Abstract
Organic agriculture has few tools against pests and diseases and is constantly looking for effective and sustainable products such as geomaterials, i.e., zeolite. This study evaluates the physiological and morphological responses of olive plants (Olea europaea) to foliar applications of different geo-materials, specifically kaolin, natural zeolite, and ammonium-enriched zeolite. The research examines leaf anatomical modifications, including internal tissue structures, trichome and stomatal density, chlorophyll content, and gas exchange parameters, alongside the impact on fruit development and extra virgin olive oil (EVOO) quality. Results indicate that kaolin application negatively influenced transpiration and stomatal conductance, an effect corroborated by increased xylem vessel wall thickness. However, the reduction in stomatal conductance was attributed to a functional rather than morphological adaptation, as no significant changes in stomatal density or size were observed. Both geo-material treatments altered leaf surface properties, particularly peltate trichome characteristics. Notably, ammonium-enriched zeolite application enhanced photosynthetic rate during early olive development, likely due to its nutritional role, and contributed to increased fruit size and oil yield. These findings highlight the potential of geo-material-based foliar treatments as an effective strategy to optimize plant physiological performance and improve olive oil production in sustainable agricultural systems.
1. Introduction
Climate change has also led, in olive growing, to the introduction of new diseases that can compromise the overall quality of production [1,2,3]; at the same time, the ban imposed by the European Community for the use of some chemical products (i.e., the recent ban of dimethoate by the Reg EU2019/1090 [4]) has forced the olive growers to adopt alternative products for the control of olive diseases. Moreover, both in the national and international scenarios, olive growers and consumers are becoming increasingly aware and attentive to the production and consumption of organic extra virgin olive oil (EVOO). Due to climate change, in recent years winter temperatures (usually low in olive-growing areas in northern Italy) have been higher than seasonal averages, and rainfall events are characterized by high intensity and variable frequency [5]. This situation has led to an increase in the incidence of some olive tree diseases, mainly related to fungal, bacterial, and insect attacks. The events just described have led olive growers to change their cultivation methods, moving mainly towards eco-friendly products like biostimulants, rock dusts, natural plant extracts, biochar, etc. [6,7,8,9].
As a result of the situation described above, olive fruit fly (Bactrocera oleae Rossi) attacks are much more frequent in olive cultivation but also attacks by fungi such as peacock’s eye (Spilocea oleaginea Castagne) or bacteria such as olive knot (Pseudomonas savastanoi). Many olive growers (both organic and IPM) have started using foliar application products based on kaolin or zeolite to cope with this emergency.
The spraying of “rock dust” (e.g., kaolin) as a foliar treatment in olive growing to reduce the negative impact of environmental stresses and to protect fruits from insect pests is a well-established approach [6]. At present, the effects of these treatments on plant physiology (e.g., canopy temperatures, gas exchanges with the atmosphere, metabolic processes, growth yield, and production quality) are not yet fully known.
The effect of the application of these products depends on several factors, such as, applied concentration [10], plant species and cultivar [11], environmental conditions [12,13,14], dimension of the canopy and plant age [12,15], and number of years of continuous treatment [16].
Recent evidence has revealed an added benefit of kaolin in the degradation of ozone and increased surface area in the particle film to provide a habitat for microbial populations, which may further facilitate ozone degradation on plant surface [17].
Similarly, natural zeolites can be used both as particle films for crop protection [18] and as corroborants for plants. Morrone and collaborators [8] showed the efficiency of zeolite-based foliar treatments on olive fruit fly control (Bactrocera oleae Rossi) and the positive effect of the use of zeolite enriched with nitrogen to increase the photosynthetic rate and fruit size.
Moreover, Rotondi et al. [19] carried out trials on olive trees treated with kaolin and zeolite and found that the intercellular CO2 concentration was positively influenced by zeolite application, while kaolin application decreased photosynthesis activity, stomatal conductance and transpiration rates.
Compared to kaolin, zeolites can potentially also be also used: as carriers for nutrients to promote nutrient use efficiency and, as a result, the chemical composition of olive fruits and olive oils [20] as carriers for pesticide and antagonistic microorganisms for pesticide reduction and disease control [21].
Microfighter project results demonstrate the efficiency of a new natural and environmentally sustainable Zeo-Biopesticide, composed of Italian natural Chabasite zeolites and a specific mBCA (Pseudomonas sp. DLS65) as an alternative to copper-based products for the control of olive knot disease in IPM farms in Italy [22,23].
Although kaolin has been studied in a large number of fruit tree species, the plant-induced changes are still not fully understood. In addition, there are few recorded results concerning different crops and under different stress prevalence or intensities [13]. Kaolin may counteract the effect of water shortage and high irradiation on leaf sclerophylly and structural traits; in fact it provides changes in leaf internal tissues and on leaf surface properties [14]. Therefore, the adoption of alternative, eco-friendly treatments like kaolin and zeolite-based foliar sprays presents an opportunity to mitigate these economic risks and enhance the sustainability of olive farming. By reducing reliance on synthetic pesticides and promoting integrated pest management strategies, growers can minimize environmental impacts and meet consumer demand for organic products [24]. Few works have studied the effect of different geomaterial-based foliar treatments on physiological plant parameters, still fewer studies have analyzed the anatomical changes in leaves after different applications of rock dusts.
