Lycopene and Other Bioactive Compounds’ Extraction from Tomato Processing Industry Waste: A Comparison of Ultrasonication Versus a Conventional Stirring Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solvents, Reagents, and Materials
2.2. Instrumentation
2.3. Extraction Process
2.3.1. Ultrasonic Bath-Assisted Extraction (UBAE)
2.3.2. Stirring Extraction (STE)
2.4. Experimental Design and Response-Surface Methodology (RSM) Optimization
2.5. Quantification of Bioactive Compounds
2.5.1. Determination of Total Polyphenol Content (TPC)
2.5.2. Individual Polyphenol Quantification
2.5.3. Determination of TCC
2.6. In Vitro Antioxidant Capacity Assessment
2.6.1. DPPH• Scavenging Activity
2.6.2. Ferric-Reducing Antioxidant Power (FRAP)
2.7. Statistics
3. Results and Discussion
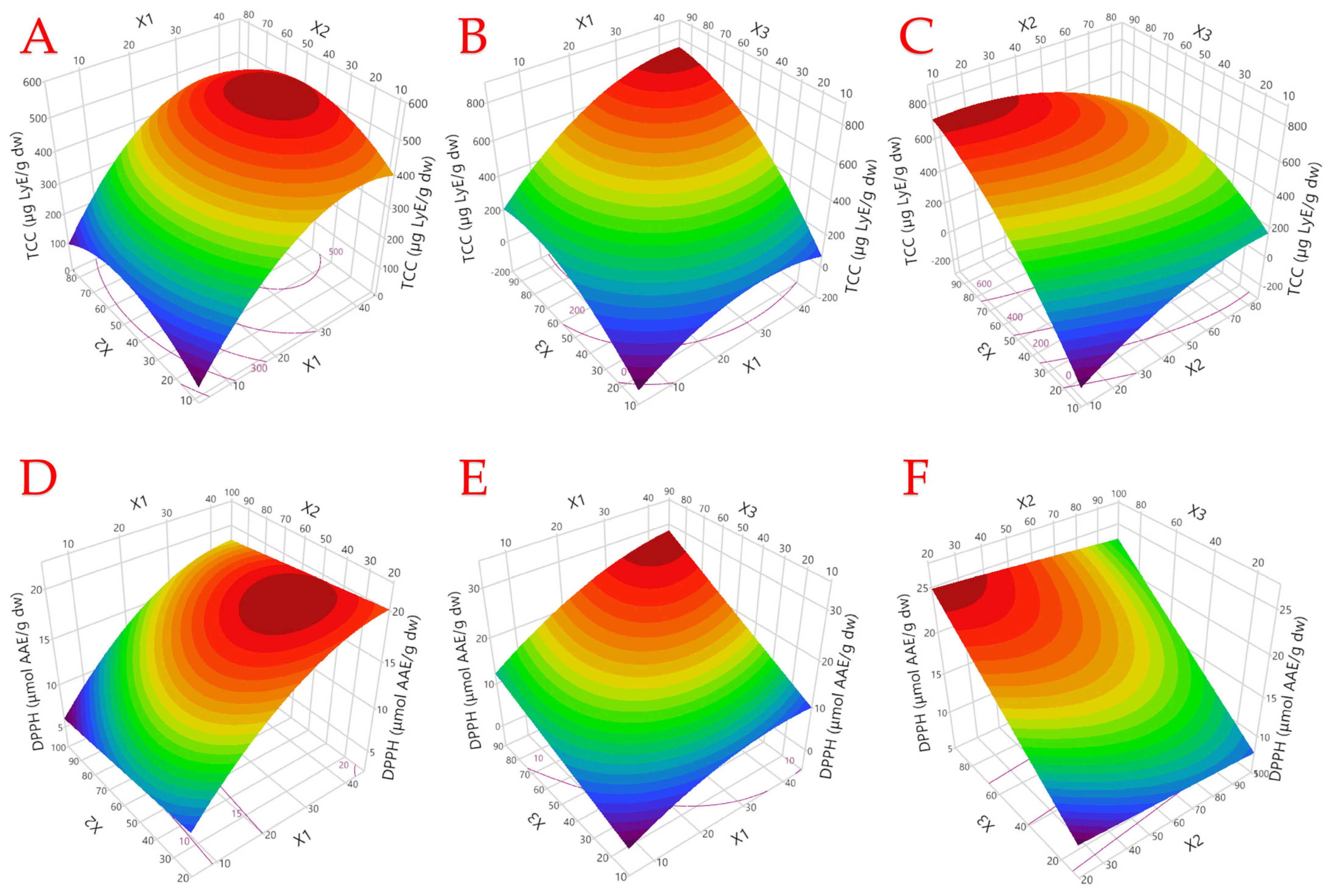
3.1. Optimization of Extraction Parameters
3.2. Model Analysis
3.3. Pareto Plot Analysis of the Effect of Extraction Parameters on Assays
3.4. Investigating Optimal Extraction Conditions Through Partial Least Squares (PLS) Analysis
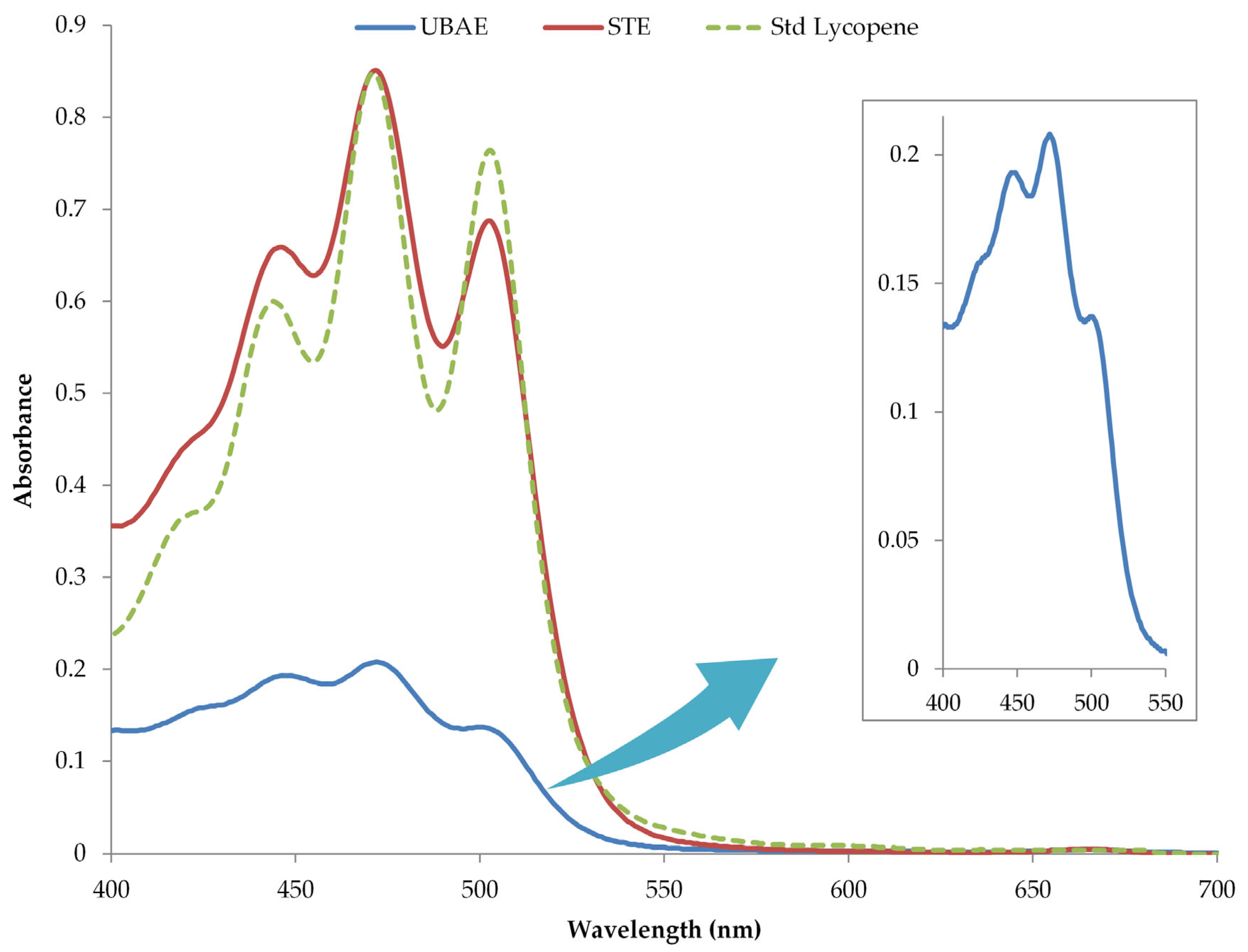
3.5. Comparison of Two Extraction Techniques
3.5.1. Antioxidant Activity of Extracts
3.5.2. Individual Polyphenols Composition
3.6. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trombino, S.; Cassano, R.; Procopio, D.; Di Gioia, M.L.; Barone, E. Valorization of Tomato Waste as a Source of Carotenoids. Molecules 2021, 26, 5062. [Google Scholar] [CrossRef] [PubMed]
- Radić, K.; Galić, E.; Vinković, T.; Golub, N.; Vitali Čepo, D. Tomato Waste as a Sustainable Source of Antioxidants and Pectins: Processing, Pretreatment and Extraction Challenges. Sustainability 2024, 16, 9158. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of Phytochemicals Present in Tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Santagata, R.; Ripa, M.; Genovese, A.; Ulgiati, S. Food Waste Recovery Pathways: Challenges and Opportunities for an Emerging Bio-Based Circular Economy. A Systematic Review and an Assessment. J. Clean. Prod. 2021, 286, 125490. