Nutrient Balance of Citrus Across the Mandarin Belts of India
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset
2.2. Methods of Tissue Analysis
2.3. The Sample Space
2.3.1. Need for Log-Ratio Transformation
2.3.2. Centered Log-Ratio Transformation (clr)
2.3.3. Weighted Log-Ratio Transformation
2.4. Statistical Analysis
3. Results
3.1. Gain Ratio
3.2. Machine Learning (ML) Classification Models
3.3. Nutrient Standards by Cultivars
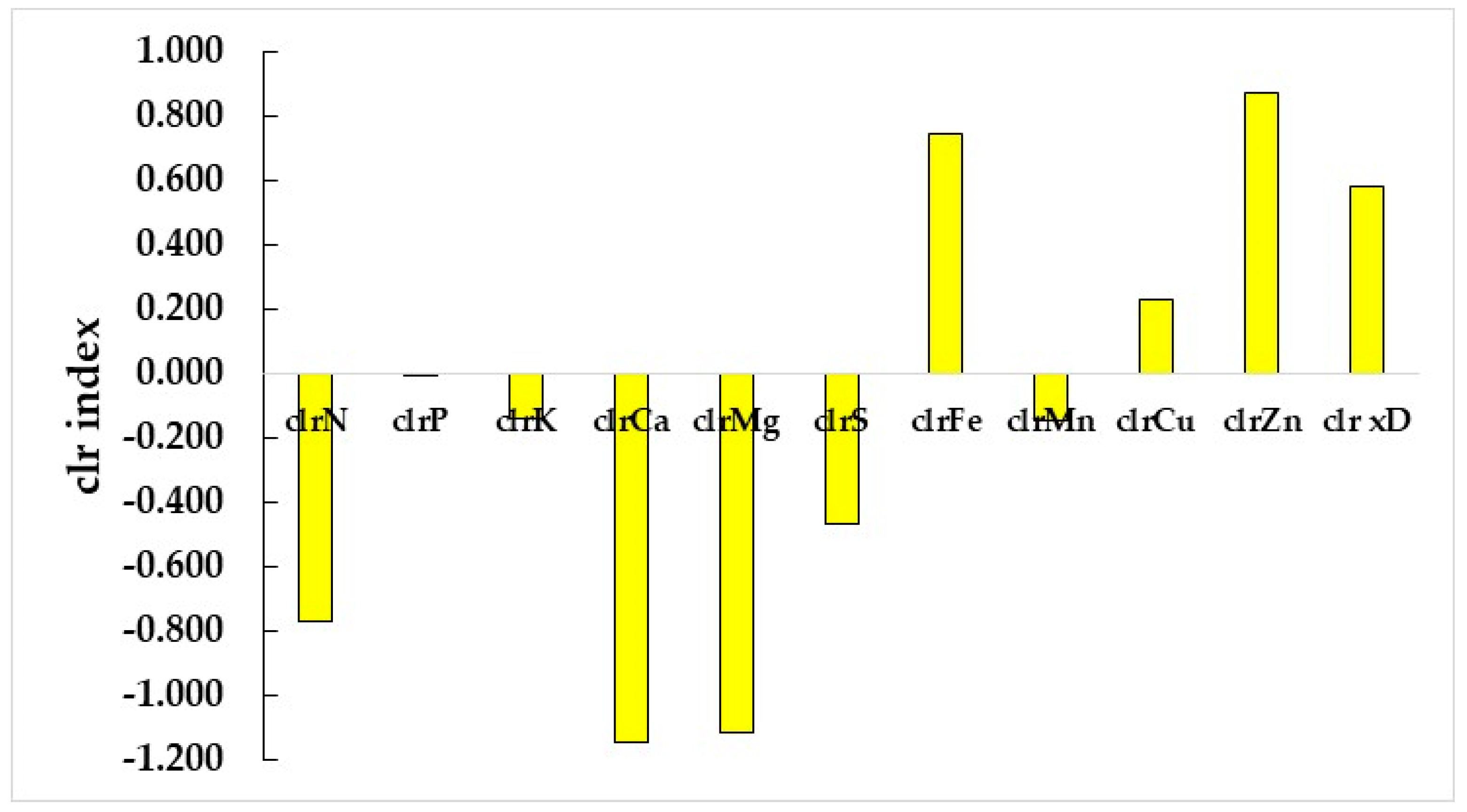
3.4. Nutrient Diagnosis
4. Discussion
4.1. Model Accuracy
4.2. Key Nutrients
4.3. Additional Diagnostic Tools
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uzun, A.; Yesiloglu, T. Genetic Diversity in Citrus. Chap 11. In Genetic Diversity in Plants; Çalişkan, M., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- FAO. Statistical Year Book of Food and Agricultural Organization; FAO: Rome, Italy, 2023; pp. 108–113. [Google Scholar]
- Malik, S.K.; Rohini, M.R.; Kumar, S.; Choudhary, R.; Par, D.; Chaudhury, R. Assessment of Genetic Diversity in Sweet Orange [Citrus sinensis (L.) Osbeck] Cultivars of India Using Morphological and RAPD Markers. Agric. Res. 2012, 1, 317–324. [Google Scholar] [CrossRef]
- Dubey, A.; Singh, A. Evaluation of rootstocks of different mandarins (Citrus reticulata) under foot-hills conditions of Arunachal Pradesh. J. Agric. Sci. 2003, 73, 527–529. [Google Scholar]
- Singh, S.; Singh, J.; Mirza, A. Evaluation of mandarin cultivars on different rootstocks-a review. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1213–1222. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Singh, J.; Singh, Y. Biodiversity of Citrus Fruits. Nat. Sem. Smart Horti. 2020, 329–334. [Google Scholar]
- Srivastava, A.K.; Singh, S. Citrus nutrition in India: Current status and future strategies. Indian J. Agric. Sci. 2008, 78, 3–16. [Google Scholar]
- Srivastava, A.K.; Singh, S. DRIS norms and their validation in Nagpur mandarin (Citrus reticulata Blanco). J. Plant Nutri. 2008, 31, 1091–1107. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Singh, S.; Albrigo, L.G. Diagnosis and remediation of nutrient constraints in citrus. Hort. Rev. 2008, 34, 277–363. [Google Scholar]
- Srivastava, A.K.; Singh, S. Soil analysis based diagnostic norms for Indian Citrus cultivars. Commun. Soil Sci. Plant Anal. 2002, 33, 1689–1706. [Google Scholar] [CrossRef]
