cell differentiation, hormone signaling, growth and development regulation, and responses to biotic and abiotic stresses [9,10]. 3R-MYB (R1R2R3-MYB) is present in most eukaryotic genomes [11,12]. 4R-MYB (R1R2R2R1/2-MYB) is the least common [13]. Limited studies have explored the functions of the R4-MYB proteins in plants [14,15].
An increasing number of studies have indicated that genes from the MYB family play crucial roles in both plant growth and development and in responding to stresses. For instance, in pumpkin, CmoMYB99 and CmoMYB144 are specifically involved in response to drought stress [16]. In ginger, ZoMYB53 and ZoMYB118 are substantially induced by various stresses, including salt, Abscisic acid (ABA), cold, and drought [17]. By contrast, the overexpression of AtMYB75 in transgenic lines significantly increased flavonoid accumulation, antioxidant activity, and tolerance to abiotic stresses, including oxidative and drought stress [18]. In Arabidopsis thaliana, AtMYB11, AtMYB12, and AtMYB111 regulate the expression of key genes that influence flavonol biosynthesis [19,20,21]. MYB family genes play a crucial role in hormone signaling during plant development, particularly in regulating ABA levels and responding to abiotic stresses, such as drought, salinity, and cold. In addition, these genes affect the growth and development of plant organs and regulate various plant life processes [22]. Furthermore, MYB family genes are integral to phenylalanine metabolism, a key aspect of plant growth and development. Phenylpropanoid metabolism, one of the most critical metabolic pathways in plants, is vital for antistress and antioxidant activities, enhancing plant stress tolerance. This pathway also produces diverse secondary metabolites, including flavonoids and phenolics, which are essential for the plants’ vital functions [23].
Anthocyanidin, a key product of phenylpropane metabolism, is a primary pigment contributing to plant color during growth and development. As a secondary metabolite, anthocyanins are part of the flavonoid metabolism pathway and are water-soluble flavonoid compounds [24,25]. They enhance the antioxidant capacity of plants, contributing to their health and stress resilience [26,27]. Research indicates that the biosynthesis of plant anthocyanins is regulated by numerous genes, with the MYB genes family playing a particularly prominent role [28,29]. Within this family, the R2R3-MYB TFs are crucial for regulating anthocyanin accumulation [30]. For example, in Arabidopsis, the R2R3-MYB TFs AtMYB113 and AtMYB114 are specifically responsible for regulating anthocyanin levels [31]. The R2R3-MYB TF GhMYB1a regulates anthocyanin accumulation in Gerbera hybrida [32]. In apples, MdMYB10 affect anthocyanin levels [33,34]. In grapes, the skin color is modulated by the R2R3-MYB TFs VvMYBA1 and VvMYBA2 [35]. In kiwifruit, AcMYB10 has been shown to play an important role in anthocyanin biosynthesis [36]. However, not all R2R3-MYB TFs promote anthocyanin production; some act as inhibitors. For instance, PlgMYBR1 serves as a negative regulator of anthocyanin biosynthesis in Platycodon grandiflorus [37]. Similarly, in peach, PpMYB10 prompts the negative regulator PpMYB18 to balance the accumulation of anthocyanins and proanthocyanins in peach [38]. Overall, R2R3-MYB TFs play a pivotal role in regulating anthocyanin effects across various members of the Rosaceae family [39].
The ‘NanGuo’ pear (Pyrus ussuriensis ‘NanGuo’ pear) is a special fruit grown in Liaoning Province, China [40]. It is one of the four famous pears in China and is a rare pear resource in the world. The ‘NanHong’ pear is a red bud variety of the ‘NanGuo’ pear. In this study, we identified 146 R2R3-MYB TFs by analyzing the genome of the ‘NanGuo’ pear. We comprehensively analyzed their homology, conserved structural domains, motifs, gene structure, physicochemical properties, and cis-acting response elements. In addition, 68 genes and 3 anthocyanin-related structural genes were selected for qRT-PCR, comparing Pyrus ussuriensis, the ‘NanGuo’ pear, with its red bud variety, the ‘NanHong’ pear [41,42]. Previous studies have demonstrated that anthocyanins substantially affect the coloration of plant fruits [43,44]. Thus, in this study, we specifically investigated the role of PuMYBs (“Pu” for Pyrus ussuriensis) in regulating anthocyanin accumulation in red-skinned pears. We conducted qRT-PCR analyses to identify 12 R2R3-MYB TFs affecting anthocyanin accumulation. In this study, key PuMYBs affecting anthocyanin accumulation in red pears were screened to provide a theoretical basis for the future cultivation of red pears and to improve their economic and food values.
2. Materials and Methods
2.1. Plant Material and Treatment
The experimental materials used for this study were the ‘NanGuo’ pear and its bud sport variety, the ‘NanHong’ pear, grown in QianShan District, Anshan City, Liaoning Province. The fruits were harvested at 30 d (20 May 2021), 60 d (20 June 2021), 90 d (20 July 2021), and 120 d (20 August 2021) after full bloom (DAFB). Following harvest, the skin and pulp of the fruits were collected separately and stored at −80 °C using liquid nitrogen quick-freezing.
2.2. Identification of MYB Gene Family Members in ‘NanGuo’ Pear
For gene analysis, the hidden Markov model mapping of the MYB DNA structural domain (PF00249) was queried using the Pfam database (http://pfam.xfam.org/search, accessed on 1 September 2023). TBtools (v2.119) softwarewas employed to compare similar sequences with known R2R3-MYB gene sequences. This combined approach was used to screen candidate genes. Protein sequences of Arabidopsis MYB family genes were obtained from the Arabidopsis Genome Database (TAIR, https://www.arabidopsis.org/, accessed on 1 September 2023). To assess the homology between the two species, a phylogenetic tree was constructed. The comparison utilized Blastp, and a local protein sequence database was created. After the identification of conserved structural domains, the sequences were sequentially renamed starting with PuMYB1. Noncompliant sequences were excluded based on the MYB Typical Structural Domains model, and the remaining genes were retained for further analysis.
2.3. Phylogenetic Analysis
A phylogenetic tree was constructed using the protein sequences of MYB genes from the ‘NanGuo’ pear and Arabidopsis thaliana. This tree was generated in MEGA11 by using the maximum likelihood (ML) method, with a model assumption of the JTT matrix, and 1000 bootstrap repeats to infer evolutionary history. The tree was then enhanced visually using iTOL (https://itol.embl.de/#/, accessed on 1 September 2023).
2.4. Conserved Structural Domains, Conserved Motif Identification, Gene Structure Analysis, and Physicochemical Property Analysis
To analyze the structure of the ‘NanGuo’ pear MYB genes, we identified conserved motifs by using the MEME tool (https://meme-suite.org/meme/tools/meme, accessed on 1 September 2023) [45], with a maximum of seven motifs allowed. The conserved structural domains were then analyzed using the NCBI’s Conserved Domain Database (CDD, http://www.ncbi.nlm.nih.gov/cdd/, accessed on 1 September 2023). The exon–intron structures of PuMYBs were delineated using the Gene Structure Display Server (http://gsds.cbi.pku.edu.cn/, accessed on 1 September 2023), and both conserved motifs and structural domains were visualized using TBtools software. The theoretical isoelectric point (PI) and molecular weight (MW) of PuMYB proteins were predicted using the ExPASy server (http://web.expasy.org/compute_pi/, accessed on 1 September 2023).
2.5. Promoter Cis-Element Analysis and Subcellular Localization Prediction
The promoter sequences of PuMYBs genes (2000 bp upstream of the start codon) were collected for cis-element prediction by using PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 1 September 2023). Subsequently, the data were sorted using Excel and visualized with TBtools. The subcellular localization of the ‘NanGuo’ pear MYB genes was predicted using the subcellular localization prediction locus (https://services.healthtech.dtu.dk/, accessed on 1 September 2023).
2.6. Analysis of PuMYBs Gene Expression
Total RNA was extracted from the ‘NanGuo’ pear and ‘NanHong’ pear at 90 and 120 d after flowering by using a modified CTAB (Cetyltrimethyl ammonium bromide) method, and the samples were stored at −80 °C [46,47]. cDNA was synthesized using the PrimeScript TM RT Reagent Kit with gDNA Eraser (Takara, Japan). The PuActin detected cDNA was stored at −20 °C [48,49]. The structural genes related to anthocyanin biosynthesis were selected according to previous research results [50] and downloaded from pear genome (https://www.rosaceae.org/species/pyrus/all, accessed on 1 September 2023). Primer3 (v. 0.4.0) software was employed to design expression primers (Table S1), and gene expression levels were analyzed using semi-quantitative PCR and qRT-PCR [51].
2.7. Determination of Anthocyanin Content in Pear Fruits
The fruit anthocyanin content was measured using a previously reported method [50], with slight modification. The procedure involved weighing 0.2 g samples of ‘NanGuo’ pear and ‘NanHong’ pear at four developmental stages: 30, 60, 90, and 120 d after flowering. Each sample was placed in a 5 mL centrifuge tube and treated with 3 mL of hydrochloric acid-ethanol extract (95% anhydrous ethanol + 5% of 1.5 M HCl); this process was repeated three times. After the reaction was completed, the supernatant was collected through centrifugation. The absorbance at 530, 620, and 650 nm was measured using a spectrophotometer. Anthocyanin content was calculated using the following formula: ([A530 − A620] − 0.1 × [A650 − A620])/4.62 × 104 × V/M × 106, where V denotes the volume of the extracted liquid and M is the mass of the sample.
