Physiological and Biochemical Effects of Potassium Deficiency on Apple Tree Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Preparatory Activities
2.2. Experimental Procedure and K Treatments
2.3. Experimental Duration and Data Collection
2.4. Plant Growth
2.5. Photosynthesis
2.6. Chlorophyll Fluorescence Analysis
2.7. Chlorophylls and Carotenoids
2.8. Electrolyte Leakage
2.9. Carbohydrates
2.10. Statistical Data Analysis
3. Results
3.1. Potassium Deficiency Symptoms and Plant Growth Measurements
3.2. Impact of Potassium on Photosynthetic Parameters and Water Use Efficiency
3.3. Potassium Levels Affect Leaf Photosynthetic Pigments and Electrolyte Leakage
3.4. Potassium Affects Glucose and the Sucrose-to-Hexose Ratio in Leaves
3.5. Potassium Deficiency Inhibits the Photochemical Performance of Apple Trees
4. Discussion
5. Conclusions
- Symptoms of K deficiency: Prolonged K deficiency led to visible symptoms, including chlorosis and necrosis, which initially appeared in the basal leaves and then spread upwards. This pattern is indicative of the mobility of K, as K is efficiently transported from older to newer tissues via the phloem.
- Effects on photosynthesis: The photosynthetic capacity of K-deficient trees was significantly impaired due to both stomatal and non-stomatal factors:
- ○
- Stomatal limitations: Reduced stomatal conductance limited CO2 uptake, directly hindering photosynthesis.
- ○
- Non-stomatal limitations: Decreased concentrations of chlorophyll and carotenoids, key pigments for light absorption, led to compromised light energy capture. Additionally, chlorophyll fluorescence analysis revealed decreased electron transport rates, PSII efficiency, and photochemical quenching, particularly under high light conditions. These findings suggest that both light-dependent reactions and carbon fixation were negatively affected by K deficiency.
- Sugar metabolism: Despite the significant impairment in photosynthetic processes, K deficiency did not cause major alterations in overall leaf sugar metabolism, suggesting that apple trees can adjust sugar production and transport even under K-limited conditions to maintain carbohydrate stability. However, there was a notable increase in the sucrose-to-hexose ratio, indicating a shift in the partitioning and utilization of sugars.