The multiple aims of the work are to evaluate the effect of kaolin and zeolite on olive leaves, in addition, considering the carrier potentiality of zeolites, the effect of NH4+-enriched zeolite compared to a natural zeolite was also evaluated. A multidisciplinary approach was adopted in order to identify leaf anatomical adaptations and leaf physiology, olive fruits and extra virgin olive oil quality derived from the treated plants were also evaluated. To achieve the objectives, the two experimental trials were conducted independently in two different olive orchards in the province of Bologna (Italy).
2. Materials and Methods
2.1. Experimental Design
The study was carried out on olive orchards (Olea europaea L. “Correggiolo”) located in two different areas of Emilia-Romagna Region in Italy:
- Bologna site (44°31′28.9″ N 11°20′21.1″ E; 54 m ASL), in this site 25 trees for each thesis were treated with three different foliar application: Kaolin (K-B) in water solution at a dosage of 3.0 kg/hL of H2O; Natural Zeolite (NZ-B) at a dosage of 0.6 kg/hL of H2O; Test (T-B) where olive trees were not treated with any compound.
- San Lazzaro di Savena site (44°27′00″ N, 11°23′33″ E; 216 m ASL in Bologna province), in this olive orchard 25 plants for each thesis were treated with three different foliar applications: Natural Zeolite (NZ-SL) dissolved in water at a dosage of 0.6 kg/hL of H2O; Zeolite Enriched with ammonium (EZ-SL) dissolved in water at a dosage of 0.6 kg/hL of H2O; Test (T-SL) where olive trees were not treated with any compound.
The natural zeolite and kaolin were supplied by Balco S.p.A company (Sassuolo, MO, Italy) and its mineralogical composition is reported in Galamini et al. [25]. The zeolite used in the EZ treatment was previously enriched with NH4+ prepared with the procedure reported in Morrone et al. [8]. The applications were carried out using a portable sprayer (flow max 50 L/min, capacity 300 L; G.R. Gamberini, Bologna, Italy). Foliar applications began at the end of flowering/fruit set stage (phenological growth stage N.69 according to BBCH-identification keys of olive tree [26]) and were subsequently repeated every 20 days, with a total of six applications until harvest. The foliar treatments applied during the experiment were conducted over a period of three growing seasons in both sites (Bologna (B) and San Lazzaro di Savena (SL)). Olive trees under study were twenty years old, not irrigated and managed with the same agronomic techniques.
2.2. Leaf Samples Collection
Five plants were selected for each treatment and from each plant, healthy and fully expanded leaves were collected; leaf samples were taken from the medial part of one-year-old shoots exposed to the western sun.
For the evaluation of the effect of kaolin and zeolite (Bologna-site-B) on external and internal leaf anatomical properties, leaf colour and chlorophyll content, leaf samples were taken at the end of flowering/fruit set stage and at the beginning of fruit colouring stage (respectively, at phenological growth stage N.69 and N.81) of the third year.
For the evaluation of the nutritional effect of NH4+-enriched zeolite (San Lazzaro site-SL), leaf samples were taken only at the beginning of fruit colouring stage (phenological growth stage N.81).
2.3. Anatomical Analyses
2.3.1. Leaves’ Morphology Analyses
To determine the leaf morphology, leaf length, leaf width, leaf perimeter, leaf area and length/width ratio, measurements were carried out on twenty healthy leaves in triplicates. The leaves were positioned underneath a transparent plexiglass plane in order to avoid perspective deformations, they were then photographed by a camera that was mounted on a tripod, finally, the leaves were analyzed and measured by a video image analysis software (Leica Application Suite—Leica—Hamburg, Germany).
2.3.2. Light Microscopy Analysis
Ten olive leaf samples subjected to each different treatments were placed in a FAA solution (in the following concentrations: formalin, acetic acid 60%, ethanol solution 2:1:17 v/v) (Merk KGaA, Darmstadt, Germany) [27]. Subsequently, the samples were dehydrated by immersing them in solutions with increasing concentrations of alcohol (Merk KGaA, Darmstadt, Germany). In the last step, the inclusion was made in a methacrylate resin (Technovit 7100, Heraeus Kulzer & Co., Wehrheim, Germany). The samples were cut with transversal cuts obtaining sections with a thickness of 3 µm using a microtome Leitz (Wetzlar, Germany). The sections were stained with PAS (Bio-Optica, Milan, Italy) -Amido Black (Sigma-Aldrich, St. Louis, Mo, USA) following the methodology proposed by Ruzin [27]; subsequently stained sections were observed with a Leica DM 4000 optical microscope (Leica Imaging Systems Ltd., Wetzlar, Germany) equipped with a Leica DC 100 digital camera. For each treatment, five replicates were made.
