Influence of Pre-Harvest Factors on the Storage of Fresh Basil (Ocimum basilicum L.): A Review
Abstract
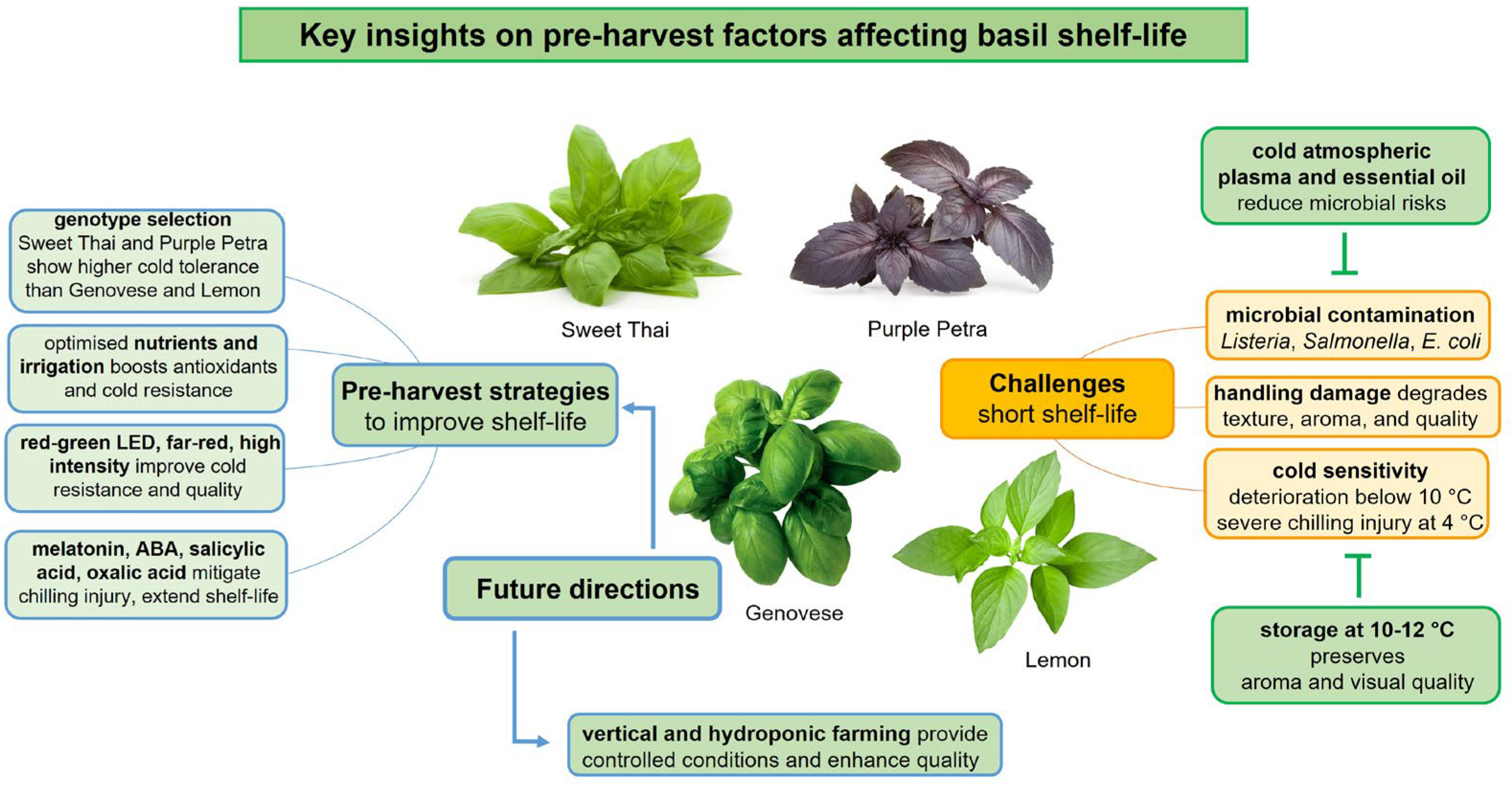
:1. Introduction
2. Genetic Material
3. Nutrition and Irrigation Management
4. Application of Chemical Substances
5. Light Management
6. Microbiological Safety
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Santos, J.; Herrero, M.; Mendiola, J.; Oliva-Teles, M.; Ibáñez, E.; Delerue-Matos, C.; Oliveira, M. Fresh-cut aromatic herbs: Nutritional quality stability during shelf-life. LWT-Food Sci. Technol. 2014, 59, 101–107. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic potential of phenolic compounds in medicinal plants—Natural health products for human health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Spence, C. Sweet basil: An increasingly popular culinary herb. Int. J. Gastron. Food Sci. 2024, 36, 100927. [Google Scholar] [CrossRef]
- Carvalho, S.D.; Schwieterman, M.L.; Abrahan, C.E.; Colquhoun, T.A.; Folta, K.M. Light quality dependent changes in morphology, antioxidant capacity, and volatile production in sweet basil (Ocimum basilicum). Front. Plant Sci. 2016, 7, 1328. [Google Scholar] [CrossRef]
- Maggio, A.; Roscigno, G.; Bruno, M.; De Falco, E.; Senatore, F. Essential-oil variability in a collection of Ocimum basilicum L. (Basil) cultivars. Chem. Biodivers. 2016, 13, 1357–1368. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; El-Nakhel, C.; Kyriacou, M.C.; Soteriou, G.A.; Pizzolongo, F.; Romano, R.; De Pascale, S.; Rouphael, Y. Genotype and successive harvests interaction affects phenolic acids and aroma profile of genovese basil for pesto sauce production. Foods 2021, 10, 278. [Google Scholar] [CrossRef]
- Camlica, M.; Yaldiz, G. Basil (Ocimum basilicum L.): Botany, genetic resource, cultivation, conservation, and stress factors. In Sustainable Agriculture in the Era of the OMICs Revolution; Springer: Berlin/Heidelberg, Germany, 2023; pp. 135–163. [Google Scholar]
- Farneti, B.; Alarcón, A.A.; Papasotiriou, F.G.; Samudrala, D.; Cristescu, S.M.; Costa, G.; Harren, F.J.; Woltering, E.J. Chilling-induced changes in aroma volatile profiles in tomato. Food Bioprocess Technol. 2015, 8, 1442–1454. [Google Scholar] [CrossRef]
- Mulaosmanovic, E.; Lindblom, T.; Windstam, S.; Bengtsson, M.; Rosberg, A.K.; Mogren, L.; Alsanius, B. Processing of leafy vegetables matters: Damage and microbial community structure from field to bag. Food Control. 2021, 125, 107894. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 2017, 64, 23–38. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Brindisi, L.J.; Simon, J.E. Preharvest and postharvest techniques that optimize the shelf life of fresh basil (Ocimum basilicum L.): A review. Front. Plant Sci. 2023, 14, 1237577. [Google Scholar] [CrossRef]
- Cozzolino, R.; Pace, B.; Cefola, M.; Martignetti, A.; Stocchero, M.; Fratianni, F.; Nazzaro, F.; De Giulio, B. Assessment of volatile profile as potential marker of chilling injury of basil leaves during postharvest storage. Food Chem. 2016, 213, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Głuchowski, A.; Czarniecka-Skubina, E.; Tambor, K.; Jariené, E. Fresh Basil Infusion: Effect of Sous-Vide Heat Treatment on Their Volatile Composition Profile, Sensory Profile, and Color. Molecules 2021, 27, 5. [Google Scholar] [CrossRef] [PubMed]
