An Effective Agrobacterium-Mediated Transient Transformation System for Studying the Lead-Tolerance Genes in Hydrangea
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Transformation and Growth Conditions of Agrobacterium
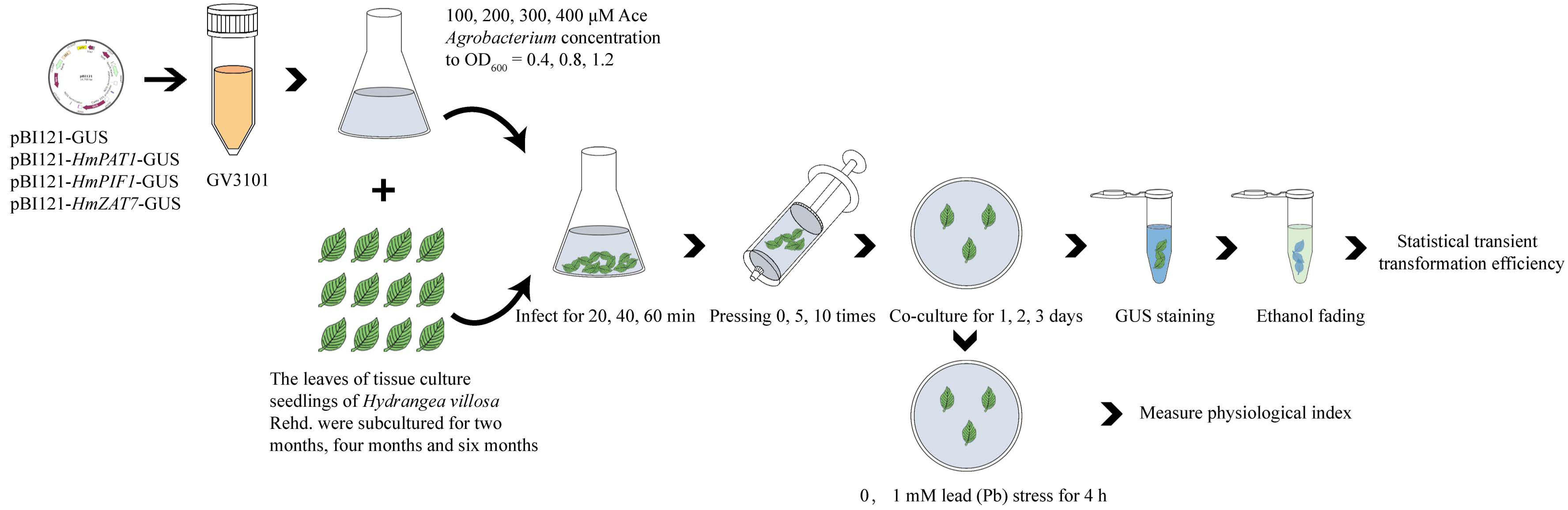
2.3. Establishing and Optimizing the Transient Transformation System
2.4. β-Glucuronidase Tissue Chemical Stain Detection of Transient Transformation Gene Expression
2.5. qRT-PCR Analysis of Pb-Tolerant Gene Screen
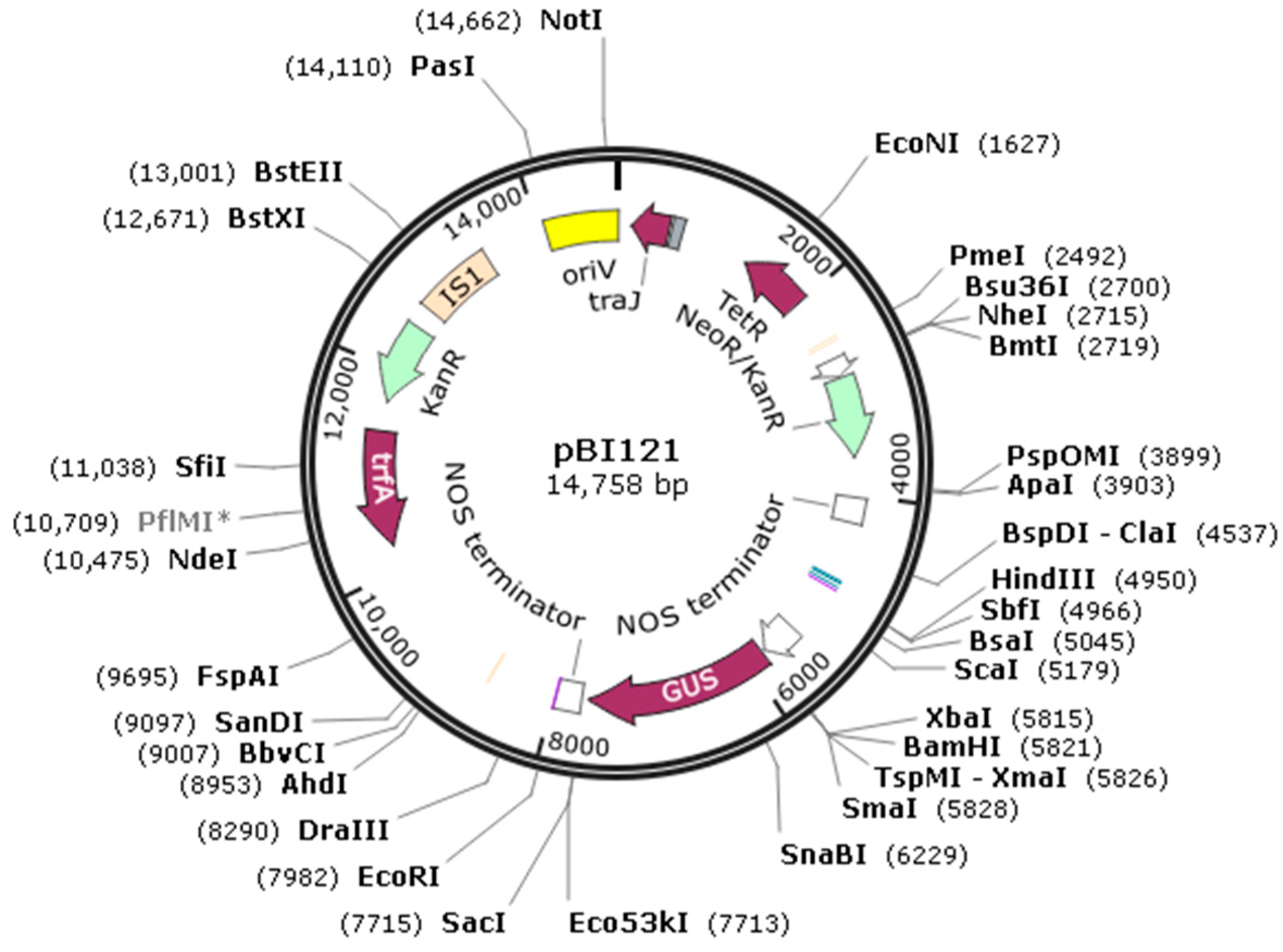
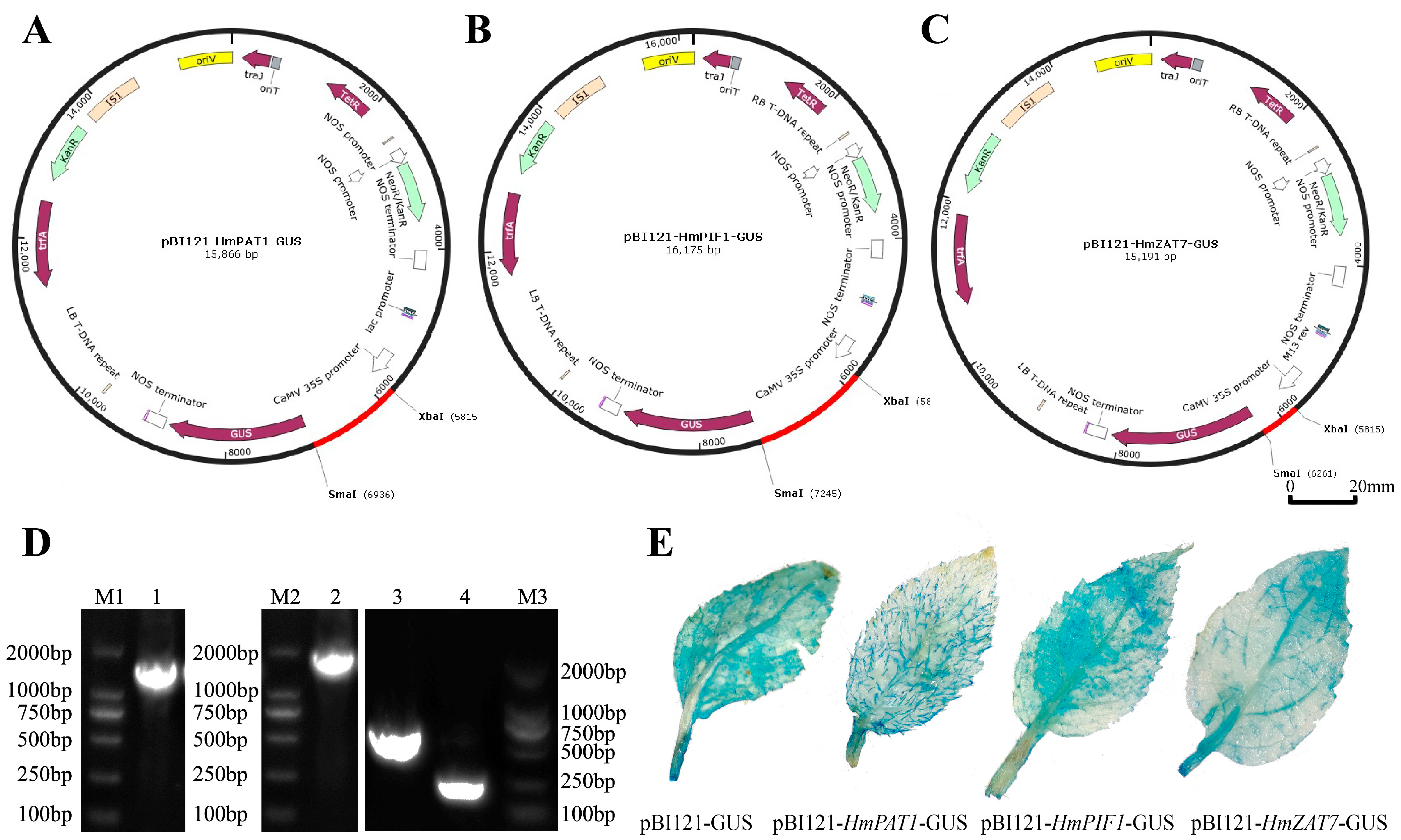
2.6. Plasmid Construction and Transient Transformation of Plant Acquisition
2.7. Pb Stess Transient Transgenic H. villosa Leaves
2.8. Transient Expression Analysis of Three Pb-Resistant Genes
2.9. Measurement of Biochemical Parameters
2.10. Statistical Analysis
3. Results
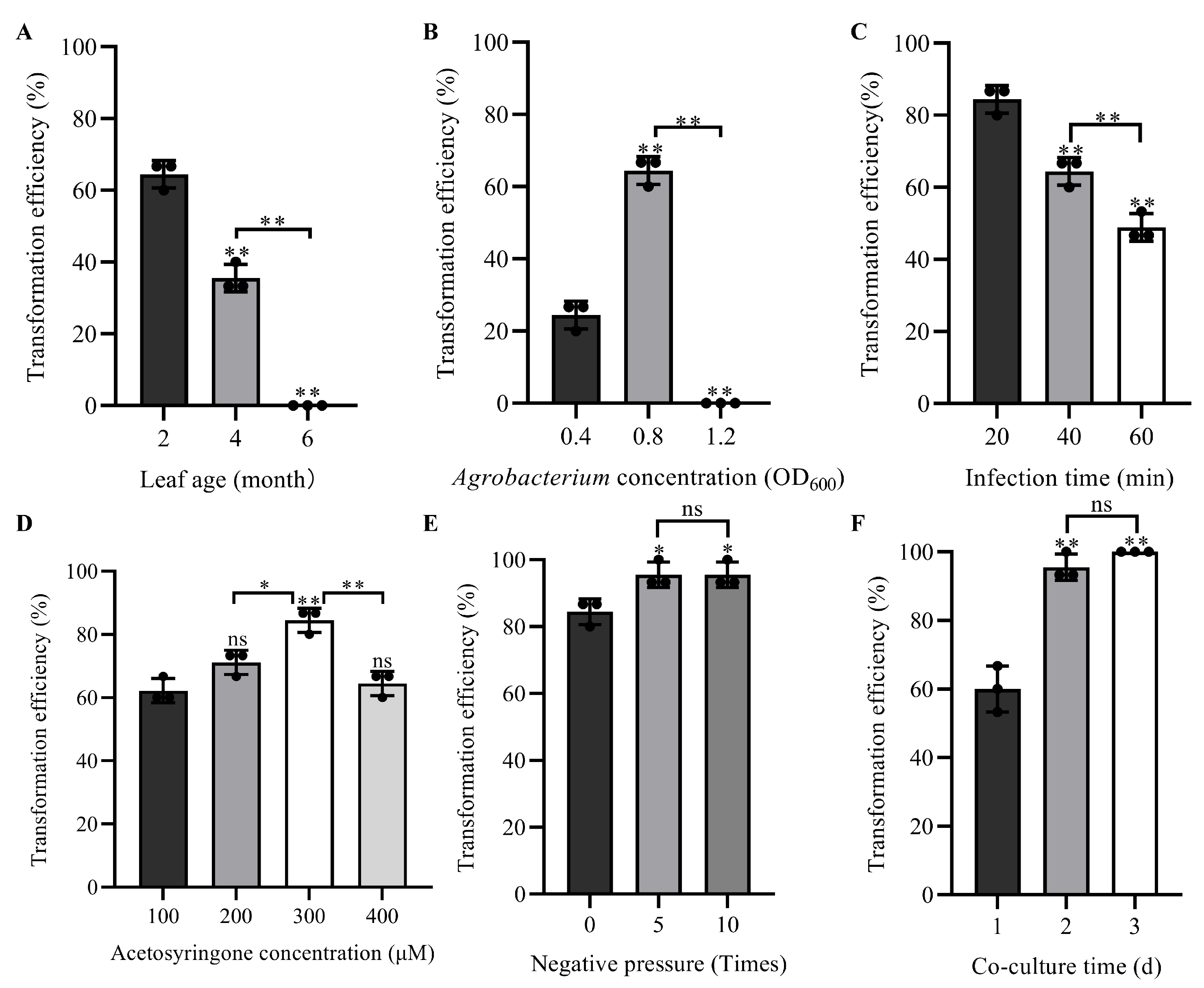
3.1. Effects of Leaf Age on Transient Transformation Efficiency of H. villosa
3.2. Effects of Agrobacterium Concentration on Transient Transformation Efficiency of H. villosa
3.3. Effects of Infection Time on Transient Transformation Efficiency of H. villosa
3.4. Effects of Ace Concentration on Transient Transformation Efficiency of H. villosa
3.5. Effects of Negative Pressure on Transient Transformation Efficiency of H. villosa
3.6. Effects of Co-Culture Time on Transient Transformation Efficiency of H. villosa
3.7. Identification of Pb Stress Resistance Genes in H. macrophylla
3.8. Transient Overexpression of Three Pb-Resistant Genes in H. villosa
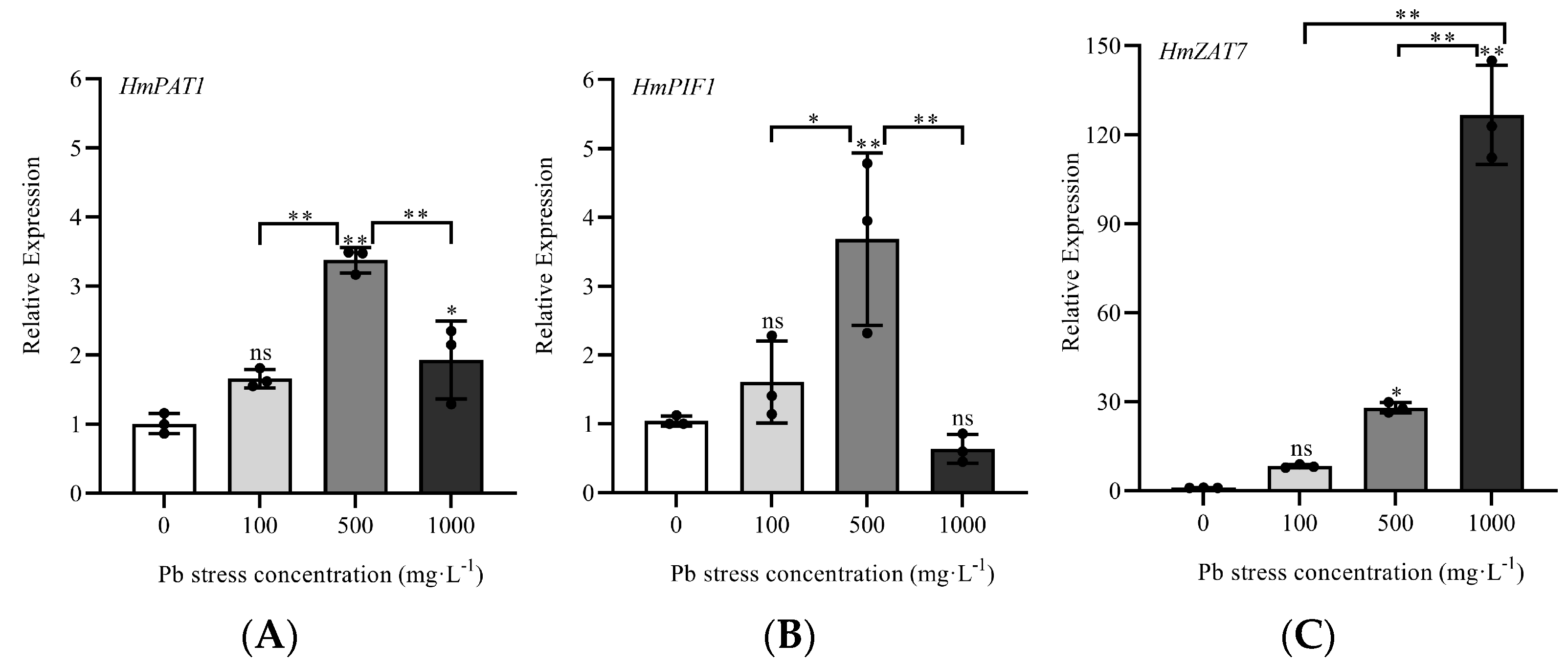
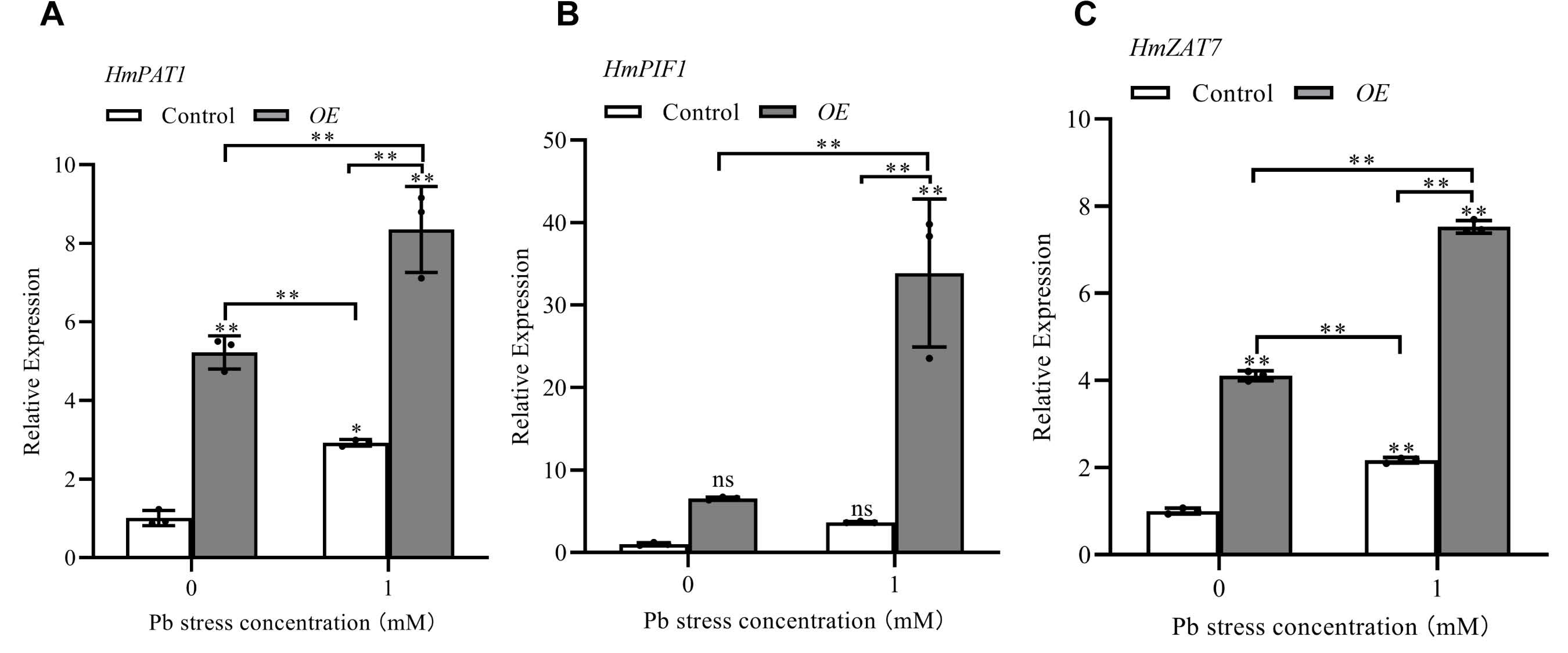
3.9. qRT-PCR Analysis of Three Pb-Resistant Genes Transiently Overexpressed in H. villosa Under Pb Stress
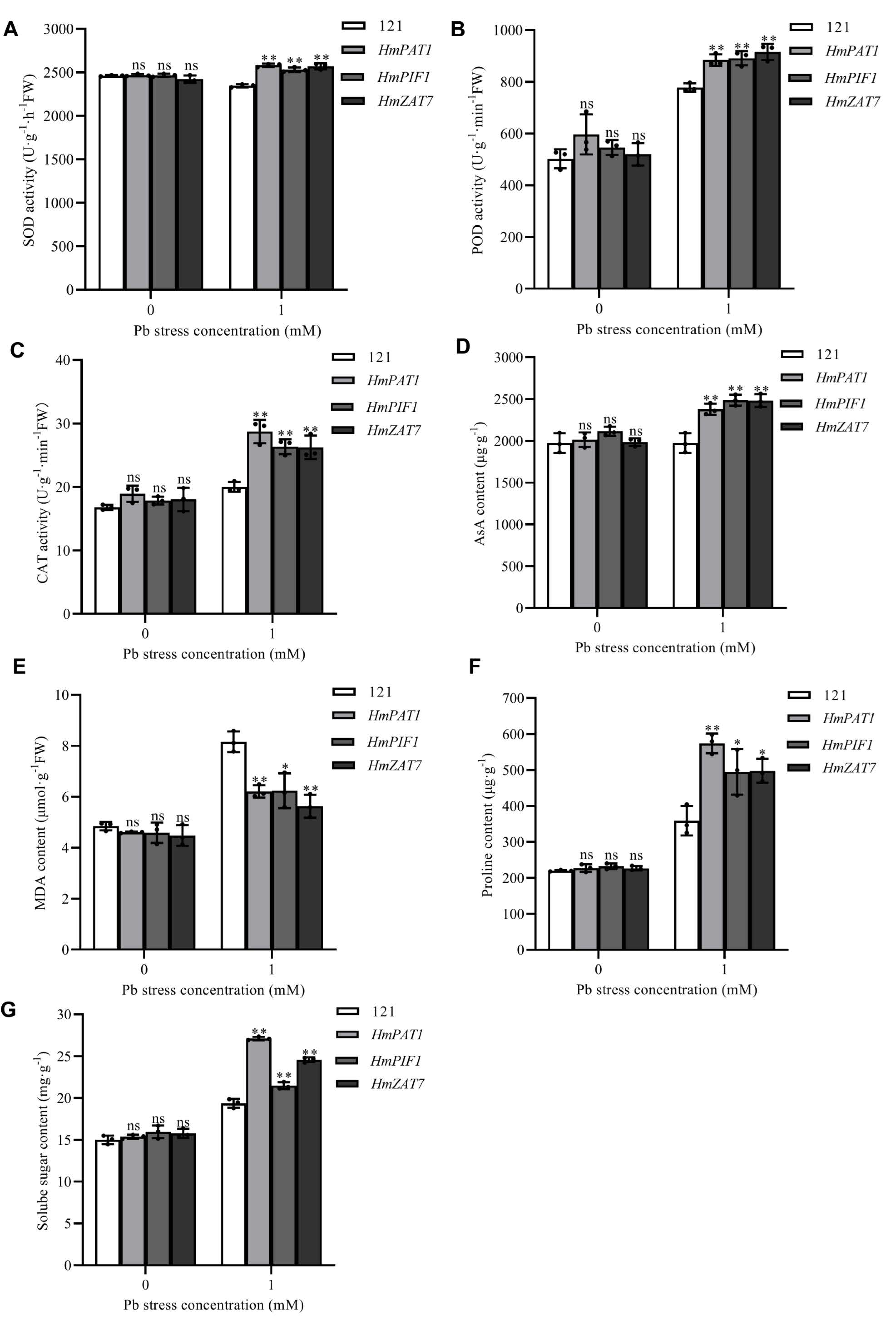
3.10. Analysis of Biochemical Parameters in H. villosa Transiently Overexpressing Three Pb-Resistant Genes Under Pb Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Yan, X.; Zhang, M.; Sun, Q.; Zhu, X. Microbial Remediation Technology for Heavy Metal Contamination of Mine Soil. Chemoecology 2024, 34, 47–59. [Google Scholar] [CrossRef]
- Su, R.K.; Xie, T.Z.; Yao, H.S.; Chen, Y.; Wang, H.; Dai, X.R.; Wang, Y.Y.; Shi, L.; Luo, Y.T. Lead Responses and Tolerance Mechanisms of Koelreuteria paniculata: A Newly Potential Plant for Sustainable Phytoremediation of Pb-Contaminated Soil. Int. J. Environ. Res. Public Health 2022, 19, 14968. [Google Scholar] [CrossRef] [PubMed]