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sarsaiya, S.; Patel, A.; Juneja, A.; Singh, R.P.; Yan, B.; Awasthi, S.K.; Jain, A.; Liu, T.; Duan, Y.; et al. Refining Biomass Residues for Sustainable Energy and Bio-Products: An Assessment of Technology, Its Importance, and Strategic Applications in Circular Bio-Economy. Renew. Sustain. Energy Rev. 2020, 127, 109876. [Google Scholar] [CrossRef]
- Morón-Ortiz, Á.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J. Sustainable Green Extraction of Carotenoid Pigments: Innovative Technologies and Bio-Based Solvents. Antioxidants 2024, 13, 239. [Google Scholar] [CrossRef]
- Giovanoudis, I.; Athanasiadis, V.; Chatzimitakos, T.; Gortzi, O.; Nanos, G.D.; Lalas, S.I. Development of a Cloud Point Extraction Technique Based on Lecithin for the Recovery of Carotenoids from Liquid Tomato Wastewater. Waste 2022, 1, 105–114. [Google Scholar] [CrossRef]
- Vlachoudi, D.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Lalas, S.I. Enhanced Extraction of Carotenoids from Tomato Industry Waste Using Menthol/Fatty Acid Deep Eutectic Solvent. Waste 2023, 1, 977–992. [Google Scholar] [CrossRef]
- Kultys, E.; Kurek, M.A. Green Extraction of Carotenoids from Fruit and Vegetable Byproducts: A Review. Molecules 2022, 27, 518. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Sit, N. Extraction of Bioactive Compounds from Plant Materials Using Combination of Various Novel Methods: A Review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public. Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Strati, I.F.; Oreopoulou, V. Effect of Extraction Parameters on the Carotenoid Recovery from Tomato Waste. Int. J. Food Sci. Technol. 2011, 46, 23–29. [Google Scholar] [CrossRef]
- Strati, I.F.; Oreopoulou, V. Process Optimisation for Recovery of Carotenoids from Tomato Waste. Food Chem. 2011, 129, 747–752. [Google Scholar] [CrossRef]
- Szabo, K.; Teleky, B.-E.; Ranga, F.; Roman, I.; Khaoula, H.; Boudaya, E.; Ltaief, A.B.; Aouani, W.; Thiamrat, M.; Vodnar, D.C. Carotenoid Recovery from Tomato Processing By-Products through Green Chemistry. Molecules 2022, 27, 3771. [Google Scholar] [CrossRef]
