The Effect of Light Intensity and Polyethylene-Glycol-Induced Water Stress on the Growth, Mitragynine Accumulation, and Total Alkaloid Content of Kratom (Mitragyna speciosa)
Abstract
:1. Introduction
2. Materials and Methods
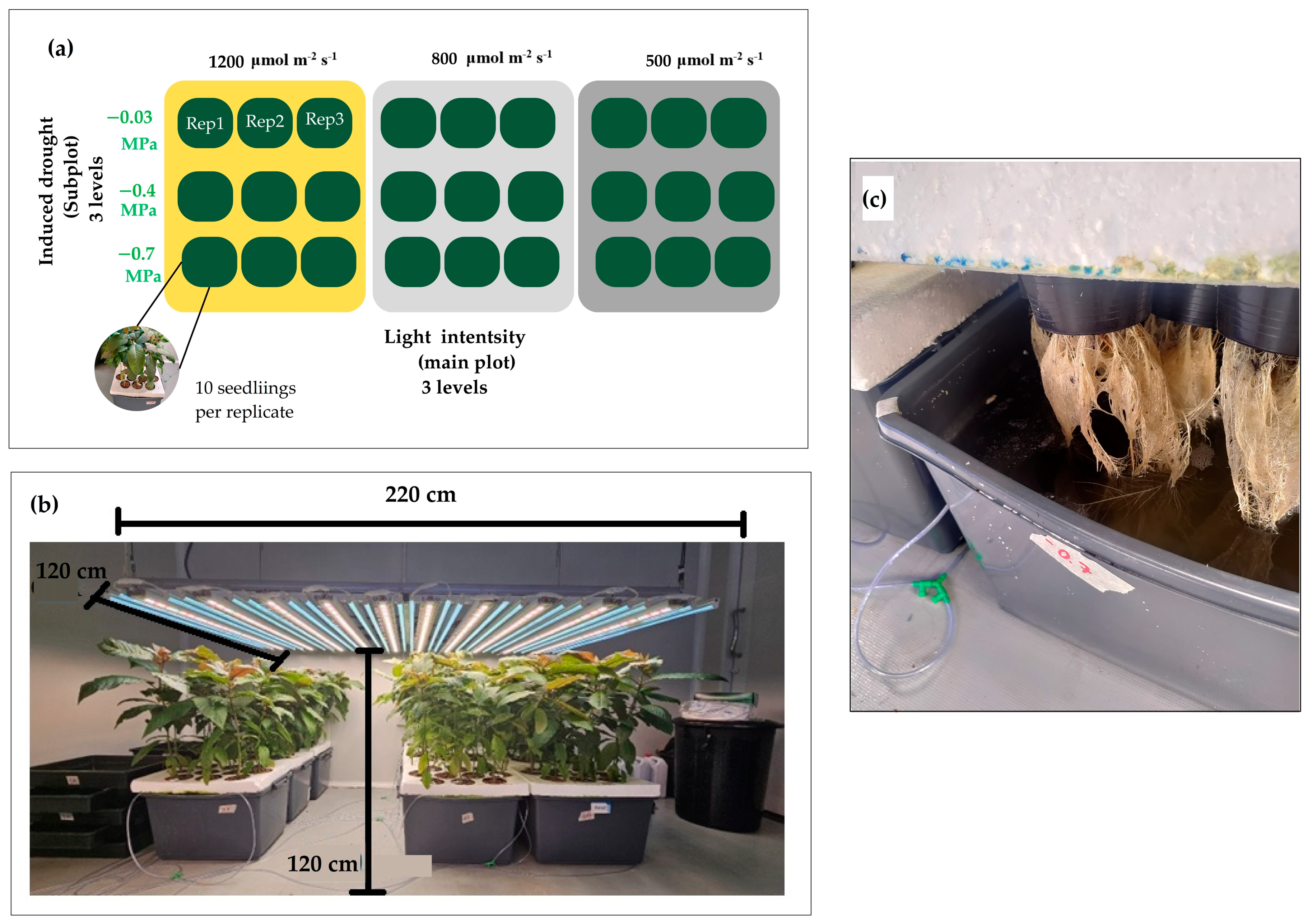
2.1. Seedling Preparation and Treatments
2.2. Growth and Leaf Trait Measurements
2.3. Seedling Harvesting
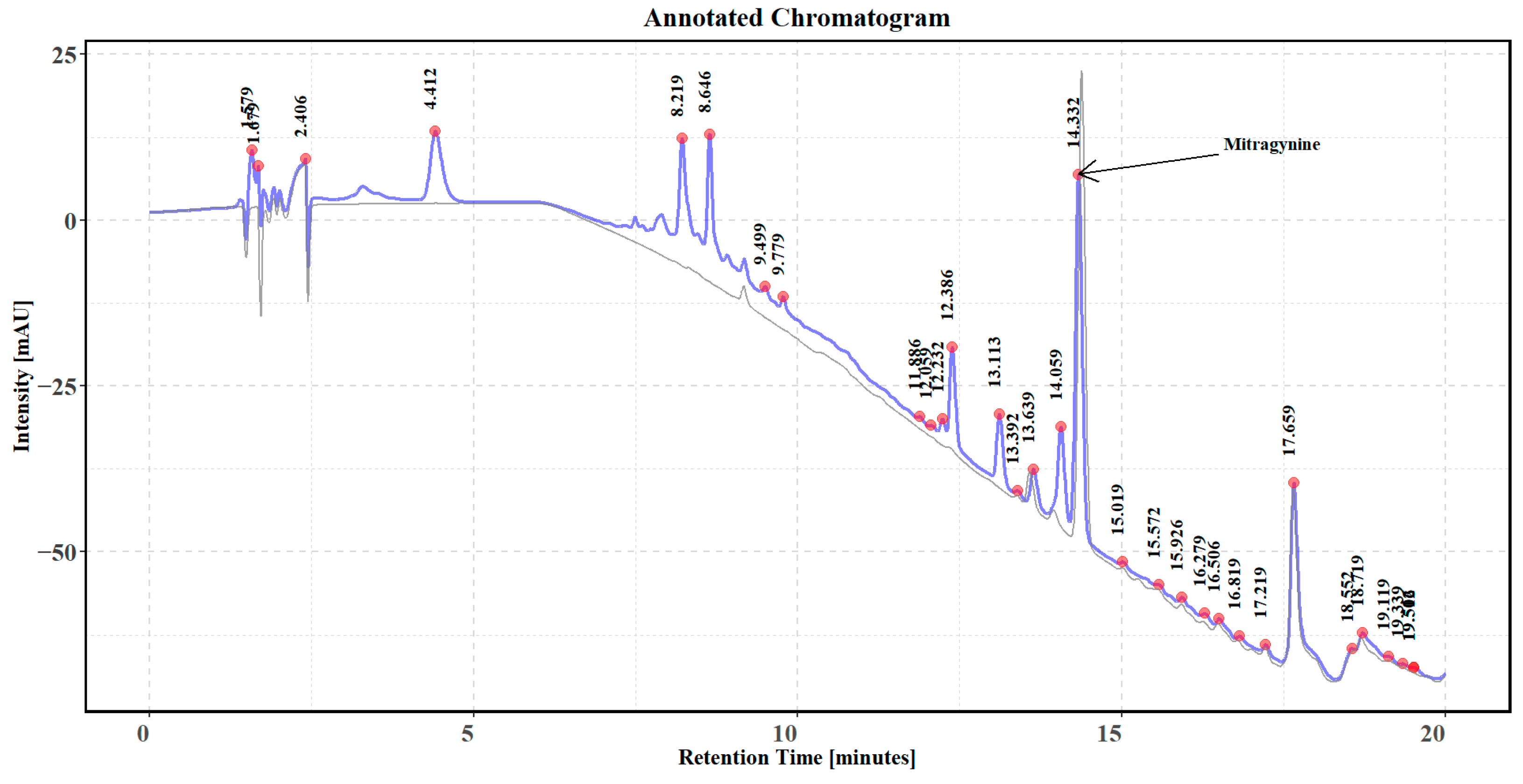
2.4. Determination of Mitragynine Content
2.5. Determination of Total Alkaloid Content
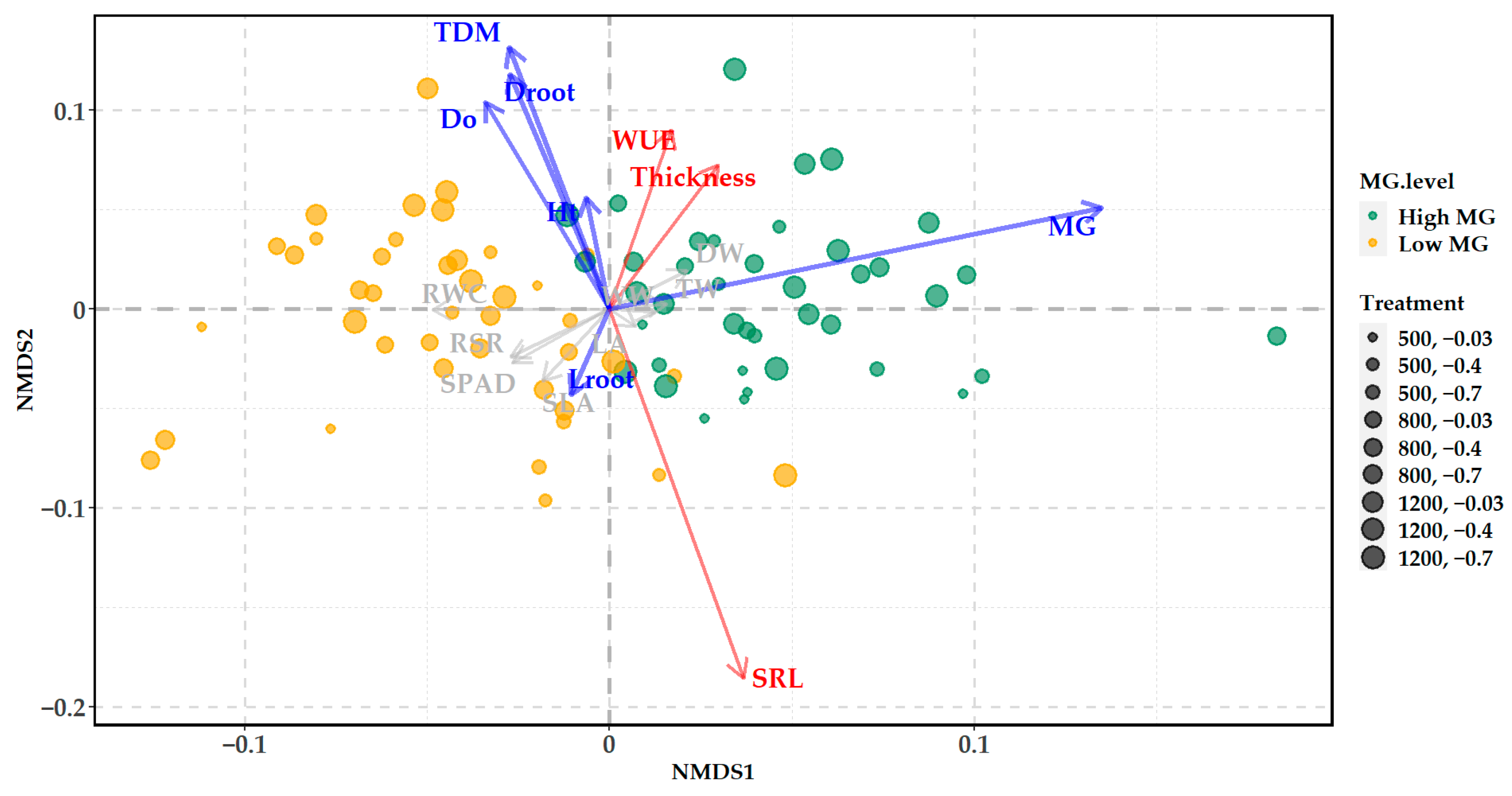
2.6. Constrained Ordination Analysis
2.7. Mean Comparison Through Analysis of Variance
3. Results
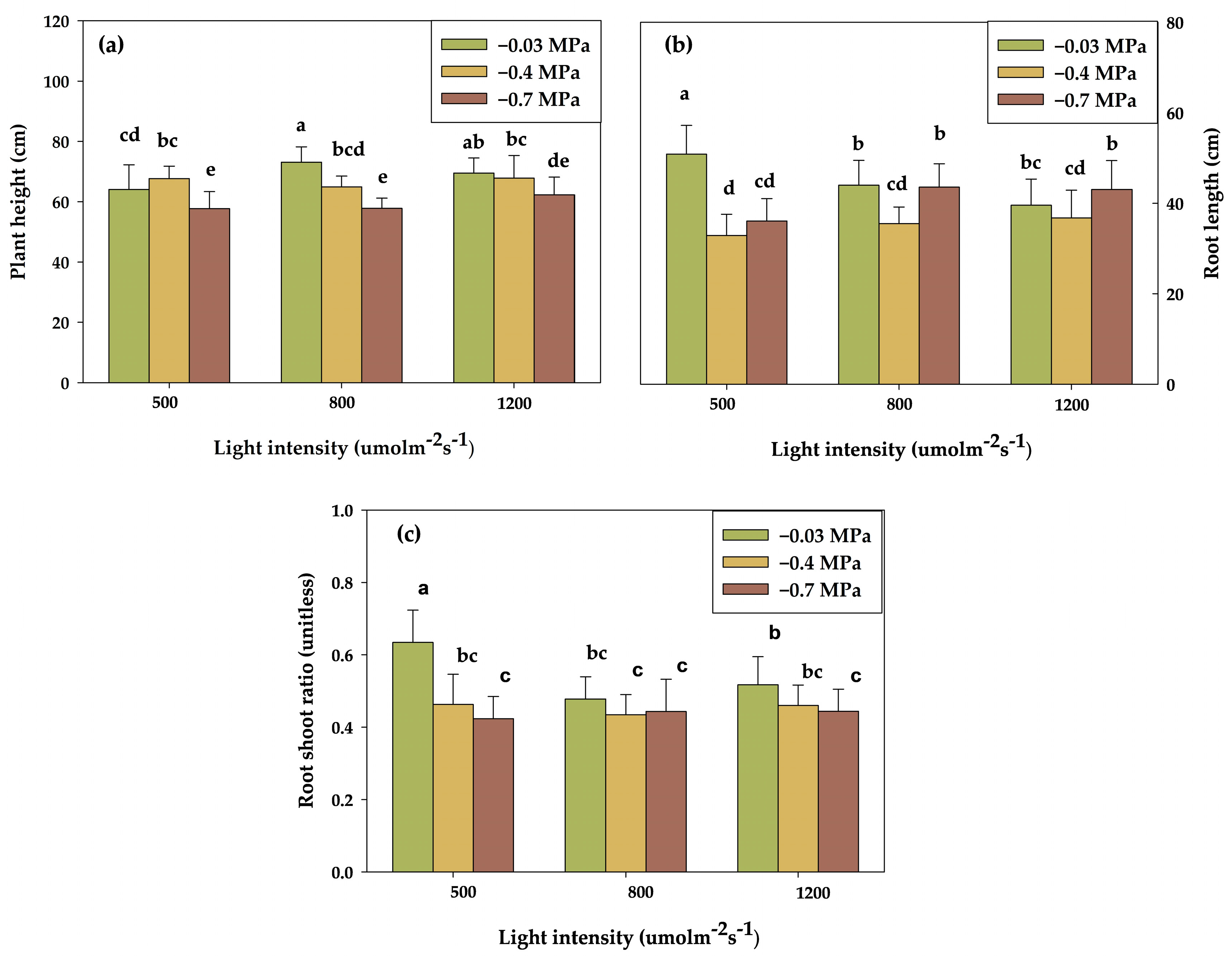
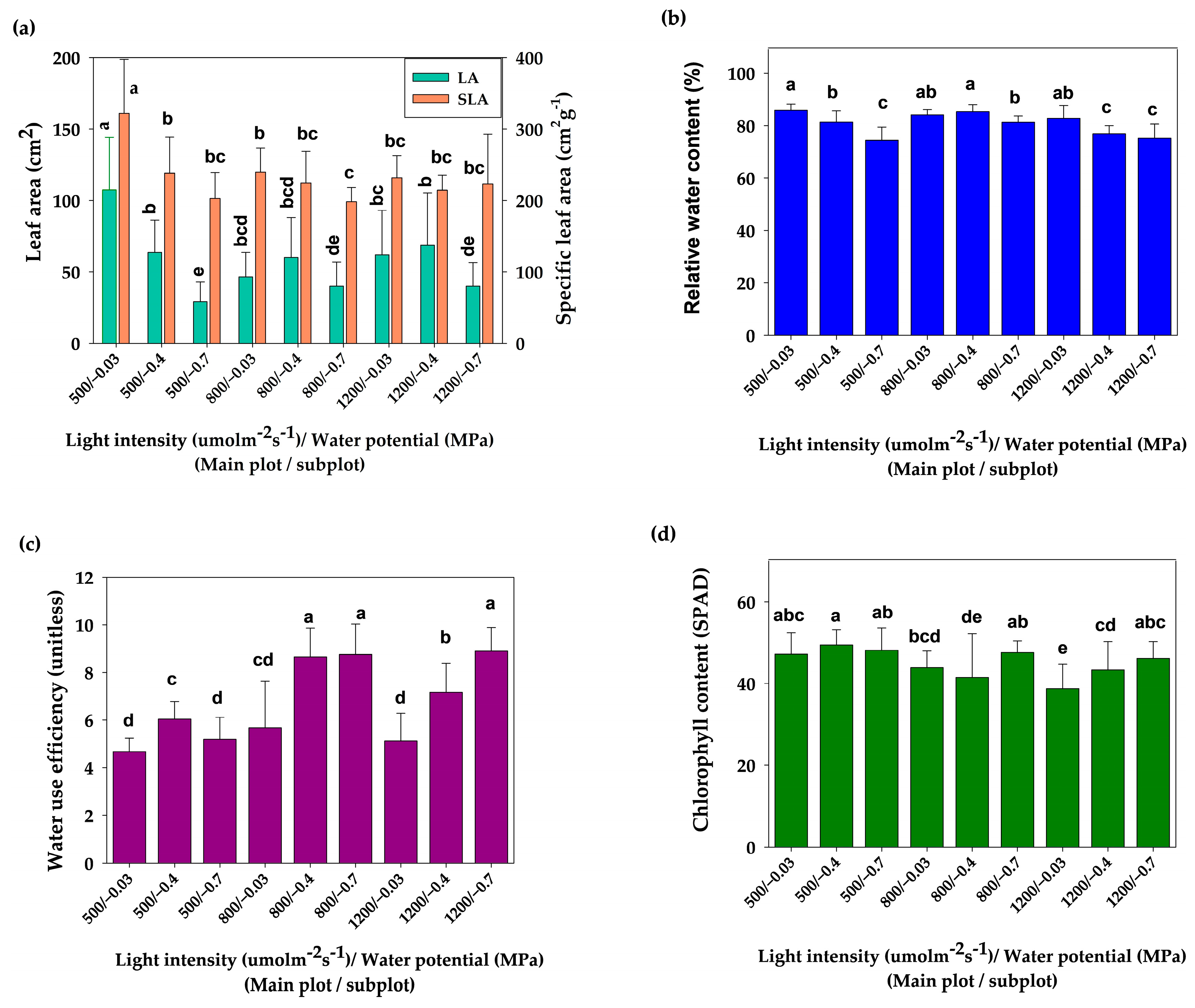
3.1. Effect of Light and Water Potential on Growth and Physiological Parameters
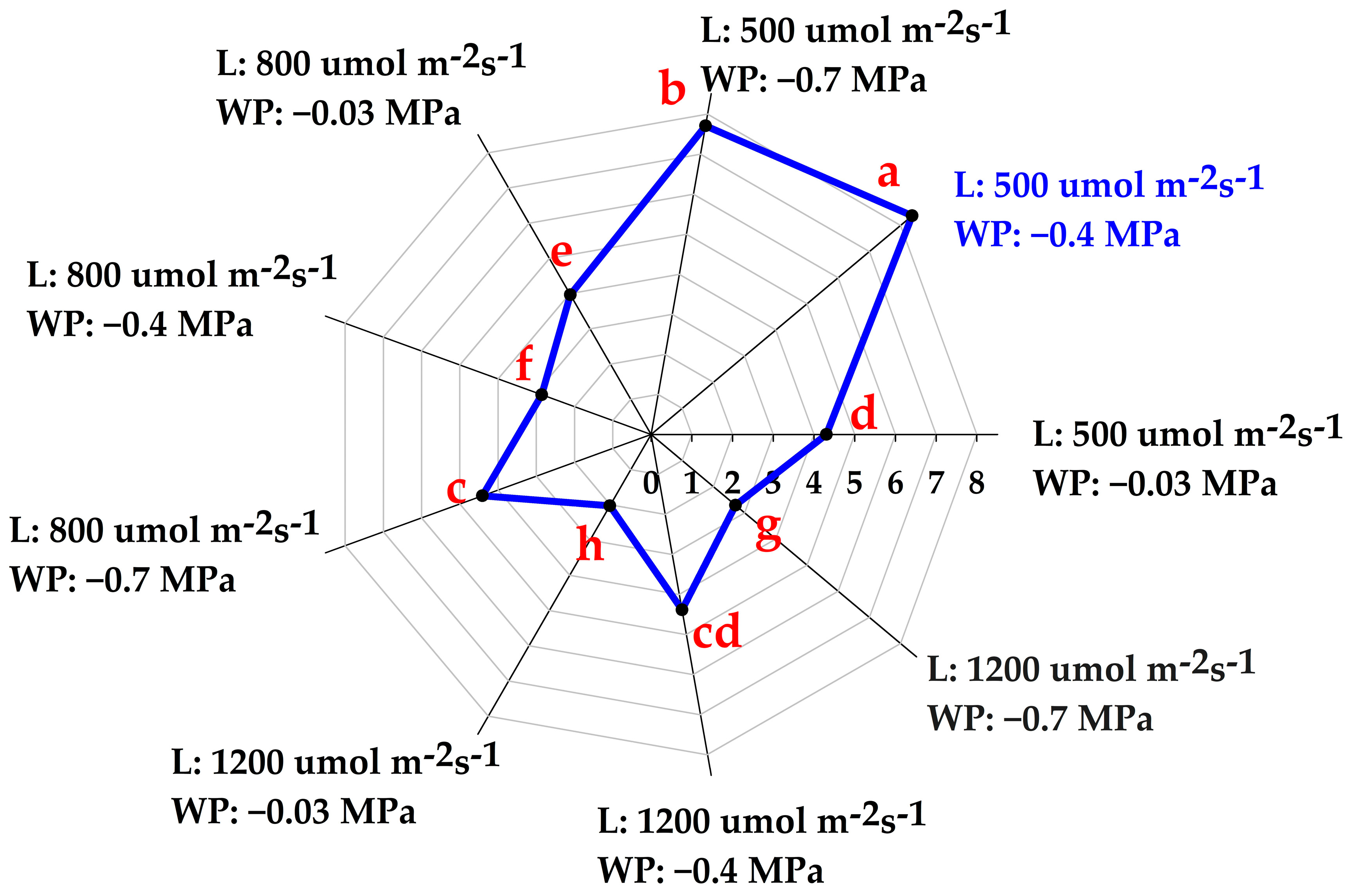
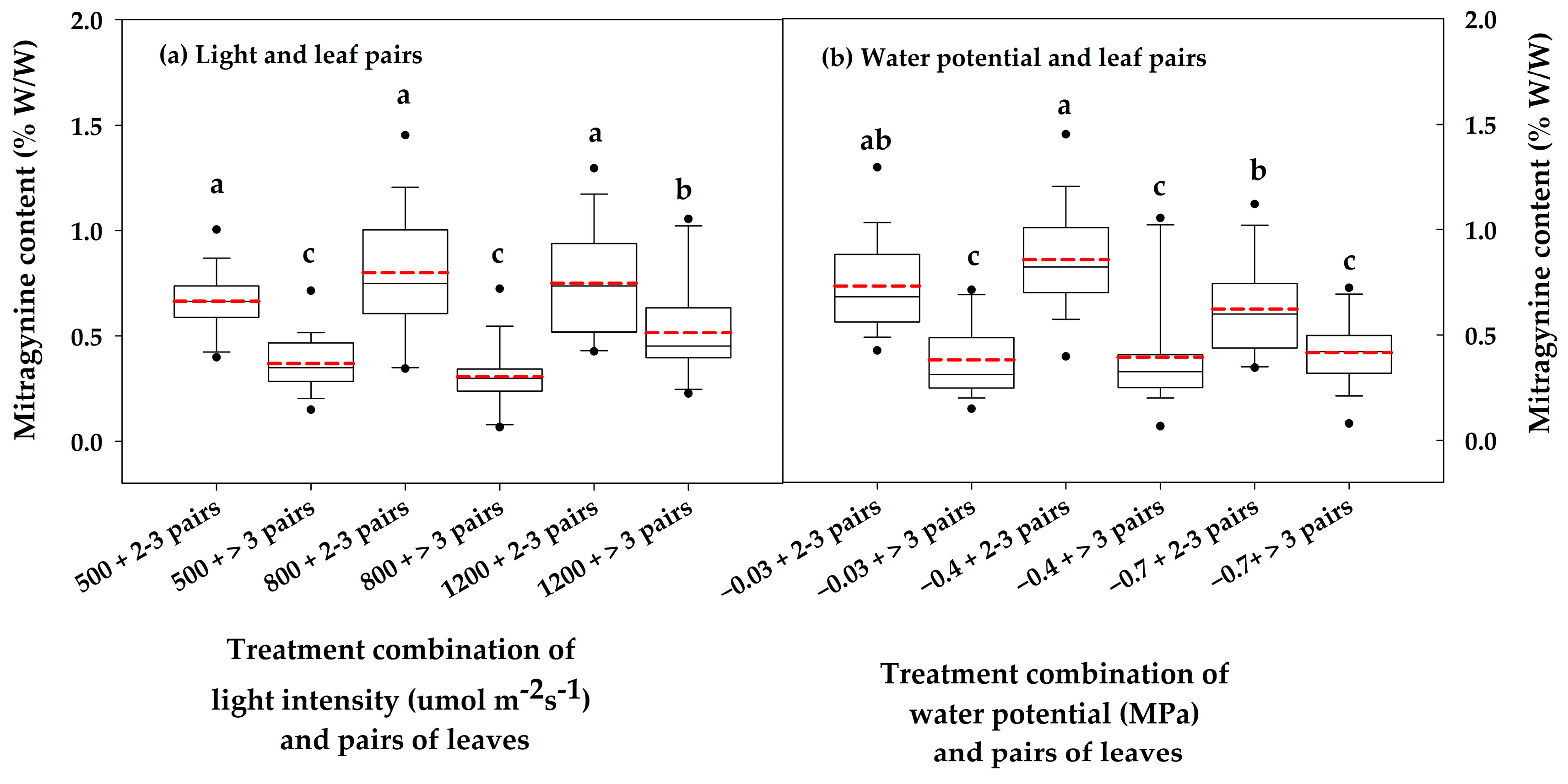
3.2. Variation in Total Alkaloid Content and Mitragynine Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cinosi, E.; Martinotti, G.; Simonato, P.; Singh, D.; Demetrovics, Z.; Roman-Urrestarazu, A.; Bersani, F.S.; Vicknasingam, B.; Piazzon, G.; Li, J.-H.; et al. Following “the Roots” of Kratom (Mitragyna speciosa): The Evolution of an Enhancer from a Traditional Use to Increase Work and Productivity in Southeast Asia to a Recreational Psychoactive Drug in Western Countries. Biomed. Res. Int. 2015, 2015, 968786. [Google Scholar] [CrossRef]
- Ngernsaengsaruay, C.; Leksungnoen, N.; Boonthasak, W.; Utharatsamee, S.; Racharak, P.; Leetanasakskul, K.; Pongamorn, P.; Saengbuapuean, A. Additional Knowledge on the Genus mitragyna (Rubiaceae) in Thailand. Thai For. Bull. 2022, 50, 20–39. [Google Scholar] [CrossRef]
- Leksungnoen, N.; Andriyas, T.; Ngernsaengsaruay, C.; Uthairatsamee, S.; Racharak, P.; Sonjaroon, W.; Kjelgren, R.; Pearson, B.J.; McCurdy, C.R.; Sharma, A. Variations in Mitragynine Content in the Naturally Growing Kratom (Mitragyna speciosa) Population of Thailand. Front. Plant Sci. 2022, 13, 1028547. [Google Scholar] [CrossRef]
- Limcharoen, T.; Pouyfung, P.; Ngamdokmai, N.; Prasopthum, A.; Ahmad, A.R.; Wisdawati, W.; Prugsakij, W.; Warinhomhoun, S. Inhibition of α-Glucosidase and Pancreatic Lipase Properties of Mitragyna speciosa (Korth.) Havil. (Kratom) Leaves. Nutrients 2022, 14, 3909. [Google Scholar] [CrossRef]
- Grundmann, O.; Garcia-Romeu, A.; McCurdy, C.R.; Sharma, A.; Smith, K.E.; Swogger, M.T.; Weiss, S.T. Not All Kratom Is Equal: The Important Distinction between Native Leaf and Extract Products. Addiction 2024, 119, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Laforest, L.C.; Kuntz, M.A.; Kanumuri, S.R.R.; Mukhopadhyay, S.; Sharma, A.; O’Connor, S.E.; McCurdy, C.R.; Nadakuduti, S.S. Metabolite and Molecular Characterization of Mitragyna speciosa Identifies Developmental and Genotypic Effects on Monoterpene Indole and Oxindole Alkaloid Composition. J. Nat. Prod. 2023, 86, 1042–1052. [Google Scholar] [CrossRef]
- Phromchan, W.; Defri, I.; Saensano, C.; Chookaew, A.; Chiarawipa, R.; Sriwiriyajan, S. Morphological and Physiological Properties of Kratom (Mitragyna speciosa) Leaves: Macronutrients, Phytochemicals, Antioxidants, and Mitragynine Content. Plant Sci. Today 2024, 11, 762–770. [Google Scholar] [CrossRef]