- Biomass allocation: A redistribution of plant biomass was observed under K deficiency. Specifically, K-deficient plants exhibited a decreased shoot-to-root ratio mainly due to the increased root biomass. This redistribution of biomass towards roots likely represents an adaptive strategy to enhance K uptake from the soil, helping the plant to cope with limited K availability.
- Future directions: Further research is needed to explore how rootstock−scion interactions might influence K utilization in apple trees. These interactions could play a significant role in optimizing K uptake and improving tree vigor and productivity under conditions of nutrient deficiency.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; pp. 178–189. [Google Scholar]
- Pettigrew, W.T. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant. 2008, 133, 670–681. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.K.; Mahiwal, S. Potassium in plant growth and development. In Role of Potassium in Plants; Springer: Cham, Switzerland, 2020; pp. 37–43. [Google Scholar]
- Templalexis, D.; Tsitsekian, D.; Liu, C.; Daras, G.; Šimura, J.; Moschou, P.; Ljung, K.; Hatzopoulos, P.; Rigas, S. Potassium transporter TRH1/KUP4 contributes to distinct auxin-mediated root system architecture responses. Plant Physiol. 2022, 188, 1043–1060. [Google Scholar] [CrossRef] [PubMed]
- Leigh, R.A.; Wyn Jones, R.G. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 1984, 97, 1–13. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A. Potassium. In Principles of Plant Nutrition; Mengel, K., Kirkby, E.A., Eds.; International Potash Institute: Worblaufeu-Bern, Switzerland, 1982; pp. 335–368. [Google Scholar]
- Xiao-Li, T.; Gang-Wei, W.; Rui, Z.; Pei-Zhu, Y.; Liu-Sheng, D.; Zhao-Hu, L. Conditions and indicators for screening cotton (Gossypium hirsutum L.) varieties tolerant to low potassium. Acta Agric. Sin. 2008, 34, 1435–1443. [Google Scholar]