- Zaman, W.; Ali, S.; Akhtar, M.S. Harnessing the Power of Plants: Innovative Approaches to Pollution Prevention and Mitigation. Sustainability 2024, 16, 10587. [Google Scholar] [CrossRef]
- Rocha, C.S.; Rocha, D.C.; Kochi, L.Y.; Carneiro, D.N.M.; dos Reis, M.V.; Gomes, M.P. Phytoremediation by Ornamental Plants: A Beautiful and Ecological Alternative. Environ. Sci. Pollut. Res. 2022, 29, 3336–3354. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, Y.; Zhao, B. Lead, Zinc Tolerance Mechanism and Phytoremediation Potential of Alcea rosea (Linn.) Cavan. and Hydrangea macrophylla (Thunb.) Ser. and Ethylenediaminetetraacetic Acid Effect. Environ. Sci. Pollut. Res. 2022, 29, 41329–41343. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, H.Q.; Zhao, B. Exploring the Remediation Potential of Hydrangea macrophylla (Thunb.) Ser. in Cadmium-Contaminated Soil by Comparing Cultivars and Seedling Age. Environ. Technol. Innov. 2024, 33, 103474. [Google Scholar] [CrossRef]
- Forte, J.; Mutiti, S. Phytoremediation Potential of Helianthus annuus and Hydrangea paniculata in Copper and Lead-Contaminated Soil. Water Air Soil. Pollut. 2017, 228, 77. [Google Scholar] [CrossRef]
- Chen, H.X.; Wang, D.H.; Zhu, Y.L.; Li, W.F.; Chen, J.R.; Li, Y.F. Integrative Transcriptomics and Proteomics Elucidate the Regulatory Mechanism of Hydrangea macrophylla Flower-Color Changes Induced by Exogenous Aluminum. Agronomy 2022, 12, 969. [Google Scholar] [CrossRef]
- Jin, J.; Song, Z.Y.; Zhao, B.; Zhang, Y.Y.; Wang, R.R. Physiological and Metabolomics Responses of Hydrangea macrophylla (Thunb.) Ser. and Hydrangea strigosa Rehd. to Lead Exposure. Ecotoxicol. Environ. Saf. 2022, 243, 113960. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Z.; Zhao, H.; Chen, H.; Zhao, B. Integrative Physiological, Transcriptomic and Metabolomic Analysis Reveals How the Roots of Two Ornamental Hydrangea macrophylla Cultivars Cope with Lead (Pb) Toxicity. Sci. Total Environ. 2024, 910, 168615. [Google Scholar] [CrossRef]