- Rodeo, A.J.D.; Mitcham, E.J. Basil postharvest chilling sensitivity is modulated by the dynamics between antioxidant enzymes and metabolites. Postharvest Biol. Technol. 2024, 211, 112805. [Google Scholar] [CrossRef]
- Larsen, D.H.; Marcelis, L.F.; van Kempen, D.; Kohlen, W.; Nicole, C.C.; Woltering, E.J. Far-red light during cultivation improves postharvest chilling tolerance in basil. Postharvest Biol. Technol. 2023, 198, 112232. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Maalekuu, K.; Elkind, Y.; Leikin-Frenkel, A.; Lurie, S.; Fallik, E. The relationship between water loss, lipid content, membrane integrity and LOX activity in ripe pepper fruit after storage. Postharvest Biol. Technol. 2006, 42, 248–255. [Google Scholar] [CrossRef]
- Wongsheree, T.; Ketsa, S.; van Doorn, W.G. The relationship between chilling injury and membrane damage in lemon basil (Ocimum × citriodourum) leaves. Postharvest Biol. Technol. 2009, 51, 91–96. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Caleb, O.J.; Gil, M.I.; Izumi, H.; Colelli, G.; Watkins, C.B.; Zude, M. Quality and safety of fresh horticultural commodities: Recent advances and future perspectives. Food Packag. Shelf Life 2017, 14, 2–11. [Google Scholar] [CrossRef]
- Mashabela, M.; Mahajan, P.V.; Sivakumar, D. Influence of different types of modified atmosphere packaging films and storage time on quality and bioactive compounds in fresh-cut cauliflower. Food Packag. Shelf Life 2019, 22, 100374. [Google Scholar] [CrossRef]
- Jalali, A.; Linke, M.; Geyer, M.; Mahajan, P.V. Shelf life prediction model for strawberry based on respiration and transpiration processes. Food Packag. Shelf Life 2020, 25, 100525. [Google Scholar] [CrossRef]
- Li, L.; Lv, F.-Y.; Guo, Y.-Y.; Wang, Z.-Q. Respiratory pathway metabolism and energy metabolism associated with senescence in postharvest Broccoli (Brassica oleracea L. var. italica) florets in response to O2/CO2 controlled atmospheres. Postharvest Biol. Technol. 2016, 111, 330–336. [Google Scholar] [CrossRef]
- Silva, F.d.; Santos, R.H.S.; Andrade, N.J.d.; Barbosa, L.C.A.; Casali, V.W.D.; Lima, R.R.d.; Passarinho, R.V.d.M. Basil conservation affected by cropping season, harvest time and storage period. Pesqui. Agropecuária Bras. 2005, 40, 323–328. [Google Scholar] [CrossRef]
- Delbeke, S.; Ceuppens, S.; Jacxsens, L.; Uyttendaele, M. Microbiological analysis of pre-packed sweet basil (Ocimum basilicum) and coriander (Coriandrum sativum) leaves for the presence of Salmonella spp. and Shiga toxin-producing E. coli. Int. J. Food Microbiol. 2015, 208, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, C.; Fava, P.; Pulvirenti, A.; Antonelli, A.; Licciardello, F. Domestic use simulation and secondary shelf life assessment of industrial Pesto alla genovese. Foods 2021, 10, 1948. [Google Scholar] [CrossRef]
- Göbel, C.; Langen, N.; Blumenthal, A.; Teitscheid, P.; Ritter, G. Cutting food waste through cooperation along the food supply chain. Sustainability 2015, 7, 1429–1445. [Google Scholar] [CrossRef]
- Ciriello, M.; Cirillo, V.; Formisano, L.; El-Nakhel, C.; Pannico, A.; De Pascale, S.; Rouphael, Y. Productive, morpho-physiological, and postharvest performance of six basil types grown in a floating raft system: A comparative study. Plants 2023, 12, 486. [Google Scholar] [CrossRef]