- White, P.J.; Karley, A.J. Potassium. In Cell Biology of Metals and Nutrients; Hell, R., Mendel, R.-R., Eds.; Springer: Heidelberg, Germany, 2010; pp. 199–224. [Google Scholar]
- Kanai, S.; Ohkura, K.; Adu-Gyamfi, J.J.; Mohapatra, P.K.; Nguyen, N.T.; Saneoka, H.; Fujita, K. Depression of sink activity precedes the inhibition of biomass production in tomato plants subjected to potassium deficiency stress. J. Exp. Bot. 2007, 58, 2917–2928. [Google Scholar] [CrossRef]
- Juniper, B.E.; Mabberley, D.J. The Story of the Apple; Timber Press, Inc.: Portland, OR, USA, 2006; 240p. [Google Scholar]
- Bai, Q.; Shen, Y.; Huang, Y. Advances in mineral nutrition transport and signal transduction in Rosaceae fruit quality and postharvest storage. Front. Plant Sci. 2021, 12, 620018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, X.; Wang, Y.; Zhang, N.; Guo, Y.; Ren, X.; Zhao, Z. K fertilization arrests malate accumulation and alters soluble sugar metabolism in apple fruit. Biol. Open 2018, 7, bio024745. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Du, X.; Wang, F.; Sha, J.; Chen, Q.; Tian, G.; Zhu, Z.; Ge, S.; Jiang, Y. Effects of potassium levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Front. Plant Sci. 2020, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Nachtigall, G.R.; Dechen, A.R. Seasonality of nutrients in leaves and fruits of apple trees. Sci. Agric. 2006, 63, 493–501. [Google Scholar] [CrossRef]
- Brunetto, G.; de Melo, G.W.B.; Toselli, M.; Quartieri, M.; Tagliavini, M. The role of mineral nutrition on yields and fruit quality in grapevine, pear and apple. Rev. Bras. Frutic. 2015, 37, 1089–1104. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 23–32. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Jákli, B.; Tavakol, E.; Tränkner, M.; Senbayram, M.; Dittert, K. Quantitative limitations to photosynthesis in K deficient sunflower and their implications on water-use efficiency. J. Plant Physiol. 2017, 209, 20–30. [Google Scholar] [CrossRef]