- Chen, C.; Lu, L.L.; Ma, S.Y.; Zhao, Y.P.; Wu, N.; Li, W.J.; Ma, L.; Kong, X.H.; Xie, Z.M.; Hou, Y.X. Analysis of PAT1 Subfamily Members in the GRAS Family of Upland Cotton and Functional Characterization of GhSCL13-2A in Verticillium dahliae Resistance. Plant Cell Rep. 2023, 42, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Wong, D.C.J.; Wang, Y.; Xu, G.Z.; Ren, C.; Liu, Y.F.; Kuang, Y.F.; Fan, P.G.; Li, S.H.; Xin, H.P.; et al. GRAS-Domain Transcription Factor PAT1 Regulates Jasmonic Acid Biosynthesis in Grape Cold Stress Response. Plant Physiol. 2021, 186, 1660–1678. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.; Lim, C.W.; Lee, S.C. GRAS-Type Transcription Factor CaGRAS1 Functions as a Positive Regulator of the Drought Response in Capsicum annuum. Environ. Exp. Bot. 2022, 198, 104853. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, M.Q.; Zhang, M.J.; Jiang, W.; Ren, X.; Liang, E.X.; Zhang, D.; Zhang, C.Q.; Xiao, N.; Li, Y.; et al. A Maize Phytochrome-interacting Factors Protein ZmPIF1 Enhances Drought Tolerance by Inducing Stomatal Closure and Improves Grain Yield in Oryza sativa. Plant Biotechnol. J. 2018, 16, 1375–1387. [Google Scholar] [CrossRef]
- Qiu, J.R.; Xiang, X.Y.; Wang, J.T.; Xu, W.X.; Chen, J.; Xiao, Y.; Jiang, C.-Z.; Huang, Z. MfPIF1 of Resurrection Plant Myrothamnus flabellifolia Plays a Positive Regulatory Role in Responding to Drought and Salinity Stresses in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 3011. [Google Scholar] [CrossRef]
- Huo, J.X. Cloning and Characterization of Stress Resistance-Related Genes IbPIF1 and IbPIF3 from Sweetpotato (Ipomoea batatas (L.) Lam.). Ph.D Thesis, China Agricultural University, Beijing, China, 2018. [Google Scholar]
- Ciftci-Yilmaz, S.; Morsy, M.R.; Song, L.; Coutu, A.; Krizek, B.A.; Lewis, M.W.; Warren, D.; Cushman, J.; Connolly, E.L.; Mittler, R. The EAR-Motif of the Cys2/His2-Type Zinc Finger Protein Zat7 Plays a Key Role in the Defense Response of Arabidopsis to Salinity Stress. J. Biol. Chem. 2007, 282, 9260–9268. [Google Scholar] [CrossRef]
- Rizhsky, L.; Davletova, S.; Liang, H.J.; Mittler, R. The Zinc Finger Protein Zat12 Is Required for Cytosolic Ascorbate Peroxidase 1 Expression during Oxidative Stress in Arabidopsis. J. Biol. Chem. 2004, 279, 11736–11743. [Google Scholar] [CrossRef]
- Ali-Benali, M.A.; Badawi, M.; Houde, Y.; Houde, M. Identification of Oxidative Stress-Responsive C2H2 Zinc Fingers Associated with Al Tolerance in near-Isogenic Wheat Lines. Plant Soil. 2013, 366, 199–212. [Google Scholar] [CrossRef]