- Ahmad, N.; Hussain, S.; Ali, M.A.; Minhas, A.; Waheed, W.; Danish, S.; Fahad, S.; Ghafoor, U.; Baig, K.S.; Sultan, H.; et al. Correlation of soil characteristics and citrus leaf nutrients contents in current scenario of Lyyah district. Horticulturae 2022, 8, 61. [Google Scholar] [CrossRef]
- Raghupathi, H.B.; Ganeshamurthy, A.N.; Ravishankar, H. Comparison of DRIS ratio norms of selected fruit crops. Indian J. Hort. 2014, 71, 168–175. [Google Scholar]
- Rymbai, H.; Deshmukh, N.A.; Ramesh, T.; Verma, V.K.; Baiswar, P.; Mawlein, J.; Talang, H.D.; Dutta, S.K.; Choudhury, B.U.; Hazarika, S.; et al. DRIS standards for identifying yield-limiting nutrients in Khasi Mandarin and their relationship with fruit yield on acidic soil of the eastern Himalayas, India. J. Plant Nutri. 2024, 47, 1331–1346. [Google Scholar] [CrossRef]
- Parent, L.E. Compositional and machine learning tools to model plant nutrition: Overview and perspectives. Horticulturae 2025, 11, 161. [Google Scholar] [CrossRef]
- Yamane, D.R.; Parent, S.-É.; Natale, W.; Cecílio Filho, A.B.; Rozane, D.E.; Nowaki, R.H.D.; Mattos Junior, D.d.; Parent, L.E. Site-Specific Nutrient Diagnosis of Orange Groves. Horticulturae 2022, 8, 1126. [Google Scholar] [CrossRef]
- Ulrich, A.; Hills, F.J. Principles and practices of plant analysis. In Soil Testing and Plant Analysis, Part II; Stelly, M., Hamilton, H., Eds.; Soil Science Society of America: Madison, WI, USA, 1967; pp. 359–387. [Google Scholar]
- Sharma, L.D.; Singh, S.K.; Oinam, N.; Das, H.; Sarkar, A.; Singh, L.B.; Choudhury, R.; Anal, H.J.; Bhupenchandra, I.; Sadhukhan, R. Citrus nutrition in India-A snapshot of up-to-date progress. Anal. Plant Soil Res. 2024, 26, 372–386. [Google Scholar] [CrossRef]
- Wilkinson, S.R.; Grunes, D.L.; Sumner, M.E. Nutrient interactions in soil and plant nutrition. In Handbook of Soil Science; Sumner, M.E., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. D89–D112. [Google Scholar]
- Fageria, N.K.; Baligar, V.C. Improving nutrient use efficiency of annual crops in Brazilian acid soils for sustainable crop. Commun. Soil Sci. Plant Anal. 2001, 32, 1303–1319. [Google Scholar] [CrossRef]