- Anarjan, N. Evaluation the Effects of Ultrasonic Parameters on Simultaneously Extraction and Size Reduction of Lycopene from Tomato Processing Waste. Waste Biomass Valorization 2020, 11, 1929–1940. [Google Scholar] [CrossRef]
- Solaberrieta, I.; Mellinas, C.; Jiménez, A.; Garrigós, M.C. Recovery of Antioxidants from Tomato Seed Industrial Wastes by Microwave-Assisted and Ultrasound-Assisted Extraction. Foods 2022, 11, 3068. [Google Scholar] [CrossRef]
- Silva, Y.P.A.; Ferreira, T.A.P.C.; Jiao, G.; Brooks, M.S. Sustainable Approach for Lycopene Extraction from Tomato Processing By-Product Using Hydrophobic Eutectic Solvents. J. Food Sci. Technol. 2019, 56, 1649–1654. [Google Scholar] [CrossRef]
- Popescu, M.; Iancu, P.; Pleșu, V.; Bîldea, C.S.; Todasca, C.M. Different Spectrophotometric Methods for Simultaneous Quantification of Lycopene and β-Carotene from a Binary Mixture. LWT 2022, 160, 113238. [Google Scholar] [CrossRef]
- Kalompatsios, D.; Athanasiadis, V.; Mantiniotou, M.; Lalas, S.I. Optimization of Ultrasonication Probe-Assisted Extraction Parameters for Bioactive Compounds from Opuntia macrorhiza Using Taguchi Design and Assessment of Antioxidant Properties. Appl. Sci. 2024, 14, 10460. [Google Scholar] [CrossRef]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction Optimisation Using Water/Glycerol for the Efficient Recovery of Polyphenolic Antioxidants from Two Artemisia Species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Thoo, Y.; Ng, S.Y.; Khoo, M.; Mustapha, W.; Ho, C. A Binary Solvent Extraction System for Phenolic Antioxidants and Its Application to the Estimation of Antioxidant Capacity in Andrographis paniculata Extracts. Int. Food Res. J. 2013, 20, 1103. [Google Scholar]
- Athanasiadis, V.; Chatzimitakos, T.; Makrygiannis, I.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Antioxidant-Rich Extracts from Lemon Verbena (Aloysia citrodora L.) Leaves through Response Surface Methodology. Oxygen 2024, 4, 1–19. [Google Scholar] [CrossRef]