- Krenek, P.; Samajova, O.; Luptovciak, I.; Doskocilova, A.; Komis, G.; Samaj, J. Transient Plant Transformation Mediated by Agrobacterium Tumefaciens: Principles, Methods and Applications. Biotechnol. Adv. 2015, 33, 1024–1042. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, G.F.; Meng, X.; Li, Y.B.; Wang, Y.C. A Versatile Agrobacterium-Mediated Transient Gene Expression System for Herbaceous Plants and Trees. Biochem. Genet. 2012, 50, 761–769. [Google Scholar] [CrossRef]
- Yin, X.D.; Luo, X.; Yang, F.; Wang, Y.F.; Song, L. An Effective Transient Expression System for Gene Function Identification in Lotus japonicus. Plant Cell Tiss. Organ. Cult. 2024, 156, 57. [Google Scholar] [CrossRef]
- Li, Y.; Chen, T.; Wang, W.; Liu, H.; Yan, X.; Wu-Zhang, K.; Qin, W.; Xie, L.; Zhang, Y.; Peng, B.; et al. A High-Efficiency Agrobacterium-Mediated Transient Expression System in the Leaves of Artemisia annua L. Plant Methods 2021, 17, 106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qiu, L.; Zhang, Y.; Wang, Y.; Fu, C.; Dai, S.; Sun, M. A High-Efficiency Transient Expression System Mediated by Agrobacterium Tumefaciens in Spinacia oleracea Leaves. Plant Methods 2024, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Xian, B.; Xi, Z.Q.; Ren, C.X.; Yan, J.; Chen, J.; Pei, J. The Establishment of Transient Expression Systems and Their Application for Gene Function Analysis of Flavonoid Biosynthesis in Carthamus tinctorius L. BMC Plant Biol. 2023, 23, 186. [Google Scholar] [CrossRef]
- Guan, S.X.; Kang, X.N.; Ge, J.Y.; Fei, R.W.; Duan, S.Y.; Sun, X.M. An Efficient Agrobacterium-Mediated Transient Transformation System and Its Application in Gene Function Elucidation in Paeonia lactiflora Pall. Front. Plant Sci. 2022, 13, 999433. [Google Scholar] [CrossRef]
- Wen, X.J.; Yuan, J.X.; Bozorov, T.A.; Waheed, A.; Kahar, G.; Haxim, Y.; Liu, X.J.; Huang, L.L.; Zhang, D.Y. An efficient screening system of disease-resistant genes from wild apple, Malus sieversii in response to Valsa mali pathogenic fungus. Plant Methods 2023, 19, 138. [Google Scholar] [CrossRef]
- Han, X.; Rong, H.; Feng, Y.N.; Xin, Y.; Luan, X.Y.; Zhou, Q.; Xu, M.; Xu, L. Protoplast Isolation and Transient Transformation System for Ginkgo biloba L. Front. Plant Sci. 2023, 14, 1145754. [Google Scholar] [CrossRef]