- Liu, Y.; Singh, S.K.; Pattanaik, S.; Wang, H.; Yuan, L. Light Regulation of the Biosynthesis of Phenolics, Terpenoids, and Alkaloids in Plants. Commun. Biol. 2023, 6, 1055. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Gómez-Cadenas, A.; Mittler, R. Plant Responses to Climate Change: Metabolic Changes under Combined Abiotic Stresses. J. Exp. Bot. 2022, 73, 3339–3354. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Fang, C.; Fernie, A.R.; Luo, J. Exploring the Diversity of Plant Metabolism. Trends Plant Sci. 2019, 24, 83–98. [Google Scholar] [CrossRef]
- Siddiqui, M.W.; Prasad, K. Plant Secondary Metabolites, Volume One; Biological and Therapeutic Significance; Apple Academic Press: Palm Bay, FL, USA, 2016. [Google Scholar] [CrossRef]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Agati, G. Differential Accumulation of Flavonoids and Hydroxycinnamates in Leaves of Ligustrum Vulgare under Excess Light and Drought Stress. New Phytol. 2004, 163, 547–561. [Google Scholar] [CrossRef]
- Czepiel, K.; Krajewski Pawełand Wilczura, P.; Bielecka, P.; Święcicki, W.; Kroc, M. Expression Profiles of Alkaloid-Related Genes across the Organs of Narrow-Leafed Lupin (Lupinus angustifolius L.) and in Response to Anthracnose Infection. Int. J. Mol. Sci. 2021, 22, 2676. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M.; et al. Plant Secondary Metabolites: The Weapons for Biotic Stress Management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef]
- Schumann, T.; Paul, S.; Melzer, M.; Dörmann, P.; Jahns, P. Plant Growth under Natural Light Conditions Provides Highly Flexible Short-Term Acclimation Properties toward High Light Stress. Front. Plant Sci. 2017, 8, 681. [Google Scholar] [CrossRef]
- Herrmann, H.A.; Schwartz, J.-M.; Johnson, G.N. From Empirical to Theoretical Models of Light Response Curves-Linking Photosynthetic and Metabolic Acclimation. Photosynth. Res. 2020, 145, 5–14. [Google Scholar] [CrossRef]
- Darko, E.; Hamow, K.A.; Marček, T.; Dernovics, M.; Ahres, M.; Galiba, G. Modulated Light Dependence of Growth, Flowering, and the Accumulation of Secondary Metabolites in Chilli. Front. Plant Sci. 2022, 13, 801656. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Wang, Y.; Wang, X.; Peng, N.; Yang, S.-S.; Shao, H.-H.; Jiao, X.-L.; Gao, W.-W. Effect of Light Intensity on Growth, Accumulation of Ginsenosides, and Expression of Related Enzyme Genes of Panax Quinquefolius. China J. Chin. Mater. Medica 2022, 47, 4877–4885. [Google Scholar] [CrossRef]
- Godoy-Hernández, G.; Loyola-Vargas, V.M. Effect of Fungal Homogenate, Enzyme Inhibitors and Osmotic Stress on Alkaloid Content of Catharanthus roseus Cell Suspension Cultures. Plant Cell Rep. 1991, 10, 537–540. [Google Scholar] [CrossRef]
- Ashihara, H.; Sano, H.; Crozier, A. Caffeine and Related Purine Alkaloids: Biosynthesis, Catabolism, Function and Genetic Engineering. Phytochemistry 2008, 69, 841–856. [Google Scholar] [CrossRef]
- Keleş, Y.; Öncel, I. Response of Antioxidative Defence System to Temperature and Water Stress Combinations in Wheat Seedlings. Plant Sci. 2002, 163, 783–790. [Google Scholar] [CrossRef]