- Papadakis, I.E.; Ladikou, E.-V.; Oikonomou, A.; Chatzistathis, T.; Chatziperou, G. Exploring the impact of potassium on growth, photosynthetic performance, and nutritional status of lemon trees (cv. Adamopoulou) grafted onto sour orange and volkamer lemon rootstocks. Sustainability 2023, 15, 15858. [Google Scholar] [CrossRef]
- Sustr, M.; Soukup, A.; Tylova, E. Potassium in root growth and development. Plants 2019, 8, 435. [Google Scholar] [CrossRef]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Oxidative stress and antioxidant responses in young leaves of mulberry plants grown under nitrogen, phosphorus or potassium deficiency. J. Integr. Plant Biol. 2007, 49, 313–322. [Google Scholar] [CrossRef]
- Chen, D.; Cao, B.; Wang, S.; Liu, P.; Deng, X.; Yin, L.; Zhang, S. Silicon moderated the K deficiency by improving the plant-water status in sorghum. Sci. Rep. 2016, 6, 22882. [Google Scholar] [CrossRef] [PubMed]
- Martineau, E.; Domec, J.C.; Bosc, A.; Dannoura, M.; Gibon, Y.; Benard, C.; Jordan-Meille, L. The role of potassium on maize leaf carbon exportation under drought condition. Acta Physiol. Plant. 2017, 39, 219. [Google Scholar] [CrossRef]
- Voitsekhovskaja, O.V.; Tyutereva, E.V. Chlorophyll b in angiosperms: Functions in photosynthesis, signaling and ontogenetic regulation. J. Plant Physiol. 2015, 189, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Koshino, Y.; Sawa, S.; Ishiguro, S.; Okada, K.; Tanaka, A. Overexpression of chlorophyllide a oxygenase (CAO) enlarges the antenna size of photosystem II in Arabidopsis thaliana. Plant J. 2001, 26, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J. Potassium control of plant functions: Ecological and agricultural implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Gerardeaux, E.; Jordan-Meille, L.; Constantin, J.; Pellerin, S.; Dingkuhn, M. Changes in plant morphology and dry matter partitioning caused by potassium deficiency in Gossypium hirsutum (L.). Environ. Exp. Bot. 2010, 67, 451–459. [Google Scholar] [CrossRef]
- Coffey, O.; Bonfield, R.; Corre, F.; Sirigiri, J.A.; Meng, D.; Fricke, W. Root and cell hydraulic conductivity, apoplastic barriers and aquaporin gene expression in barley (Hordeum vulgare L.) grown with low supply of potassium. Ann. Bot. 2018, 122, 1131–1141. [Google Scholar] [CrossRef]