- Huang, L.L. Study on the Establishment of Regeneration System and Genetic Transformation System of Hydrangea macrophylla. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2010. [Google Scholar]
- Hamama, L.; Voisine, L.; Peltier, D.; Boccon-Gibod, J. Shoot Regeneration and Genetic Transformation by Agrobacterium tumefaciens of Hydrangea macrophylla Ser. Leaf Discs. Sci. Hortic. 2011, 127, 378–387. [Google Scholar] [CrossRef]
- Negishi, T.; Oshima, K.; Hattori, M.; Kanai, M.; Mano, S.; Nishimura, M.; Yoshida, K. Tonoplast- and Plasma Membrane-Localized Aquaporin-Family Transporters in Blue Hydrangea Sepals of Aluminum Hyperaccumulating Plant. PLoS ONE 2012, 7, e43189. [Google Scholar] [CrossRef]
- Qin, B.; Fan, S.-L.; Yu, H.-Y.; Lu, Y.-X.; Wang, L.-F. HbMYB44, a Rubber Tree MYB Transcription Factor with Versatile Functions in Modulating Multiple Phytohormone Signaling and Abiotic Stress Responses. Front. Plant Sci. 2022, 13, 893896. [Google Scholar] [CrossRef]
- Azizi-Dargahlou, S.; Pouresmaeil, M. Agrobacterium Tumefaciens-Mediated Plant Transformation: A Review. Mol. Biotechnol. 2024, 66, 1563–1580. [Google Scholar] [CrossRef] [PubMed]
- Lao, X.; Jin, P.; Yang, R.; Liang, Y.; Zhang, D.; Zeng, Y.; Li, X. Establishment of Agrobacterium-Mediated Transient Transformation System in Desert Legume Eremosparton songoricum (Litv.) Vass. Int. J. Mol. Sci. 2024, 25, 11934. [Google Scholar] [CrossRef] [PubMed]
- Swackhammer, A.; Provencher, E.A.P.; Donkor, A.K.; Garofalo, J.; Dowling, S.; Garchitorena, K.; Phyo, A.; Ramírez Veliz, N.; Karen, M.; Kwon, A.; et al. Mechanistic Analysis of the VirA Sensor Kinase in Agrobacterium Tumefaciens Using Structural Models. Front. Microbiol. 2022, 13, 898785. [Google Scholar] [CrossRef]
- Toh, W.K.; Loo, E.P.-I.; Tee, C.S.; Loh, P.C.; Wong, H.L. Development of Acetosyringone-Inducible Gateway® and Golden Gate Expression Vectors for Heterologous Gene Expression in Agrobacterium tumefaciens. In Vitr. Cell. Dev. Biol.-Plant 2020, 56, 578–587. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Hu, R.; Yang, L.; Zuo, Z.J. Establishment of a Transient Transformation Protocol in Cinnamomum camphora. Forests 2023, 14, 1872. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, B.; Li, Y. Research on Tissue Culture and Rapid Propagation Techniques of Two Wild Hydrangea Species. Seed 2020, 39, 60–63, 70. [Google Scholar] [CrossRef]
