Abstract
Neopestalotiopsis spp. are emerging fungal pathogens associated with leaf spot, fruit rot, crown rot, and root rot in strawberries. Despite their endophytic lifestyle, global outbreaks of these pathogens have been reported over the past few years, causing severe economic losses in commercial strawberry production. Resistance has been reported against the chemical fungicides used to manage Neopestalotiopsis spp. This review comprehensively examines the epidemiology, biology, and infection mechanisms of Neopestalotiopsis spp. on strawberries. Recent advances in management strategies, including biological control agents and resistant strawberry cultivars, have also been highlighted. By integrating these approaches, this work aims to provide a foundation for sustainable management practices to mitigate the impact of Neopestalotiopsis spp. on strawberry production.
1. Introduction
Strawberry (Fragaria × ananassa), a hybrid of the wild octoploid (2n = 8x = 56 chromosomes) species F. virginiana and F. chiloensis, is considered one of the major economically important berry crops worldwide. As members of the family Rosaceae, strawberries are a key component of the Mediterranean diet and are widely consumed because of their nutritional value and excellent source of antioxidants. Strawberries are high in vitamin C, folate, and minerals such as magnesium, calcium, chloride, and fluoride, and are appreciated for their taste, texture, and flavor. They are also low in calories and sugars (i.e., sucrose and glucose) and contain approximately 90% water [1,2,3]. The consumption of strawberries is expected to increase due to their functional food properties. This increase is observed in all forms of strawberries, including fresh, processed (frozen or canned), and those incorporated into jams, juices, chocolates, ice creams, and similar products. Strawberries contain bioactive compounds such as anthocyanins, alkaloids, flavonols, and ellagitannins [4], which promote health and wellness. These compounds exhibit cardioprotective, anti-inflammatory, and antioxidant properties [5]; mitigate the effects of heavy metal toxicity (e.g., cadmium in the lungs) [6]; and show promise in cancer treatments [7]. Furthermore, strawberries are a rich source of antioxidants with antimicrobial activity [8]. With regular consumption, the benefits of strawberries can be long-lasting, as antioxidants can remain in the bloodstream for more than a month [9].
The cultivation of strawberries is an extensive global practice, with this agricultural activity being observed in more than 76 countries. This practice encompasses a wide array of growing systems, including conventional and organic methods, as well as open field and greenhouse settings. China is the leading producer, followed by the United States, Mexico, Turkey, and Spain [10,11]. Strawberries thrive in temperate climates, with the optimal growth temperatures ranging from 26 to 34 °C. Based on their flowering habits, strawberry cultivars are classified as long-day (producing two harvests per season), short-day (producing a single harvest per season), or day-neutral in which the onset of flowering depends on temperature differences [10].
Despite their economic importance, strawberry production faces significant challenges from weeds, pests, and diseases, which reduce yields, affect fruit quality, and shorten postharvest lifespans. In particular, viral, fungal, and oomycete diseases pose major threats. These pathogens cause diseases in both nurseries and fields, affecting roots, crowns, the vascular system, and leaves. For example, Colletotrichum spp. are responsible for fruit anthracnose [12,13,14,15]. On the other hand, postharvest losses during storage are caused primarily by fungi such as Botrytis spp., Penicillium spp., and Rhizopus spp. [16]. Although comprehensive reports on the economic impact of strawberry diseases are limited, in Spain alone, yield losses due to root and crown rot are estimated to cause economic losses of between EUR 40 and 100 million per year [17].
Recently, Neopestalotiopsis spp. have emerged as a significant threat to strawberry production worldwide. These pathogens are characterized by their ability to cause leaf, root, crown, and fruit rot, as well as leaf spot, resulting in severe economic losses. Outbreaks affecting more than 30% of plants after transplanting were reported in Mexico, the United States, China, Taiwan, and Egypt between 2018 and 2021. New outbreaks continue to occur around the world, facilitated by the rapid spread of the pathogens through infected runner plants, their endophytic lifestyle [18,19,20,21,22], and their cosmopolitan nature, as they persist in the soil and affect not only strawberries but also other economically important crops such as mango, avocado, blueberry, tea plants, camedor palms, grapevine, and apple [23,24,25,26,27,28].
This review highlights recent advances in understanding the epidemiology, biology and infection mechanism of Neopestalotiopsis spp. in strawberry plants, resistant strawberry cultivars, and biological control agents used to mitigate their impact on strawberry production.
2. Overview of Neopestalotiopsis Infection in Strawberries
Strawberry fruit rot caused by Pestalotia longisetula was first reported in Florida in 1973, where it caused severe losses in both experimental plots and commercial fields [29]. Earlier, Pestalotia fruit rot due to P. longisetula was reported in Israel in 1968 [30]. Subsequently, Steyaert [31] proposed a classification system for the genus Pestalotia on the basis of the number of internal spore cells, dividing it into three genera: Pestalotia (six cells), Pestalotiopsis (five cells), and Truncatella (four cells). As a result, P. longisetula was reclassified as Pestalotiopsis longisetula. Further classification was based on morphological characteristics, particularly the color of the median spore cells (versicolorous or concolorous) [31,32]. Today, Pestalotiopsis is divided into three genera: Pestalotiopsis, Neopestalotiopsis, and Pseudopestalotiopsis. This classification is based on molecular analyses of the large subunit of the nuclear ribosomal RNA region (LSU), the internal transcribed spacer (ITS) and the partial β-tubulin (TUB), and translation elongation factor 1-alpha (TEF) gene regions, combined with their morphological characteristics [33]. According to the Mycobank Database [34], 635 species names have been recorded across these three genera (https://www.mycobank.org/; accessed 30 November 2024). While early classifications of Pestalotiopsis-like fungi were based on the host from which they were isolated, recent species identification has been based on phylogenetic and morphological analyses [35,36,37]. Although Pestalotiopsis-like fungi are generally considered secondary, weak, and opportunistic pathogens, they are capable of infecting a wide range of crops. Their lifestyle allows them to persist as endophytes, and they have also been isolated as saprobes from soil, dead leaves, paper, twigs, wood, and polluted stream water [24,38].
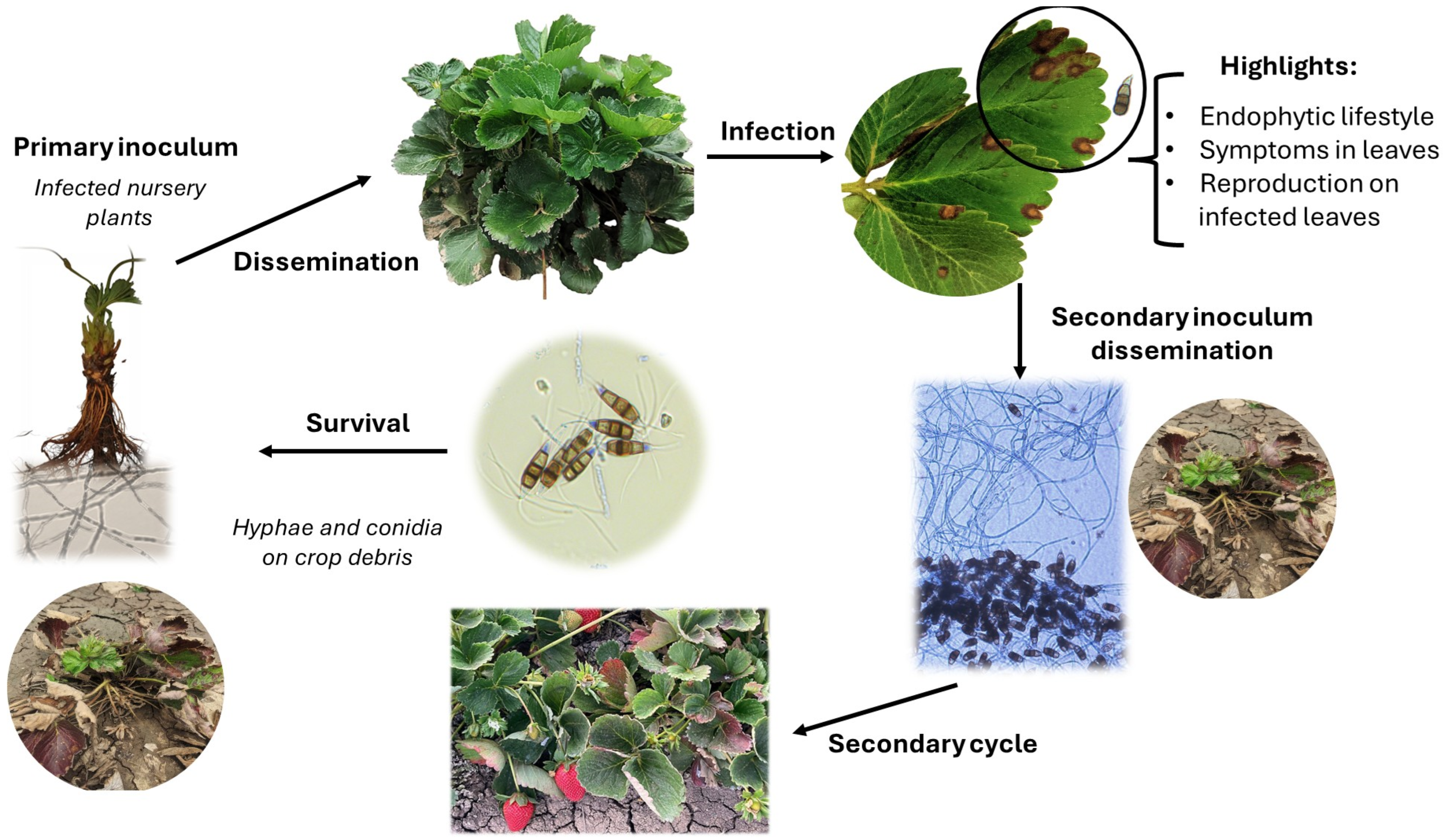
Infection by Pestalotiopsis-like fungi typically occurs when plants are injured or stressed, especially on the leaves. Common causes of injury include insect damage, mechanical damage from tools, sun scorching, and handling during harvest. However, infection can also occur without causing tissue damage through foliar spore sprays [33,39,40]. The asexual stage of these fungi is the primary source of inoculum, as little is known about their sexual stage. To date, only two sexual stages have been described: Pestalosphaeria and Neobroomella [24]. Successful infection depends on the environmental conditions, including high humidity (>80%), warm temperatures (between 20 °C and 30 °C), prolonged wetting times (over 72 h), and host susceptibility. Spore dispersal is facilitated by infected runner plants, contaminated nursery tools, rain, and wind (Figure 1) [39,40,41].