- Lee, J.-E.; Jayakody, J.T.M.; Kim, J.-I.; Jeong, J.-W.; Choi, K.-M.; Kim, T.-S.; Seo, C.; Azimi, I.; Hyun, J.; Ryu, B. The Influence of Solvent Choice on the Extraction of Bioactive Compounds from Asteraceae: A Comparative Review. Foods 2024, 13, 3151. [Google Scholar] [CrossRef]
- More, P.R.; Jambrak, A.R.; Arya, S.S. Green, Environment-Friendly and Sustainable Techniques for Extraction of Food Bioactive Compounds and Waste Valorization. Trends Food Sci. Technol. 2022, 128, 296–315. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef]
- Campos-Lozada, G.; Pérez-Marroquín, X.A.; Callejas-Quijada, G.; Campos-Montiel, R.G.; Morales-Peñaloza, A.; León-López, A.; Aguirre-Álvarez, G. The Effect of High-Intensity Ultrasound and Natural Oils on the Extraction and Antioxidant Activity of Lycopene from Tomato (Solanum lycopersicum) Waste. Antioxidants 2022, 11, 1404. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Ninčević Grassino, A.; Ostojić, J.; Miletić, V.; Djaković, S.; Bosiljkov, T.; Zorić, Z.; Ježek, D.; Rimac Brnčić, S.; Brnčić, M. Application of High Hydrostatic Pressure and Ultrasound-Assisted Extractions as a Novel Approach for Pectin and Polyphenols Recovery from Tomato Peel Waste. Innov. Food Sci. Emerg. Technol. 2020, 64, 102424. [Google Scholar] [CrossRef]




| Design Point | Independent Variables | Responses | |||||
|---|---|---|---|---|---|---|---|
| TCC (μg LyE/g dw) | DPPH (μmol AAE/g dw) | ||||||
| X1 (R, mL/g) | X2 (t, min) | X3 (E, %) | Actual * | Predicted | Actual * | Predicted | |
| 1 | 1 (40) | −1 (5) | 0 (80) | 164.9 ± 6.6 | 241.7 | 6.56 ± 0.33 | 6.52 |
| 2 | −1 (10) | 1 (25) | 0 (80) | 325.4 ± 18.2 | 287.3 | 4.28 ± 0.27 | 4.40 |
| 3 | 0 (25) | 0 (15) | 0 (80) | 115.4 ± 5.7 | 122.0 | 5.30 ± 0.12 | 5.24 |
| 4 | 0 (25) | 1 (25) | −1 (60) | 148.8 ± 8.6 | 228.7 | 5.69 ± 0.33 | 6.11 |
| 5 | −1 (10) | 0 (15) | −1 (60) | 294 ± 20 | 222.7 | 3.06 ± 0.09 | 2.56 |
| 6 | 1 (40) | 0 (15) | −1 (60) | 121.6 ± 8.8 | 71.0 | 6.84 ± 0.44 | 6.41 |
| 7 | 0 (25) | 0 (15) | 0 (80) | 119.8 ± 6.9 | 122.0 | 5.36 ± 0.24 | 5.24 |