- Li, G.; Song, P.L.; Wang, X.; Ma, Q.C.; Zhang, H.X.; Zhang, Y.X.; Xu, J.F.; Qi, B.X. Establishment of Agrobacterium tumefaciens Mediated Transient Transformation System in Young Leaves of Duli pear (Pyrus betulifolia). J. Fruit Sci. 2021, 38, 2006–2013. [Google Scholar] [CrossRef]
- An, W.; Zhang, Y.; Yuan, H. A System Establishment of Transient Expression on Iris foetidissima L. Mol. Plant Breed. 2020, 18, 6359–6363. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hu, B.; Shao, S.; Ni, H.; Fu, Z.Y.; Hu, L.; Zhou, Y.; Min, X.X.; She, S.F.; Chen, S.C.; Huang, M.X.; et al. Current Status, Spatial Features, Health Risks, and Potential Driving Factors of Soil Heavy Metal Pollution in China at Province Level. Environ. Pollut. 2020, 266, 114961. [Google Scholar] [CrossRef]
- Miao, R.Q.; Zang, W.; Yuan, Y.; Zhang, Y.; Zhang, A.; Pang, Q.Y. The Halophyte Gene ScVTC2 Confers Resistance to Oxidative Stress via AsA-Mediated Photosynthetic Enhancement. Plant Physiol. Biochem. 2021, 169, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Manuka, R.; Saddhe, A.A.; Srivastava, A.K.; Kumar, K.; Penna, S. Overexpression of Rice OsWNK9 Promotes Arsenite Tolerance in Transgenic Arabidopsis Plants. J. Biotechnol. 2021, 332, 114–125. [Google Scholar] [CrossRef]
- Meng, L.D.; Yang, Y.P.; Ma, Z.W.; Jiang, J.W.; Zhang, X.M.; Chen, Z.R.; Cui, G.; Yin, X.J. Integrated Physiological, Transcriptomic and Metabolomic Analysis of the Response of Trifolium pratense L. to Pb Toxicity. J. Hazard. Mater. 2022, 436, 129128. [Google Scholar] [CrossRef]
- Ye, L.; Liao, T.C.; Deng, X.; Long, H.; Liu, G.; Ke, W.T.; Huang, K.Y. Establishment of an RNA-Based Transient Expression System in the Green Alga Chlamydomonas reinhardtii. New Biotechnol. 2024, 83, 175–187. [Google Scholar] [CrossRef]
- Xiao, Y.X.; Zhang, J.; Li, Y.T.; Hsiang, T.; Zhang, X.P.; Zhu, Y.X.; Du, X.Y.; Yin, J.; Li, J.K. An Efficient Overexpression Method for Studying Genes in Ricinus That Transport Vectorized Agrochemicals. Plant Methods 2022, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Wixom, A.Q.; Casavant, N.C.; Kuhl, J.C.; Xiao, F.; Dandurand, L.-M.; Caplan, A.B. Solanum sisymbriifolium Plants Become More Recalcitrant to Agrobacterium Transfection as They Age. Physiol. Mol. Plant Pathol. 2018, 102, 209–218. [Google Scholar] [CrossRef]