- Zobayed, S.M.A.; Afreen, F.; Kozai, T. Phytochemical and Physiological Changes in the Leaves of St. John’s Wort Plants under a Water Stress Condition. Environ. Exp. Bot. 2007, 59, 109–116. [Google Scholar] [CrossRef]
- Helander, A.; Rylski, A. Drug Testing for Mitragynine and Kratom: Analytical Challenges and Medico-Legal Considerations. Drug Test. Anal. 2023, 15, 213–219. [Google Scholar] [CrossRef]
- Charoenratana, S.; Anukul, C.; Aramrattana, A. Attitudes towards Kratom Use, Decriminalization and the Development of a Community-Based Kratom Control Mechanism in Southern Thailand. Int. J. Drug Policy 2021, 95, 103197. [Google Scholar] [CrossRef]
- Khalil, S.; Abdullah, S.A.J.; Ahmad, R. Enforcement Status of the Poison Act 1952 against Offences Related to Kratom (Mitragyna speciosa Korth) Misuse in Malaysia. UUM J. Leg. Stud. 2020, 11, 75–93. [Google Scholar] [CrossRef]
- Henningfield, J.E.; Grundmann, O.; Huestis, M.A.; Smith, K.E. Kratom Safety and Toxicology in the Public Health Context: Research Needs to Better Inform Regulation. Front. Pharmacol. 2024, 15, 1403140. [Google Scholar] [CrossRef]
- Prozialeck, W.C. Update on the Pharmacology and Legal Status of Kratom. J. Osteopath. Med. 2016, 116, 802–809. [Google Scholar] [CrossRef]
- Talamond, P.; Mondolot, L.; Gargadennec, A.; de Kochko, A.; Hamon, S.; Fruchier, A.; Campa, C. First Report on Mangiferin (C-Glucosyl-Xanthone) Isolated from Leaves of a Wild Coffee Plant, Coffea pseudozanguebariae (Rubiaceae). Acta Bot. Gall. 2008, 155, 513–519. [Google Scholar] [CrossRef]
- Campa, C.; Mondolot, L.; Rakotondravao, A.; Bidel, L.P.R.; Gargadennec, A.; Couturon, E.; La Fisca, P.; Rakotomalala, J.-J.; Jay-Allemand, C.; Davis, A.P. A Survey of Mangiferin and Hydroxycinnamic Acid Ester Accumulation in Coffee (Coffea) Leaves: Biological Implications and Uses. Ann. Bot. 2012, 110, 595–613. [Google Scholar] [CrossRef]
- Resh, H.M. Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Fujiwara, K.; Kubo, S.; Eijima, K.; Matsuda, R.; Yano, A. Improved LED Artificial Sunlight Source System Available for Sunlight-Effect Research in Plant Sciences. J. Agric. Meteorol. 2022, 78, 101–112. [Google Scholar] [CrossRef]
- Zhang, M.; Sharma, A.; León, F.; Avery, B.; Kjelgren, R.; McCurdy, C.R.; Pearson, B.J. Plant Growth and Phytoactive Alkaloid Synthesis in Kratom [Mitragyna speciosa (Korth.)] in Response to Varying Radiance. PLoS ONE 2022, 17, 4. [Google Scholar] [CrossRef]
- Maggini, R.; Kiferle, C.; Guidi, L.; Pardossi, A.; Raffaelli, A. Growing Medicinal Plants in Hydroponic Culture. Acta Hortic. 2011, 952, 697–704. [Google Scholar] [CrossRef]
- Koriesh, E.M.; Abo El-Soud, I.H. Medicinal Plants in Hydroponic System under Water-Deficit Conditions—A Way to Save Water. In Technological and Modern Irrigation Environment in Egypt; Best Management Practices & Evaluation; Springer: Berlin/Heidelberg, Germany, 2020; pp. 131–153. [Google Scholar] [CrossRef]
- Michel, B.E.; Kaufmann, M.R. The Osmotic Potential of Polyethylene Glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Atherton, H.R.; Li, P. Hydroponic Cultivation of Medicinal Plants—Plant Organs and Hydroponic Systems: Techniques and Trends. Horticulturae 2023, 9, 349. [Google Scholar] [CrossRef]