- Kays, S.J. Postharvest Physiology and Handling of Perishable Plant Products; Van Nostrand Reinhold Company: New York, NY, USA, 1991. [Google Scholar]
- Campos, P.S.; Quartin, V.N.; Ramalho, J.C.; Nunes, M.A. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves ofCoffea sp. plants. J. Plant Physiol. 2003, 160, 283–292. [Google Scholar] [CrossRef]
- West, C. Terpene biosynthesis and metabolism. In Plant Physiology Biochemistry and Molecular Biology; Dennis, D.T., Turpin, D.H., Eds.; Longman Scientific & Technical: London, UK, 1990; pp. 353–369. [Google Scholar]
- Cantwell, M.I.; Reid, M.S. Postharvest physiology and handling of fresh culinary herbs. J. Herbs Spices Med. Plants 1993, 1, 93–127. [Google Scholar] [CrossRef]
- Fratianni, F.; Cefola, M.; Pace, B.; Cozzolino, R.; De Giulio, B.; Cozzolino, A.; d’Acierno, A.; Coppola, R.; Logrieco, A.F.; Nazzaro, F. Changes in visual quality, physiological and biochemical parameters assessed during the postharvest storage at chilling or non-chilling temperatures of three sweet basil (Ocimum basilicum L.) cultivars. Food Chem. 2017, 229, 752–760. [Google Scholar] [CrossRef]
- López-Blancas, E.; Martínez-Damián, M.T.; Colinas-León, M.T.; Martínez Solís, J.; Rodríguez-Pérez, J.E. Basil’Nufar’ (Ocimum basilicum L.) post-harvest quality under refrigeration. Rev. Chapingo Ser. Hortic. 2014, 20, 187–200. [Google Scholar] [CrossRef]
- IS Mahlangu, R.; Maboko, M.M.; Mudau, F.N.; Amoo, S.O. Nitrogen levels, plant density and postharvest storage duration affect phytochemical and antioxidant properties of field-grown basil and rocket crops. Int. J. Veg. Sci. 2021, 27, 515–525. [Google Scholar] [CrossRef]
- Li, Y.; Heckman, J.; Wyenandt, A.; Mattson, N.; Durner, E.; Both, A. Potential benefits of silicon nutrition to hydroponically grown sweet basil. HortScience 2020, 55, 1799–1803. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium enrichment enhances the quality and shelf life of basil leaves. Plants 2020, 9, 801. [Google Scholar] [CrossRef]
- Bekhradi, F.; Delshad, M.; Marín, A.; Luna, M.C.; Garrido, Y.; Kashi, A.; Babalar, M.; Gil, M.I. Effects of salt stress on physiological and postharvest quality characteristics of different Iranian genotypes of basil. Hortic. Environ. Biotechnol. 2015, 56, 777–785. [Google Scholar] [CrossRef]
- Jordán, M.J.; Quílez, M.; Luna, M.C.; Bekhradi, F.; Sotomayor, J.A.; Sánchez-Gómez, P.; Gil, M.I. Influence of water stress and storage time on preservation of the fresh volatile profile of three basil genotypes. Food Chem. 2017, 221, 169–177. [Google Scholar] [CrossRef]
- Luna, M.C.; Bekhradi, F.; Ferreres, F.; Jordan, M.J.; Delshad, M.; Gil, M.I. Effect of water stress and storage time on anthocyanins and other phenolics of different genotypes of fresh sweet basil. J. Agric. Food Chem. 2015, 63, 9223–9231. [Google Scholar] [CrossRef]
- Suamuang, N.; Poolpukdee, A.; Herkhuntod, W.; Pholpakdee, R.; Intarated, S.; Supapvanich, S. Chilling injury alleviation of holy basil by preharvest salicylic acid and oxalic acid application. In Proceedings of the Burapha University International Conference, Pattaya, Thailand, 28–29 July 2016; pp. 158–164. [Google Scholar]