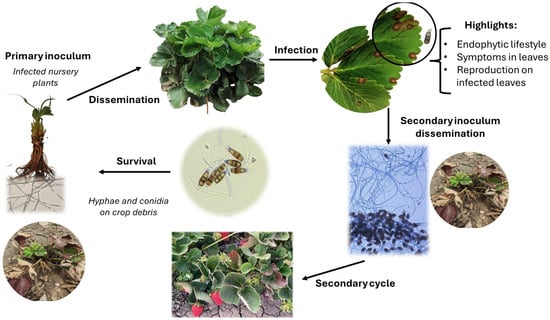
Figure 1.
Life cycle of Neopestalotiopsis spp. on strawberries.
Watanabe et al. [42] described the conidial adhesion mechanism of Pestalotiopsis neglecta as a four-stage process: (I) weak adhesion through the mucilaginous coating surrounding the spore; (II) strong attachment by substances issued from the base of the pedicel or the extremities of the apical appendage; (III) enhanced adhesion with adhesive material released from lower pigmented cells, and (IV) adhesive matter released from the emerging germ tube. The tight anchorage to the host surface enables successful host infection. In contrast to other strawberry fungal or oomycete pathogens such as Phytophthora spp. which exhibit a more gradual infection process, Neopestalotiopsis species cause rapid and aggressive infections. Rodrigues et al. [43] documented the infection process of P. longisetula (reclassified as N. rosae by Baggio et al. [41]) on cv. Camarosa strawberry leaves over six days. Conidia germinated within 48 h after inoculation (HAI), and at 72 HAI, large germ tubes formed on the epidermal cells without penetration. At 96 HAI, hyphae developed inter- and intracellularly in the lacunous parenchyma and tracheary elements, and pycnidia were produced on the leaf surface. At 144 HAI, the conidia were released.
Neopestalotiopsis spp. cause significant economic damage to strawberry plants, leading to reduced yields, plant death (Figure 2), and postharvest diseases in pseudocarps. Spores can be dispersed by the wind and rain, serving as a secondary inoculum that can infect nearby nurseries and persist in soil and crop residue for more than a year [44]. Other fungi commonly associated with Pestalotiopsis-like infections in strawberries include Curvularia spp. and Alternaria spp. on leaves and stems; Aspergillus niger, Colletotrichum spp., and Rhizopus stolonifer on fruit; and Rhizoctonia spp. and Fusarium spp. on roots [45].
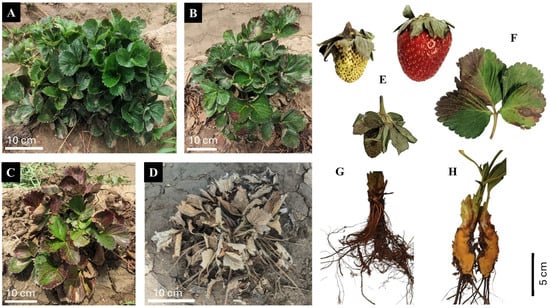
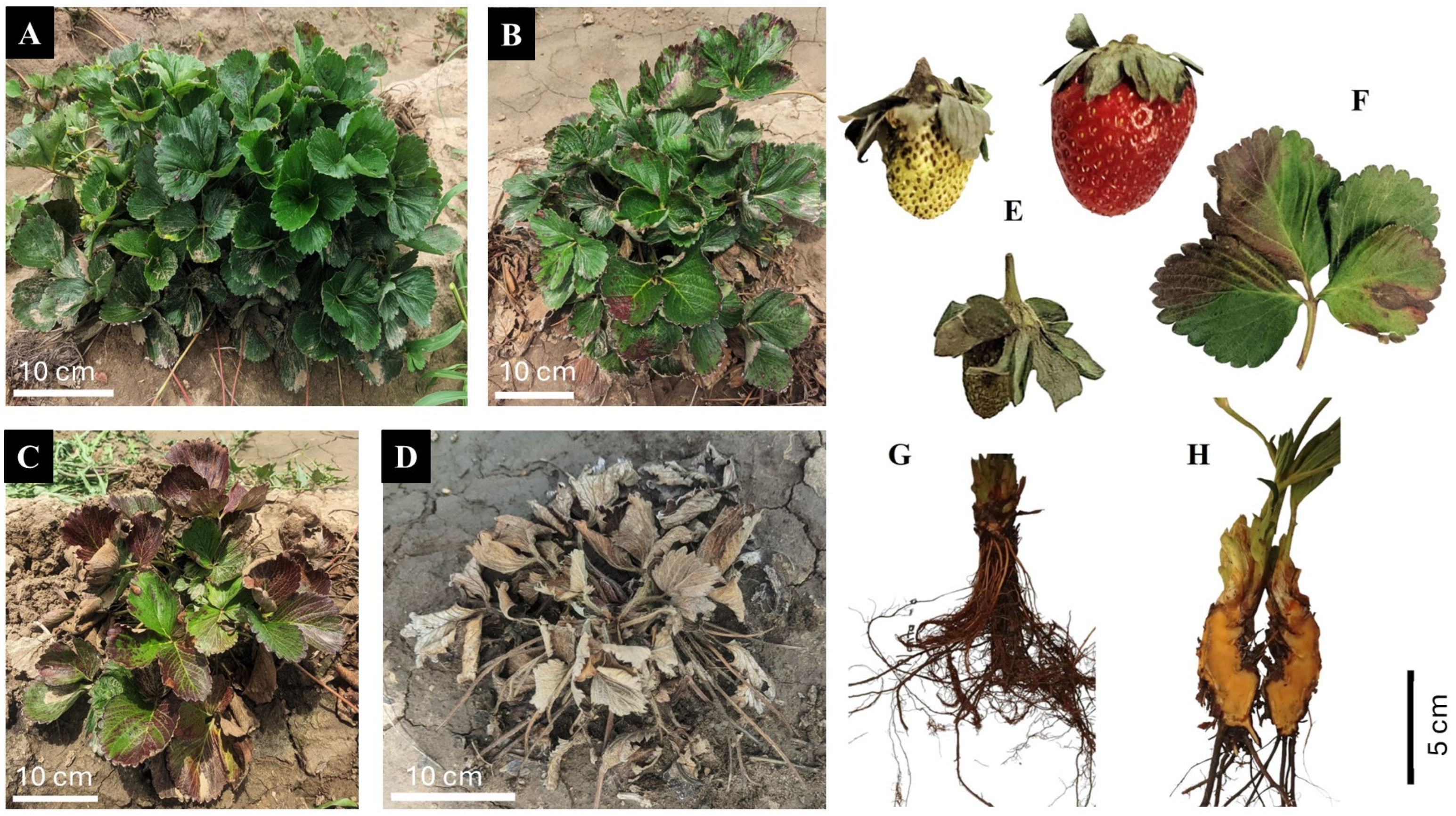
Figure 2.
Symptoms caused by Neopestalotiopsis spp. on strawberry crops: symptomless plant (A); leaf spots with a diffuse black halo surrounding a light brown center (B,F); leaf necrosis and wilting (C); death of infected plant (D); and fruit (E), root (G), and crown (H) rot.
Symptoms and signs of infection caused by Neopestalotiopsis spp. vary among plant tissues: In leaves, small dark brown spots with pycnidia and conidia develop, progressing concentrically to cause leaf blight; in petioles, sunken, dark brown lesions form at the base, affecting the crown and causing leaf wilt; in crowns, irregular reddened areas with dark brown edges appear, and in roots, infected areas turn dark brown. Severe infections can stunt plant growth and lead to death within a month [19]. Fruit symptoms include dry, light brown, slightly sunken lesions that become covered with black pycnidia, eventually affecting the entire fruit (Figure 2) [41].
Pestalotiopsis and related genera produce over 300 metabolites, some of which are of medical importance (e.g., the anticancer agent Taxol) and others facilitate infection by damaging plant tissues. These include organic acids, amino acids, and esters [46]. Additionally, Pestalotiopsis spp. produce carbohydrate-active enzymes (CAZymes), such as cutinases and pectinases, which degrade plant cell wall components for nutrition [47,48]. Phytotoxins, which kill plant tissues during infection, are also produced by Neopestalotiopsis spp. For example, N. clavispora releases oxysporone, afritoxinone A, and afritoxinone B, which cause necrotic lesions on non-host plants such as Ipomea batatas, Mangifera indica, and Bauhinia purpurea [49]. Other reported phytotoxins from Pestalotiopsis spp. include (+)-epiepoxydon 54 and pesthetoxin (PT-toxin), pestalopyrone, nectriapyrone, pestalotin, hydroxypestalotin, hydroxypestalopyrone, and pestaloside, all of which contribute to necrotic leaf spot formation [50].