- Perez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. Corrigendum to: New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2016, 64, 715–716. [Google Scholar] [CrossRef]
- Mustafa, R.R.; Sukor, R.; Nor, S.M.M.; Saari, N.; Azri, F.A. Enhancing Extraction Yield and Purity of Mitragynine from Mitragyna speciosa through Sequential Solvent Extraction and Characterisation Using NMR Technique. Int. J. Sci. Technol. Res. 2020, 9, 3846–3854. [Google Scholar]
- Shamsa, F.; Monsef, H.; Ghamooshi, R.; Verdian-Rizi, M. Spectrophotometric Determination of Total Alkaloids in Some Iranian Medicinal Plants. Thai J. Pharm. Sci. 2008, 32, 17–20. [Google Scholar] [CrossRef]
- McCune, B.P.; Grace, J. Analysis of Ecological Communities. J. Exp. Marine Bio. Ecol. 2002, 289, 303–305. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-Parametric Multivariate Analyses of Changes in Community Structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. The Vegan Package. Community Ecology Package, Version 10; R Foundation for Statistical Computing: Vienna, Austria, 2007; p. 719. [Google Scholar]
- de Mendiburu, F.; de Mendiburu, M.F. Package ‘Agricolae’. R Package, Version 1; R Foundation for Statistical Computing: Vienna, Austria, 2019; pp. 1143–1149. [Google Scholar]
- Johnson, D.M.; Smith, W.K. Limitations to Photosynthetic Carbon Gain in Timberline Abies Lasiocarpa Seedlings during Prolonged Drought. Can. J. For. Res. 2007, 37, 568–579. [Google Scholar] [CrossRef]
- Irving, L.J. Carbon Assimilation, Biomass Partitioning and Productivity in Grasses. Agriculture 2015, 5, 1116–1134. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Yamamoto, H.; Allakhverdiev, S.I.; Inaba, M.; Yokota, A.; Murata, N. Oxidative Stress Inhibits the Repair of Photodamage to the Photosynthetic Machinery. EMBO J. 2001, 20, 5587–5594. [Google Scholar] [CrossRef]
- Urban, L.; Aarrouf, J.; Bidel, L.P.R. Assessing the Effects of Water Deficit on Photosynthesis Using Parameters Derived from Measurements of Leaf Gas Exchange and of Chlorophyll a Fluorescence. Front. Plant Sci. 2017, 8, 2068. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Bi, M.-H.; Nie, Z.-F.; Jiang, H.; Liu, X.-D.; Fang, X.-W.; Brodribb, T.J. Evolution of Stomatal Closure to Optimize Water-Use Efficiency in Response to Dehydration in Ferns and Seed Plants. New Phytol. 2021, 230, 2001–2010. [Google Scholar] [CrossRef]
- Zhang, L.X.; Guo, Q.S.; Chang, Q.S.; Zhu, Z.B.; Liu, L.; Chen, Y.H. Chloroplast Ultrastructure, Photosynthesis and Accumulation of Secondary Metabolites in Glechoma Longituba in Response to Irradiance. Photosynthetica 2015, 53, 144–153. [Google Scholar] [CrossRef]
- Fanourakis, D.; Hyldgaard, B.; Giday, H.; Aulik, I.; Bouranis, D.; Körner, O.; Ottosen, C.-O. Stomatal Anatomy and Closing Ability Is Affected by Supplementary Light Intensity in Rose (Rosa hybrida L.). Hortic. Sci. 2019, 46, 81. [Google Scholar] [CrossRef]