| 8 | 0 (25) | −1 (5) | −1 (60) | 121.2 ± 4.1 | 146.0 | 5.15 ± 0.18 | 5.67 |
| 9 | −1 (10) | −1 (5) | 0 (80) | 406.6 ± 13.4 | 393.5 | 5.31 ± 0.39 | 5.26 |
| 10 | 0 (25) | −1 (5) | 1 (100) | 475.7 ± 28.1 | 387.2 | 6.59 ± 0.45 | 6.17 |
| 11 | 0 (25) | 0 (15) | 0 (80) | 113.5 ± 4.9 | 122.0 | 5.07 ± 0.21 | 5.24 |
| 12 | 1 (40) | 1 (25) | 0 (80) | 143.9 ± 3.6 | 135.5 | 5.68 ± 0.36 | 5.65 |
| 13 | 1 (40) | 0 (15) | 1 (100) | 141.1 ± 4.1 | 123.2 | 2.50 ± 0.05 | 3.00 |
| 14 | −1 (10) | 0 (15) | 1 (100) | 152.4 ± 7.3 | 275.0 | 3.91 ± 0.28 | 4.34 |
| 15 | 0 (25) | 1 (25) | 1 (100) | 125.5 ± 7.5 | 92.1 | 4.51 ± 0.12 | 4.00 |
| Design Point | Independent Variables | Responses | |||||
|---|---|---|---|---|---|---|---|
| TCC (μg LyE/g dw) | DPPH (μmol AAE/g dw) | ||||||
| X1 (R, mL/g) | X2 (t, min) | X3 (T, °C) | Actual * | Predicted | Actual * | Predicted | |
| 1 | 1 (40) | −1 (30) | 0 (50) | 442.1 ± 24.3 | 504.0 | 21.01 ± 1.16 | 19.61 |
| 2 | −1 (10) | 1 (90) | 0 (50) | 224.9 ± 13.5 | 198.0 | 8.46 ± 0.27 | 9.03 |
| 3 | 0 (25) | 0 (60) | 0 (50) | 491.6 ± 16.7 | 481.1 | 16.13 ± 0.52 | 15.96 |
| 4 | 0 (25) | 1 (90) | −1 (20) | 191.8 ± 5.6 | 232.2 | 9.61 ± 0.44 | 9.79 |
| 5 | −1 (10) | 0 (60) | −1 (20) | 97 ± 5.3 | 101.0 | 5.84 ± 0.33 | 5.18 |
| 6 | 1 (40) | 0 (60) | −1 (20) | 247.1 ± 17.5 | 241.0 | 12.55 ± 0.7 | 12.32 |
| 7 | 0 (25) | 0 (60) | 0 (50) | 471 ± 17.4 | 481.1 | 14.85 ± 0.65 | 15.96 |
| 8 | 0 (25) | −1 (30) | −1 (20) | 177.7 ± 10.1 | 139.4 | 10.41 ± 0.26 | 11.00 |
| 9 | −1 (10) | −1 (30) | 0 (50) | 254 ± 8.6 | 270.8 | 10.76 ± 0.57 | 10.24 |
| 10 | 0 (25) | −1 (30) | 1 (80) | 691.3 ± 37.3 | 650.9 | 21.69 ± 1.17 | 22.13 |
| 11 | 0 (25) | 0 (60) | 0 (50) | 480.6 ± 26 | 481.1 | 15.88 ± 0.86 | 15.96 |
| 12 | 1 (40) | 1 (90) | 0 (50) | 483 ± 25.6 | 431.2 | 17.81 ± 0.73 | 18.40 |
| 13 | 1 (40) | 0 (60) | 1 (80) | 684.1 ± 30.8 | 680.1 | 24.63 ± 0.49 | 25.68 |
| 14 | −1 (10) | 0 (60) | 1 (80) | 347.6 ± 22.6 | 353.7 | 13.49 ± 0.35 | 14.10 |
| 15 | 0 (25) | 1 (90) | 1 (80) | 374 ± 20.6 | 412.4 | 23.15 ± 1.74 | 20.92 |
| Factor | UBAE | STE | ||
|---|---|---|---|---|
| TCC | DPPH | TCC | DPPH | |