3. Neopestalotiopsis Outbreaks in Strawberry Are Extending Worldwide: Epidemiology and Most Affected Cultivars
Neopestalotiopsis spp. is a critical pathogen that causes severe economic losses by reducing both the runner plants’ population per hectare and overall yield. Reports of Neopestalotiopsis spp. infection in blueberries and strawberries have been consistent worldwide [25,26,35,51,52,53]. Mexico, the United States, and China are among the most affected strawberry-producing countries. However, Egypt, a significant producer and exporter of strawberries, reported N. rosae infections in the Festival cultivar in 2017, causing wilting and mortality. Symptoms including leaf wilt, crown necrosis, and vascular tissue damage also developed in the Fortuna cultivar [22]. In Taiwan, Wu et al. [21] identified N. rosae as the primary fungus responsible for reducing more than 30% of strawberry plants (cv. Xiang-Shui) after transplanting during the 2019–2020 season. Despite being an anthracnose-tolerant cultivar, symptoms included necrotic lesions on leaves (with visible black pycnidia), stolons, crowns, and fruits, which significantly affected the production and yield. Similarly, Fernández-Ozuna et al. [54] reported a greater than 30% disease incidence caused by N. rosae in strawberry plants (cv. Sweet Charlie) in Paraguay, with symptoms including necrotic lesions on leaves, crowns, and roots and the formation of sunken spots and pycnidia on fruit.
In Germany and India, N. rosae has been identified as the causal agent of wilting and necrosis in strawberries [52,55]. In contrast, Ecuador experienced outbreaks in 2014 and 2020, with a 50% yield reduction in strawberry crops (cv. Albion) due to root and crown rot caused by N. mesopotamica. The symptoms included reddish discoloration in new leaves, browning of the leaf apices and petioles of older leaves, and eventual plant death [56]. By 2019, the disease incidence in Ecuadorian strawberry fields had reached 70% [57]. In Argentina [58], Korea [59], Spain [60], Italy [61,62], Uruguay [63], and China [64], N. clavispora has been reported to cause root and crown rot, discoloration, stunted growth, poor rooting, and plant death in more than 50% of strawberry plantations, resulting in significant yield losses. In China, N. rosae and N. fragariae have also been responsible for root rot and leaf spot symptoms in strawberry crops since 2018–2019 and 2021, respectively [20,65]. Notably, Baggio et al. [41] reclassified N. clavispora (previously reported in Spain by Chamorro et al. [60]) as N. rosae on the basis of its 100% nucleotide identity in three gene regions (i.e., ITS, β-tubulin, and tef1).
Mexico, the fifth largest producer of strawberries in the world, produced 641,552 tons in 2023, which were exported mainly to the United States along with more than ten other countries. China leads in terms of global production, with more than 3.4 million tons produced [66]. From 2017 to 2020, Mexico experienced losses exceeding 50% of established transplants, with more than 60% of strawberry plants showing symptoms of anthracnose on the leaves and stems. The symptoms included leaf spots, root rot, and crown rot, which resulted in plant death, postharvest diseases, and poor fruit quality. The cultivars most affected were Camino Real, Albion, and Festival, and N. rosae was identified as the causal agent [18,19]. Camino Real, which was once the primary cultivar grown by family farmers in Guanajuato, has since been replaced by other cultivars due to its susceptibility. The origin of the pathogen in Mexico remains unclear, with uncertainty as to whether it was introduced via contaminated runner plants or was already present in the soil as an opportunistic pathogen. The initial reports were linked to open-field nurseries in the state of Michoacán [19].
In the United States, Florida experienced several outbreaks of Neopestalotiopsis species during the 2019–2020 season, resulting in the destruction of more than 80 hectares of strawberry fields and significant economic losses. Phylogenetic analysis identified the Neopestalotiopsis sp. as the causal agent, with the source of the inoculum traced to nurseries in North Carolina. The symptoms caused by the Neopestalotiopsis sp. are more severe than those caused by N. rosae, affecting the fruit, leaves, crowns, petioles, and roots [41]. In California, Lawrence et al. [67] reported that 60% of the disease incidence was caused by N. rosae, with symptoms of crown and root rot. Since 2020, reports of Neopestalotiopsis sp. infections have been consistent in the United States, with documented cases in Indiana [68], Georgia [69], and Ohio, where the disease incidence has exceeded 50% [70]. Canadian strawberry producers have also been affected by a Neopestalotiopsis sp., with a 40% disease incidence. The pathogen is believed to have originated from a nursery in North Carolina that supplies plants to Ontario [41,71]. None of the cultivars affected by Neopestalotiopsis spp. have demonstrated resistance to these pathogens. In this context, Guan et al. [68] evaluated the susceptibility of 22 strawberry cultivars to leaf spot disease caused by a Neopestalotiopsis sp. They reported that commercial cultivars such as Camino Real, Albion, San Andreas, Evie II, Florida Brilliance, and Florida Sensation were the most affected, followed by Camarosa, Monterey, Chandler, and Sweet Charlie. Conversely, cultivars such as Galletta, AC Valley, and Flavorfest presented no symptoms, irrespective of the type of plant material used (bare-root or plug), and were therefore classified as resistant.
4. The Pathogen Neopestalotiopsis: Molecular and Morphological Characterization
Historically, the identification of Pestalotiopsis spp. has relied on the morphological characteristics of colonies and spore measurements. However, Neopestalotiopsis is a genus with cryptic species that are not host-specific and cannot be differentiated solely by conidial morphology. Molecular strategies are needed for accurate identification [24,72]. In this context, Maharachchikumbura et al. [33] classified Pestalotiopsis, Pseudopestalotiopsis, and Neopestalotiopsis on the basis of phylogenetic and morphological data.
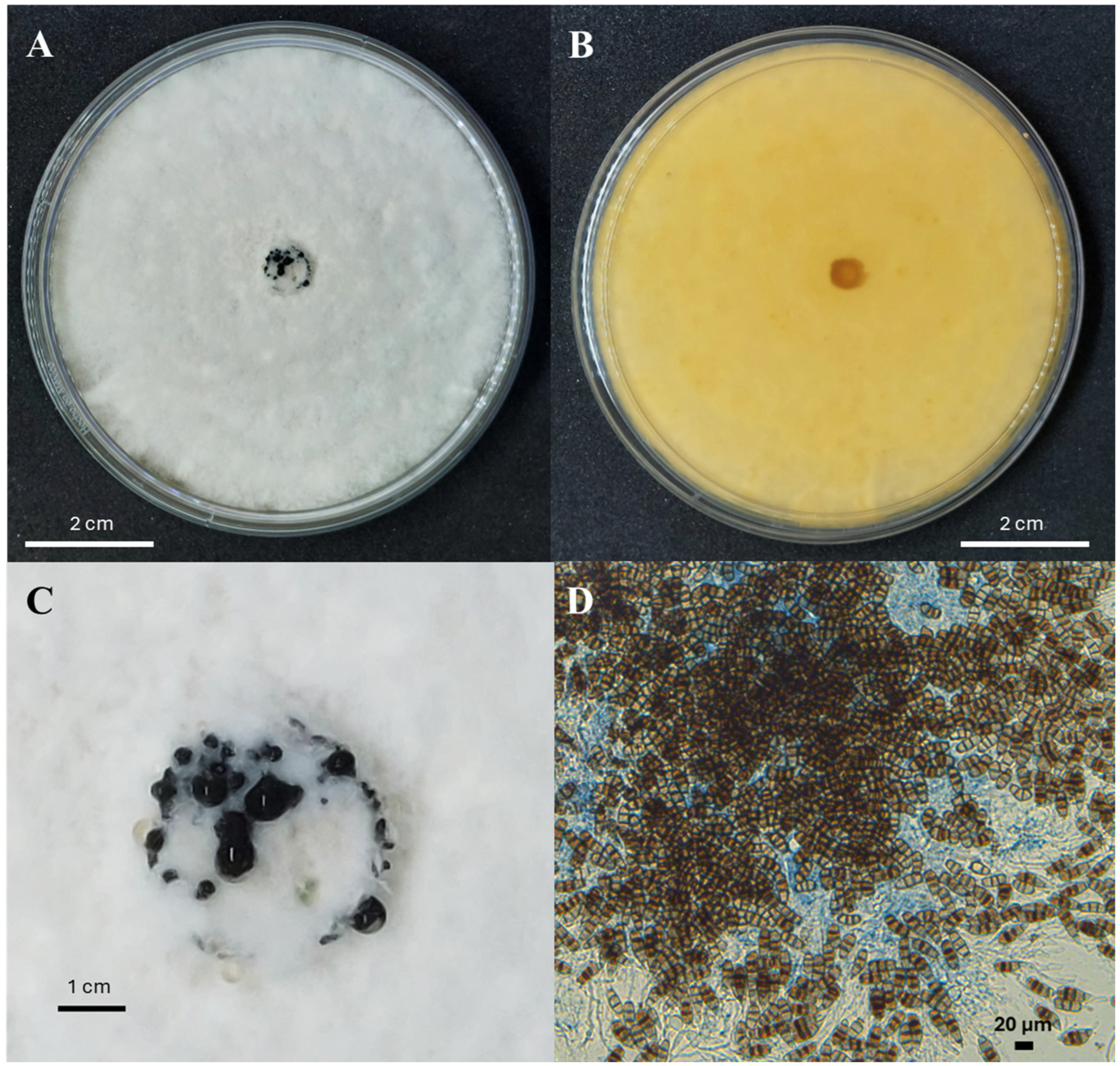
According to Sun et al. [20] and Maharachchikumbura et al. [33], N. rosae grows optimally at 25 °C on potato dextrose agar (PDA). The colony morphology features a white or pale-yellow front (similar to the reverse side), with a lobate edge and aerial mycelium on the surface. The colony is filamentous and circular, with black, concentric, and glistening conidiomata (Figure 3A,B).