- Ali, Z.; Merrium, S.; Habib-ur-Rahman, M.; Hakeem, S.; Saddique, M.A.B.; Sher, M.A. Wetting Mechanism and Morphological Adaptation; Leaf Rolling Enhancing Atmospheric Water Acquisition in Wheat Crop—A Review. Environ. Sci. Pollut. Res. 2022, 29, 30967–30985. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Correia, O.; Beyschlag, W. Two Different Strategies of Mediterranean Macchia Plants to Avoid Photoinhibitory Damage by Excessive Radiation Levels during Summer Drought. Acta Oecologica 1999, 20, 15–23. [Google Scholar] [CrossRef]
- Hazrati, S.; Tahmasebi-Sarvestani, Z.; Nicola, S.; Kashkooli, A.B.; Habibzadeh, F.; Mohammadi, H.; Mokhtassi-Bidgoli, A. Effect of Light and Water Deficiency on Growth and Concentration of Various Primary and Secondary Metabolites of Aloe vera L. J. Agric. Sci. Technol. 2020, 22, 1343–1358. [Google Scholar]
- Macáková, K.; Afonso, R.; Saso, L.; Mladěnka, P. The Influence of Alkaloids on Oxidative Stress and Related Signaling Pathways. Free Radic. Biol. Med. 2019, 134, 429–444. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Stress Enhances the Synthesis of Secondary Plant Products: The Impact of Stress-Related over-Reduction on the Accumulation of Natural Products. Plant Cell Physiol. 2013, 54, 817–826. [Google Scholar] [CrossRef]
- Shil, S.; Dewanjee, S. Impact of Drought Stress Signals on Growth and Secondary Metabolites (SMs) in Medicinal Plants. J. Phytopharm. 2022, 11, 371–376. [Google Scholar] [CrossRef]
- Hiba, H.; Janeeshma, E.; Puthur, J.T. Dynamic Alterations of Metabolites in Plectranthus amboinicus (Lour.) Spreng. to Encounter Drought and Zn Toxicity. Braz. J. Bot. 2021, 44, 587–599. [Google Scholar] [CrossRef]
- Jansen, M.A.K.; Hectors, K.; O’Brien, N.M.; Guisez, Y.; Potters, G. Plant Stress and Human Health: Human Consumers Benefit from UV-B Acclimated Crops? Plant Sci. 2008, 175, 449–458. [Google Scholar] [CrossRef]
- Mewis, I.; Schreiner, M.; Nguyen, C.N.; Krumbein, A.; Ulrichs, C.; Lohse, M.; Zrenner, R. UV-B Irradiation Changes Specifically the Secondary Metabolite Profile in Broccoli Sprouts: Induced Signaling Overlaps with Defense Response to Biotic Stressors. Plant Cell Physiol. 2012, 53, 1546–1560. [Google Scholar] [CrossRef]
- Bandurska, H.; Niedziela, J.; Chadzinikolau, T. Separate and Combined Responses to Water Deficit and UV-B Radiation. Plant Sci. 2013, 213, 98–105. [Google Scholar] [CrossRef]
- Ncise, W.; Daniels, C.W.; Nchu, F. Effects of Light Intensities and Varying Watering Intervals on Growth, Tissue Nutrient Content and Antifungal Activity of Hydroponic Cultivated Tulbaghia violacea L. under Greenhouse Conditions. Heliyon 2020, 6, e03906. [Google Scholar] [CrossRef]
- Kleinwächter, M.; Selmar, D. New Insights Explain That Drought Stress Enhances the Quality of Spice and Medicinal Plants: Potential Applications. Agron. Sustain. Dev. 2015, 35, 121–131. [Google Scholar] [CrossRef]
- Haghpanah, M.; Hashemipetroudi, S.; Arzani, A.; Araniti, F. Drought Tolerance in Plants: Physiological and Molecular Responses. Plants 2024, 13, 2962. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Gopi, R.; Manivannan, P.; Panneerselvam, R. Antioxidative Potentials as a Protective Mechanism in Catharanthus roseus (L.) G. Don. Plants under Salinity Stress. Turk. J. Bot. 2007, 31, 245–251. [Google Scholar]