2.8. Statistical Analysis
The qRT-PCR data were analyzed using Microsoft Excel (2016), and the results are expressed as the mean ± SE (±1). Statistical significance between the means was assessed using a t test, with significance thresholds set at * p < 0.05 and ** p < 0.01. All data were compiled using Origin2016.
3. Results
3.1. Phylogenetic Analysis of the MYB Family of ‘NanGuo’ Pear
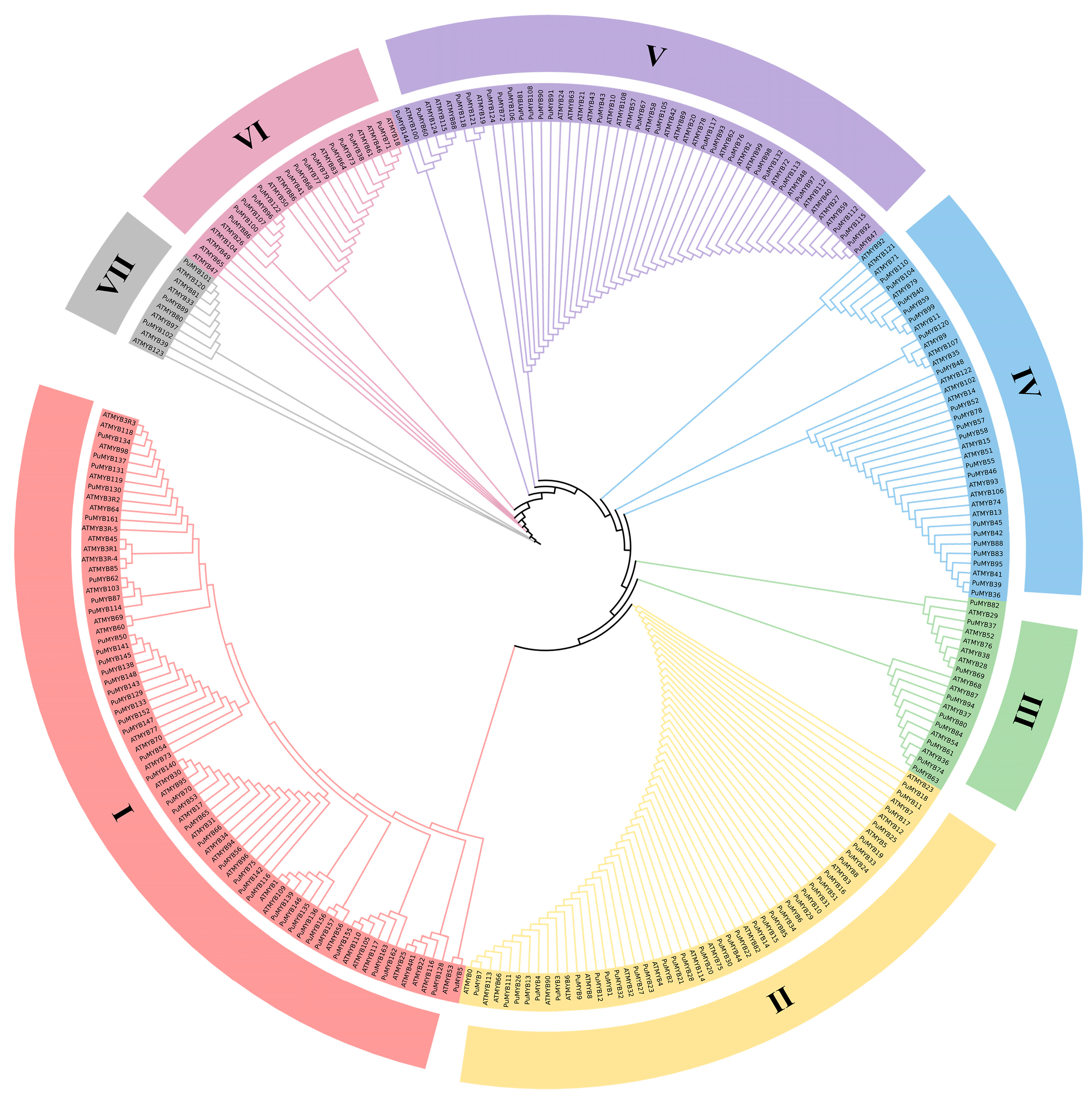
To investigate the evolutionary relationships of MYB family genes in the ‘NanGuo’ pear, a phylogenetic tree was constructed using 146 identified R2R3-MYB genes from the ‘NanGuo’ pear and MYB genes from Arabidopsis (Figure 1). MEGA11 was used to draw the phylogenetic tree with the ML method. The resulting phylogenetic tree revealed that these genes were distributed across seven subfamilies. Subfamily S1 contains the most genes (74), including 39 PuMYBs. Subfamily S2 has 53 genes (including 37 PuMYBs), subfamily S5 has 51 genes (including 25 PuMYBs), subfamily S4 has 38 genes (including 20 PuMYBs), and subfamily S6 has 24 genes (including 13 PuMYBs). Subfamily S3 has 19 genes (including 9 PuMYBs) and subfamily S7 has 10 genes (including 3 PuMYBs).
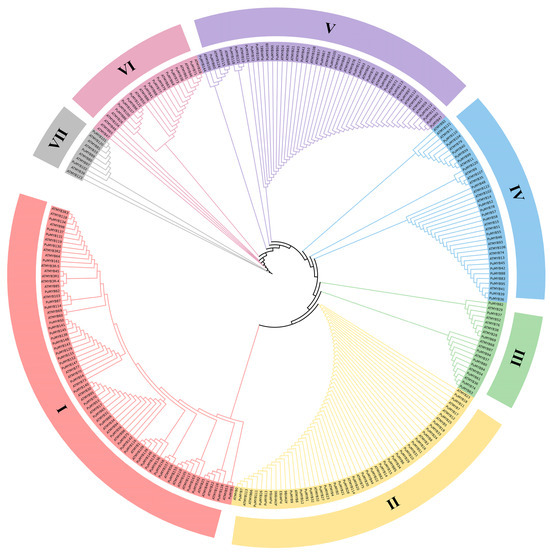
Figure 1.
MEGA11 was applied to the MYB protein sequences of Arabidopsis and ‘NanGuo’ pear using maximum likelihood method (ML). The phylogenetic evolutionary tree was constructed and divided into 7 subfamilies.
3.2. Conserved Domain, Motif Analysis, and Gene Structure of MYB TFs from ‘NanGuo’ Pear
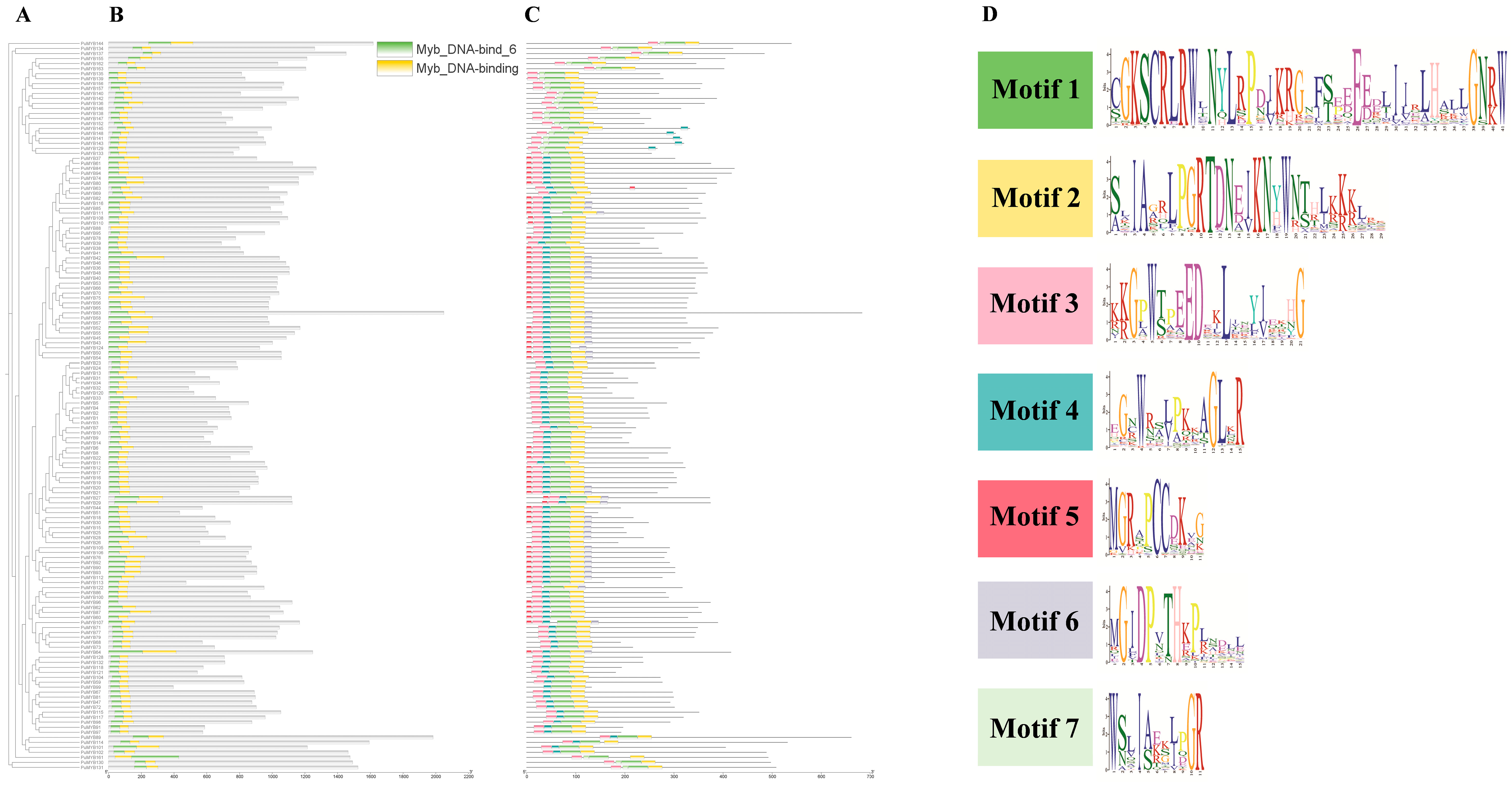
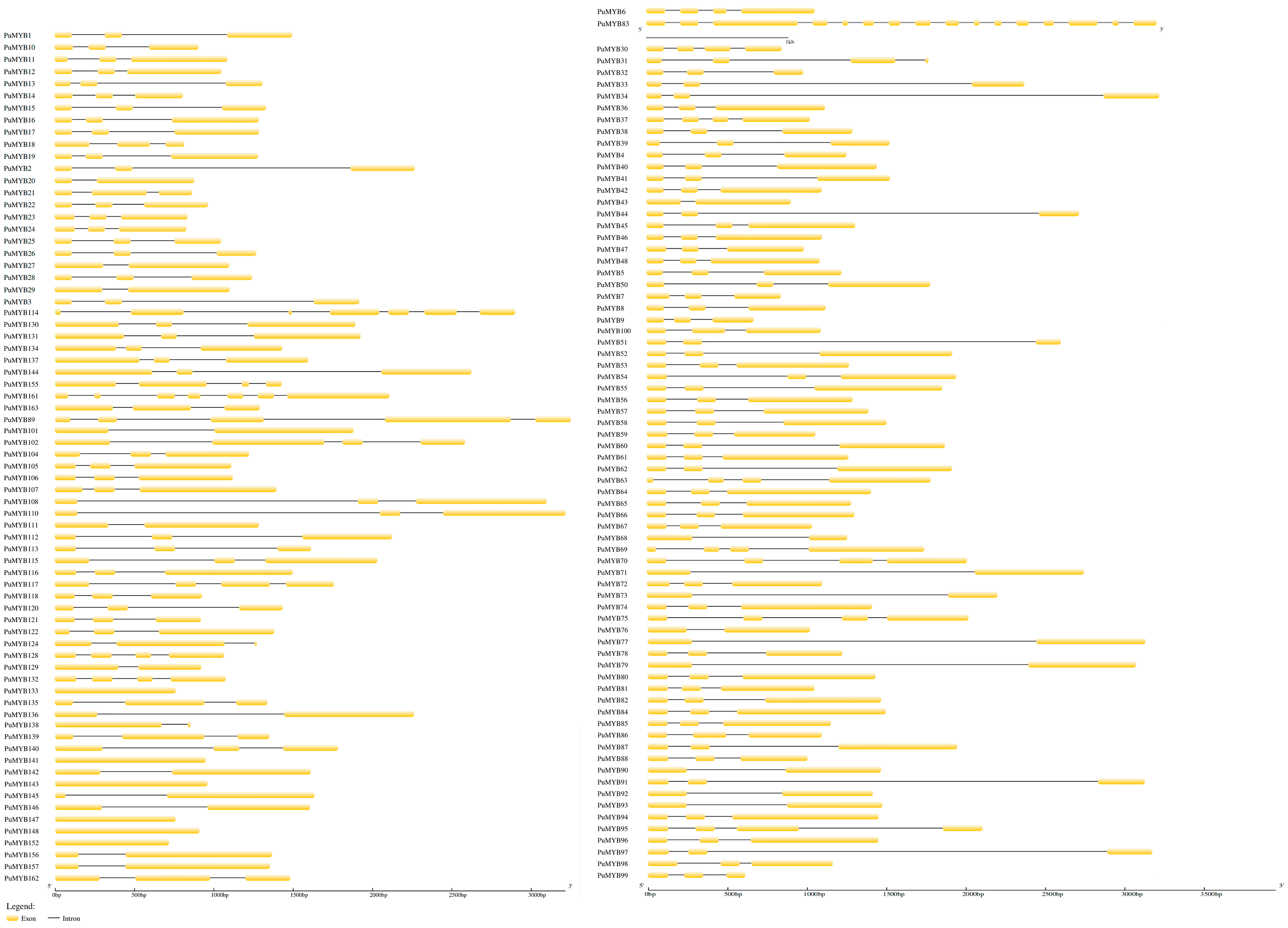
To identify conserved domains within the ‘NanGuo’ pear, the protein sequences of 146 PuMYBs genes were analyzed using the NCBI CDD and visualized with TBtools. The results of the study showed that all sequences contained two MYB DNA-binding structural domains (Figure 2B). The domains were distributed not only in the N-terminal region but also in the middle and C-terminal regions of the protein. Further analysis of the conserved motifs of PuMYBs in the ‘NanGuo’ pear