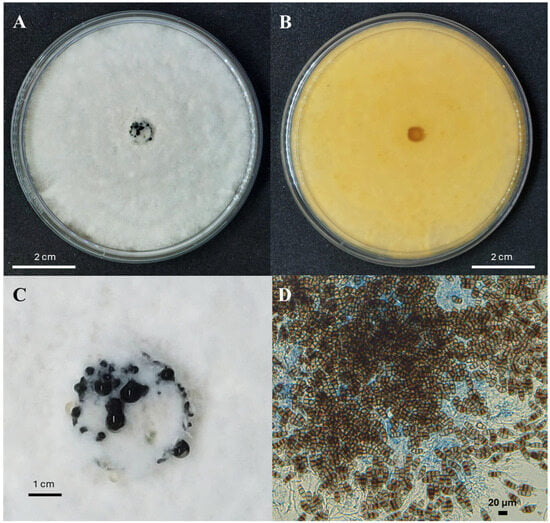
Figure 3.
Features of typical Neopestalotiopsis spp. mycelial growth on potato dextrose agar media: the front (A) and reverse (B) sides after 7 days at 28 °C, the conidiomata formation in the center of the colony (C), and the conidia (D).
Conidiomata (asexual morphology): Pycnidial, globose, solitary, and semi-immersed; dark brown to black; and approximately 100–300 µm in diameter. The exuded conidial masses are globose, dark brown, and glistening (Figure 3C).
Conidiogenous cells: Discrete, simple, cylindrical, hyaline, smooth-walled, and proliferate 2–4 times percurrently. They taper toward a truncate apex with visible periclinal thickening, measuring approximately 5–20 × 2–8 µm. The conidiophores are indistinct and are often reduced to conidiogenous cells.
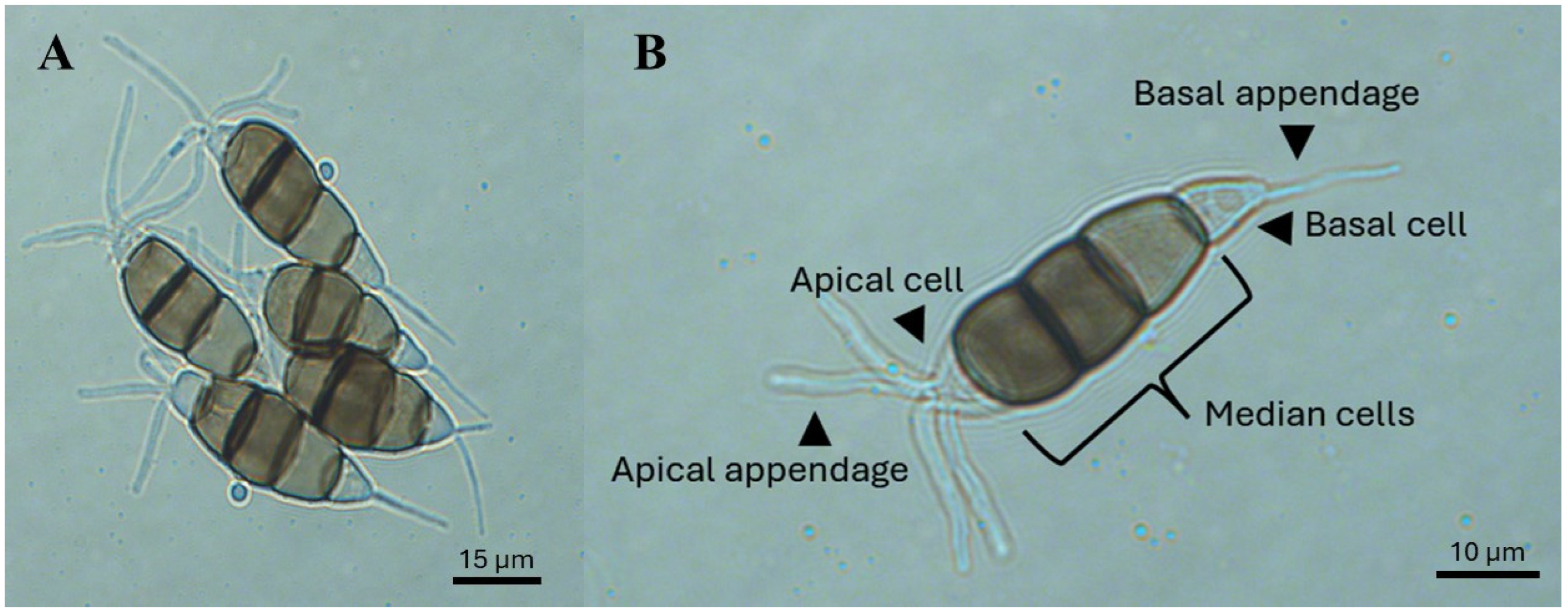
Conidia: Long fusoid (approximately 3–7.2 µm long), five-celled, ellipsoid, and straight to slightly curved, with four septa, approximately 24.8 × 8.5 µm long. The basal cell (first cell) is conic to obconic with a truncate base, and is hyaline, rugose, and thin-walled, often with a short oblique appendage projecting from the base. The three median cells are doliiform (approximately 13.6–19.9 µm long), rugose, and versicolored, with septa darker than the rest of the cells: the second cell from the base is pale brown (approximately 4.5–8 µm long), the third cell is honey-brown (approximately 4.3–7.4 µm long), and the fourth cell is brown (approximately 3.4–7.1 µm in length). The apical cell (approximately 3–6 µm in length) is hyaline, cylindrical, and thin and smooth walled, with 2–5 tubular apical appendages (approximately 23.4–42.4 µm long) inserted at different loci in the upper half of the apical cell. These appendages are unbranched and filiform. The basal appendage (approximately 3.8–8.7 µm long) is single, tubular, unbranched, and centric, arising from the basal cell (Figure 4B).
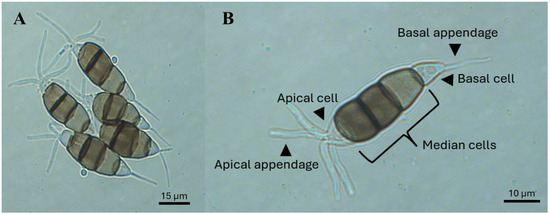
Figure 4.
Conidia of Neopestalotiopsis spp. (A,B) and their structures with versicolorous median cells.
At the morphological level, one way to differentiate Pestalotiopsis-like fungi, although not definitively, is on the basis of the color of the median cells of the conidia. For example, Neopestalotiopsis spp. have versicolorous median cells, whereas Pestalotiopsis spp. and Pseudopestalotiopsis spp. have light and dark concolorous median cells, respectively (Figure 4A). However, conidial size (length and width) is not a reliable characteristic for taxonomic identification, as species within these genera have similar dimensions [33]. The color of the median cells is due to melanin, a pigment that protects spores from biotic and abiotic stresses, plays a role in plant–pathogen interactions, and is essential for spore structure development [73,74].
Although PDA and water agar are routinely used for the recovery and identification of Neopestalotiopsis spp., Zuniga et al. [44] recently developed a semi-selective medium (NSM) based on a selective medium for B. cinerea. This medium contains NaNO3, K2HPO4, MgSO4, KCl, CuSO4, glucose, streptomycin, chloramphenicol, penthiopyrad, tannic acid, and agar. NSM can differentiate and quantify Neopestalotiopsis spp. spores from other fungi isolated from soil and plant residue (e.g., leaves, crowns) after incubation at 24 °C for 5–7 days. It inhibits the growth of other strawberry pathogens such as Colletotrichum spp., Botrytis spp., Alternaria spp., and Phytophthora spp. In this medium, the growth of Neopestalotiopsis spp. causes the color of NSM medium to change from milky-white to brown, and colonies appear irregular with white mycelium.
The molecular screening of Pestalotiopsis-like fungi was reported by Maharachchikumbura et al. [38], who tested ten gene regions to clarify species boundaries: nuclear ribosomal large subunit rDNA (LSU), nuclear ribosomal small subunit rDNA (SSU), partial actin (ACT), glutamine synthase (GS), glyceraldehyde-3-phosphate dehydrogenase (GPDH), RNA polymerase II (RPB1), calmodulin (CAL), β-tubulin, ITS, and tef1. The most suitable markers were ITS, tef1, and β-tubulin, as the others failed to resolve the species and were less successful in PCR amplification. The authors recommend the use of these three markers together, as ITS alone does not satisfactorily resolve species. Baggio et al. [41] subsequently confirmed these findings, suggesting that additional gene loci are needed to clarify species separation in Neopestalotiopsis spp. Their study identified a Neopestalotiopsis sp. as the causal agent of strawberry disease in Florida, which was genetically distinct and more aggressive than N. rosae. This species can be isolated from leaves and fruit. To distinguish between a Neopestalotiopsis sp. and N. rosae, Kaur et al. [75] developed a polymerase chain reaction coupled with a restriction fragment length polymorphism (PCR/RFLP) assay. This method targets a β-tubulin gene fragment (using the Bt2a + Bt2b primer set) and digests the PCR product with the restriction enzyme BsaWI. The assay produces two bands (290 and 130 bp) for the Neopestalotiopsis sp. and one band (420 bp) for N. rosae, due to a mutation in the β-tubulin gene of the former. The authors recommended the use of this reliable and accurate test only for symptomatic strawberry plants.
On the other hand, Rebello et al. [76] also contributed to the molecular identification of these pathogens by developing a high-resolution melting (HRM) assay to differentiate Neopestalotiopsis species based on the polymorphisms in a partial region of the β-tubulin gene. This method is effective for symptomatic strawberry samples in which either spores or crude leaf extracts are used and allows the differentiation of N. rosae from the aggressive Neopestalotiopsis sp.