- Courbet, G.; Gallardo, K.; Vigani, G.; Brunel-Muguet, S.; Trouverie, J.; Salon, C.; Qurry, A. Disentangling the complexity and diversity of crosstalk between sulfur and other mineral nutrients in cultivated plants. J. Expt. Bot. 2019, 70, 4183–4196. [Google Scholar] [CrossRef]
- Parent, S.É. Why We should use balances and machine learning to diagnose ionomes. Authorea 2020, 10. [Google Scholar] [CrossRef]
- Parent, S.E. Compositional and machine learning tools to manage plant nutrition: A reiew with examples. Horticulturae, 2025; in review. [Google Scholar]
- Reetika; Rana, G.S.; Komal; Kumar, S.; Saini, P. Influence of foliar sprays of nutrients on yields and yield-accrediting characters of kinnow mandarin; a review. Agric. Rev. 2020, 41, 297–302. [Google Scholar]
- Huchche, A.D.; Ram, L.; Srivastava, A.K.; Kohli, R.R.; Dass, H.C. Evaluation of the nutritional status of Nagpur mandarin (Citrus reticulata Blanco ) by foliar sampling. Trop. Agric. 1999, 72, 93–98. [Google Scholar]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis of Soils, Plants, and Waters; University of California, Division of Agriculture Sciences: Oakland, CA, USA, 1978; pp. 82–96. [Google Scholar]
- Greenacre, M. Compositional data analysis. Annu. Rev. Stat. Its Appl. 2021, 8, 271–299. [Google Scholar] [CrossRef]
- Aitchison, J. The Statistical Analysis of Compositional Data; Chapman and Hall: London, UK, 1986. [Google Scholar]
- Jarrel, W.M.; Beverly, R.B. The dilution effect in plant nutrition studies. Adv. Agron. 1981, 34, 197–223. [Google Scholar]
- Chayes, F. Correlation between variables of constant sum. J. Geophys. Res. 1960, 65, 4185–4193. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics. A Biometricla Approach; McGraw-Hill: New-York, NY, USA, 1997. [Google Scholar]
- Kenworthy, A.L. Plant analysis and interpretation of analysis for horticultural crops. In Soil Testing and Plant Analysis, Part II; Stelly, M., Hamilton, H., Eds.; Soil Science Society of America: Madison, WI, USA, 1967; pp. 59–75. [Google Scholar]
- Greenacre, M.; Gunsky, E.; Bacon-Shone, J.; Erb, I.; Quinn, T. Aitchison’s Compositional Data Analysis 40 Years On: A Reappraisal. Stat. Sci. 2023, 38, 386–410. [Google Scholar] [CrossRef]
- Lagatu, H.; Maume, L. Le diagnostic foliaire de la pomme de terre. Ann. L’Ecole Natl. D’Agronomie Montp. 1934, 22, 50–158. [Google Scholar]