2.3.3. ESEM Analysis
The environmental scanning electron microscope (ESEM) provides a highly relevant and controllable environment in which to study hydrated systems without the artefacts of other highly prepared specimens [28]. In high pressure conditions, very wet non-conductive samples can be observed free of charging artefacts without a conductive coating covering their surface [29]. For each treatment three leaves were collected, a small area (10 mm2) of the central part (both upper and lower side) of the leaves was mounted on aluminum stubs; in order to see the stomata clearly, the trichome layers of the lower side were removed using adhesive tape. Leaves were observed by ESEM (Zeiss, EVO LS 10, Oberkochen, Germany) and the ultrastructural parameters were evaluated on both abaxial and adaxial leaf side. Fifteen images of the upper and lower side of each leaf of ESEM analysis were evaluated by a video image analysis software (Leica Application Suite—Leica—Hamburg, Germany).
Both on the upper and lower side of the leaves, trichome density and trichome size were evaluated. On the lower side peltate trichome density was also determined by counting of trichome stalks remaining after the trichome layers removal.
In addition, on the abaxial surface, the stomatal density, stomatal width, and stomatal length were measured in order to provide a comprehensive dataset for further analysis.
All parameters were calculated in micrometres (µm) and stomatal density (stoma/mm2) was determined by proportioning it to 1 mm2.
2.4. Ecophysiological Measurements, Chlorophyll’s Content and Colour Leaf Measurements
Leaf gas exchange measurements: photosynthesis (A), stomatal conductance (g), intercellular CO2 concentration (Ci) and transpiration rate (E) were measured using a LiCor portable photosynthesis system (LiCor 6400 Nebr., Lincoln, NE, USA) operating at 400 µmol m−2 s−1 flow rate. For each treatment twenty five leaves were measured in the morning (10:00 a.m. to 12:00 a.m.), according to the protocols of Denaxa et al. [11] and Jifon and Syvertsen [30], on twenty-four undamaged, sun-exposed mature leaves collected from the central part of the one-year-old shoot, according to Larbi et al. [31].
Chlorophyll was extracted according to Arnon et al. [32] from 0.3 g of leaves grinded under liquid nitrogen using 30 mL of ethanol at 95% (Merk KGaA, Darmstadt, Germany). Measurements were made at 663 nm and 645 nm via spectrophotometer (Jasco V-500 Spectrophotometer, Tokyo, Japan).
For each treatment leaves’ colour was recorded at three random points of the adaxial surface of each of twenty leaves using Konica Minolta CR-400 Chroma Meter (Konica Minolta, Inc., Osaka, Japan) calibrated with a standard white plate at room temperature. The data collected are L* (lightness) and a* (red-green scale).
2.5. Fruit Weight, Oil Content and Olive Oil Quality
2.5.1. Fruit Weight and Oil Content
Fruit fresh weights trends were monitored in samples consisting of 100 olives per thesis, collected every 7 days, from the 21 July to olive harvest (October).
Olive fruits were collected at fruit colouring stage (phenological growth stage N.85) 50 fruits were weighted and then milled using a grinder (IKA MF 10 basic Microfine grinder drive, Breisgau, Germany) and oil quantity was gravimetrically determined on 6 g of olive paste using Randall extraction method [33] on a SER 148 Solvent Extractor (Velp Scientifica, Milan, Italy). The extraction was carried on dried olive paste over a 30 min period, with thimbles immersed in boiling hexane (Merk KGaA, Darmstadt, Germany) (69 °C) and 30 min of reflux washing, where the sample is removed from liquid hexane and fat content absorption continues only by reflux; finally hexane is collected and then dried to determine gravimetrically oil content. The oil content was expressed as percent of dry matter (W/W). Analysis of fruit oil content was carried out in triplicate.
2.5.2. Olive Oil Production
Olive samples were obtained harvesting five plants for each thesis. Olives, processed within 24 h from the harvest, were defoliated, washed, and milled using a low-scale continuous mill (Oliomio®; Toscana Enologica Mori, Firenze, Italy) equipped with a blade crusher, a horizontal malaxator and a two-phase decanter.
For each sample, the technological settings, such as temperature (below 27 °C), time of malaxation (20 min), speed of the decanter (4200 rpm) and flux of water in the separator (0.8 L h−1) were standardized and kept identical to minimize variability due to extraction procedures. Oil samples were filtered through cotton filters, poured into dark glass bottles, keeping the headspace to a minimum, and stored in a temperature-controlled cupboard set at 15 ± 1 °C until analysis.
2.5.3. Chemical Analysis of EVOO (Extra Virgin Olive Oil)
Free acidity were determined by titration using sodium hydroxide solution (Merck KGaA, Darmstadt, Germany); peroxide value were determined by titrating with sodium thiosulphate (VWR International Srl, Milan, Italy) a solution of oil in acetic acid and chloroform treated with potassium iodide (Merk KGaA, Darmstadt, Germany) and UV-spectrophotometric indices (K232, K270) were determined dissolving oil sample in iso-octane Merck KGaA, Darmstadt, Germany) and measure the adsorbance at 232 nm and 268 nm using a Jasco Spectrophotometer (V-500, Tokyo, Japan) following the official methodologies) [34].