Accurate detection methods for Neopestalotiopsis spp. are essential for timely diagnosis and effective management of this disease in strawberry fields. Specific molecular markers for identifying Neopestalotiopsis spp. should be designed for use in PCR techniques to streamline diagnosis. Once developed, these markers could be incorporated into isothermal DNA amplification techniques, such as loop-mediated isothermal amplification (LAMP) or recombinase polymerase amplification (RPA), enabling their field use without specialized PCR equipment. Similar approaches have been successfully applied to other strawberry pathogens, such as P. fragariae, P. cactorum [77,78], and B. fragariae [79].
5. Neopestalotiopsis Disease Management in Strawberry Crops
The infection of strawberry crops by Neopestalotiopsis species depends on several factors, including cultivar susceptibility, the use of disease-free plants from nurseries, and effective field management practices by growers. Key strategies include the removal of infested plant residues, ensuring the purchase of healthy runner plants, and, in the case of growers producing their own runner plants, the testing of these runner plants by reliable and authorized laboratories. Coordination with trained crop management technicians is also critical. Early detection and diagnosis can save money and plants by allowing a quick response to treatment. Recent studies have indicated that Neopestalotiopsis spp. spores can survive in soil and plant residues even in beds covered with black mulch and tilled soil after multiple strawberry harvest seasons. Zuniga et al. [44] reported that Neopestalotiopsis spp. can tolerate high temperatures (≤40 °C) but preplant soil fumigation can effectively reduce the amount of soil inoculum. In this context, soil treatments with alternatives to methyl bromide—such as 1,3-dichloropropene (1,3-D), chloropicrin (Pic), dazomet (DZ), metam sodium (MS), and metam potassium (MP)—have been proposed. Other methods include solarization, steam treatment, anaerobic soil disinfestation, and cover cropping. These approaches not only target fungal pathogens but also help to manage pests such as aphids, lepidopteran leafrollers, weevils, lygus bugs, spider mites, and thrips [10].
Chemical treatments applied to the soil before transplanting have reduced the inoculum of Neopestalotiopsis spp. using 1,3-D, Pic, and MP alone or in combination with 1,3-D and Pic at ratios of 63:35 [80]. In addition, Tran et al. [81] reported that cinnamon essential oil-encapsulated lipid nanoemulsions inhibited the growth of N. rosae even at high dilutions (up to 500 times) from stock solutions (200 or 400 µg/mL), either alone or in combination with other essential oils. Cinnamon essential oil reduced the appearance of strawberry leaf spot by 79% within 7 days of treatment at a 500-fold dilution (200 µg/mL), preventing disease without causing phytotoxicity. On the other hand, Darapanit et al. [82] reported that clove and turmeric extracts at 10,000 mg/L completely inhibited the growth of Neopestalotiopsis spp. and Pseudopestalotiopsis spp. isolated from strawberry and other fruits. Clove extract at 1000 mg/L also resulted in the 100% inhibition of Pestalotiopsis-like fungi, whereas the ginger, lemongrass, and roselle extracts resulted in a greater than 75% inhibition at the same concentration.
The chemical fungicides tested to control N. rosae infection in strawberries (Table 1) included thiram (1000 ppm) and hymexazole (1250 ppm), which inhibited over 90% of mycelial growth in vitro. Under greenhouse conditions, thiram at 500, 750, 1000, and 1250 ppm/liter reduced root and crown symptoms by 80% in the Fortuna and Festival cultivars, whereas hymexazole achieved 79% efficacy in the cv. Festival [22]. Baggio et al. [83] evaluated 30 commercial fungicides in vitro against Neopestalotiopsis spp. and reported that fludioxonil, fluazinam, difenoconazole, fluotriafol, myclobutanil, propiconazole, tetraconazole, and triflumizole (at 1 and 100 µg/mL), and captan, thiram, and chlorothalonil (at 100 and 1000 µg/mL) were the most effective at inhibiting fungal growth. Field trials have shown that alternating cyprodinil and fludioxonil with thiram is a reliable program for controlling fruit rot caused by Neopestalotiopsis sp. However, the use of these fungicides in commercial fields requires careful consideration, as some fungicides are not registered for strawberry pest management programs or are restricted to nursery use. Baggio et al. [83] noted that quinone outside inhibitor (QoI) fungicides such as azoxystrobin should be avoided because of resistance conferred by the G143A mutation in the cytb gene. Similar resistance has been observed in QoI-resistant isolates of Colletotrichum acutatum on strawberries [84].

Table 1.
Active ingredients, Fungicide Resistance Action Committee (FRAC) codes, and modes of action of chemical fungicides used to control Neopestalotiopsis spp. infections in strawberries.
Acosta-González et al. [86] showed that preventive root dipping with combinations of pydiflumetofen + fludioxonil, cyprodinil + fludioxonil, and prochloraz + thiram reduced the incidence of N. rosae infection and plant mortality. Biological control agents such as Trichoderma asperellum and T. koningiopsis can also be included in management programs, either alone or in combination with synthetic fungicides, under greenhouse conditions. Amrutha and Vijayaraghavan [87] reported that seven fungicides—carbendazim 12% + mancozeb 63% (Saaf), cymoxanil 8% + mancozeb 64% (Curzate M8), copper hydroxide 77WP (Kocide), copper oxychloride 50WP (Fytolan), propineb 70WP (Antracol), and the Bordeaux mixture—achieved the 100% inhibition of mycelial growth in vitro. Propineb and carbendazim + mancozeb reduced disease incidence by 78.03% and 74.39%, respectively, in an in vivo study. However, carbendazim should be used with caution, as Kummanid et al. [88] identified a mutation in the β-tubulin gene in Pestalotiopsis sp. isolates that confers resistance to this fungicide.
In contrast, T. asperellum alone reduced leaf blight by 74%. In addition to Trichoderma spp., bacteria such as Bacillus cereus (Bce-2) have shown promise in inhibiting N. clavispora mycelial growth by up to 79%. Fermentation extracts of B. cereus prevent leaf spot by inducing the expression of defense-related enzymes and genes, including peroxidase (POD), superoxide dismutase (SOD), catalase (CAT), and phenylalanine ammonia-lyase (PAL), in strawberry leaves [89]. Similarly, Yang et al. [90] reported that B. amyloliquefaciens isolates not only promoted growth but also exhibited antifungal activity against F. solani, reduced root rot in strawberry plants by more than 57%, and induced defense-related enzymes such as SOD, CAT, in addition to malondialdehyde in leaves.
More reports on the evaluation of chemical fungicides, botanical fungicides, and biological control agents under field conditions are needed worldwide to consider them in the management of Neopestalotiopsis spp. diseases.
The management of Neopestalotiopsis spp. needs to be addressed both in nurseries and in the field. In the context of nurseries, effective surveillance and management strategies for diseases are paramount. In particular, when importing runner plants, it is essential to consider the purchase of disease-free material. To date, Neopestalotiopsis spp. have not been classified as a quarantine pest by global plant health authorities. Therefore, it is essential for growers to conduct tests in certified laboratories to ensure the introduction of Neopestalotiopsis spp. free runner plants in the field. Similarly, it is imperative to disinfect both the propagation trays and the substrate to be used. In this regard, it is essential that the runner plants are treated with chemical fungicides and subsequently with effective biological agents that have been rigorously evaluated in real field conditions. Implementing precise management strategies throughout the strawberry production chain will ensure enhanced crop yields and minimize economic losses for the grower.
6. Perspectives and Concluding Remarks
One of the most significant challenges facing the strawberry industry is the development of cultivars resistant to Neopestalotiopsis infection. Until such cultivars are widely available, ensuring that plants from commercial nurseries are disease-free is essential to prevent substantial economic losses. While some cultivars have been evaluated for resistance or susceptibility [68], an integrated disease management approach incorporating biological controls is necessary to meet the market’s demands for fruit quality and safety.
Strawberry plants rely on a combination of immune system efficacy, physical defenses, secondary metabolites, and pathogenesis-related proteins to combat pathogens. Further research into their defense genes is crucial for enhancing plant protection against pathogen attacks [91]. For example, the application of bio-stimulants could be explored to promote plant health and resilience [92]. A study by Alam et al. [93] on the genetic resistance of Florida germplasm and wild relatives identified a novel resistance allele in the cultivar Yasmin, which confers resistance to a Neopestalotiopsis sp. infection. These findings highlight the potential for developing resistant cultivars in breeding programs.
The publication of the draft genome of Neopestalotiopsis spp. [94] marks a significant step toward understanding their pathogenic mechanisms. However, until more comprehensive insights are gained, growers must adhere to institutional plant health recommendations. Governments and agricultural authorities should provide clear guidelines on pathogen management, including whether Neopestalotiopsis spp. should be classified as quarantine pests, and implement monitoring systems to track their distribution within and across countries. Further research is needed to fully understand the lifestyle and infection processes of Neopestalotiopsis spp. in strawberries.
This review addresses the most critical aspects of Neopestalotiopsis infection, offering an overview of how growers can manage the disease and highlighting advances in diagnostic methods on the basis of published information. By integrating these findings into practical strategies, the strawberry industry can better mitigate the impact of this pathogen and ensure sustainable production.
Author Contributions
Conceptualization, J.G.Á.-H., A.M.-A. and P.A.-Z.; writing—original draft preparation, J.G.Á.-H.; writing—review and editing, J.G.Á.-H., C.G.L.-R., M.d.R.A.-J., B.T.-B., V.O.-P., J.P.D.-F., A.M.-A. and P.A.-Z.; supervision, A.M.-A. and P.A.-Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work is part of the project 22427.25-P Encapsulación como Herramienta de Conservación de Hongos Entomopatógenos: Evaluación de Parámetros de Calidad y Efectividad en el Control de Plagas funded by Tecnológico Nacional de México.