- Beaufils, E.R. Diagnosis and recommendation integrated system (DRIS). In Soil Science Bulletin 132; University of Natal: Pietermaritzburg, South Africa, 1973. [Google Scholar]
- Walworth, J.L.; Sumner, M.E. The Diagnosis and Recommendation Integrated System (DRIS). Adv. Soil Sci. 1987, 6, 149–188. [Google Scholar] [CrossRef]
- Chlningaryan, A.; Sukkarieh, S.; Whelan, B. Machine learning approaches for crop yield prediction and nitrogen status estimation in precision agriculture: A review. Comput. Electron. Agric. 2018, 151, 61–69. [Google Scholar] [CrossRef]
- Huynh-Thu, V.A.; Geurts, P. Unsupervised Gene Network Inference with Decision Trees and Random Forests. In Gene Regulatory Networks; Methods in Molecular Biology; Sanguinetti, G., Huynh-Thu, V., Eds.; Humana Press: New York, NY, USA, 2019; Volume 1883. [Google Scholar]
- Martinez, D.A.; Loening, U.E.; Graham, M.C.; Gathorne-Hardy, A. When the medicine feeds the problem; Do nitrogen fertilizers and pesticides enhance the nutritional quality of crops for their pests and pathogens? Front. Sustain. Food Syst. 2021, 5, 701310. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: Boston, MA, USA, 1986. [Google Scholar]
- Liu, S.; Yang, X.; Guan, Q.; Lu, Z.; Lu, J. An ensemble modeling framework for distinguishing nitrogen, phosphorous and potassium deficiencies in winter oilseed Rape (Brassica napus L.) using hyperspectral data. Remote Sens. 2020, 12, 4060. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Seligman, N. Criteria for publishing papers on crop modeling. Field Crops Res. 2000, 8, 165–172. [Google Scholar] [CrossRef]


| Log Ratio | Gain Ratio | Log Ratio | Gain Ratio | Log Ratio | Gain Ratio | Log Ratio | Gain Ratio | Log Ratio | Gain Ratio |
|---|---|---|---|---|---|---|---|---|---|
| N/P | 0.072 | P/Ca | 0.054 | K/Fe | 0.052 | Ca/xD | 0.044 | S/xD | 0.080 |
| N/K | 0.042 | P/Mg | 0.059 | K/Mn | 0.048 | Mg/S | 0.056 | Fe/Mn | 0.057 |
| N/Ca | 0.051 | P/S | 0.082 | K/Cu | 0.059 | Mg/Fe | 0.046 | Fe/Cu | 0.058 |
| N/Mg | 0.050 | P/Fe | 0.073 | K/Zn | 0.055 | Mg/Mn | 0.062 | Fe/Zn | 0.056 |
| N/S | 0.080 | P/Mn | 0.074 | K/xD | 0.037 | Mg/Cu | 0.075 | Fe/xD | 0.061 |
| N/Fe | 0.066 | P/Cu | 0.078 | Ca/Mg | 0.048 | Mg/Zn | 0.054 | Mn/Cu | 0.063 |