The phenolic fraction was extracted in triplicate on 8 g of oil sample using 8 mL of a methanol/water (60:40, v:v) solution [35]. The Folin–Ciocalteu reagent assay was used for the determination of total phenol content. Briefly on 0.1 mL of oil sample extract 0.5 mL of Folin–Ciocalteu reagent and 2 mL of saturated solution of Na2CO3 was added, the mixture was then incubated in the dark for 60 min and the absorbance was measured at 750 nm [36] using a Jasco Spectrophotometer (V-500, Tokyo, Japan). The results were expressed as equivalents of gallic acid. All analysis were carried out in three replicates.
2.5.4. Sensory Analysis of EVOO
Sensory analyses were carried out by an analytical taste panel recognized by the International Olive Oil Council (IOOC) of Madrid and by the Italian Ministry of Agriculture, Food Sovereignty and Forests.
The panel evaluated all oil samples following an incomplete randomized block design. Olive oil samples were placed in blue tasting glasses, with a temperature of 15–18 °C. A panel test was established using a standard profile sheet (IOOC/T20) modified by IBE-CNR, following Rotondi et al. [37].
The tasters evaluated direct or retro nasal aromatic olfactory sensations (olive fruity, green/leaf and secondary positive flavours), gustatory sensations (olive fruity, bitterness and secondary positive flavours) and tactile/kinaesthetic sensation (pungency). The tasters had to rate the intensity of the different descriptors on a continuous 0–10 cm scale. Median of sensory data and robust standard deviations were calculated.
2.6. Statistical Analysis