- Cao, S.; Shi, L.; Shen, Y.; He, L.; Meng, X. Ecological Roles of Secondary Metabolites of Saposhnikovia Divaricata in Adaptation to Drought Stress. PeerJ 2022, 10, e14336. [Google Scholar] [CrossRef]
- Brechner, M.L.; Albright, L.D.; Weston, L.A. Effects of UV-B on Secondary Metabolites of St. John’s Wort (Hypericum perforatum L.) Grown in Controlled Environments. Photochem. Photobiol. 2011, 87, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Li, W.; Zheng, Q.; Wang, W.; Xiao, B.; Xing, D. Effect of Low Light Intensity on Growth and Accumulation of Secondary Metabolites in Roots of Glycyrrhiza Uralensis Fisch. Biochem. Syst. Ecol. 2010, 38, 160–168. [Google Scholar] [CrossRef]
- Coelho, G.C.; Rachwal, M.F.G.; Dedecek, R.A.; Curcio, G.R.; Nietsche, K.; Schenkel, E.P. Effect of Light Intensity on Methylxanthine Contents of Ilex paraguariensis A. St. Hil. Biochem. Syst. Ecol. 2007, 35, 75–80. [Google Scholar] [CrossRef]
- Chauser-Volfson, E.; Gutterman, Y. Content and Distribution of Anthrone C-Glycosides in the South African Arid Plant SpeciesAloe Mutabilisgrowing in Direct Sunlight and in Shade in the Negev Desert of Israel. J. Arid Environ. 1998, 40, 441–451. [Google Scholar] [CrossRef]
- Höft, M.; Verpoorte, R.; Beck, E. Growth and Alkaloid Patterns of Roots of Tabernaemontana Pachysiphon and Rauvolfia Mombasiana as Influenced by Environmental Factors. Bot. Acta 1998, 111, 222–230. [Google Scholar] [CrossRef]
- Cai, Z.Q.; Wang, W.H.; Yang, J.; Cai, C.T. Growth, Photosynthesis and Root Reserpine Concentrations of Two Rauvolfia Species in Response to a Light Gradient. Ind. Crops Prod. 2009, 30, 220–226. [Google Scholar] [CrossRef]
- Idris, A.; Linatoc, A.C.; Muhammad, S.M.; Aliyu, A.M.; Bakar, M.F.A. Effect of Light Intensity on the Total Flavonoid and Total Phenolic Contents of Mikania Micrantha and Tridax Procumbens. J. Sci. Technol. 2018, 10, 1–7. [Google Scholar] [CrossRef]
- Men, Y.; Wang, D.; Li, B.; Su, Y.; Chen, G. Effects of Drought Stress on the Antioxidant System, Osmolytes and Secondary Metabolites of Saposhnikovia Divaricata Seedlings. Acta Physiol. Plant. 2018, 40, 191. [Google Scholar] [CrossRef]
- Bandurska, H.; Cieślak, M. The Interactive Effect of Water Deficit and UV-B Radiation on Salicylic Acid Accumulation in Barley Roots and Leaves. Environ. Exp. Bot. 2013, 94, 9–18. [Google Scholar] [CrossRef]
- Rajabbeigi, E.; Eichholz, I.; Beesk, N.; Ulrichs, C.; Kroh, L.W.; Rohn, S.; Huyskens-Keil, S. Interaction of Drought Stress and UV-B Radiation-Impact on Biomass Production and Flavonoid Metabolism in Lettuce (Lactuca sativa L.). J. Appl. Bot. Food Qual. 2013, 86, 190–197. [Google Scholar] [CrossRef]
- Savickiene, N.; Raudone, L. Trends in Plants Phytochemistry and Bioactivity Analysis. Plants 2024, 13, 3173. [Google Scholar] [CrossRef]
- Reifenrath, K.; Müller, C. Species-Specific and Leaf-Age Dependent Effects of Ultraviolet Radiation on Two Brassicaceae. Phytochemistry 2007, 68, 875–885. [Google Scholar] [CrossRef]
- Wahid, A. Physiological Implications of Metabolite Biosynthesis for Net Assimilation and Heat-Stress Tolerance of Sugarcane (Saccharum officinarum) Sprouts. J. Plant Res. 2007, 120, 219–228. [Google Scholar] [CrossRef]