Acknowledgments
José Guadalupe Ávila-Hernández thanks Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI, México) and Cinvestav Unidad Irapuato for the financial support for his PhD studies.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Parra-Palma, C.; Úbeda, C.; Gil, M.; Ramos, P.; Castro, R.I.; Morales-Quintana, L. Comparative study of the volatile organic compounds of four strawberry cultivars and it relation to alcohol acyltransferase enzymatic activity. Sci. Hortic. 2019, 251, 65–72. [Google Scholar] [CrossRef]
- Hasnaa, S.; Chadia, O.; Abdellah, M.; Azzeddine, E.R.; Asmae, T.; Abdallah, D.; Lhoussine, B. Study of the physicochemical characteristics of different strawberries consumed in Morocco. Curr. Res. Nutr. 2023, 11, 339–350. [Google Scholar] [CrossRef]
- Salas-Arias, K.; Irías-Mata, A.; Sánchez-Kopper, A.; Hernández-Moncada, R.; Salas-Morgan, B.; Villalta-Romero, F.; Calvo-Castro, L.A. Strawberry Fragaria x ananassa cv. Festival: A polyphenol-based phytochemical characterization in fruit and leaf extracts. Molecules 2023, 28, 1865. [Google Scholar] [CrossRef]
- Ko, M.J.; Jayaramaiah, R.H.; Gupta, R.; Kim, S.W.; An, J.U.; Wang, Z.; Li, M.; Kang, N.J.; Hong, K.P.; Kang, J.S.; et al. Evaluation of bioactive compounds in strawberry fruits by a targeted metabolomic approach. Korean J. Hortic. Sci. Technol. 2017, 35, 805–819. [Google Scholar] [CrossRef]
- Basu, A.; Izuora, K.; Betts, N.M.; Ebersole, J.L.; Scofield, R.H. Dietary strawberries improve biomarkers of antioxidant status and endothelial function in adults with cardiometabolic risks in a randomized controlled crossover trial. Antioxidants 2021, 10, 1730. [Google Scholar] [CrossRef]
- Nofal, A.E.; AboShabaan, H.S.; Fayyad, R.M.; Ereba, R.E.; Omar, N.A.; Elsharkawy, S.M.; Elberri, A.I. Immunostimulatory and anti-inflammatory impact of Fragaria ananassa methanol extract in a rat model of cadmium chloride-induced pulmonary toxicity. Front. Immunol. 2023, 14, 1297315. [Google Scholar] [CrossRef]
- Amatori, S.; Mazzoni, L.; Alvarez-Suarez, J.M.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Errico Provenzano, A.; Persico, G.; Mezzetti, B.; et al. Polyphenol-rich strawberry extract (PRSE) shows in vitro and in vivo biological activity against invasive breast cancer cells. Sci. Rep. 2016, 6, 30917. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, X.; Chen, H.; Liu, Y.; Xiao, Y.; Chen, H.; Tang, Z.; Li, Q.; Yao, H. Evaluation of a strawberry fermented beverage with potential health benefits. PeerJ 2021, 9, e11974. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Miller, M.G.; Thangthaeng, N.; Scott, T.M.; Shukitt-Hale, B.; Edirisinghe, I.; Burton-Freeman, B. Metabolic fate of strawberry polyphenols after chronic intake in healthy older adults. Food Funct. 2018, 9, 96–106. [Google Scholar] [CrossRef]
- Hernández-Martínez, N.R.; Blanchard, C.; Wells, D.; Salazar-Gutiérrez, M.R. Current state and future perspectives of commercial strawberry production: A review. Sci. Hortic. 2023, 312, 111893. [Google Scholar] [CrossRef]
- González-Ramírez, M.G.; Santoyo-Cortés, V.H.; Arana-Coronado, J.J.; Muñoz-Rodríguez, M. The insertion of Mexico into the global value chain of berries. World Dev. Perspect. 2020, 20, 100240. [Google Scholar] [CrossRef]
- Narro-Sánchez, J.; Dávalos-González, P.A.; Velásquez-Valle, R.; Castro-Franco, J. Main strawberry diseases in Irapuato, Guanajuato and Zamora, Michoacan, Mexico. Acta Hortic. 2006, 708, 167–172. [Google Scholar] [CrossRef]
- Takeda, F.; Janisiewicz, W.J.; Smith, B.J.; Nichols, B. A New approach for strawberry disease control. Eur. J. Hortic. Sci. 2019, 84, 3–13. [Google Scholar] [CrossRef]
- Pastrana, A.M.; Borrero, C.; Pérez, A.G.; Avilés, M. Soilborne pathogens affect strawberry fruit flavor and quality. Plant Sci. 2023, 326, 111533. [Google Scholar] [CrossRef]
- Villarino, M.; Larena, I.; Melgarejo, P.; De Cal, A. Effect of chemical alternatives to methyl bromide on soil-borne disease incidence and fungal populations in Spanish strawberry nurseries: A long-term study. Pest Manag. Sci. 2021, 77, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhang, W.; Zhu, N.; Mao, S.; Tu, K. Early detection and classification of pathogenic fungal disease in post-harvest strawberry fruit by electronic nose and gas chromatography-mass spectrometry. Food Res. Int. 2014, 62, 162–168. [Google Scholar] [CrossRef]
- Avilés, M.; Pastrana, A.M.; Borrero, C. Emerging diseases in Spain strawberry crops: Neopestalotiopsis leaf and crown rot and Fusarium wilt. Plants 2024, 13, 3441. [Google Scholar] [CrossRef]
- Morales-Mora, L.A.; Martínez-Salgado, S.J.; De Ita, M.A.V.; Andrade-Hoyos, P.; Silva-Rojas, H.V.; Romero-Arenas, O. First report of leaf spot and anthracnosis caused by Pestalotiopsis sp. on strawberry in Puebla, Mexico. Plant Dis. 2019, 103, 2668. [Google Scholar] [CrossRef]
- Rebollar-Alviter, A.; Silva-Rojas, H.V.; Fuentes-Aragón, D.; Acosta-González, U.; Martínez-Ruiz, M.; Parra-Robles, B.E. An emerging strawberry fungal disease associated with root rot, crown rot and leaf spot caused by Neopestalotiopsis rosae in Mexico. Plant Dis. 2020, 104, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Harishchandra, D.; Jia, J.; Zuo, Q.; Zhang, G.; Wang, Q.; Yan, J.; Zhang, W.; Li, X. Role of Neopestalotiopsis rosae in causing root rot of strawberry in Beijing, China. Crop Prot. 2021, 147, 105710. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Tsai, C.-Y.; Wu, Y.-M.; Ariyawansa, H.-A.; Chung, C.-L.; Chung, P.-C. First report of Neopestalotiopsis rosae causing leaf blight and crown rot on strawberry in Taiwan. Plant Dis. 2021, 105, 487. [Google Scholar] [CrossRef]
- Essa, T.A.; Kamel, S.M.; Ismail, A.M.; El-Ganainy, S.M. Characterization and chemical control of Neopestalotiopsis rosae the causal agent of strawberry root and crown rot in Egypt. Egypt. J. Phytopathol. 2018, 46, 1–19. [Google Scholar] [CrossRef]
- Gerardo-Lugo, S.S.; Tovar-Pedraza, J.M.; Maharachchikumbura, S.S.N.; Apodaca-Sánchez, M.A.; Correia, K.C.; Sauceda-Acosta, C.P.; Camacho-Tapia, M.; Hyde, K.D.; Marraiki, N.; Elgorban, A.M.; et al. Characterization of Neopestalotiopsis species associated with mango grey leaf spot disease in Sinaloa, Mexico. Pathogens 2020, 9, 788. [Google Scholar] [CrossRef]
- Pandey, A.K.; Sinniah, G.D.; Yadav, S.; Maharachchikumbura, S.S.N. Pestalotiopsis-like species: Host network and lifestyle on tea crop. Fungal Biol. Rev. 2024, 47, 100340. [Google Scholar] [CrossRef]
- Jevremović, D.; Vasić, T.; Živković, S.; Vasilijević, B.; Marić, M.; Vojvodić, M.; Bulajić, A. Neopestalotiopsis clavispora: A causal agent of twig dieback on highbush blueberries in Serbia. J. Plant Dis. Prot. 2022, 129, 1277–1283. [Google Scholar] [CrossRef]
- Rodríguez-Gálvez, E.; Hilário, S.; Lopes, A.; Alves, A. Diversity and pathogenicity of Lasiodiplodia and Neopestalotiopsis species associated with stem blight and dieback of blueberry plants in Peru. Eur. J. Plant Pathol. 2020, 157, 89–102. [Google Scholar] [CrossRef]
- Sarmiento-Chacón, M.; Hernández-García, V.; Rodríguez-Larramendi, L.A.; Salas-Marina, M.Á.; Ríos-Velasco, C. Neopestalotiopsis sp. and Colletotrichum karstii, causal agents of leaf spots on camedor palm (Chamaedorea quezalteca) in Mexico. Rev. Mex. Fitopatol. 2023, 41, 165–181. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Zhang, W.; Liu, M.; Maharachchikumbura, S.S.N.; Zhou, Y.; Huang, J.B.; Nilthong, S.; Wang, Z.Y.; Li, X.H.; Yan, J.Y.; et al. Identification and characterization of pestalotiopsis-like fungi related to grapevine diseases in China. Fungal Biol. 2015, 119, 348–361. [Google Scholar] [CrossRef]
- Howard, C.M.; Albregts, E.E. A strawberry fruit rot caused by Pestalotia longisetula. Phytopathology 1973, 63, 862–863. [Google Scholar] [CrossRef]
- Kenneth, R.G.; Barkai-golan, R.; Netzer, D. A Pestalotia fruit rot of strawberries in Israel. Plant Dis. Rep. 1968, 52, 472–474. [Google Scholar]
- Steyaert, R.L. Contributions à l’étude monographique de Pestalotia de Not. et Monochaetia Sacc. (Truncatella gen. nov. et Pestalotiopsis gen. nov.). Bull. Jard. Bot. Brux. 1949, 19, 285–354. [Google Scholar] [CrossRef]
- Guba, E.F. Monograph of Monochaetia and Pestalotia; Harvard University Press: Cambridge, MA, USA, 1961; p. 342. [Google Scholar]
- Maharachchikumbura, S.S.N.; Hyde, K.D.; Groenewald, J.Z.; Xu, J.; Crous, P.W. Pestalotiopsis revisited. Stud. Mycol. 2014, 79, 121–186. [Google Scholar] [CrossRef]
- MycoBank Datbase. Available online: https://www.mycobank.org/ (accessed on 30 November 2024).