- Supapvanich, S.; Phonpakdee, R.; Wongsuwan, P. Chilling injury alleviation and quality maintenance of lemon basil by preharvest salicylic acid treatment. Emir. J. Food Agric. 2015, 27, 801–807. [Google Scholar] [CrossRef]
- Jensen, N.B.; Clausen, M.R.; Kjaer, K.H. Spectral quality of supplemental LED grow light permanently alters stomatal functioning and chilling tolerance in basil (Ocimum basilicum L.). Sci. Hortic. 2018, 227, 38–47. [Google Scholar] [CrossRef]
- Tan, T.; Li, S.; Fan, Y.; Wang, Z.; Raza, M.A.; Shafiq, I.; Wang, B.; Wu, X.; Yong, T.; Wang, X. Far-red light: A regulator of plant morphology and photosynthetic capacity. Crop J. 2022, 10, 300–309. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; Kyriacou, M.C.; Carillo, P.; Scognamiglio, L.; De Pascale, S.; Rouphael, Y. Morpho-physiological and biochemical responses of hydroponically grown basil cultivars to salt stress. Antioxidants 2022, 11, 2207. [Google Scholar] [CrossRef] [PubMed]
- Larsen, D.H.; Li, H.; van de Peppel, A.C.; Nicole, C.C.; Marcelis, L.F.; Woltering, E.J. High light intensity at End-Of-Production improves the nutritional value of basil but does not affect postharvest chilling tolerance. Food Chem. 2022, 369, 130913. [Google Scholar] [CrossRef] [PubMed]
- Albornoz, K.; Rosas, R.; López, M.D. Influence of a Preharvest Melatonin Application on Postharvest Chilling Injury in Basil (Ocimum basilicum L.). J. Hortic. Res. 2023, 31, 2. [Google Scholar]
- Satpute, A.; Meyering, B.; Albrecht, U. Preharvest abscisic acid application to alleviate chilling injury of sweet basil (Ocimum basilicum L.) during cold storage. HortScience 2019, 54, 155–161. [Google Scholar] [CrossRef]
- Sipos, L.; Balázs, L.; Székely, G.; Jung, A.; Sárosi, S.; Radácsi, P.; Csambalik, L. Optimization of basil (Ocimum basilicum L.) production in LED light environments—A review. Sci. Hortic. 2021, 289, 110486. [Google Scholar] [CrossRef]
- Verdaguer, D.; Jansen, M.A.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Kokkinou, I.; Ntoulas, N.; Nektarios, P.A.; Varela, D. Response of native aromatic and medicinal plant species to water stress on adaptive green roof systems. HortScience 2016, 51, 608–614. [Google Scholar] [CrossRef]
- Mahajan, M.; Kuiry, R.; Pal, P.K. Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100255. [Google Scholar] [CrossRef]
- Bekhradi, F.; Luna, M.; Delshad, M.; Jordan, M.; Sotomayor, J.; Martínez-Conesa, C.; Gil, M. Effect of deficit irrigation on the postharvest quality of different genotypes of basil including purple and green Iranian cultivars and a Genovese variety. Postharvest Biol. Technol. 2015, 100, 127–135. [Google Scholar] [CrossRef]
- Supapvanich, S.; Promyou, S. Efficiency of salicylic acid application on postharvest perishable crops. Salicylic Acid Plant Growth Dev. 2013, 339–355. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Wanichananan, P.; Janta, S.; Toojinda, T.; Darwell, C.T.; Mosaleeyanon, K. The influence of different light spectra on physiological responses, antioxidant capacity and chemical compositions in two holy basil cultivars. Sci. Rep. 2022, 12, 588. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, L.; Stanojević, J.; Milenković, L.; Šunić, L.; Kovač, R.; Cvetković, D.; Babić, M.; Ilić, Z. Aroma profile and antioxidant activity of sweet basil aqueous extracts affect by light modification. J. Essent. Oil Bear. Plants 2022, 25, 1131–1144. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.-O. Spectral effects of artificial light on plant physiology and secondary metabolism: A review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Larsen, D.H.; Li, H.; Shrestha, S.; Verdonk, J.C.; Nicole, C.C.; Marcelis, L.F.; Woltering, E.J. Lack of blue light regulation of antioxidants and chilling tolerance in Basil. Front. Plant Sci. 2022, 13, 852654. [Google Scholar] [CrossRef]
- Patel, M.; Lee, R.; Merchant, E.V.; Juliani, H.R.; Simon, J.E.; Tepper, B.J. Descriptive aroma profiles of fresh sweet basil cultivars (Ocimum spp.): Relationship to volatile chemical composition. J. Food Sci. 2021, 86, 3228–3239. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Larsen, D.H.; Woltering, E.J.; Nicole, C.C.; Marcelis, L.F. Response of basil growth and morphology to light intensity and spectrum in a vertical farm. Front. Plant Sci. 2020, 11, 597906. [Google Scholar] [CrossRef]
- Monaghan, J.; Augustin, J.; Bassett, J.; Betts, R.; Pourkomailian, B.; Zwietering, M. Risk assessment or assessment of risk? Developing an evidence-based approach for primary producers of leafy vegetables to assess and manage microbial risks. J. Food Prot. 2017, 80, 725–733. [Google Scholar] [CrossRef]
- Yang, S.-C.; Lin, C.-H.; Aljuffali, I.A.; Fang, J.-Y. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef]
- Gil, M.I.; Selma, M.V.; Suslow, T.; Jacxsens, L.; Uyttendaele, M.; Allende, A. Pre-and postharvest preventive measures and intervention strategies to control microbial food safety hazards of fresh leafy vegetables. Crit. Rev. Food Sci. Nutr. 2015, 55, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Chitarra, W.; Decastelli, L.; Garibaldi, A.; Gullino, M.L. Potential uptake of Escherichia coli O157: H7 and Listeria monocytogenes from growth substrate into leaves of salad plants and basil grown in soil irrigated with contaminated water. Int. J. Food Microbiol. 2014, 189, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-Eckman, A.R.; Gao, M.; Blaustein, R.A.; Tikekar, R.V. Cold atmospheric plasma treatment induces oxidative stress and alters microbial community profile in the leaves of sweet basil (Ocimum basilicum var. Kiera) plant. J. Food Sci. 2025, 90, e70066. [Google Scholar] [CrossRef]
- Xylia, P.; Chrysargyris, A.; Tzortzakis, N. The Postharvest Safety and Quality of Fresh Basil as Affected by the Use of Cypriot Oregano (Origanum dubium) Extracts. Horticulturae 2024, 10, 159. [Google Scholar] [CrossRef]
| Species/Cultivar | Treatment | Effects | References |
|---|---|---|---|
| Ocimum basilicum L. | Sub-optimal nitrogen application (60 vs. 120 kg ha−1) | Increased phenolic content, enhanced storability with economic benefits | [35] |
| Genovese basil | Integration of silicon (75 ppm) in nutrient solution | Reduced cold damage without affecting morphology | [36] |
| Genovese basil (cv. Tigullio) | Selenium biofortification (4 mg L−1 sodium selenate) | Reduced ethylene production, increased antioxidant compounds (e.g., rosmarinic acid) | [37] |
| Iranian green basil | Reduced irrigation (up to 25% of field capacity) | Maintained visual appearance during storage | [38] |