- Yang, H.; Peng, L.; Chen, L.; Zhang, L.; Kan, L.; Shi, Y.; Mei, X.; Malladi, A.; Xu, Y.; Dong, C. Efficient potassium (K) recycling and root carbon (C) metabolism improve K use efficiency in pear rootstock genotypes. Plant Physiol. Biochem. 2023, 196, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sun, S.; Zhang, L.; Yang, J.; Wang, Z.; Ma, F.; Li, M. Carbohydrate metabolism and transport in apple roots under nitrogen deficiency. Plant Physiol. Biochem. 2020, 155, 455–463. [Google Scholar] [CrossRef]

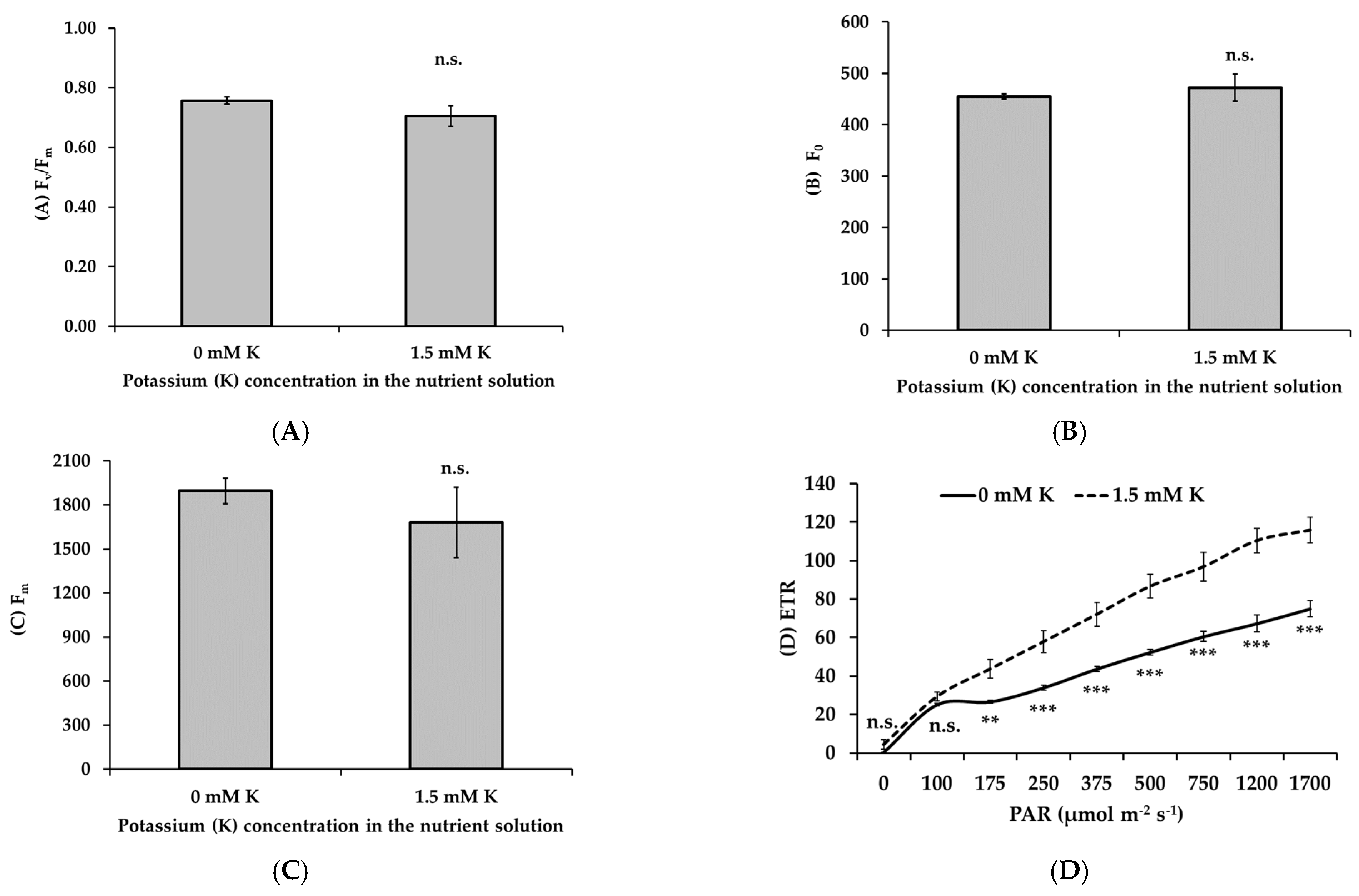
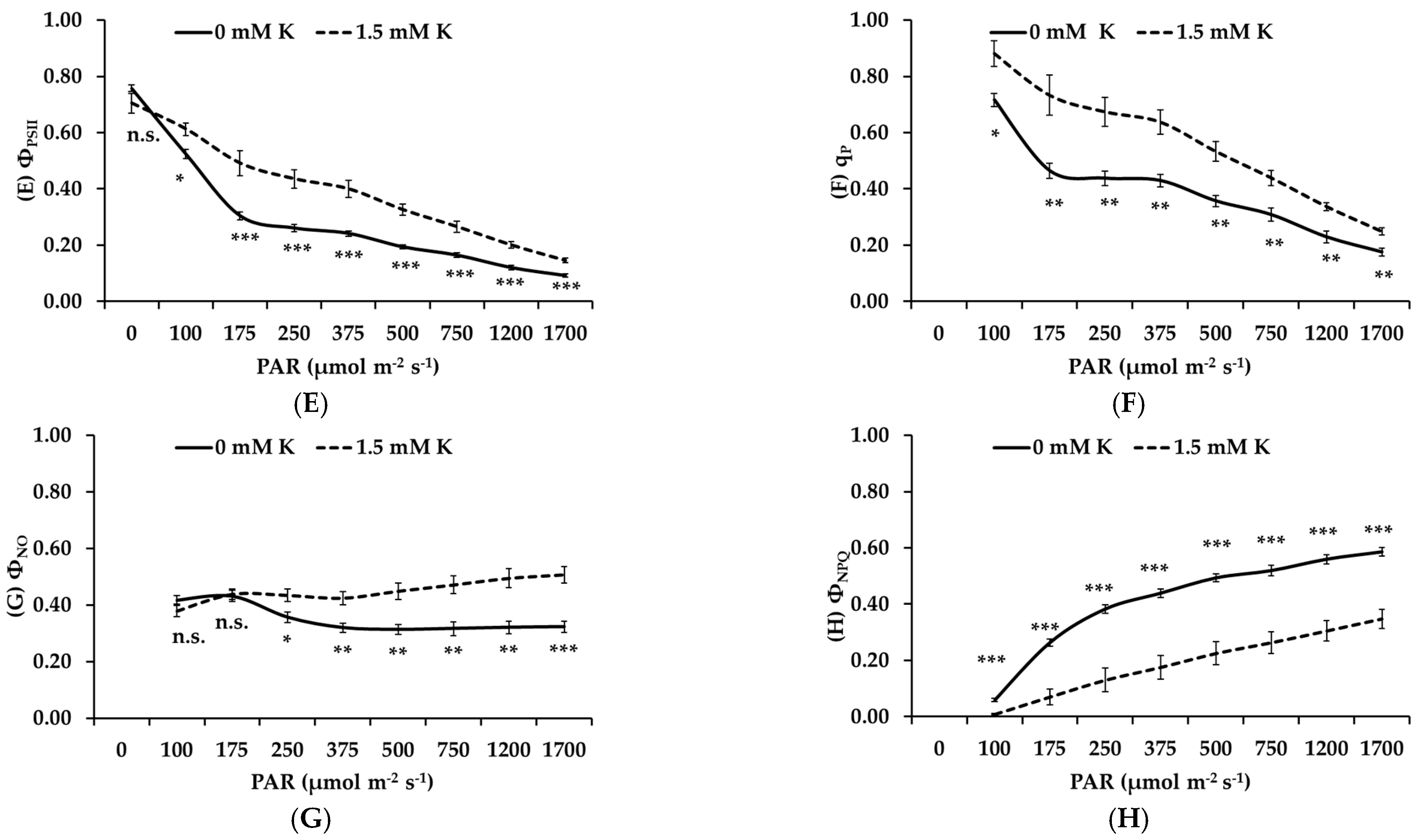
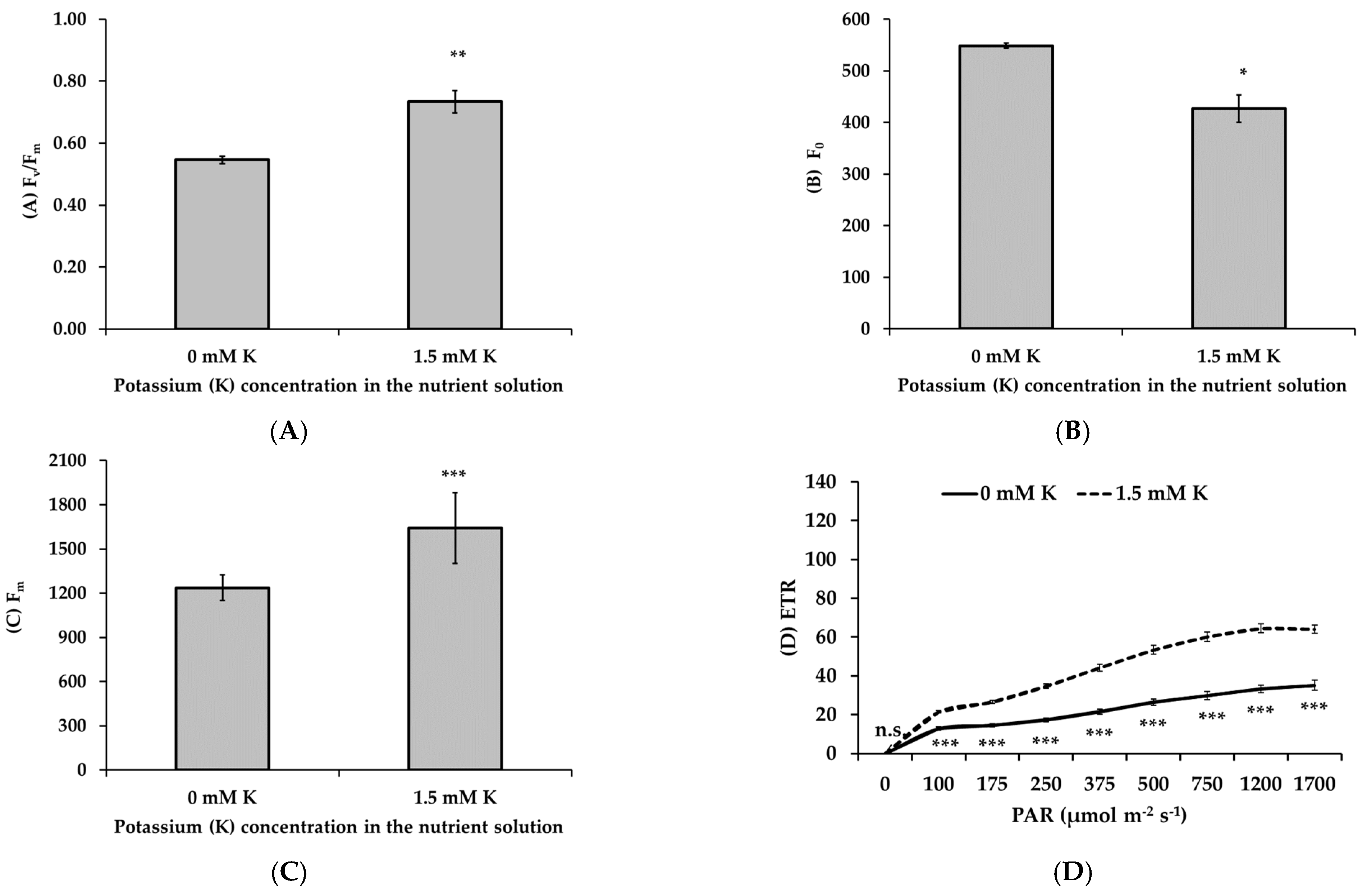
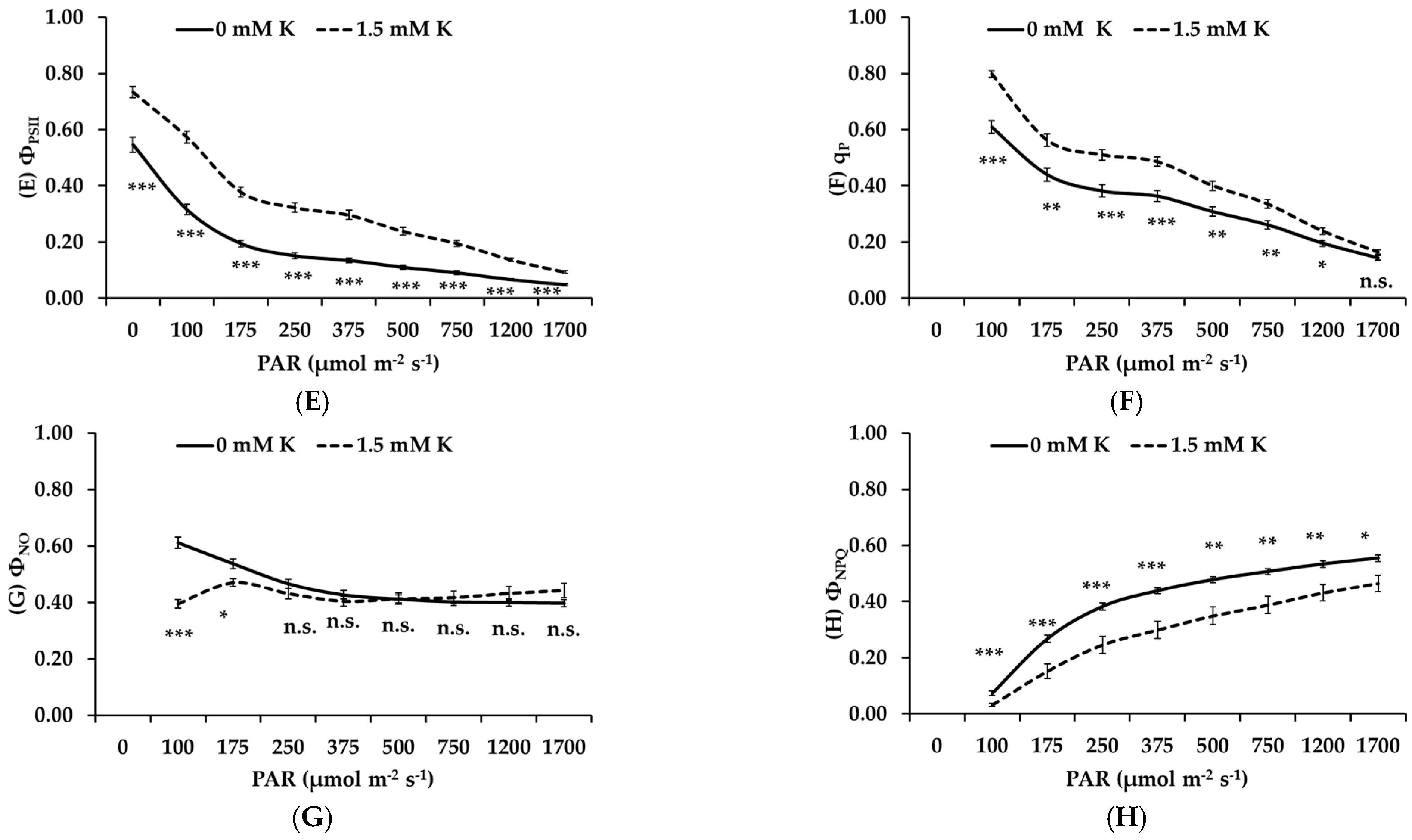
| Parameter | Plant Part | 0.00 mM K | 0.75 mM K | 1.50 mM K | 3.00 mM K |
|---|---|---|---|---|---|
| Fresh weight (FW) (g) | Leaves | 91.14 ± 2.32 a | 93.18 ± 10.79 a | 86.82 ± 5.12 a | 96.01 ± 7.35 a |
| Scion’s stems | 130.48 ± 5.03 b | 161.48 ± 5.03 a | 159.97 ± 6.91 a | 161.32 ± 2.36 a | |
| Rootstock’s stem | 113.29 ± 8.22 a | 136.1 ± 8.72 a | 144.29 ± 15.4 a | 128.41 ± 12.64 a | |
| Root | 200.17 ± 45.36 a | 138.83 ± 10.77 a | 121.44 ± 13.09 a | 121.3 ± 13.11 a | |
| Entire plant | 535.07 ± 42.68 a | 529.6 ± 24.06 a | 512.52 ± 22.4 a | 507.03 ± 23.31 a | |
| Scion | 221.62 ± 5.81 a | 254.66 ± 14.48 a | 246.79 ± 10.01 a | 257.33 ± 8.81 a | |
| Rootstock | 313.45 ± 44.36 a | 274.93 ± 12.56 a | 265.73 ± 19.15 a | 249.7 ± 20.28 a | |
| Shoot/Root ratio | 1.95 ± 0.34 b | 2.85 ± 0.15 ab | 3.36 ± 0.36 a | 3.34 ± 0.42 a | |
| Dry weight (DW) (g) | Leaves | 45.7 ± 0.52 a | 45.87 ± 3.96 a | 42.03 ± 1.9 a | 44.72 ± 2.21 a |
| Scion’s stems | 69.87 ± 2.45 b | 84.4 ± 3.12 a | 83.51 ± 3.56 a | 82 ± 1.29 a | |
| Rootstock’s stem | 57.98 ± 4.02 a | 70.83 ± 5.71 a | 76.57 ± 7.81 a | 68.14 ± 7.03 a | |
| Root | 28.87 ± 1.12 a | 23.04 ± 1.37 b | 24.83 ± 1.55 b | 24.64 ± 1.03 b | |
| Entire plant | 202.42 ± 5.43 a | 224.14 ± 9.16 a | 226.95 ± 10.09 a | 219.5 ± 8.54 a | |
| Scion | 115.57 ± 2.68 a | 130.28 ± 6.31 a | 125.54 ± 4.42 a | 126.72 ± 3.22 a | |