- Zheng, L.; Yang, J.X.; Chen, Y.J.; Ding, L.P.; Wei, J.H.; Wang, H.Z. An Improved and Efficient Method of Agrobacterium Syringe Infiltration for Transient Transformation and Its Application in the Elucidation of Gene Function in Poplar. BMC Plant Biol. 2021, 21, 54. [Google Scholar] [CrossRef]
- Kaur, M.; Manchanda, P.; Kalia, A.; Ahmed, F.K.; Nepovimova, E.; Kuca, K.; Abd-Elsalam, K.A. Agroinfiltration Mediated Scalable Transient Gene Expression in Genome Edited Crop Plants. Int. J. Mol. Sci. 2021, 22, 10882. [Google Scholar] [CrossRef]
- Missaghi, M.; Yari, F.; Mousavi, A.; Mostofi, Y.; Ofoghi, H. Optimisation of Agrobacterium-Mediated Transformation in Saintpaulia ionantha H. Wendl Using Response Surface Methodology (RSM). N. Z. J. Crop Hortic. Sci. 2024, 53, 1–12. [Google Scholar] [CrossRef]
- Mohammad, T.; Ghogare, R.; Morton, L.B.; Dhingra, A.; Potlakayala, S.; Rudrabhatla, S.; Dhir, S.K. Evaluation of Parameters Affecting Agrobacterium-Mediated Transient Gene Expression in Industrial Hemp (Cannabis sativa L.). Plants 2024, 13, 664. [Google Scholar] [CrossRef]
- Liu, S.W.; Ma, J.J.; Liu, H.M.; Guo, Y.T.; Li, W.; Niu, S.H. An Efficient System for Agrobacterium-Mediated Transient Transformation in Pinus tabuliformis. Plant Methods 2020, 16, 52. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Chen, J.B.; Huang, J.; Liu, F.; Guo, R.; Yang, L.; Grabon, A.; Zhao, K.; Kong, F.L.; et al. Agrobacterium tumefaciens-Mediated Transformation System for the Important Medicinal Plant Dendrobium catenatum Lindl. In Vitr. Cell. Dev. Biol.-Plant 2018, 54, 228–239. [Google Scholar] [CrossRef]
- Li, H.; Guo, L.H.; Yan, M.L.; Hu, J.; Lin, Q.Q.; Wang, P.; Wang, M.L.; Zhao, H.; Wang, Y.; Ni, D.J.; et al. A Rapid and Efficient Transient Expression System for Gene Function and Subcellular Localization Studies in the Tea Plant (Camellia sinensis) Leaves. Sci. Hortic. 2022, 297, 110927. [Google Scholar] [CrossRef]
- Acanda, Y.; Welker, S.; Orbović, V.; Levy, A. A Simple and Efficient Agroinfiltration Method for Transient Gene Expression in Citrus. Plant Cell Rep. 2021, 40, 1171–1179. [Google Scholar] [CrossRef]