- Santos, J.; Hilário, S.; Pinto, G.; Alves, A. Diversity and pathogenicity of pestalotioid fungi associated with blueberry plants in Portugal, with description of three novel species of Neopestalotiopsis. Eur. J. Plant Pathol. 2022, 162, 539–555. [Google Scholar] [CrossRef]
- Diogo, E.; Gonçalves, C.I.; Silva, A.C.; Valente, C.; Bragança, H.; Phillips, A.J.L. Five new species of Neopestalotiopsis associated with diseased Eucalyptus spp. in Portugal. Mycol. Prog. 2021, 20, 1441–1456. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, F.; Lu, Q.; Hao, X.; Zheng, M.; Wang, L.; Li, N.; Ding, C.; Wang, X.; Yang, Y. Diversity of Pestalotiopsis-like species causing gray blight disease of tea plants (Camellia sinensis) in China, including two novel Pestalotiopsis species, and analysis of their pathogenicity. Plant Dis. 2019, 103, 2548–2558. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Guo, L.D.; Cai, L.; Chukeatirote, E.; Wu, W.P.; Sun, X.; Crous, P.W.; Bhat, D.J.; McKenzie, E.H.C.; Bahkali, A.H.; et al. A multi-locus backbone tree for Pestalotiopsis, with a polyphasic characterization of 14 new species. Fungal Divers. 2012, 56, 95–129. [Google Scholar] [CrossRef]
- Belisário, R.; Aucique-Pérez, C.E.; Abreu, L.M.; Salcedo, S.S.; de Oliveira, W.M.; Furtado, G.Q. Infection by Neopestalotiopsis spp. occurs on unwounded eucalyptus leaves and is favoured by long periods of leaf wetness. Plant Pathol. 2020, 69, 194–204. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Guo, L.D.; Chukeatirote, E.; Bahkali, A.H.; Hyde, K.D. Pestalotiopsis-morphology, phylogeny, biochemistry and diversity. Fungal Divers. 2011, 50, 167–187. [Google Scholar] [CrossRef]
- Baggio, J.S.; Forcelini, B.B.; Wang, N.Y.; Ruschel, R.G.; Mertely, J.C.; Peres, N.A. Outbreak of leaf spot and fruit rot in Florida strawberry caused by Neopestalotiopsis spp. Plant Dis. 2021, 105, 305–315. [Google Scholar] [CrossRef]
- Watanabe, K.; Parbery, D.G.; Kobayashi, T.; Doi, Y. Conidial adhesion and germination of Pestalotiopsis neglecta. Mycol. Res. 2000, 104, 962–968. [Google Scholar] [CrossRef]
- Rodrigues, F.A.; Silva, I.T.; Antunes Cruz, M.F.; Carré-Missio, V. The infection process of Pestalotiopsis longisetula leaf spot on strawberry leaves. J. Phytopathol. 2014, 162, 690–692. [Google Scholar] [CrossRef]
- Zuniga, A.I.; Baggio, J.S.; Peres, N.A. A semi-selective medium to evaluate over-summering survival of Neopestalotiopsis sp. in Florida strawberry fields. Plant Dis. 2024, 108, 2096–2103. [Google Scholar] [CrossRef]
- Morales-Mora, L.A.; Andrade-Hoyos, P.; Valencia-de Ita, M.A.; Romero-Arenas, O.; Silva-Rojas, H.V.; Contreras-Paredes, C.A. Characterization of strawberry associated fungi and in vitro antagonistic effect of Trichoderma harzianum. Rev. Mex. Fitopatol. 2020, 38, 434–449. [Google Scholar] [CrossRef]
- Jiang, P.; Fu, X.; Niu, H.; Chen, S.; Liu, F.; Luo, Y.; Zhang, D.; Lei, H. Recent advances on Pestalotiopsis genus: Chemistry, biological activities, structure–activity relationship, and biosynthesis. Arch. Pharm. Res. 2023, 46, 449–499. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Liu, L.; Xiang, M.; Wang, W.; Sun, X.; Che, Y.; Guo, L.; Liu, G.; Guo, L.; et al. Genomic and transcriptomic analysis of the endophytic fungus reveals its lifestyle and high potential for synthesis of natural products. BMC Genom. 2015, 16, 28. [Google Scholar] [CrossRef]
- Guo, J.; Ren, H.; Ijaz, M.; Qi, X.; Ahmed, T.; You, Y.; Li, G.; Yu, Z.; Islam, M.S.; Ali, H.M.; et al. The completed genome sequence of Pestalotiopsis versicolor, a pathogenic ascomycete fungus with implications for bayberry production. Genomics 2023, 115, 110695. [Google Scholar] [CrossRef]
- Xie, J.; Wei, J.G.; Wang, K.W.; Luo, J.; Wu, Y.J.; Luo, J.T.; Yang, X.H.; Yang, X.B. Three phytotoxins produced by Neopestalotiopsis clavispora, the causal agent of ring spot on Kadsura coccinea. Microbiol. Res. 2020, 238, 126531. [Google Scholar] [CrossRef]
- Yang, X.L.; Zhang, J.Z.; Luo, D.Q. The taxonomy, biology and chemistry of the fungal Pestalotiopsis genus. Nat. Prod. Rep. 2012, 29, 622–641. [Google Scholar] [CrossRef]
- Espinoza, J.G.; Briceño, E.X.; Keith, L.M.; Latorre, B.A. Canker and twig dieback of blueberry caused by Pestalotiopsis spp. and a Truncatella sp. in Chile. Plant Dis. 2008, 92, 1407–1414. [Google Scholar] [CrossRef]
- Chandana, R.; Poonacha, T.T.; Chethan, D.; Karan, R.; Kruthika, R.; Khan, F.; Ashwini, K.S.; Bevanur, A.; Vani, Y.; Ramesh, G.V.; et al. Neopestalotiopsis rosae, a novel pathogen causing leaf blight and crown rot of strawberries in India. Physiol. Mol. Plant Pathol. 2024, 133, 102377. [Google Scholar] [CrossRef]
- Van Hemelrijck, W.; Ceustermans, A.; Van Campenhout, J.; Lieten, P.; Bylemans, D. crown rot in strawberry caused by Pestalotiopsis. Acta Hortic. 2017, 1156, 781–785. [Google Scholar] [CrossRef]
- Fernández-Ozuna, Y.A.; Gini Álvarez, A.R.; Lopez-Nicora, H.D.; Arrúa Alvarenga, A.A.; Colmán, A.A. First report of Neopestalotiopsis rosae causing leaf spot and crown rot on strawberry (Fragaria × ananassa) in Paraguay. New Dis. Rep. 2023, 48. [Google Scholar] [CrossRef]
- Schierling, T.E.; Voegele, R.T.; El-Hasan, A. First report on the emergence of Neopestalotiopsis rosae as a severe economic threat to strawberry production in Germany. Microorganisms 2025, 13, 6. [Google Scholar] [CrossRef]
- Hidrobo-Chavez, J.; Ramírez-Villacís, D.X.; Barriga-Medina, N.; Herrera, K.; León-Reyes, A. First report of Neopestalotiopsis mesopotamica causing root and crown rot on strawberry in Ecuador. Plant Dis. 2022, 106, 1066. [Google Scholar] [CrossRef]
- Intriago-Reyna, H.O.; Rivas-Figueroa, F.J.; Rivera-Casignia, Á.M.; Álvarez-Romero, P.I.; Ferreira, A.F.T.A.F.e. Outbreaks of crown rot in Fragaria x ananassa caused by Neopestalotiopsis mesopotamica in Ecuador. Emir. J. Food Agric. 2021, 33, 520–527. [Google Scholar] [CrossRef]
- Obregón, V.G.; Meneguzzi, N.G.; Ibañez, J.M.; Lattar, T.E.; Kirschbaum, D.S. First report of Neopestalotiopsis clavispora causing root and crown rot on strawberry plants in Argentina. Plant Dis. 2018, 102, 1856. [Google Scholar] [CrossRef]
- Park, K.; Han, I.; Lee, S.M.; Choi, S.L.; Kim, M.C.; Lee, H. Crown and root rot of strawberry caused by Neopestalotiopsis clavispora in Korea. Kor. J. Mycol. 2019, 47, 427–435. [Google Scholar] [CrossRef]
- Chamorro, M.; Aguado, A.; De los Santos, B. First report of root and crown rot caused by Pestalotiopsis clavispora (Neopestalotiopsis clavispora) on strawberry in Spain. Plant Dis. 2016, 100, 1495. [Google Scholar] [CrossRef]
- Gilardi, G.; Bergeretti, F.; Gullino, M.L.; Garibaldi, A. First report of Neopestalotiopsis clavispora causing root and crown rot on strawberry in Italy. Plant Dis. 2019, 103, 2959. [Google Scholar] [CrossRef]
- Sigillo, L.; Ruocco, M.; Gualtieri, L.; Pane, C.; Zaccardelli, M. First report of Neopestalotiopsis clavispora causing crown rot in strawberry in Italy. J. Plant Pathol. 2020, 102, 281. [Google Scholar] [CrossRef]
- Machín, A.; González, P.; Vicente, E.; Sánchez, M.; Estelda, C.; Ghelfi, J.; Silvera-Pérez, E. First report of root and crown rot caused by Neopestalotiopsis clavispora on strawberry in Uruguay. Plant Dis. 2019, 103, 2946. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, X.; Liu, Y.; Zhang, Z.; Wang, Z.; Xue, C.; Ma, Y.; Wang, F. First report of Neopestalotiopsis clavispora causing calyx and receptacle blight on strawberry in China. Plant Dis. 2022, 106, 1307. [Google Scholar] [CrossRef]
- Prematunga, C.J.; You, L.Q.; Gomdola, D.; Balasuriya, A.; Yang, Y.H.; Jayawardena, R.S.; Luo, M. An addition to pestalotioid fungi in china: Neopestalotiopsis fragariae sp. nov. causing leaf spots on Fragaria× ananassa. Asian J. Mycol. 2022, 5, 220–238. [Google Scholar] [CrossRef]
- SIAP (Servicio de Información Agroalimentaria y Pesquera Panorama Agroalimentario 2018–2024). Available online: https://www.gob.mx/siap/acciones-y-programas/panorama-agroalimentario-258035 (accessed on 15 December 2024).