The data collected were elaborated using Microsoft® Excel 2007/XLSTAT© (Version 2009.3.02, Addinsoft, Inc., Brooklyn, NY, USA). The significant differences among means at a 5% level were determined by ANOVA followed by Tukey’s Honestly Significant Difference (HSD) test.
3. Results and Discussion
3.1. Bologna Site
3.1.1. Anatomical Analyses
Leaves’ Morphology Analyses
Leaves from untreated trees and those treated with NZ and K were measured to assess whether the treatment could alter their size. The analysis revealed that after three years of treatment, the leaves maintained the same size and there were no significant differences between the samples. Specifically, the average leaf size was 707.69 mm2 for untreated (T-B) leaves, 696.45 mm2 for leaves treated with natural zeolite (NZ-B), and 669.33 mm2 for leaves treated with kaolin (K-B), as is shown in Table A1—Appendix A.
Light Microscopy Analysis
From an anatomical point of view, olive leaves have been studied for their structural characteristics (epidermal tissue thickness, palisade tissue thickness, and spongy tissue thickness). Additionally, differences in the conduction system (xylem cells) have been investigated. In this context, to better understand transport capacity, measurements were taken of the maximum diameter of the vessels in the vascular bundles and the thickness of the walls of xylem cells (Table 1 and Figure 1). Analyzing the size of the leaves treated with natural zeolite and with kaolin, no significant differences were observed compared to test leaves (no treatments): these results were also observed in the leaf thickness, in fact no differences were found in the thickness of epidermal, spongy and palisade parenchyma cells, as shown in Table 1. With regard to xylem measures, main vein diameter was higher in NZ-B (Natural Zeolite)-treated leaves while their wall thickness was higher in K-B (Kaolin)-treated leaves, as shown in Figure 1.