- Wang, B.Y.; Jin, G.Y.; Huo, C.H.; Chang, C.; Yu, F. Establishment of Agrobacterium-mediated transient transformation system of Camptotheca acuminata leaves. Mol. Plant Breed. 2018, 16, 5624–5630. [Google Scholar] [CrossRef]
- Li, L.; Gu, H.; Yue, Y.Z.; Yang, X.L.; Wang, L.G. Advances in Transient Transformation Systems of Woody Plants. Mol. Plant Breed. 2020, 18, 7784–7794. [Google Scholar] [CrossRef]
- Xie, Q.J.; Wang, D.N.; Ding, Y.T.; Gao, W.S.; Li, J.H.; Cao, C.W.; Sun, L.L.; Liu, Z.Y.; Gao, C.Q. The Ethylene Response Factor Gene, ThDRE1A, Is Involved in Abscisic Acid- and Ethylene-Mediated Cadmium Accumulation in Tamarix hispida. Sci. Total Environ. 2024, 937, 173422. [Google Scholar] [CrossRef]
- Karle, S.B.; Kumar, K. Rice Tonoplast Intrinsic Protein Member OsTIP1;2 Confers Tolerance to Arsenite Stress. J. Hazard. Mater. 2024, 465, 133078. [Google Scholar] [CrossRef]
- Liu, P.; An, L.J.; Ma, L.; Zou, L.J.; Du, S.Z.; Shen, Y. MTP Family Analysis and Association Study Reveal the Role of ZmMTP11 in Lead (Pb) Accumulation. Plant Physiol. Biochem. 2024, 212, 108740. [Google Scholar] [CrossRef]
- He, S.; An, R.; Yan, J.; Zhang, C.; Zhang, N.; Xi, N.; Yu, H.; Zou, C.; Gao, S.; Yuan, G.; et al. Association Studies of Genes in a Pb Response-Associated Network in Maize (Zea mays L.) Reveal That ZmPIP2;5 Is Involved in Pb Tolerance. Plant Physiol. Biochem. 2023, 195, 300–309. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, R.; Shi, L.; Zhao, B. An Effective Agrobacterium-Mediated Transient Transformation System for Studying the Lead-Tolerance Genes in Hydrangea. Horticulturae 2025, 11, 320. https://doi.org/10.3390/horticulturae11030320
Cong R, Shi L, Zhao B. An Effective Agrobacterium-Mediated Transient Transformation System for Studying the Lead-Tolerance Genes in Hydrangea. Horticulturae. 2025; 11(3):320. https://doi.org/10.3390/horticulturae11030320
Chicago/Turabian StyleCong, Rong, Liang Shi, and Bing Zhao. 2025. "An Effective Agrobacterium-Mediated Transient Transformation System for Studying the Lead-Tolerance Genes in Hydrangea" Horticulturae 11, no. 3: 320. https://doi.org/10.3390/horticulturae11030320
APA StyleCong, R., Shi, L., & Zhao, B. (2025). An Effective Agrobacterium-Mediated Transient Transformation System for Studying the Lead-Tolerance Genes in Hydrangea. Horticulturae, 11(3), 320. https://doi.org/10.3390/horticulturae11030320