was conducted using MEME software (5.5.7), and the results were also visualized using TBtools (Figure 2C), set to seven motifs (Figure 2D). The functionality of the genes was inferred by comparing conserved domains with these conserved motifs. In addition, the gene structure of selected PuMYBs was analyzed. The findings revealed that the PuMYBs typically contained fewer than five introns (Figure 3). These results indicated that although the genetic structure of PuMYBs is highly conserved, there is a high degree of sequence diversity among different subfamilies.
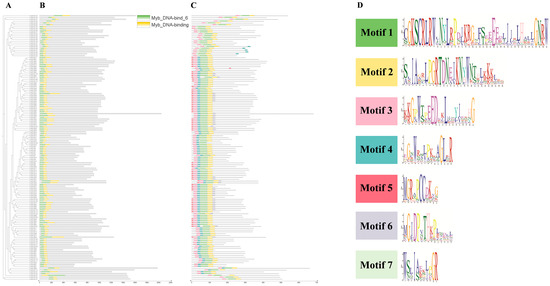
Figure 2.
Phylogenetic tree (A), conserved domain (B), conserved motif (C), and seven amino acid sequence markers (D) of R2R3-MYB gene of ‘NanGuo’ pear.
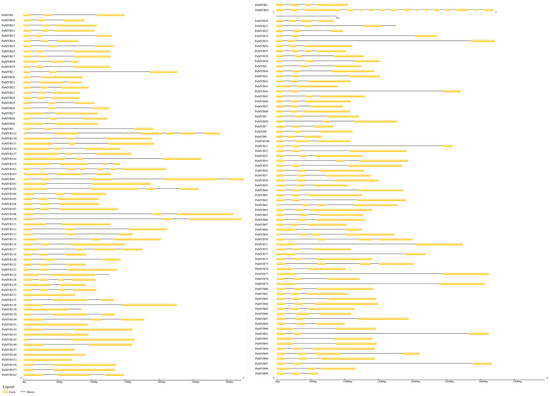
Figure 3.
Gene structure of the ‘NanGuo’ pear R2R3-MYB gene, where black lines are introns and yellow blocks are exons.
3.3. Prediction of Physicochemical Properties and Subcellular Localization of MYB TFs from ‘NanGuo’ Pear
The physicochemical properties of PuMYBs in the ‘NanGuo’ pear, namely, amino acid count, PI, MW, and hydrophilicity, were predicted using ExPASy server and are summarized in Table S2. The number of amino acid sequences (AA) of these proteins was generally less than 700, with most ranging between 200 and 400. The MW of these proteins was typically within 60,000 Daltons; however, PuMYB83, PuMYB89, and PuMYB144 were exceptions, with most others falling between 30,000 and 40,000 Daltons. The PI for most proteins was below 10, although a few genes exhibited higher PI values. Most of the PuMYBs had an instability index greater than 40, suggesting their potential instability. All PuMYBs exhibited hydrophilicity. The aliphatic index (AI), which indicates protein thermostability, ranged from 50 to 90; for instance, PuMYB3 had the highest AI at 87.7, PuMYB156 was the lowest at 48.82. Subcellular localization predictions indicated that all 145 R2R3-MYB TFs were located in the nucleus except PuMYB95, which was located in the cytoplasm (Table S3).