- Campa, C.; Urban, L.; Mondolot, L.; Fabre, D.; Roques, S.; Lizzi, Y.; Aarrouf, J.; Doulbeau, S.; Breitler, J.-C.; Letrez, C.; et al. Juvenile Coffee Leaves Acclimated to Low Light Are Unable to Cope with a Moderate Light Increase. Front. Plant Sci. 2017, 8, 1126. [Google Scholar] [CrossRef]
- Porto, D.D.; Matsuura, H.N.; Henriques, A.T.; Rosa, L.M.G.; Fett, J.P.; Fett-Neto, A.G. The Alkaloid Brachycerine Contributes to Protection against Acute UV-B Damage in Psychotria. Ind. Crops Prod. 2020, 147, 112216. [Google Scholar] [CrossRef]
- Leong Bin Abdullah, M.F.I.; Singh, D. The Adverse Cardiovascular Effects and Cardiotoxicity of Kratom (Mitragyna speciosa Korth.): A Comprehensive Review. Front. Pharmacol. 2021, 12, 726003. [Google Scholar] [CrossRef]
- Hasnain, A.; Esang, M.; Sabreen, R. A Case of Kratom-Induced Seizures. Cureus 2020, 12, e6588. [Google Scholar]
- Neerman, M.F.; Frost, R.E.; Deking, J. A Drug Fatality Involving Kratom. J. Forensic Sci. 2013, 58, S278–S279. [Google Scholar] [CrossRef]
- Yusoff, N.H.M.; Suhaimi, F.W.; Vadivelu, R.K.; Hassan, Z.; Rümler, A.; Rotter, A.; Amato, D.; Dringenberg, H.C.; Mansor, S.M.; Navaratnam, V.; et al. Abuse Potential and Adverse Cognitive Effects of Mitragynine (Kratom). Addict. Biol. 2016, 21, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Papsun, D.; Schroeder, W.; Brower, J.; Logan, B. Forensic Implications of Kratom: Kratom Toxicity, Correlation with Mitragynine Concentrations, and Polypharmacy. Curr. Addict. Rep. 2023, 10, 272–281. [Google Scholar] [CrossRef]
- Swogger, M.T.; Smith, K.E.; Garcia-Romeu, A.; Grundmann, O.; Veltri, C.A.; Henningfield, J.E.; Busch, L.Y. Understanding Kratom Use: A Guide for Healthcare Providers. Front. Pharmacol. 2022, 13, 801855. [Google Scholar] [CrossRef] [PubMed]
- Oehlert, G.W. A First Course in Design and Analysis of Experiments; W. H. Freeman and Company: New York, NY, USA, 2010; ISBN 0716735105. [Google Scholar]


| Traits | Water Potential | Light Intensity (μmol m−2 s−1) | |||
|---|---|---|---|---|---|
| 500 | 800 | 1200 | Avg | ||
| Total dry mass (g) | −0.03 MPa | 31.84 ± 7.44 | 42.55 ± 9.15 | 43.1 ± 9.92 | 39.16 ± 10.05 a |
| −0.40 MPa | 32.57 ± 11.22 | 32.47 ± 9.3 | 45.98 ± 13.32 | 37.00 ± 12.72 a | |
| −0.70 MPa | 23.36 ± 9.76 | 30.61 ± 7.75 | 32.16 ± 11.32 | 28.71 ± 10.12 b | |
| Avg | 29.26 ± 10.16 b | 35.21 ± 9.97 a | 40.41 ± 12.7 a | ||
| Stem dry mass (g) | −0.03 MPa | 16.04 ± 4.00 | 23.30 ± 6.22 | 24.53 ± 5.21 | 21.29 ± 6.30 a |
| −0.40 MPa | 19.31 ± 6.30 | 19.27 ± 5.49 | 27.25 ± 7.00 | 21.95 ± 7.16 a | |
| −0.70 MPa | 14.63 ± 5.57 | 18.16 ± 3.84 | 19.71 ± 6.84 | 17.50 ± 5.76 b | |
| Avg | 16.66 ± 5.54 c | 20.24 ± 5.55 b | 23.83 ± 6.92 a | ||
| Leaf dry mass (g) | −0.03 MPa | 5.71 ± 1.58 | 7.87 ± 1.83 | 5.89 ± 2.33 | 6.49 ± 2.11 a |
| −0.40 MPa | 4.41 ± 2.42 | 4.92 ± 1.81 | 6.08 ± 2.50 | 5.13 ± 2.29 b | |
| −0.70 MPa | 2.50 ± 1.81 | 4.24 ± 1.23 | 3.78 ± 1.91 | 3.51 ± 1.77 c | |
| Avg | 4.21 ± 2.32 b | 5.68 ± 2.25 a | 5.25 ± 2.42 ab | ||
| Root dry mass (g) | −0.03 MPa | 10.09 ± 2.41 | 11.38 ± 2.83 | 12.68 ± 3.30 | 11.39 ± 2.96 a |
| −0.40 MPa | 8.84 ± 3.09 | 8.27 ± 2.17 | 12.65 ± 4.07 | 9.92 ± 3.66 b | |