| Genovese basil (cv. Dolly) | Controlled water stress (75% and 50% of field capacity) | Depletion of aromatic profile in some cultivars, increased phenolic acids | [39,40] |
| Ocimum tenuiflorum L. (Holy basil) | Exogenous application of salicylic acid (5 mM) | Delayed degradation of phenolic compounds, improved antioxidant capacity | [41] |
| Lemon basil (Ocimum × citriodorum) | Pre-harvest application of salicylic acid (5 mM) | Reduced chilling injury, delayed electrolyte loss, and chlorophyll degradation | [42] |
| Sweet basil | LED lighting with red and green light (80:20 ratio) | Improved shelf-life, enhanced tolerance to low temperatures (6 °C) | [43] |
| Sweet basil | Far-red light integration (730 nm) | Increased cold tolerance during storage, enhanced photosynthetic and morphological traits | [44] |
| (Ocimum basilicum L. var. thyrsiflora and Cinnamon; Ocimum × citriodorum) | Saline stress (60 mM NaCl) in hydroponics | Reduced leaf browning and increased phenolic acid content during storage | [45] |
| Sweet basil | Increased light intensity (600 μmol m−2 s−1) | Improved fresh biomass, dry matter content, and visual quality during cold storage | [46] |
| Genovese basil | Pre-harvest application of melatonin (400 µM) | Reduced cold damage, preserved visual quality and colorimetric characteristics | [47] |
| Genovese basil (cv. Tigullio) | Pre-harvest application of abscisic acid (1000–1500 mg L−1) | Improved shelf-life, reduced chilling injury, and lower electrolyte loss at low temperatures | [48] |
| Holy basil (Ocimum tenuiflorum L.) | Exogenous application of oxalic acid (5 mM) | Reduced chilling injury, delayed lipid degradation, and improved antioxidant capacity | [41] |
| Lemon basil (Ocimum × citriodorum) | Pre-harvest application of salicylic acid (5 mM) | Delayed loss of electrolytes, reduced chilling injury, and preserved chlorophyll pigments | [42] |
| Sweet basil | Red and blue light supplementation in vertical farming | Enhanced production of secondary metabolites, improved post-harvest quality | [49,50] |
| Sweet basil | Integration of UV-A light in light management | Increased synthesis of phenolic acids, flavonoids, and pigments, linked to shelf-life improvement | [50,51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciriello, M.; Carillo, P.; Lentini, M.; Rouphael, Y. Influence of Pre-Harvest Factors on the Storage of Fresh Basil (Ocimum basilicum L.): A Review. Horticulturae 2025, 11, 326. https://doi.org/10.3390/horticulturae11030326
Ciriello M, Carillo P, Lentini M, Rouphael Y. Influence of Pre-Harvest Factors on the Storage of Fresh Basil (Ocimum basilicum L.): A Review. Horticulturae. 2025; 11(3):326. https://doi.org/10.3390/horticulturae11030326
Chicago/Turabian StyleCiriello, Michele, Petronia Carillo, Matteo Lentini, and Youssef Rouphael. 2025. "Influence of Pre-Harvest Factors on the Storage of Fresh Basil (Ocimum basilicum L.): A Review" Horticulturae 11, no. 3: 326. https://doi.org/10.3390/horticulturae11030326
APA StyleCiriello, M., Carillo, P., Lentini, M., & Rouphael, Y. (2025). Influence of Pre-Harvest Factors on the Storage of Fresh Basil (Ocimum basilicum L.): A Review. Horticulturae, 11(3), 326. https://doi.org/10.3390/horticulturae11030326