3.4. Analysis of Promoter Cis-Acting Elements of MYB TFs from ‘NanGuo’ Pear
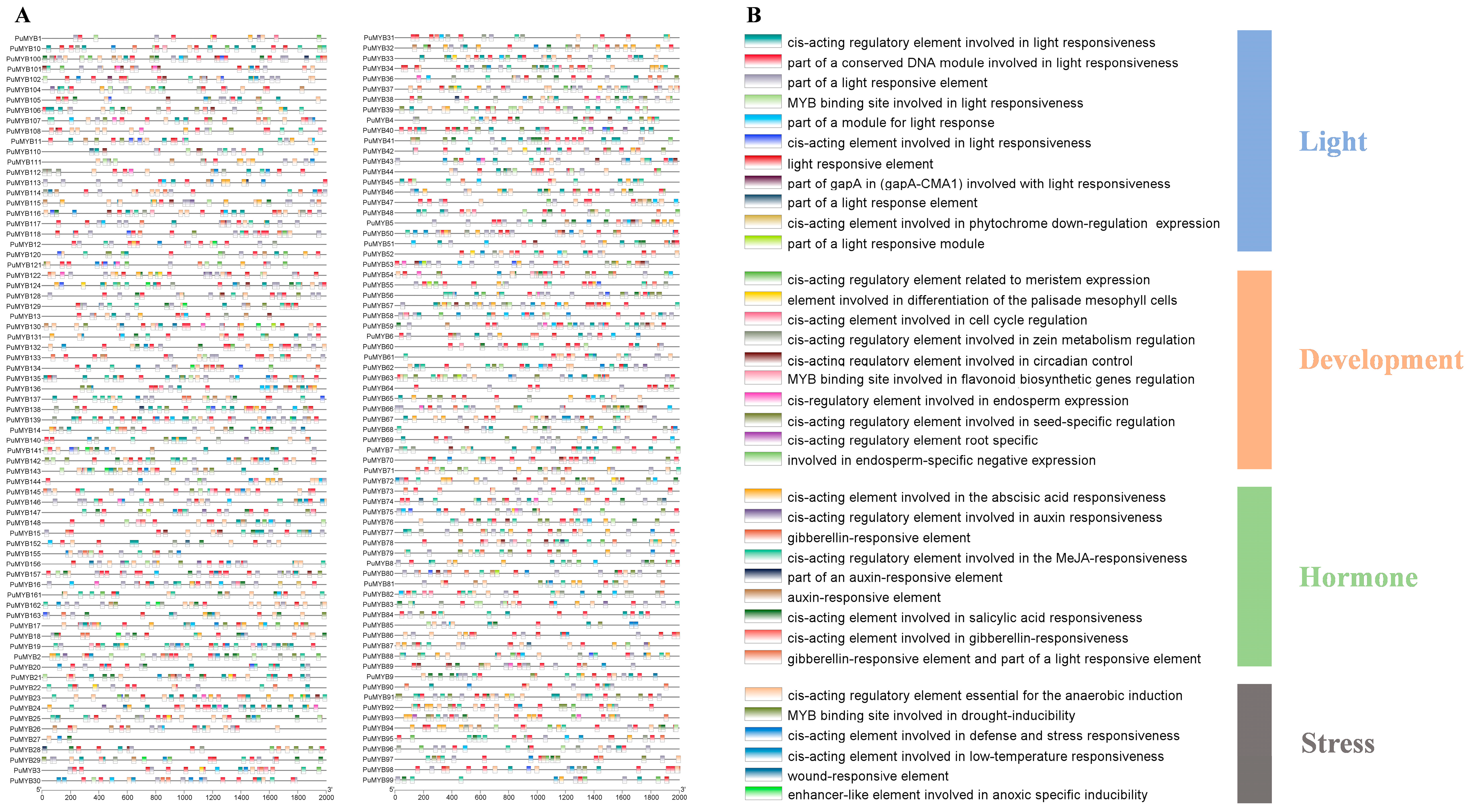
Cis-acting elements are DNA sequences within gene promoters that affect gene expression. Analysis of the promoter regions in the PuMYB family (2000 bp upstream of the ATG start codon) revealed 36 cis-acting elements that mediate various responses (Figure 4A). These elements were categorized into four groups: hormone induction, development induction, light induction, and stress induction. Specifically, there were 11 light response elements, 10 development elements, 9 hormone induction elements, and 6 stress induction elements (Figure 4B). Among the development elements, one regulatory element was involved in flavonoid synthesis (MYB binding site involved in flavonoid biosynthetic genes regulation), which is closely linked to anthocyanin production. This finding suggests that PuMYBs containing this element in their promoters play a crucial role in regulating anthocyanin synthesis. In addition, nine hormone response elements, including those for ABA (1), auxins (IAA) (3), gibberellins (GA) (3), methyl jasmonate (MeJa) (1), and salicylic acid (SA) (1), were identified (Figure 4B). These findings indicated that PuMYBs are crucial in hormone-mediated processes, affecting plant growth and development.
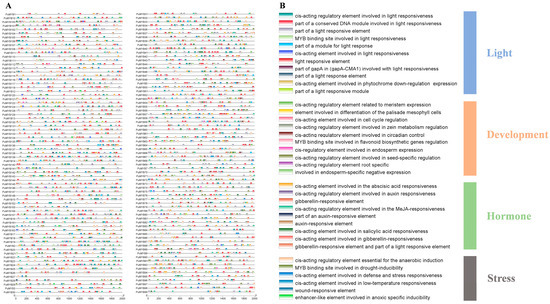
Figure 4.
Inspection of cis-acting elements in ‘Nanguo’ pear MYB genes. (A) Cis-elements with similar functions are displayed in the same color. The black line indicates the promoter length of the PuMYB genes; (B) The different colored boxes represent cis-acting elements with different functions.
3.5. Analysis of Expression of PuMYBs in ‘NanGuo’ Pear and ‘NanHong’ Pear
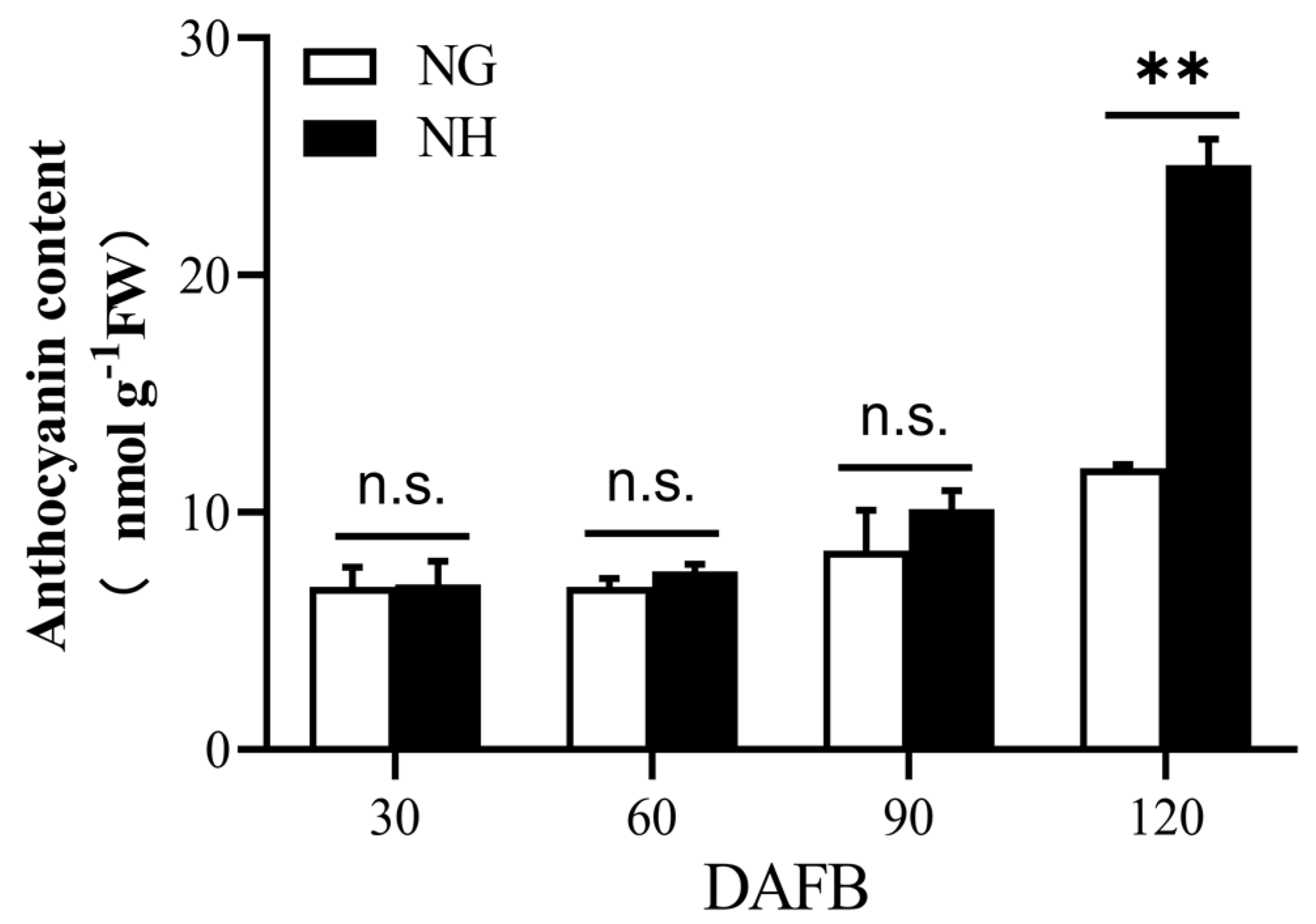
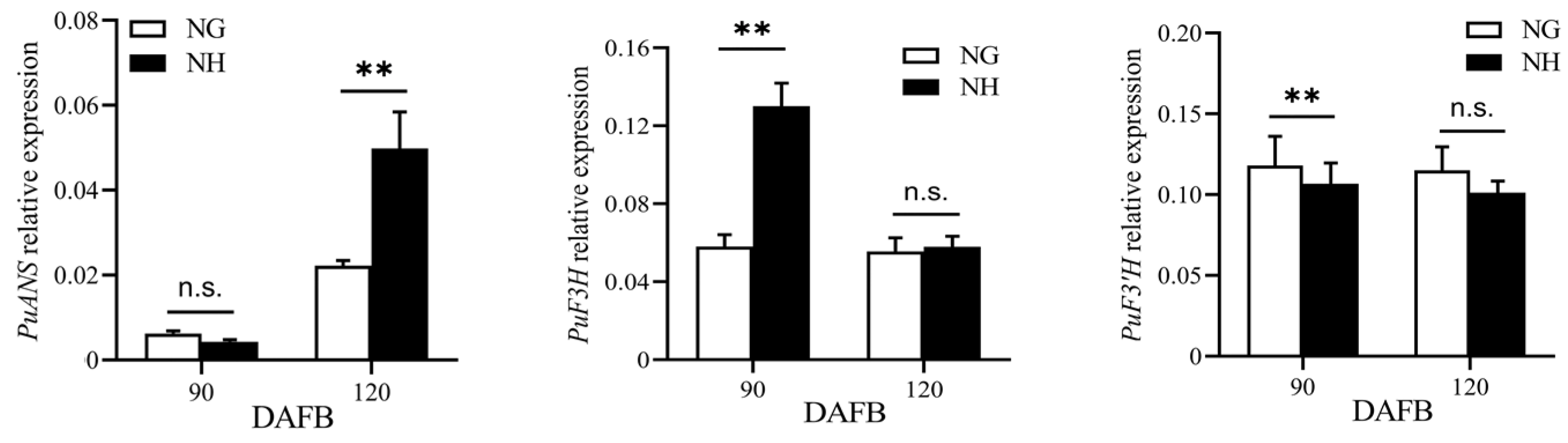
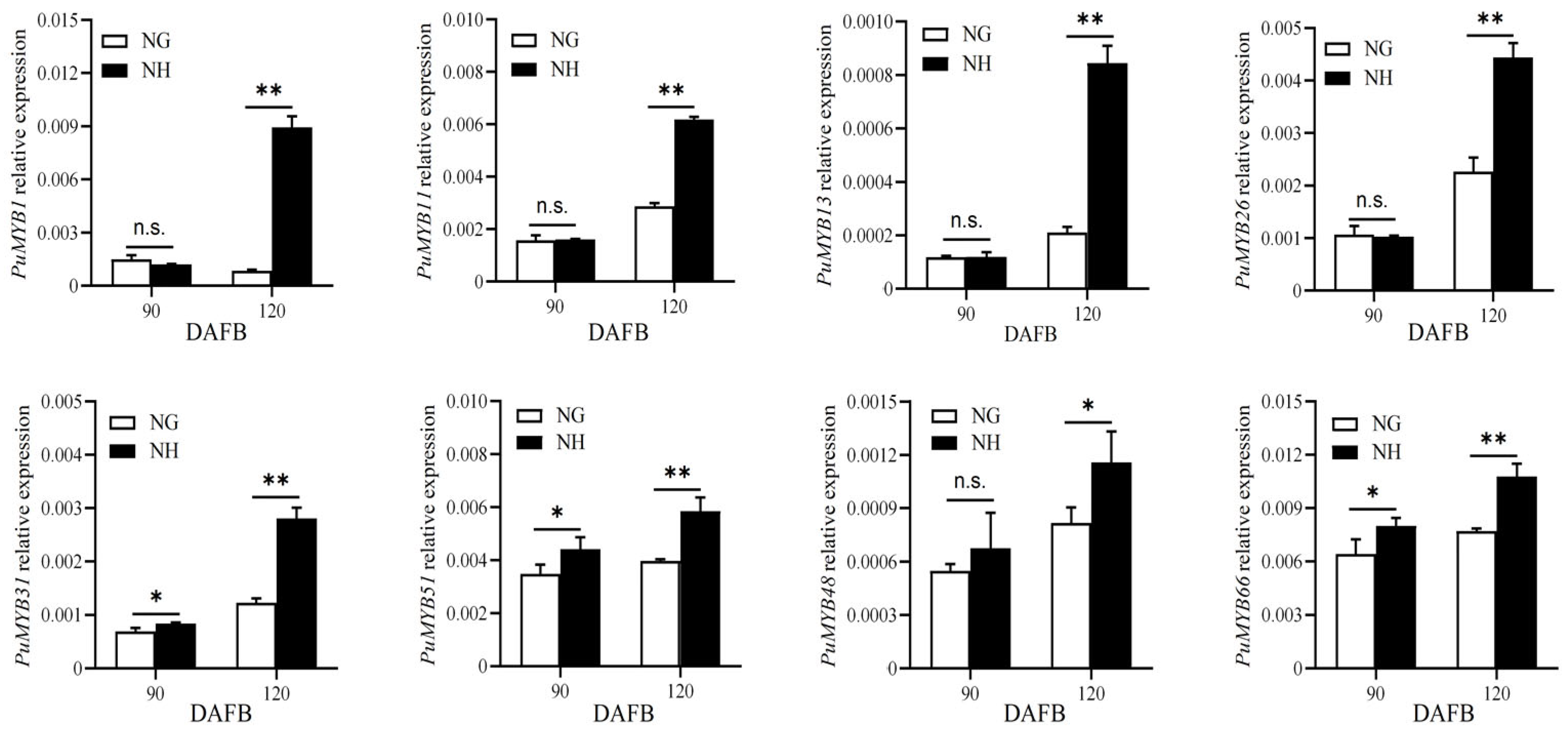
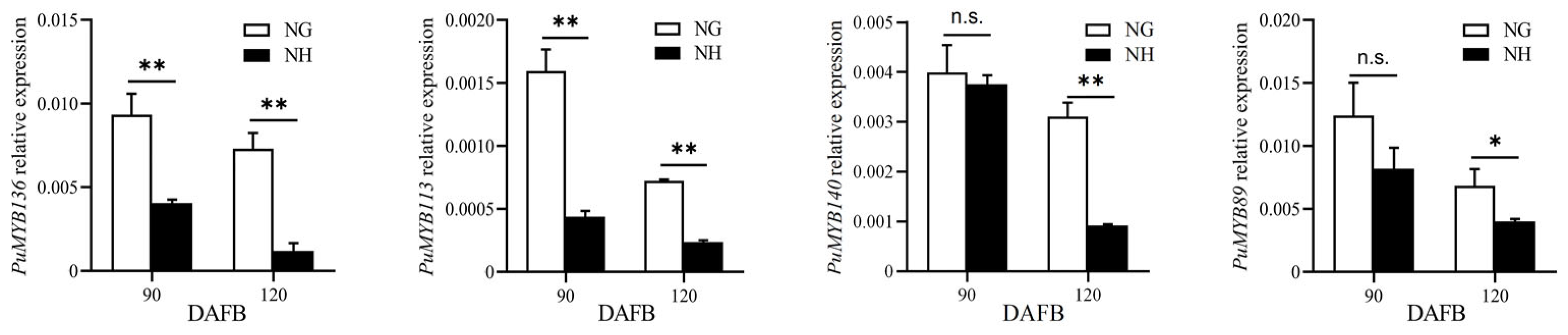
The ‘NanGuo’ pear does not change color during development, unlike the ‘NanHong’ pear, which is a bud mutant of ‘NanGuo’ and shows noticeable color changes. Analysis of anthocyanin content in both varieties during the development stage revealed significant increases at 90 and 120 DAFB, suggesting that these are the color shifting stages (Figure 5). We analyzed the structural genes associated with anthocyanin biosynthesis and compared their expression patterns in NG and NH pears during fruit development. As shown in Figure 6, PuANS were significantly expressed in NH between 90 and 120 DAFB, which was consistent with the anthocyanin accumulation pattern (Figure 6). To identify genes affecting coloration, we compared the expression of PuMYBs between these two periods. We referenced the phylogenetic tree to analyze homologous relationships and reviewed studies on MYB genes in Arabidopsis that affect anthocyanin accumulation, including AtMYB113, AtMYB114, and AtMYB4. This led to the selection of 105 R2R3-MYB TFs for semi-quantitative PCR (Figure S1). Semi-quantitative PCR revealed 56 genes whose expression levels differed significantly. The R2R3-MYB TFs demonstrating significant differences in their expression levels, which were analyzed through qRT-PCR (Figure S2). Subsequently, the selected genes were analyzed through qRT-PCR to screen genes regulating anthocyanin accumulation in the ‘NanGuo’ pear. A total of 12 genes showed significant differential expression between the ‘NanGuo’ pear and the ‘NanHong’ pear. Among them, the expression levels of PuMYB1, PuMYB11, PuMYB13, PuMYB26, PuMYB31, PuMYB48, PuMYB51, and PuMYB66 increased with anthocyanin accumulation (Figure 7), indicating their positive regulatory effect on anthocyanin accumulation. However, the expression levels of PuMYB89, PuMYB113, PuMYB136, and PuMYB140 decreased with an increase in anthocyanin accumulation, which indicated that these genes may exert negative regulatory effects on anthocyanin accumulation (Figure 8). In addition, the expression levels of numerous genes significantly differed between the ‘NanGuo’ and ‘NanHong’ pears, including PuMYB41, PuMYB78, and PuMYB85, which correlated negatively with anthocyanin accumulation. However, the changes in their expression were not significant at 90 and 120 d in ‘NanHong’, leading to their exclusion from further analysis. Similarly, some genes such as PuMYB78 showed significantly higher expressions in ‘NanHong’, exceeding the expression levels in ‘NanGuo’ at 120 days; however, they did not meet the criteria for further investigation.