| Stepwise regression coefficients | ||||
| Intercept | 121.97 * | 5.243 * | 481.07 * | 15.96 * |
| X1—liquid-to-solid ratio | −75.86 * | 0.628 * | 116.60 * | 4.681 * |
| X2—extraction time | −53.10 | −0.431 | −36.42 | −0.605 |
| X3—ultrasonic power/temperature | 26.138 | −0.404 | 172.93 * | 5.569 * |
| X1 × 2 | - | - | - | - |
| X1X3 | - | −1.298 * | 46.6 | 1.107 |
| X2X3 | −94.45 * | −0.655 | −82.85 * | - |
| X12 | 51.004 | −0.597 | −72.41 * | −1.641 * |
| X22 | 91.529 * | 0.811 * | −57.66 | - |
| X32 | - | −0.569 | −64.71 * | - |
| ANOVA | ||||
| F-value (model) | 4.236 | 8.045 | 23.25 | 68.1 |
| F-value (lack of fit) | 744.37 | 19.1 | 32.565 | 3.334 |
| p-value (model) | 0.0323 * | 0.0102 * | 0.0006 * | <0.0001 * |
| p-value (lack of fit) | 0.0013 * | 0.0504 ns | 0.0300 * | 0.2501 ns |
| R2 | 0.761 | 0.915 | 0.969 | 0.974 |
| Adjusted R2 | 0.581 | 0.801 | 0.927 | 0.960 |
| RMSE | 76.373 | 0.553 | 48.396 | 1.138 |
| CV | 59.6 | 24.55 | 47.52 | 37.71 |
| DF (total) | 14 | 14 | 14 | 14 |
| Technique | Parameters | Independent Variables | Desirability | Stepwise Regression | ||
|---|---|---|---|---|---|---|
| X1 (R, mL/g) | X2 (t, min) | X3 (E, %) | ||||
| UBAE | TCC (μg LyE/g dw) | 10 | 5 | 96 | 0.9576 | 489.9 ± 145.5 |
| DPPH (μmol AAE/g dw) | 40 | 5 | 66 | 0.9761 | 6.97 ± 1.17 | |
| X1 (R, mL/g) | X2 (t, min) | X3 (T, °C) | ||||
| STE | TCC (μg LyE/g dw) | 37 | 37 | 80 | 0.9946 | 731.1 ± 100.4 |
| DPPH (μmol AAE/g dw) | 38 | 33 | 80 | 0.9945 | 25.86 ± 1.97 | |
| Technique | Independent Variables | PLS Model Values | |||
|---|---|---|---|---|---|
| X1 (R, mL/g) | X2 (t, min) | X3 (E, %) | TCC (μg LyE/g dw) | DPPH (μmol AAE/g dw) | |
| UBAE | 10 | 5 | 100 | 514.05 | 6.24 |
| X1 (R, mL/g) | X2 (t, min) | X3 (T, °C) | |||
| STE | 40 | 30 | 80 | 741.69 | 26.28 |
| Parameters | UBAE | STE |
|---|---|---|
| TCC (μg LyE/g) | 420.8 ± 29.46 | 723.75 ± 46.32 * |
| DPPH (μmol AAE/g) | 7.5 ± 0.52 | 19.94 ± 0.98 * |
| FRAP (μmol AAE/g) | 4.76 ± 0.15 | 16.68 ± 0.82 * |
| TPC (mg GAE/g) | 2.62 ± 0.05 | 3.69 ± 0.08 * |
| Phenolic Compounds (mg/g) | UBAE | STE |