Table 1.
Anatomical parameters of leaf derived by olive trees untreated (T-B) and treated with different geomaterial: Natural Zeolite (NT-B) and Kaolin (K-B).
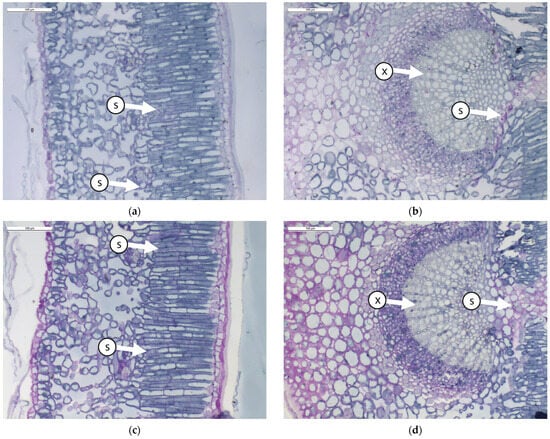
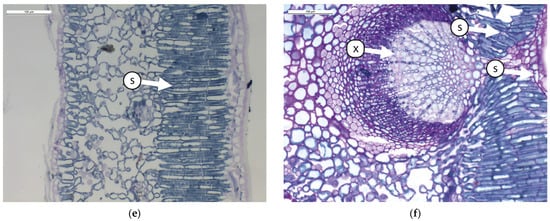
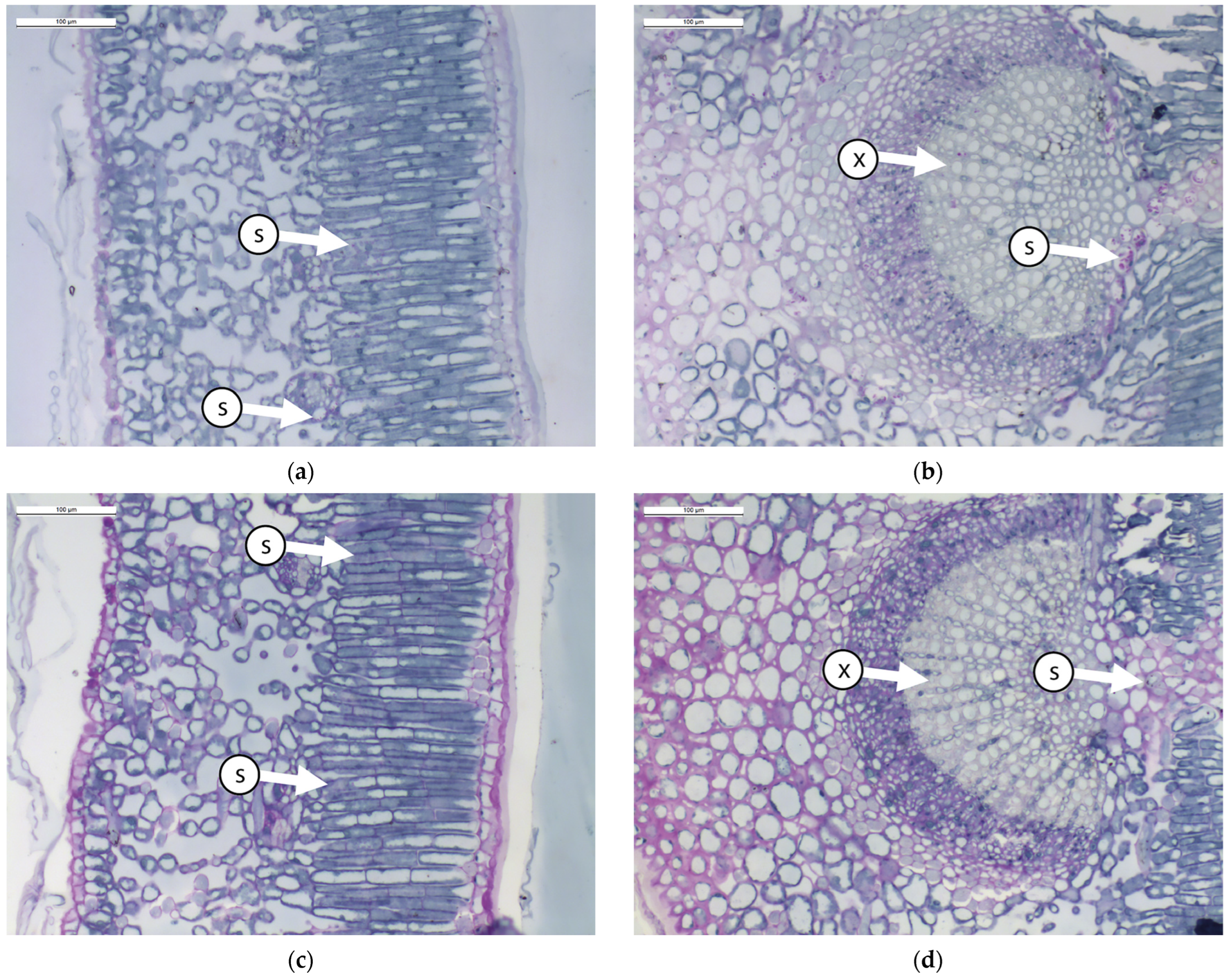
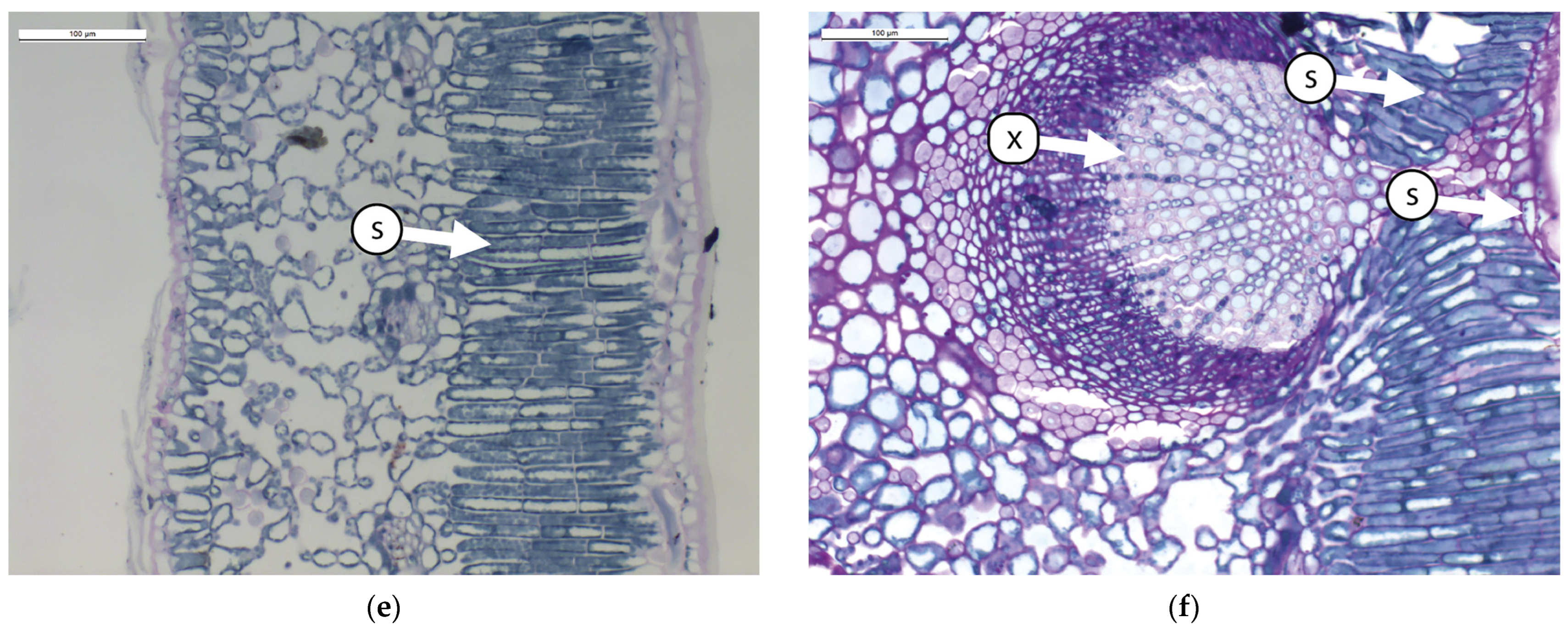
Figure 1.
Transverse sections of olive leaves samples subjected to different foliar treatment and stained with PAS-Amido Black: (a). leaf blade treated with Natural Zeolite (NZ-B); (b). leaf vein treated with Natural Zeolite (NZ-B); (c). leaf blade treated with Kaolin (K-B); (d). leaf vein treated with Kaolin (K-B); (e). leaf blade untreated (T-B); (f). leaf vein untreated (T-B). Legend: S = starch; X = xylem.
The results show that the leaves do not modify the ratio between different tissues due to the treatment (Table 1 and Figure 1). From an anatomical perspective, the leaf mesophyll consists of 3–4 layers of palisade cells (Figure 1a,c,e). In this tissue, small starch granules are present, particularly in ZN-B (Figure 1a). Below the palisade tissue, we find the spongy tissue characterized by large intercellular spaces (Figure 1a,c,e). In this tissue, no significant differences between treatments are observed. The central vein (Figure 1b,d,f) is composed of various tissues, but it causes greater wall thickening, resulting in a reduced lumen of the xylematic cells in K-B and T-B. The reduction in the diameter of xylem vessels is probably correlated with the plant’s transport capacity through the xylem. Indeed, in