| N/Mn | 0.089 | P/Zn | 0.062 | Ca/S | 0.085 | Mg/xD | 0.053 | Mn/Zn | 0.056 |
| N/Cu | 0.076 | P/xD | 0.062 | Ca/Fe | 0.064 | S/Fe | 0.070 | Mn/xD | 0.087 |
| N/Zn | 0.059 | K/Ca | 0.053 | Ca/Mn | 0.058 | S/Mn | 0.085 | Cu/Zn | 0.055 |
| N/xD | 0.086 | K/Mg | 0.049 | Ca/Cu | 0.081 | S/Cu | 0.072 | Cu/xD | 0.079 |
| P/K | 0.058 | K/S | 0.059 | Ca/Zn | 0.061 | S/Zn | 0.062 | Zn/xD | 0.060 |
| Feature | Concentration | clr | wlr |
|---|---|---|---|
| Gain ratio | |||
| N | 0.046 | 0.009 | 0.009 |
| P | 0.009 | 0.003 | 0.003 |
| K | 0.010 | 0.011 | 0.011 |
| Ca | 0.009 | 0.002 | 0.002 |
| Mg | 0.013 | 0.007 | 0.007 |
| S | 0.017 | 0.006 | 0.006 |
| Fe | 0.016 | 0.017 | 0.017 |
| Mn | 0.014 | 0.004 | 0.004 |
| Cu | 0.015 | 0.003 | 0.003 |
| Zn | 0.003 | 0.000 | 0.000 |
| xD | 0.019 | 0.021 | 0.021 |
| Model | Concentration | clr | wlr | |||
|---|---|---|---|---|---|---|
| AUC-ROC | Accuracy | AUC-ROC | Accuracy | AUC-ROC | Accuracy | |
| K-Nearest Neighbors (KNNs) | 0.770 | 0.812 | 0.736 | 0.753 | 0.736 | 0.753 |
| Support Vector Machine (SVM) | 0.745 | 0.796 | 0.721 | 0.796 | 0.717 | 0.796 |
| Random Forest | 0.788 | 0.798 | 0.743 | 0.775 | 0.745 | 0.796 |
| Naïve Bayes | 0.740 | 0.739 | 0.680 | 0.704 | 0.680 | 0.704 |
| Gradient Boosting | 0.786 | 0.781 | 0.778 | 0.800 | 0.778 | 0.802 |
| Component | ‘Nagpur’ | ‘Khasi’ | ‘Kinnow’ | ‘Nagpur’ | ‘Khasi’ | ‘Kinnow’ |
|---|---|---|---|---|---|---|
| Number of Specimens | 46 | 39 | 40 | 46 | 39 | 40 |
| Centered Log-Ratio (clr) Standards | Weighted Log-Ratio (wlr) Standards | |||||
| N | 2.755 ± 0.214 | 2.874 ± 0.128 | 2.895 ± 0.194 | 3.024 ± 0.235 | 3.161 ± 0.140 | 3.184 ± 0.213 |
| P | −0.177 ± 0.242 | −0.577 ± 0.522 | −0.277 ± 0.381 | −0.199 ± 0.266 | −0.635 ± 0.574 | −0.305 ± 0.419 |
| K | 2.265 ± 0.438 | 2.402 ± 0.155 | 2.036 ± 0.532 | 2.512 ± 0.481 | 2.643 ± 0.170 | 2.240 ± 0.586 |
| Ca | 2.627 ± 0.408 | 2.382 ± 0.411 | 2.955 ± 0.142 | 2.885 ± 0.202 | 2.620 ± 0.452 | 3.251 ± 0.156 |
| Mg | 0.591 ± 0.218 | 0.313 ± 0.537 | 0.895 ± 0.198 | 0.641 ± 0.239 | 0.344 ± 0.591 | 0.985 ± 0.217 |
| S | 0.931 ± 0.530 | 0.635 ± 0.281 | 0.753 ± 0.297 | 1.032 ± 0.583 | 0.699 ± 0.310 | 0.828 ± 0.326 |
| Fe | −2.745 ± 0.488 | −2.203 ± 0.215 | −3.088 ± 0.547 | −3.009 ± 0.537 | −2.423 ± 0.237 | −3.375 ± 0.602 |