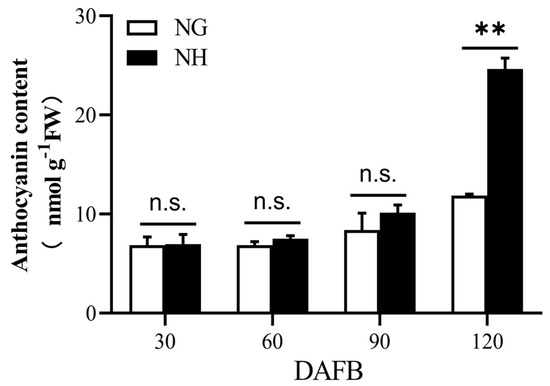
Figure 5.
Anthocyanin contents of ‘NanGuo’ pear and ‘NanHong’ pear fruits. ‘NG’ is ‘NanGuo’ pear. ‘NH’ is ‘NanHong’ pear. By t test, n.s. stands for no significant difference, ** p < 0.01.
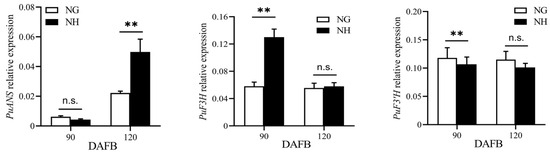
Figure 6.
qRT-PCR was used to verify the expression levels of genes related to anthocyanin synthesis in ‘NanGuo’ pear and ‘Nanhong’ pear at 90 and 120 DAFB, respectively. By t test, n.s. stands for no significant difference, ** p < 0.01.
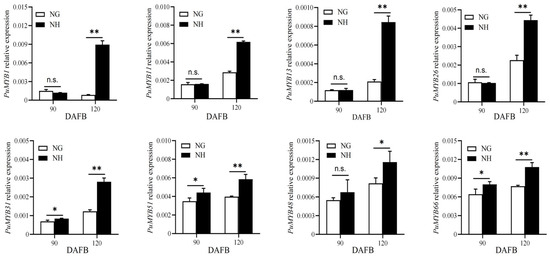
Figure 7.
qRT-PCR was used to verify the expression levels of MYB genes at 90 and 120 DAFB of ‘NanGuo’ pear and ‘NanHong’ pear, respectively (consistent with anthocyanin accumulation trend). By t test, n.s. stands for no significant difference, * p < 0.05, ** p < 0.01.
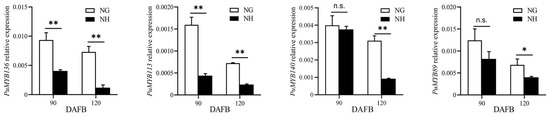
Figure 8.
qRT-PCR was used to verify the expression levels of MYB genes at 90 and 120 DAFB of ‘NanGuo’ pear and ‘NanHong’ pear, respectively (contrary to the trend of anthocyanin accumulation). By t test, n.s. stands for no significant difference, * p < 0.05, ** p < 0.01.
4. Discussion
The MYB TF family is ubiquitous in plants and represents one of the largest TF families in higher plants [52,53]. It plays a crucial role in various biological processes, including plant growth and development, hormone signaling, secondary metabolism, and resistance to both biotic and abiotic stresses [54,55].
Phylogenetic analysis is a reliable method for inferring genes function because genes clustered on the same branch typically exhibit similar functions. In this study, we constructed phylogenetic trees featuring genes from Arabidopsis thaliana and 146 ‘NanGuo’ pear genes, categorizing them into seven subfamilies to examine their functional relationships. For instance, genes such as AtMYB61, which are associated with plant growth and development, were identified as belonging to subfamily S6. By contrast, genes linked to plant resistance mechanisms, such as AtMYB60 and AtMYB96, constituted subfamily S1. The TFs AtMYB113 and AtMYB114, which regulate anthocyanin accumulation in Arabidopsis, are members of subfamily S2 [31]. AtMYB4, which inhibits the accumulation of flavonoids and other compounds, also belongs to subfamily S2 [56]. Based on these findings, we hypothesized that PuMYBs in subfamilies S2 are pivotal for regulating anthocyanin accumulation in pear fruits. Thus, 105 PuMYBs were selected for further analysis. Among these, twelve were identified as regulators of anthocyanin accumulation: six from subfamily S2, three from subfamily S1, one from subfamily S4 (PuMYB48), from subfamily S5 (PuMYB113), and one from subfamily S7 (PuMYB89). The role of MYB TFs in anthocyanin accumulation extends beyond pears. In Gerbera, GhMYB1a regulates both anthocyanin and flavonol levels, affecting flower color [32]. Similarly, in apple, MdMYB10 regulates fruit coloration by affecting anthocyanin accumulation [33,34]. By contrast, PlgMYBR1 in Eustoma flowers exerts a negative regulatory effect on anthocyanin accumulation [37]. In addition, DbMYB2 positively regulates the expression of genes related to anthocyanin biosynthesis in Dendrobium, thereby affecting anthocyanin synthesis [57]. This finding indicates the importance of MYB TFs in modulating anthocyanin accumulation. Through comparative phylogenetic analyses with Arabidopsis thaliana, multiple TFs were found on the same branch, suggesting that they share similar functions. In Arabidopsis, AtMYB60 and AtMYB61 are associated with root development and stomatal opening and closing [58,59]. Phylogenetic analysis suggests that their homologs in pear, namely PuMYB114, PuMYB71, and PuMYB38, have the same functions. Furthermore, AtMYB52 and AtMYB96 are involved in drought stress responses through ABA-mediated pathways; thus, their homologs in pear, PuMYB37 and PuMYB56, may affect plant drought stress responses, as determined through phylogenetic tree analysis [60,61]. In this study, 146 R2R3-MYB TFs were identified by analyzing the sequence of the PuMYB genes family. Of these, 12 R2R3-MYB TFs involved in anthocyanin regulation were analyzed through qRT-PCR and three structural genes were related to anthocyanin. We also analyzed the remaining 56 PuMYB genes with significant differences in their expression levels through qRT-PCR. Although their effects on anthocyanin accumulation were inconsistent, the analysis of their expression changes provides insights into their effects on other quality characteristics of the fruit, laying the foundation for subsequent experiments.
Cis-regulatory elements serve as crucial molecular switches in genes [16], playing a pivotal role in gene transcription regulation in response to external stimuli [1]. The analysis of cis-elements on the promoter of PuMYBs revealed the involvement of some TFs in stress responses. Specifically, nine hormone-related response elements, including those for ABA, IAA, GA, MeJa, and SA, were identified. For instance, the promoters of PuMYB117, PuMYB135, and PuMYB139 contain multiple hormone response elements, suggesting their potential role in hormone regulation during fruit growth and development [60,61,62]. Additionally, we identified six stress response elements related to anaerobic induction, general stress response, drought induction, low temperature response, wound response, and hypoxic induction. Moreover, we identified 11 light response elements and 10 growth and development elements involved in palisade mesophyll cell differentiation, circadian rhythm control, flavonoid biosynthesis, seed-specific regulation, and endosperm expression. For example, the promoters of PuMYB61, PuMYB63, and PuMYB65 featured multiple growth and development response elements, indicating their possible impact on plant growth and development [63,64]. These elements, present on the promoters of PuMYBs, interact synergistically in response to plant stress and various hormonal signals, highlighting the functional diversity of PuMYBs [5]. This diversity also supports the involvement of some TFs in response to low temperatures, drought, and other stressors [17], providing valuable directions for future research.
5. Conclusions
In this study, we identified 146 R2R3-MYB TFs by examining MYB structural domains and conducting similar sequence comparisons, confirming that all these genes are highly conserved. We further analyzed their expression by using qRT-PCR, which revealed that the expression patterns of PuMYB1, PuMYB11, PuMYB13, PuMYB26, PuMYB31, PuMYB48, PuMYB51, and PuMYB66 were consistent with the accumulation trends of anthocyanidins. Conversely, the expression of PuMYB89, PuMYB113, PuMYB136, and PuMYB140 showed an inverse relation with the accumulation of anthocyanidins. These findings not only aid the ongoing production and research of red pears in China but also offer new insights and foundational knowledge for understanding the functional roles of red pears in various other aspects.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae10090989/s1, Figure S1. Changes in expression of 105 PuMYBs genes analyzed by evolutionary tree homology at 90 and 120d of fruit bloom in NG and NH; Figure S2. The expression levels of MYB gene in ‘NanGuo’ pear were verified by qRT-PCR at 90 and 120 days of NG and NH, respectively; Table S1: PuMYBs gene specific primers for qRT-PCR method. Table S2: Protein physicochemical properties of 146 PuMYBs genes. Table S3: Subcellular localization prediction of PuMYBs gene. Table S4: the accession number of PuMYBs genes.
Author Contributions
H.Y. conceived and designed the experiments; X.L., Q.H., Y.L., Z.L., W.L., and H.L. participated in the experiments and data analyses; X.L. and Q.H. wrote the manuscript with inputs and guidance from H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Liaoning Province Department of Education fund (JYTMS20231278).