|---|---|---|
| Non-Flavonoids | ||
| Coniferyl alcohol | 0.32 ± 0.01 | 0.38 ± 0.02 * |
| Syringic acid | 0.11 ± 0.01 * | n.d. |
| 4-Methylcatechol | 0.03 ± 0 | 0.11 ± 0 * |
| Ferulic acid | 0.07 ± 0 | 0.29 ± 0.02 * |
| trans-Cinnamic acid | 0.06 ± 0 | 0.37 ± 0.02 * |
| 3,4,5—Trimethoxycinnamic acid | n.d. | 0.07 ± 0 * |
| ∑ Non-flavonoids | 0.59 ± 0.02 | 1.21 ± 0.08 * |
| Flavonoids | ||
| Rutin | 0.29 ± 0.01 | 0.46 ± 0.01 * |
| Naringin | 0.48 ± 0.02 | 0.92 ± 0.02 * |
| Naringin dihydrochalcone | 0.14 ± 0.01 | 0.39 ± 0.03 * |
| Naringenin | 0.12 ± 0 | 0.35 ± 0.03 * |
| ∑ Flavonoids | 1.03 ± 0.04 | 2.13 ± 0.09 * |
| Total identified phenolics (TIP) | 1.62 ± 0.06 | 3.33 ± 0.17 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavridis, K.; Todas, N.; Kalompatsios, D.; Athanasiadis, V.; Lalas, S.I. Lycopene and Other Bioactive Compounds’ Extraction from Tomato Processing Industry Waste: A Comparison of Ultrasonication Versus a Conventional Stirring Method. Horticulturae 2025, 11, 71. https://doi.org/10.3390/horticulturae11010071
Mavridis K, Todas N, Kalompatsios D, Athanasiadis V, Lalas SI. Lycopene and Other Bioactive Compounds’ Extraction from Tomato Processing Industry Waste: A Comparison of Ultrasonication Versus a Conventional Stirring Method. Horticulturae. 2025; 11(1):71. https://doi.org/10.3390/horticulturae11010071
Chicago/Turabian StyleMavridis, Konstantinos, Nikolaos Todas, Dimitrios Kalompatsios, Vassilis Athanasiadis, and Stavros I. Lalas. 2025. "Lycopene and Other Bioactive Compounds’ Extraction from Tomato Processing Industry Waste: A Comparison of Ultrasonication Versus a Conventional Stirring Method" Horticulturae 11, no. 1: 71. https://doi.org/10.3390/horticulturae11010071
APA StyleMavridis, K., Todas, N., Kalompatsios, D., Athanasiadis, V., & Lalas, S. I. (2025). Lycopene and Other Bioactive Compounds’ Extraction from Tomato Processing Industry Waste: A Comparison of Ultrasonication Versus a Conventional Stirring Method. Horticulturae, 11(1), 71. https://doi.org/10.3390/horticulturae11010071