| −0.70 MPa | 6.23 ± 2.61 | 8.21 ± 3.03 | 8.67 ± 2.96 | 7.70 ± 2.97 c | |
| Avg | 8.39 ± 3.09 b | 9.29 ± 3.01 b | 11.34 ± 3.85 a | ||
| Stem diameter (mm) | −0.03 MPa | 13.31 ± 1.53 | 14.41 ± 1.73 | 14.76 ± 1.79 | 14.16 ± 1.74 a |
| −0.40 MPa | 12.48 ± 1.51 | 13.06 ± 1.64 | 14.5 ± 1.55 | 13.35 ± 1.74 a | |
| −0.70 MPa | 11.12 ± 1.86 | 12.58 ± 1.36 | 12.44 ± 1.97 | 12.04 ± 1.81 b | |
| Avg | 12.3 ± 1.83 b | 13.35 ± 1.72 a | 13.9 ± 2.01 a | ||
| Root diameter (mm) | −0.03 MPa | 14.02 ± 1.26 | 15.54 ± 1.87 | 17.74 ± 5.08 | 15.77 ± 3.46 a |
| −0.40 MPa | 12.98 ± 1.93 | 13.72 ± 2.05 | 16.03 ± 1.80 | 14.24 ± 2.28 b | |
| −0.70 MPa | 11.36 ± 1.64 | 13.24 ± 1.18 | 14.33 ± 2.35 | 12.98 ± 2.13 c | |
| Avg | 12.79 ± 1.93 c | 14.17 ± 1.95 b | 16.03 ± 3.56 a | ||
| Specific root length (cm g−1) | −0.03 MPa | 5.35 ± 1.5 | 4.05 ± 0.99 | 3.32 ± 1.02 | 4.24 ± 1.43 b |
| −0.40 MPa | 4.30 ± 2.24 | 4.63 ± 1.48 | 3.16 ± 1.09 | 4.03 ± 1.73 b | |
| −0.70 MPa | 6.53 ± 2.23 | 5.80 ± 1.68 | 5.42 ± 1.65 | 5.92 ± 1.86 a | |
| Avg | 5.39 ± 2.15 a | 4.83 ± 1.55 a | 3.97 ± 1.62 b | ||
| Leaf thickness (mm) | −0.03 MPa | 0.17 ± 0.03 | 0.18 ± 0.02 | 0.21 ± 0.02 | 0.18 ± 0.03 |
| −0.40 MPa | 0.19 ± 0.03 | 0.19 ± 0.02 | 0.20 ± 0.03 | 0.19 ± 0.03 | |
| −0.70 MPa | 0.17 ± 0.03 | 0.18 ± 0.02 | 0.18 ± 0.03 | 0.18 ± 0.03 | |
| Avg | 0.18 ± 0.03 b | 0.18 ± 0.02 b | 0.20 ± 0.03 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leksungnoen, N.; Andriyas, T.; Ku-Or, Y.; Chongdi, S.; Tansawat, R.; Aramrak, A.; Ngernsaengsaruay, C.; Uthairatsamee, S.; Sonjaroon, W.; Thongchot, P.; et al. The Effect of Light Intensity and Polyethylene-Glycol-Induced Water Stress on the Growth, Mitragynine Accumulation, and Total Alkaloid Content of Kratom (Mitragyna speciosa). Horticulturae 2025, 11, 272. https://doi.org/10.3390/horticulturae11030272
Leksungnoen N, Andriyas T, Ku-Or Y, Chongdi S, Tansawat R, Aramrak A, Ngernsaengsaruay C, Uthairatsamee S, Sonjaroon W, Thongchot P, et al. The Effect of Light Intensity and Polyethylene-Glycol-Induced Water Stress on the Growth, Mitragynine Accumulation, and Total Alkaloid Content of Kratom (Mitragyna speciosa). Horticulturae. 2025; 11(3):272. https://doi.org/10.3390/horticulturae11030272
Chicago/Turabian StyleLeksungnoen, Nisa, Tushar Andriyas, Yongkriat Ku-Or, Suthaporn Chongdi, Rossarin Tansawat, Attawan Aramrak, Chatchai Ngernsaengsaruay, Suwimon Uthairatsamee, Weerasin Sonjaroon, Phatthareeya Thongchot, and et al. 2025. "The Effect of Light Intensity and Polyethylene-Glycol-Induced Water Stress on the Growth, Mitragynine Accumulation, and Total Alkaloid Content of Kratom (Mitragyna speciosa)" Horticulturae 11, no. 3: 272. https://doi.org/10.3390/horticulturae11030272
APA StyleLeksungnoen, N., Andriyas, T., Ku-Or, Y., Chongdi, S., Tansawat, R., Aramrak, A., Ngernsaengsaruay, C., Uthairatsamee, S., Sonjaroon, W., Thongchot, P., Ardsiri, S., & Pongchaidacha, P. (2025). The Effect of Light Intensity and Polyethylene-Glycol-Induced Water Stress on the Growth, Mitragynine Accumulation, and Total Alkaloid Content of Kratom (Mitragyna speciosa). Horticulturae, 11(3), 272. https://doi.org/10.3390/horticulturae11030272