| Rootstock | 86.85 ± 3.04 a | 93.87 ± 5.95 a | 101.4 ± 6.88 a | 92.78 ± 6.94 a | |
| Shoot/root ratio | 6.08 ± 0.45 b | 8.79 ± 0.32 a | 8.35 ± 0.92 a | 7.96 ± 0.5 a | |
| Water content (% FW) | Leaves | 49.76 ± 1.10 a | 50.02 ± 1.69 a | 51.43 ± 0.70 a | 52.92 ± 1.71 a |
| Scion’s stems | 46.42 ± 0.34 c | 47.76 ± 0.58 b | 47.78 ± 0.46 b | 49.17 ± 0.30 a | |
| Rootstock’s stem | 48.72 ± 1.14 a | 48.12 ± 1.32 a | 46.82 ± 0.37 a | 47.04 ± 0.40 a | |
| Root | 83.47 ± 2.48 a | 83.3 ± 0.61 a | 79.1 ± 1.41 a | 78.47 ± 2.93 a | |
| Entire plant | 61.28 ± 2.89 a | 57.6 ± 1.00 a | 55.67 ± 1.01 a | 56.6 ± 1.16 a | |
| Scion | 47.82 ± 0.55 b | 48.73 ± 0.70 b | 49.08 ± 0.57 ab | 50.69 ± 0.62 a | |
| Rootstock | 70.47 ± 3.38 a | 65.81 ± 1.63 a | 61.65 ± 1.48 a | 62.55 ± 2.33 a | |
| Total length of the scion’s stems (cm) | 260 ± 18.68 a | 223.8 ± 27.61 a | 248.6 ± 19.61 a | 235.2 ± 8.1 a | |
| Plant Part | Parameter | 0.00 mM K | 0.75 mM K | 1.50 mM K | 3.00 mM K |
| Top leaves | Pn | 6.17 ± 0.19 b | 6.64 ± 0.32 b | 9.08 ± 0.53 a | 8.82 ± 0.45 a |
| gs | 0.09 ± 0.01 b | 0.08 ± 0.01 b | 0.12 ± 0.01 a | 0.09 ± 0.01 b | |
| Ci | 261.46 ± 6.12 a | 246.56 ± 12.34 a | 250.9 ± 10.44 a | 205.82 ± 15.5 b | |
| E | 1.77 ± 0.18 a | 1.57 ± 0.07 a | 2.16 ± 0.09 a | 1.83 ± 0.25 a | |
| Pn/gs | 72.15 ± 4.23 b | 81.76 ± 7.36 b | 76.38 ± 5.83 b | 105.03 ± 10.29 a | |
| Pn/Ci | 0.02 ± 0 b | 0.03 ± 0 b | 0.04 ± 0 a | 0.04 ± 0 a | |
| Pn/E | 3.64 ± 0.37 b | 4.25 ± 0.16 ab | 4.2 ± 0.12 ab | 4.99 ± 0.43 a | |
| Basal leaves | Pn | 5.32 ± 0.4 c | 7.73 ± 0.31 b | 8.22 ± 0.27 ab | 9.25 ± 0.46 a |
| gs | 0.07 ± 0 b | 0.09 ± 0.01 ab | 0.11 ± 0.01 a | 0.12 ± 0.01 a | |
| Ci | 248.41 ± 10.18 a | 234.55 ± 13.7 a | 242.52 ± 15.04 a | 238.59 ± 10.39 a | |
| E | 1.46 ± 0.09 a | 2.02 ± 0.23 a | 2.05 ± 0.26 a | 2.3 ± 0.29 a | |
| Pn/gs | 81.1 ± 6.12 a | 87.05 ± 8.78 a | 82.35 ± 9.78 a | 83.32 ± 6.83 a | |
| Pn/Ci | 0.02 ± 0 b | 0.03 ± 0 a | 0.03 ± 0 a | 0.04 ± 0 a | |
| Pn/E | 3.67 ± 0.28 a | 4.09 ± 0.49 a | 4.43 ± 0.58 a | 4.3 ± 0.39 a |
| Plant Part | Parameter | 0.00 mM K | 0.75 mM K | 1.50 mM K | 3.00 mM K |
|---|---|---|---|---|---|