- Lawrence, D.P.; Brittain, G.D.; Aglave, B.; Sances, F.V. first report of Neopestalotiopsis rosae causing crown and root rot of strawberry in California. Plant Dis. 2023, 107, 566. [Google Scholar] [CrossRef]
- Guan, W.; Bonkowski, J.; Creswell, T.; Egel, D.S. Strawberry cultivar susceptibility to Neopestalotiopsis leaf spot in Indiana. Plant Health Prog. 2023, 24, 135–139. [Google Scholar] [CrossRef]
- Madrid, A.M.J.; Munoz, G.; Collins, C.; Brannen, P. First report of the new Neopestalotiopsis species causing strawberry leaf spot and fruit rot in Georgia. Plant Dis. 2024, 108, 2574. [Google Scholar] [CrossRef]
- Rotondo, F.; Klass, T.L.; Scott, K.; McCartney, M.; Jacobs, J.M.; Lewis Ivey, M.L. First report of Neopestalotiopsis disease in Ohio caused by an emerging and novel species of Neopestalotiopsis on strawberry. Plant Dis. 2022, 107, 940. [Google Scholar] [CrossRef]
- McNally, J.; Prapagar, K.; Goldenhar, K.; Pate, E.; Shan, S.; Kalischuk, M. First report of an aggressive species of Neopestalotiopsis affecting strawberry in Canada. New Dis. Rep. 2023, 48, e12210. [Google Scholar] [CrossRef]
- Tovar-Pedraza, J.M.; Solano-Báez, A.R.; Leyva-Mir, S.G.; Tlapal-Bolaños, B.; Camacho-Tapia, M.; García-León, E.; Ayala-Escobar, V.; Nava-Díaz, C.; Quezada-Salinas, A.; Santiago-Santiago, V.; et al. The need and opportunity to update the inventory of plant pathogenic fungi and oomycetes in Mexico. J. Fungi 2024, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, Y.; Yang, Y. Pestalotiopsis diversity: Species, dispositions, secondary metabolites, and bioactivities. Molecules 2022, 27, 8088. [Google Scholar] [CrossRef]
- Yu, X.; Huo, L.; Liu, H.; Chen, L.; Wang, Y.; Zhu, X. Melanin is required for the formation of the multi-cellular conidia in the endophytic fungus Pestalotiopsis microspora. Microbiol. Res. 2015, 179, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Gelain, J.; Marin, M.V.; Peres, N.A.; Schnabel, G. Development of a molecular tool for identification of a new Neopestalotiopsis sp. associated with disease outbreaks on strawberry. Plant Dis. 2023, 107, 1544–1549. [Google Scholar] [CrossRef]
- Rebello, C.S.; Wang, N.-Y.; Marin, M.V.; Baggio, J.S.; Peres, N.A. Detection and species differentiation of Neopestalotiopsis spp. from strawberry (Fragaria × ananassa) in Florida using a high-resolution melting analysis. PhytoFrontiers 2023, 3, 156–163. [Google Scholar] [CrossRef]
- Munawar, M.A.; Toljamo, A.; Martin, F.; Oksanen, E.; Kokko, H. Development and evaluation of a recombinase polymerase amplification assay for rapid detection of strawberry red stele pathogen. Phytopathol. Res. 2020, 2, 26. [Google Scholar] [CrossRef]
- Lu, X.; Xu, H.; Song, W.; Yang, Z.; Yu, J.; Tian, Y.; Jiang, M.; Shen, D.; Dou, D. Rapid and simple detection of Phytophthora cactorum in strawberry using a coupled recombinase polymerase amplification–lateral flow strip assay. Phytopathol. Res. 2021, 3, 12. [Google Scholar] [CrossRef]
- Vielba-Fernández, A.; Dowling, M.; Schnabel, G.; Fernández-Ortuño, D. A loop-mediated isothermal amplification assay for the identification of Botrytis fragariae in strawberry. Plant Dis. 2023, 107, 3414–3421. [Google Scholar] [CrossRef]
- Alonzo, G.; Baggio, J.S.; Peres, N.A. Effect of fumigants on inoculum of Neopestalotiopsis spp. in strawberry crowns and soil. Plant Dis. 2024. [Google Scholar] [CrossRef]
- Tran, T.N.M.; Vu, N.B.D.; Nguyen, M.H. Antifungal activity of essential oil-encapsulated lipid nanoemulsions against Neopestalotiopsis rosae causing leaf spot on strawberry. J. Plant Dis. Prot. 2023, 130, 823–832. [Google Scholar] [CrossRef]
- Darapanit, A.; Boonyuen, N.; Leesutthiphonchai, W.; Nuankaew, S.; Piasai, O. Identification, pathogenicity and effects of plant extracts on Neopestalotiopsis and Pseudopestalotiopsis causing fruit diseases. Sci. Rep. 2021, 11, 22606. [Google Scholar] [CrossRef]
- Baggio, J.S.; Rebello, C.S.; de Morais, M.B.; Marin, M.V.; Gama, A.B.; Forcelini, B.B.; Mertely, J.C.; Peres, N.A. Efficacy of single- and multi-site fungicides against Neopestalotiopsis spp. of strawberry. Plant Dis. 2023, 107, 2177–2184. [Google Scholar] [CrossRef]
- Forcelini, B.B.; Seijo, T.E.; Amiri, A.; Peres, N.A. Resistance in strawberry isolates of Colletotrichum acutatum from Florida to quinone-outside inhibitor fungicides. Plant Dis. 2016, 100, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Fungicide Resistance Action Committee (FRAC). Frac List 2024 FRAC Code List©* 2024: Fungal Control Agents Sorted by Cross-Resistance Pattern and Mode of Action (Including Coding for FRAC Groups on Product Labels). Available online: https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2024.pdf (accessed on 21 January 2024).
- Acosta-González, U.; Leyva-Mir, S.G.; Silva-Rojas, H.V.; Rebollar-Alviter, A. Preventive and curative effects of treatments to manage strawberry root and crown rot caused by Neopestalotiopsis rosae. Plant Dis. 2024, 108, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Amrutha, P.; Vijayaraghavan, R. Evaluation of fungicides and biocontrol agents against Neopestalotiopsis clavispora causing leaf blight of strawberry (Fragaria × ananassa Duch.). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 622–628. [Google Scholar] [CrossRef]
- Kummanid, J.; Akimitsu, K.; Nalumpang, S. Mutations of the β-tubulin gene fragments from carbendazim-resistant isolates of Pestalotiopsis sp. causing strawberry leaf blight in Chiang Mai, Thailand. J. Phytopathol. 2017, 165, 515–521. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, J.; Chen, J.; Jun, S.; Yuan, Y.; Dai, X.; Wang, F.; Ma, Y. The biological control effect of Bacillus cereus on strawberry leaf spot disease caused by Neopestalotiopsis clavispora. Sci. Hortic. 2024, 327, 112841. [Google Scholar] [CrossRef]
- Yang, R.; Liu, P.; Ye, W.; Chen, Y.; Wei, D.; Qiao, C.; Zhou, B.; Xiao, J. Biological control of root rot of strawberry by Bacillus amyloliquefaciens strains CMS5 and CMR12. J. Fungi 2024, 10, 410. [Google Scholar] [CrossRef]
- Amil-Ruiz, F.; Blanco-Portales, R.; Muñoz-Blanco, J.; Caballero, J.L. The strawberry plant defense mechanism: A molecular review. Plant Cell Physiol. 2011, 52, 1873–1903. [Google Scholar] [CrossRef]
- Garza-Alonso, C.A.; Olivares-Sáenz, E.; González-Morales, S.; Cabrera-De la Fuente, M.; Juárez-Maldonado, A.; González-Fuentes, J.A.; Tortella, G.; Valdés-Caballero, M.V.; Benavides-Mendoza, A. Strawberry biostimulation: From mechanisms of action to plant growth and fruit quality. Plants 2022, 11, 3463. [Google Scholar] [CrossRef]
- Alam, E.; Moyer, C.; Verma, S.; Peres, N.A.; Whitaker, V.M. Exploring the genetic basis of resistance to Neopestalotiopsis species in strawberry. Plant Genome 2024, 17, e20477. [Google Scholar] [CrossRef]
- Hsu, S.Y.; Lin, Y.C.; Xu, Y.C.; Chang, H.X.; Chung, P.C.; Ariyawansa, H.A. High-quality genome assembly of Neopestalotiopsis rosae ML1664, the pathogen causing strawberry leaf blight and crown rot. Mol. Plant Microbe Interact. 2022, 35, 949–953. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).