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author. The accession number of PuMYBs genes used in this study were uploaded to the Genebank under accession number PQ178308-PQ178453 and were listed in Table S4.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, Z.; Liu, Z.; Wu, H.; Xu, Z.; Zhang, H.; Qian, W.; Gao, W.; She, H. Genome-Wide Identification and Characterization of MYB Gene Family and Analysis of Its Sex-Biased Expression Pattern in Spinacia oleracea L. Int. J. Mol. Sci. 2024, 25, 795. [Google Scholar] [CrossRef] [PubMed]
- Chanwala, J.; Khadanga, B.; Jha, D.K.; Sandeep, I.S.; Dey, N. MYB Transcription Factor Family in Pearl Millet: Genome-Wide Identification, Evolutionary Progression and Expression Analysis under Abiotic Stress and Phytohormone Treatments. Plants 2023, 12, 355. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gu, C.; Fu, Z.; Wang, Z. Genome-Wide Identification and Analysis of MYB Transcription Factor Family in Hibiscus hamabo. Plants 2023, 12, 1429. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tang, L.; Tang, X.; Mao, Y. Genome-Wide Identification and Analysis of MYB Transcription Factors in Pyropia yezoensis. Plants 2023, 12, 3613. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Luo, R.; Sun, Y.; Yang, C.; Li, X.; Gao, A.; Pu, J. Genome-Wide Characterization, Identification and Expression Profile of MYB Transcription Factor Gene Family during Abiotic and Biotic Stresses in Mango (Mangifera indica). Plants 2022, 11, 3141. [Google Scholar] [CrossRef]
- Ai, P.; Xue, J.; Shi, Z.; Liu, Y.; Li, Z.; Li, T.; Zhao, W.; Khan, M.A.; Kang, D.; Wang, K.; et al. Genome-wide characterization and expression analysis of MYB transcription factors in Chrysanthemum nankingense. BMC Plant Biol. 2023, 23, 140. [Google Scholar] [CrossRef]
- Hou, X.J.; Li, S.B.; Liu, S.R.; Hu, C.G.; Zhang, J.Z. Genome-Wide Classification and Evolutionary and Expression Analyses of Citrus MYB Transcription Factor Families in Sweet Orange. PLoS ONE 2014, 9, e112375. [Google Scholar] [CrossRef]
- Liu, L.; Chao, N.; Yidilisi, K.; Kang, X.; Cao, X. Comprehensive analysis of the MYB transcription factor gene family in Morus alba. BMC Plant Biol. 2022, 22, 281. [Google Scholar] [CrossRef]
- Lu, M.; Chen, Z.; Dang, Y.; Li, J.; Wang, J.; Zheng, H.; Li, S.; Wang, X.; Du, X.; Sui, N. Identification of the MYB gene family in Sorghum bicolor and functional analysis of SbMYBAS1 in response to salt stress. Plant Mol. Biol. 2023, 113, 249–264. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, K.; Khurshid, M.; Li, J.; He, M.; Georgiev, M.I.; Zhang, X.; Zhou, M. MYB Transcription Repressors Regulate Plant Secondary Metabolism. Crit. Rev. Plant Sci. 2019, 38, 159–170. [Google Scholar] [CrossRef]
- Roy, S. Function of MYB domain transcription factors in abiotic stress and epigenetic control of stress response in plant genome. Plant Signal Behav. 2016, 11, e1117723. [Google Scholar] [CrossRef] [PubMed]
- Baldoni, E.; Genga, A.; Cominelli, E. Plant MYB Transcription Factors: Their Role in Drought Response Mechanisms. Int. J. Mol. Sci. 2015, 16, 15811–15851. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Fan, P.; Mo, Z.; Tan, P.; Feng, G.; Li, F.; Peng, F. Identification, Expression and Co-Expression Analysis of R2R3-MYB Family Genes Involved in Graft Union Formation in Pecan (Carya illinoinensis). Forests 2020, 11, 917. [Google Scholar] [CrossRef]
- Qing, X.; Jie, H.; Jianhui, D.; Xiaojin, H.; Xian, Z. Genomic Survey and Expression Profiling of the MYB Gene Family in Watermelon. Hortic. Plant J. 2018, 4, 1–15. [Google Scholar] [CrossRef]
- Leng, L.; Zhang, X.; Liu, W.; Wu, Z. Genome-Wide Identification of the MYB and bHLH Families in Carnations and Expression Analysis at Different Floral Development Stages. Int. J. Mol. Sci. 2023, 24, 9499. [Google Scholar] [CrossRef]
- Xu, M.; Fu, J.; Ni, Y.; Zhang, C. Genome wide analysis of the MYB gene family in pumpkin. PeerJ 2024, 12, e17304. [Google Scholar] [CrossRef]
- Xing, H.-T.; Shi, J.-Y.; Yin, S.-Q.; Wu, Q.-H.; Lv, J.-L.; Li, H.-L. The MYB family and their response to abiotic stress in ginger (Zingiber officinale Roscoe). BMC Genom. 2024, 25, 460. [Google Scholar] [CrossRef]
- Teng, S.; Keurentjes, J.; Bentsink, L.; Koornneef, M.; Smeekens, S. Sucrose-Specific Induction of Anthocyanin Biosynthesis in Arabidopsis Requires the MYB75/PAP1 Gene. Plant Physiol. 2005, 4, 1840–1852. [Google Scholar] [CrossRef]
- Pandey, A.; Misra, P.; Trivedi, P.K. Constitutive expression of Arabidopsis MYB transcription factor, AtMYB11, in tobacco modulates flavonoid biosynthesis in favor of flavonol accumulation. Plant Cell Rep. 2015, 34, 1515–1528. [Google Scholar] [CrossRef]
- Stracke, R.; Ishihara, H.; Huep, G.; Barsch, A.; Mehrtens, F.; Niehaus, K.; Weisshaar, B. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 2007, 50, 660–677. [Google Scholar] [CrossRef]
- Mehrtens, F.; Kranz, H.; Bednarek, P.; Weisshaar, B. The Arabidopsis Transcription Factor MYB12 Is a Flavonol-Specific Regulator of Phenylpropanoid Biosynthesis. Plant Physiol. 2005, 138, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, X.; Gao, X.; Wu, W.; Zhou, B. Light Induced Regulation Pathway of Anthocyanin Biosynthesis in Plants. Int. J. Mol. Sci. 2021, 22, 11116. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.-J.; Zheng, Q.-D.; Yao, Y.-H.; Ou, Y.; Chen, J.-Y.; Wang, M.-J.; Lai, H.-P.; Yan, L.; Liu, Z.-J.; Ai, Y. Genome-Wide Identification of the MYB Gene Family in Cymbidiumensifolium and Its Expression Analysis in Different Flower Colors. Int. J. Mol. Sci. 2021, 22, 13245. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Li, X.; Fu, Y.; Li, C. Environmental Stimuli and Phytohormones in Anthocyanin Biosynthesis: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 16415. [Google Scholar] [CrossRef]
- Holton, T.A.; Cornish, E.C. Genetics and Biochemistry of Anthocyanin Biosynthesis. Plant Cell 1995, 7, 1071–1083. [Google Scholar] [CrossRef]
- Kay, C.D.; Kroon, P.A.; Cassidy, A. The bioactivity of dietary anthocyanins is likely to be mediated by their degradation products. Mol. Nutr. Food Res. 2010, 53, S92–S101. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.; Holcroft, D.M.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef]
- Mu, Z.; Yang, Y.; Yusuyin, A.; Xu, Y.; Yuan, H.; Liu, C. Transcriptome Analysis Provides Insights into Anthocyanin Synthesis in Blueberry. Horticulturae 2023, 9, 1036. [Google Scholar] [CrossRef]
- Yang, L.; Liu, H.; Hao, Z.; Zong, Y.; Xia, H.; Shen, Y.; Li, H. Genome-Wide Identification and Expression Analysis of R2R3-MYB Family Genes Associated with Petal Pigment Synthesis in Liriodendron. Int. J. Mol. Sci. 2021, 22, 11291. [Google Scholar] [CrossRef]
- Yan, H.; Pei, X.; Zhang, H.; Li, X.; Zhang, X.; Zhao, M.; Chiang, V.L.; Sederoff, R.R.; Zhao, X. MYB-mediated regulation of anthocyanin biosynthesis. Int. J. Mol. Sci. 2021, 22, 3013. [Google Scholar] [CrossRef]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008, 53, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Tang, Y.; Pang, B.; Li, X.; Yang, Y.; Deng, J.; Feng, C.; Li, L.; Ren, G.; Wang, Y.; et al. The R2R3-MYB transcription factor GhMYB1a regulates flavonol and anthocyanin accumulation in Gerbera hybrida. Hortic. Res. 2020, 7, 78. [Google Scholar] [CrossRef]