| Top leaves | [Chla] (μg mg−1 FW) | 2.23 ± 0.05 a | 2.38 ± 0.1 a | 2.42 ± 0.18 a | 2.56 ± 0.15 a |
| [Chlb] (μg mg−1 FW) | 0.76 ± 0.02 a | 0.8 ± 0.03 a | 0.83 ± 0.05 a | 0.8 ± 0.05 a | |
| [Chla] + [Chlb] (μg mg−1 FW) | 2.99 ± 0.07 a | 3.18 ± 0.13 a | 3.25 ± 0.23 a | 3.36 ± 0.19 a | |
| [Carotenoids] (μg mg−1 FW) | 0.53 ± 0.02 a | 0.59 ± 0.01 a | 0.58 ± 0.04 a | 0.62 ± 0.04 a | |
| [Chla] (μg cm−2) | 42.49 ± 1.96 b | 48.91 ± 1.45 ab | 49.5 ± 3.09 ab | 53.63 ± 2.52 a | |
| [Chlb] (μg cm−2) | 14.46 ± 0.64 a | 16.41 ± 0.55 a | 17.1 ± 0.89 a | 16.89 ± 0.84 a | |
| [Chla] + [Chlb] (μg cm−2) | 56.95 ± 2.56 b | 65.32 ± 1.97 ab | 66.59 ± 3.95 a | 70.52 ± 3.36 a | |
| [Carotenoids] (μg cm−2) | 10 ± 0.3 b | 12.2 ± 0.23 a | 11.82 ± 0.56 a | 13.07 ± 0.57 a | |
| [Chla]/[Chl b] | 2.94 ± 0.06 b | 2.98 ± 0.04 b | 2.89 ± 0.05 b | 3.18 ± 0.01 a | |
| SPAD values | 56.46 ± 1.17 a | 55.71 ± 1.66 a | 57.13 ± 0.46 a | 53.92 ± 1.6 a | |
| Electrolyte leakage (%) | 28.47 ± 1.88 a | 19.66 ± 1.57 bc | 24.43 ± 3.13 ab | 13.94 ± 1.22 c | |
| Basal leaves | [Chla] (μg mg−1 FW) | 1.23 ± 0.05 b | 2.03 ± 0.12 a | 2.22 ± 0.08 a | 2 ± 0.05 a |
| [Chlb] (μg mg−1 FW) | 0.46 ± 0.01 c | 0.75 ± 0.04 ab | 0.81 ± 0.03 a | 0.7 ± 0.02 b | |
| [Chla] + [Chlb] (μg mg−1 FW) | 1.69 ± 0.06 b | 2.78 ± 0.16 a | 3.03 ± 0.11 a | 2.7 ± 0.07 a | |
| [Carotenoids] (μg mg−1 FW) | 0.34 ± 0.01 b | 0.49 ± 0.02 a | 0.5 ± 0.01 a | 0.46 ± 0.01 a | |
| [Chla] (μg cm−2) | 23.78 ± 1.04 b | 43.28 ± 3 a | 42.34 ± 0.98 a | 41.61 ± 0.66 a | |
| [Chlb] (μg cm−2) | 8.86 ± 0.38 b | 16.04 ± 1.1 a | 15.48 ± 0.28 a | 14.51 ± 0.2 a | |
| [Chla] + [Chlb] (μg cm−2) | 32.64 ± 1.4 b | 59.32 ± 4.09 a | 57.81 ± 1.23 a | 56.12 ± 0.85 a | |
| [Carotenoids] (μg cm−2) | 6.49 ± 0.34 b | 10.47 ± 0.68 a | 9.57 ± 0.25 a | 9.46 ± 0.08 a | |
| [Chla]/[Chlb] | 2.69 ± 0.04 b | 2.7 ± 0.02 b | 2.74 ± 0.03 b | 2.87 ± 0.01 a | |
| SPAD values | 38.27 ± 0.99 c | 49.79 ± 1.7 a | 48.1 ± 1.63 ab | 44.34 ± 1.22 b | |
| Electrolyte leakage (%) | 34.73 ± 2.08 a | 25.67 ± 1.65 b | 26.38 ± 1 b | 20.96 ± 1.25 c |
| Parameter | 0.00 mM K | 0.75 mM K | 1.50 mM K | 3.00 mM K |