a study conducted by Martin-Benito et al. [38] on stressed pine plants, it was shown that drought induced the plants to allocate less carbon to the formation of tracheid cell walls, with a consequent increase in the diameter of the tracheid lumen. The authors hypothesized that this process potentially maximized hydraulic conductivity by reducing resistance to embolism. Some authors [39,40] have highlighted the positive effect of Kaolin on water stress tolerance.
Regarding the presence of starch inclusions in the leaf vein, this compound appears more abundant in NZ, indicating that the starch differs in leaves treated with NZ.
ESEM Observations
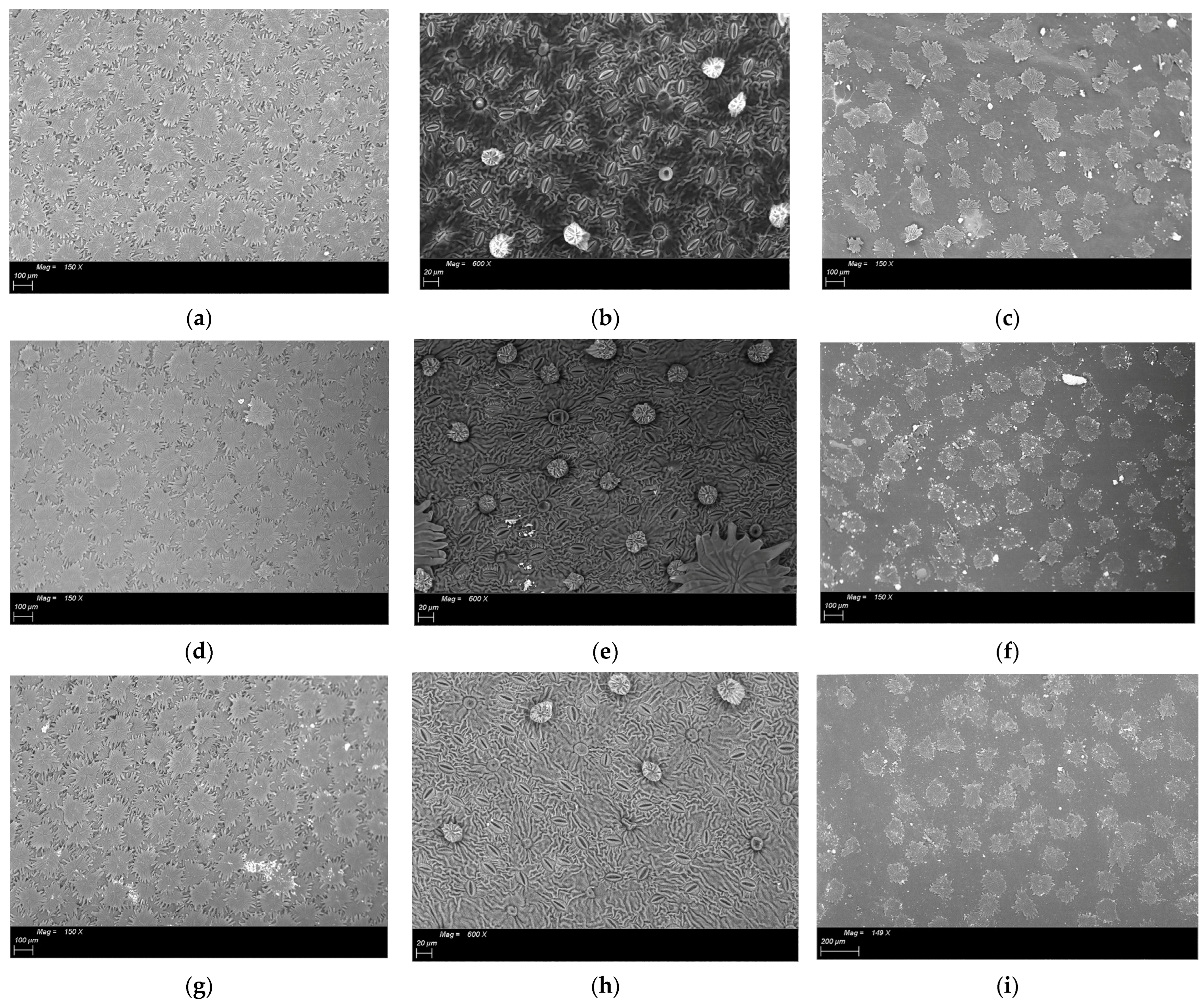
Olive tree is an evergreen xerophytic plant carrying hypostomatic leaves with trichomes on both sides. Olive trichomes are non-glandular peltate trichomes and are specialized cell types representing a protective barrier against acute environmental conditions: control water loss and leaf temperature protective mechanism against solar radiation [41]. Peltate trichome number on the abaxial surface of leaves treated with both geomaterials (natural zeolite and kaolin), are statistically less numerous compared to T leaves, these differences were already noticed since the first sampling (fruit set stage, N.69 on the BBCH scale). Even if our study was conducted in areas characterized by no drought conditions and mild climate, the minor density of peltate trichomes in NZ and K leaves were ascribable to the presence of powder on the surface which already acts like a protective film against high temperatures.
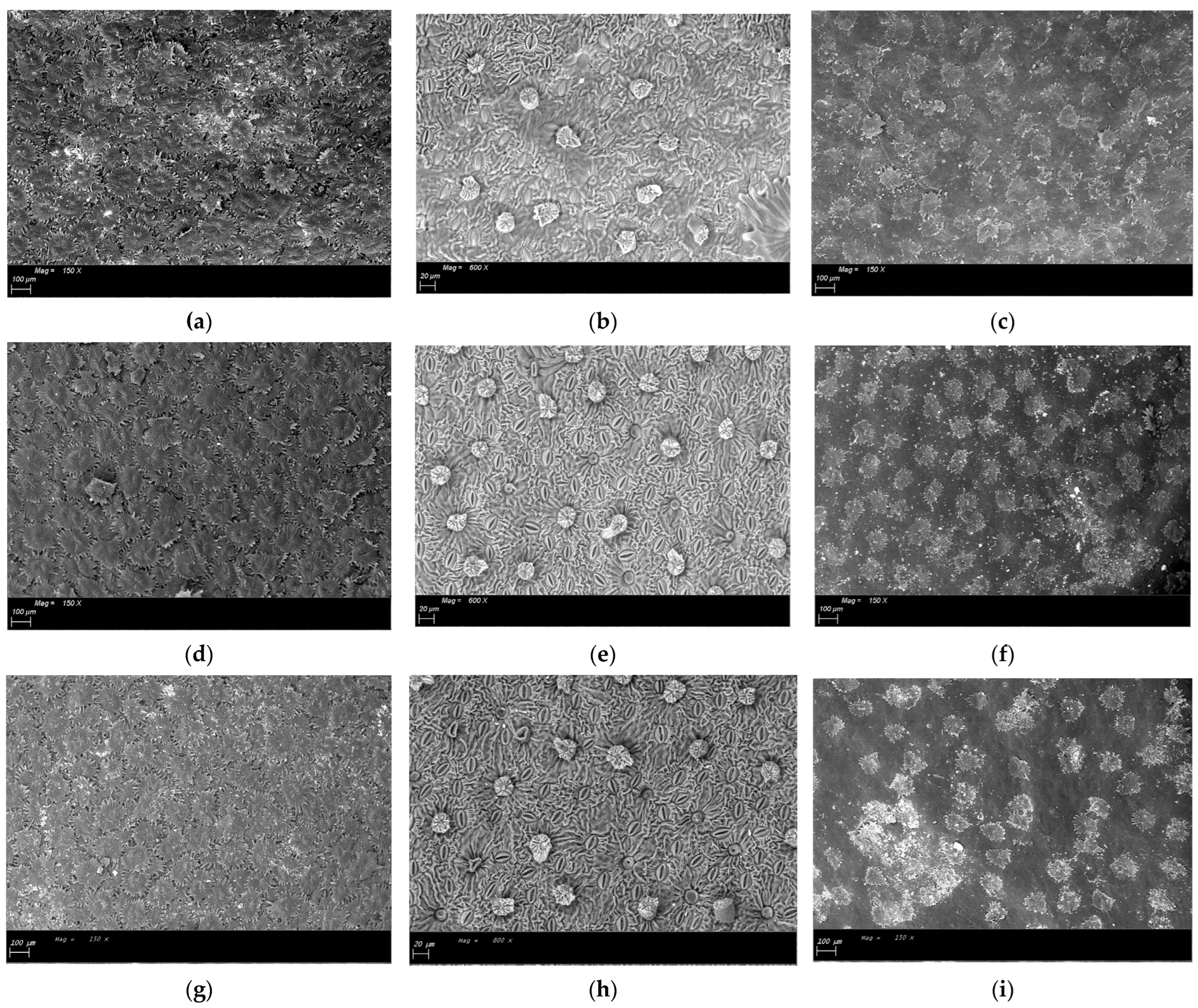