- Espley, R.V.; Hellens, R.P.; Putterill, J.; Stevenson, D.E.; Kutty-Amma, S.; Allan, A.C. Red colouration in apple fruit is due to the activity of the MYB transcription factor MsMYB10. Plant J. 2007, 49, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Telias, A.; Lin-Wang, K.; Stevenson, D.E.; Cooney, J.M.; Hellens, R.P.; Allan, A.C.; Hoover, E.E.; Bradeen, J.M. Apple skin patterning is associated with differential expression of MYB10. BMC Plant Biol. 2011, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Goto-Yamamoto, N.; Hirochika, H. Retrotransposon-Induced Mutations in Grape Skin Color. Science 2004, 304, 982. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Man, Y.; Wang, Y. Light- and Temperature-Induced Expression of an R2R3-MYB Gene Regulates Anthocyanin Biosynthesis in Red-Fleshed Kiwifruit. Int. J. Mol. Sci. 2019, 20, 5228. [Google Scholar] [CrossRef]
- Kim, E.; Hyun, T.K. PlgMYBR1, an R2R3-MYB transcription factor, plays as a negative regulator of anthocyanin biosynthesis in Platycodon grandiflorus. 3 Biotech. 2023, 13, 75. [Google Scholar] [CrossRef]
- Zhou, H.; Lin-Wang, K.; Wang, F.; Espley, R.V.; Ren, F.; Zhao, J.; Ogutu, C.; He, H.; Jiang, Q.; Allan, A.C.; et al. Activator-type R2R3-MYB genes induce a repressor-type R2R3-MYB gene to balance anthocyanin and proanthocyanidin accumulation. New Phytol. 2019, 221, 1919–1934. [Google Scholar] [CrossRef]
- Li, X.; Guo, C.; Ahmad, S.; Wang, Q.; Yu, J.; Liu, C.; Guo, Y. Systematic Analysis of MYB Family Genes in Potato and Their Multiple Roles in Development and Stress Responses. Biomolecules 2019, 9, 317. [Google Scholar] [CrossRef]
- Liyong, Q.; Xiaojing, L.; Nannan, Z.; Zhuoran, Z.; Yueming, Y.; Yuqi, D.; Jianan, S.; Islam, M.; Zepeng, Y.; Aide, W. Genome-wide identification of CXE and PuCXE15 functions in the catabolism of volatile ester in ‘Nanguo’ pear fruit. Plant Physiol. Biochem. 2023, 203, 107996. [Google Scholar] [CrossRef]
- Luo, M.; Ge, W.; Sun, H.; Yang, Q.; Sun, Y.; Zhou, X.; Zhou, Q.; Ji, S. Salicylic acid treatment alleviates diminished ester production in cold-stored ‘Nanguo’ pear by promoting the transcription of PuAAT. Postharvest Biol. Tec. 2022, 187, 111849. [Google Scholar] [CrossRef]
- Xiaojing, L.; Liyong, Q.; Nannan, Z.; Lihong, Z.; Yiqing, S.; Xuanting, H.; Hongyu, W.; Zepeng, Y.; Aide, W. Integrated metabolome and transcriptome analysis of the regulatory network of volatile ester formation during fruit ripening in pear. Plant Physiol. Biochem. 2022, 185, 80–90. [Google Scholar] [CrossRef]
- Xinyue, L.; Wei, G.; Mingyang, X.; Jiaming, Z.; Guan, W.; Hui, Y.; Aide, W. PuWRKY31 affects ethylene production in response to sucrose signal in pear fruit. Hortic. Res. 2022, 9, 156. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, D.; Wang, A.; Li, T.; Jiang, S.; Cong, P.; Li, T. The methylation of the PcMYB10 promoter is associated with green-skinned sport in Max Red Bartlett pear. Plant Physiol. 2013, 162, 885–896. [Google Scholar] [CrossRef]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1994, 2, 28–36. [Google Scholar] [PubMed]
- Tong, L.; Yaxiu, X.; Lichao, Z.; Yinglin, J.; Dongmei, T.; Hui, Y.; Aide, W. The Jasmonate-Activated Transcription Factor MdMYC2 Regulates ETHYLENE RESPONSE FACTOR and Ethylene Biosynthetic Genes to Promote Ethylene Biosynthesis during Apple Fruit Ripening. Plant Cell 2017, 29, 1316–1334. [Google Scholar] [CrossRef]
- Bu, H.; Yu, W.; Yuan, H.; Yue, P.; Wei, Y.; Wang, A. Endogenous auxin content contributes to larger size of apple fruit. Front. Plant Sci. 2020, 11, 592540. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tan, D.; Liu, Z.; Jiang, Z.; Wei, Y.; Zhang, L.; Li, X.; Yuan, H.; Wang, A. Apple MdACS6 regulates ethylene biosynthesis during fruit development involving ethylene-responsive factor. Plant Cell Physiol. 2015, 56, 1909–1917. [Google Scholar] [CrossRef]
- Li, T.; Jiang, Z.; Zhang, L.; Tan, D.; Wei, Y.; Yuan, H.; Li, T.; Wang, A. Apple MdERF2 negatively affects ethylene biosynthesis during fruit ripening by suppressing MdACS1 transcription. Plant J. 2016, 88, 735–748. [Google Scholar] [CrossRef]
- Liu, W.; Wei, Y.; Sha, S.; Xu, Y.; Li, H.; Yuan, H.; Wang, A. The mechanisms underpinning anthocyanin accumulation in a red-skinned bud sport in pear (Pyrus ussuriensis). Plant Cell Rep. 2023, 42, 1089–1105. [Google Scholar] [CrossRef]
- Tan, D.; Li, T.; Wang, A. Apple 1-Aminocyclopropane-1-Carboxylic Acid Synthase Genes, MdACS1 and MdACS3a, are Expressed in Different Systems of Ethylene Biosynthesis. Plant Mol. Biol. Rep. 2013, 31, 204–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Butelli, E.; Stefano, R.D.; Schoonbeek, H.J.; Magusin, A.; Pagliarani, C.; Wellner, N.; Hill, L.; Orzaez, D.; Granell, A.; et al. Anthocyanins double the shelf life of tomatoes by delaying overripening and reducing susceptibility to gray mold. Curr. Biol. 2013, 23, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tian, C.; Zhang, Y.; Li, C.; Li, X.; Yu, Q.; Wang, S.; Wang, X.; Chen, X.; Feng, S. Transcriptomic and metabolomic analysis provides insights into anthocyanin and procyanidin accumulation in pear. BMC Plant Biol. 2020, 20, 129. [Google Scholar] [CrossRef]
- Luan, X.; Xu, W.; Zhang, J.; Shen, T.; Chen, C.; Xi, M.; Zhong, Y.; Xu, M. Genome-Scale Identification, Classification, and Expression Profiling of MYB Transcription Factor Genes in Cinnamomum camphora. Int. J. Mol. Sci. 2022, 23, 14279. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, X.; Niyitanga, S.; He, Q.; Chen, S.; Xu, J.; Qi, J.; Tao, A.; Fang, P.; Zhang, L. Transcriptomes of Different Tissues for Expression Characteristics Analysis of MYB gene Family in Kenaf (Hibiscus cannabinus L.). Tropical Plant Biol. 2022, 15, 261–275. [Google Scholar] [CrossRef]
- Wang, X.-C.; Wu, J.; Guan, M.-L.; Zhao, C.-H.; Geng, P.; Zhao, Q. Arabidopsis MYB4 plays dual roles in flavonoid biosynthesis. Plant J. 2020, 101, 637–652. [Google Scholar] [CrossRef]
- Lim, G.-H.; Kim, S.W.; Ryu, J.; Kang, S.-Y.; Kim, J.-B.; Kim, S.H. Upregulation of the MYB2 Transcription Factor is Associated with Increased Accumulation of Anthocyanin in the Leaves of Dendrobium bigibbum. Int. J. Mol. Sci. 2020, 21, 5653. [Google Scholar] [CrossRef]
- Simeoni, F.; Skirycz, A.; Simoni, L.; Castorina, G.; de Souza, L.P.; Fernie, A.R.; Alseekh, S.; Giavalisco, P.; Conti, L.; Tonelli, C.; et al. The AtMYB60 transcription factor regulates stomatal opening by modulating oxylipin synthesis in guard cells. Sci. Rep. 2022, 12, 533. [Google Scholar] [CrossRef]
- Liang, Y.-K.; Dubos, C.; Dodd, I.C.; Holroyd, G.H.; Hetherington, A.M.; Campbell, M.M. AtMYB61, an R2R3-MYB Transcription Factor Controlling Stomatal Aperture in Arabidopsis thaliana. Curr. Biol. 2005, 15, 1201–1206. [Google Scholar] [CrossRef]
- Park, M.Y.; Kang, J.-Y.; Kim, S.Y. Overexpression of AtMYB52 Confers ABA Hypersensitivity and Drought Tolerance. Mol. Cells 2011, 31, 447–454. [Google Scholar] [CrossRef]
- Seo, P.J.; Xiang, F.; Qiao, M.; Park, J.-Y.; Na Lee, Y.; Kim, S.-G.; Lee, Y.-H.; Park, W.J.; Park, C.-M. The MYB96 Transcription Factor Mediates Abscisic Acid Signaling during Drought Stress Response in Arabidopsis. Plant Physiol. 2009, 151, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, W.; Jiang, H.; Mao, Z.; Wang, N.; Jiang, S.; Xu, H.; Yang, G.; Zhang, Z.; Chen, X. The R2R3-MYB transcription factor MdMYB24-like is involved in methyl jasmonate-induced anthocyanin biosynthesis in apple. Plant Physiol. Biochem. 2019, 139, 0981–9428. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 1360–1385. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lee, C.; Zhong, R.; Ye, Z.-H. MYB58 and MYB63 Are Transcriptional Activators of the Lignin Biosynthetic Pathway during Secondary Cell Wall Formation in Arabidopsis. Plant Cell 2009, 21, 248–266. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).