|---|---|---|---|---|
| Sucrose (Suc) | 3.32 ± 0.7 a | 2.41 ± 0.28 a | 3.09 ± 0.07 a | 2.79 ± 0.15 a |
| Glucose (Glu) | 0.7 ± 0.04 b | 0.38 ± 0.07 c | 0.65 ± 0.05 b | 1.07 ± 0.09 a |
| Fructose (Fru) | 0.59 ± 0.25 a | 1.84 ± 0.44 a | 1.61 ± 0.15 a | 1.29 ± 0.29 a |
| Sorbitol (Sorb) | 11.58 ± 0.43 a | 10.3 ± 0.7 a | 11.64 ± 0.17 a | 12.36 ± 0.61 a |
| Total sugars | 16.19 ± 1 a | 14.94 ± 1.38 a | 16.99 ± 0.29 a | 17.51 ± 0.52 a |
| Translocating sugars | 14.9 ± 0.8 a | 12.71 ± 0.94 a | 14.73 ± 0.2 a | 15.15 ± 0.69 a |
| Non-translocating sugars | 1.29 ± 0.21 a | 2.23 ± 0.5 a | 2.26 ± 0.15 a | 2.36 ± 0.24 a |
| Trans/Total | 0.92 ± 0.01 a | 0.86 ± 0.03 a | 0.87 ± 0.01 a | 0.86 ± 0.02 a |
| Non-trans/Total | 0.08 ± 0.01 a | 0.14 ± 0.03 a | 0.13 ± 0.01 a | 0.14 ± 0.02 a |
| Trans/Non-trans | 12.16 ± 1.25 a | 7.23 ± 2.3 a | 6.57 ± 0.39 a | 6.65 ± 0.77 a |
| Sucr/Fru + Glu | 2.54 ± 0.15 a | 1.28 ± 0.3 b | 1.38 ± 0.1 b | 1.23 ± 0.15 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladikou, E.-V.; Daras, G.; Landi, M.; Chatzistathis, T.; Sotiropoulos, T.; Rigas, S.; Papadakis, I.E. Physiological and Biochemical Effects of Potassium Deficiency on Apple Tree Growth. Horticulturae 2025, 11, 42. https://doi.org/10.3390/horticulturae11010042
Ladikou E-V, Daras G, Landi M, Chatzistathis T, Sotiropoulos T, Rigas S, Papadakis IE. Physiological and Biochemical Effects of Potassium Deficiency on Apple Tree Growth. Horticulturae. 2025; 11(1):42. https://doi.org/10.3390/horticulturae11010042
Chicago/Turabian StyleLadikou, Evangelia-Vasiliki, Gerasimos Daras, Marco Landi, Theocharis Chatzistathis, Thomas Sotiropoulos, Stamatis Rigas, and Ioannis E. Papadakis. 2025. "Physiological and Biochemical Effects of Potassium Deficiency on Apple Tree Growth" Horticulturae 11, no. 1: 42. https://doi.org/10.3390/horticulturae11010042
APA StyleLadikou, E.-V., Daras, G., Landi, M., Chatzistathis, T., Sotiropoulos, T., Rigas, S., & Papadakis, I. E. (2025). Physiological and Biochemical Effects of Potassium Deficiency on Apple Tree Growth. Horticulturae, 11(1), 42. https://doi.org/10.3390/horticulturae11010042