| Mn | −3.632 ± 0.444 | −3.068 ± 0.193 | −3.275 ± 0.528 | −4.002 ± 0.488 | −3.375 ± 0.212 | −3.603 ± 0.581 |
| Cu | −4.858 ± 0.394 | −5.195 ± 0.320 | −4.804 ± 0.972 | −5.370 ± 0.434 | −5.714 ± 0.352 | −5.284 ± 1.069 |
| Zn | −4.211 ± 0.331 | −4.128 ± 0.213 | −4.494 ± 0.934 | −4.591 ± 0.364 | −4.541 ± 0.234 | −4.944 ± 1.027 |
| xD | 6.453 ± 0.125 | 6.565 ± 0.093 | 6.384 ± 0.167 | 7.098 ± 0.137 | 7.221 ± 0.102 | 7.023 ± 0.183 |
| Average variance | 0.137 | 0.099 | 0.276 | 0.166 | 0.120 | 0.334 |
| Component | Back-Transformed clr Means | Literature | ||||
|---|---|---|---|---|---|---|
| ‘Nagpur’ | ‘Khasi’ | ‘Kinnow’ | ‘Nagpur’ § | ‘Khasi’ § | ‘Kinnow’ † | |
| N | 23.2 | 26.1 | 26.6 | 17–28 | 19.6–25.6 | 22.8–25.3 |
| P | 1.2 | 0.8 | 1.1 | 0.8–1.5 | 0.8–1.0 | 1.1–1.5 |
| K | 14.2 | 16.3 | 11.3 | 10.1–25.9 | 9.8–19.3 | 13.4–15.7 |
| Ca | 20.4 | 15.9 | 28.3 | 17.9–32.8 | 19.6–24.9 | - |
| Mg | 2.7 | 2.0 | 3.6 | 4.2–9.2 | 2.3–4.8 | - |
| S | 3.7 | 2.8 | 3.1 | - | - | - |
| Fe | 0.095 | 0.163 | 0.068 | 0.075–0.113 | 0.084–0.249 | 0.082–0.103 |
| Mn | 0.039 | 0.068 | 0.056 | 0.055–0.085 | 0.042–0.088 | 0.038–0.041 |
| Cu | 0.011 | 0.008 | 0.012 | 0.010–0.018 | 0.002–0.014 | 0.005–0.010 |
| Zn | 0.022 | 0.024 | 0.016 | 0.014–0.030 | 0.016–0.027 | 0.015–0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, A.K.; Huchche, A.D.; Parent, L.-E.; Malhotra, S.K.; Ziogas, V.; Baishya, L.K. Nutrient Balance of Citrus Across the Mandarin Belts of India. Horticulturae 2025, 11, 254. https://doi.org/10.3390/horticulturae11030254
Srivastava AK, Huchche AD, Parent L-E, Malhotra SK, Ziogas V, Baishya LK. Nutrient Balance of Citrus Across the Mandarin Belts of India. Horticulturae. 2025; 11(3):254. https://doi.org/10.3390/horticulturae11030254
Chicago/Turabian StyleSrivastava, Anoop Kumar, Ambadas Dattatray Huchche, Leon-Etienne Parent, Suresh Kumar Malhotra, Vasileios Ziogas, and Lohit Kumar Baishya. 2025. "Nutrient Balance of Citrus Across the Mandarin Belts of India" Horticulturae 11, no. 3: 254. https://doi.org/10.3390/horticulturae11030254
APA StyleSrivastava, A. K., Huchche, A. D., Parent, L.-E., Malhotra, S. K., Ziogas, V., & Baishya, L. K. (2025). Nutrient Balance of Citrus Across the Mandarin Belts of India. Horticulturae, 11(3), 254. https://doi.org/10.3390/horticulturae11030254