Abaxial surfaces of leaves treated with different geomaterial, collected before the first application, were characterized by the same number and size of stomata (area and max diameters), as shown in Table 2 and Figure 2. At the end of cycle applications (beginning of fruit colouring stage—N.81) NZ leaves exhibited higher stomatal density characterized by bigger stomata (max diameter and area) compared to K and T leaves (Table 2—Figure 3).

Table 2.
Stomatal parameters and number of peltate trichomes measured on the lower (abaxial) side of leaves collected in different phenological phases. Different treatments: natural zeolite (NZ-B), Kaolin (K-B), control (T-B).
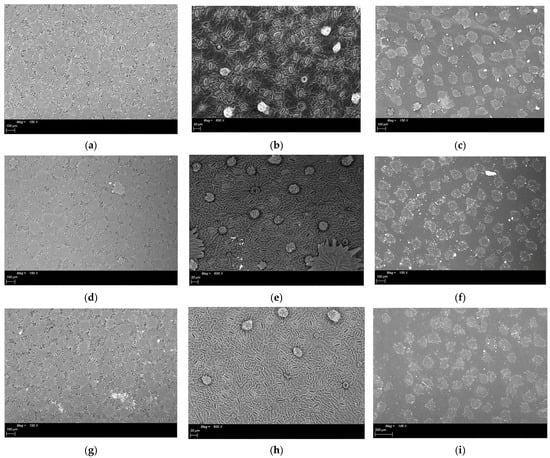
Figure 2.
ESEM images of the fruit set phase sampling of leaves treated with different geomaterials: (a). Abaxial surface of leave treated with K (Kaolin)—150×; (b). Abaxial surface of dehaired leaves treated with K (Kaolin)—600×; (c). Adaxial surface of leaves treated with K (Kaolin)—150×; (d). Abaxial surface of untreated (T) leaves—150×; (e). Abaxial surface of dehaired untreated (T) leaves—600×; (f). Adaxial surface of untreated (T) leaves—150×; (g). Abaxial surface of leaves treated with NZ (natural zeolite)—150×; (h). Abaxial surface of dehaired leaves treated with NZ (natural zeolite)—600×; (i). Adaxial surface of leaves treated with NZ (natural zeolite)—150×.
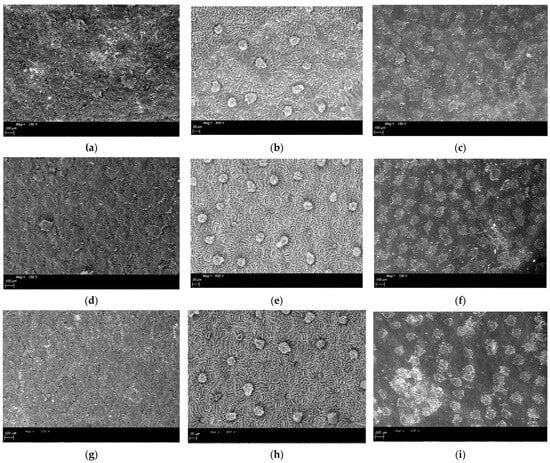
Figure 3.
ESEM images of the fruit colouring phase sampling of leaves treated with different geomaterials: (a). Abaxial surface of leaves treated with K (Kaolin)—150×; (b). Abaxial surface of dehaired leaves treated with K (Kaolin)—600×; (c). Adaxial surface of leaves treated with K (Kaolin)—150×; (d). Abaxial surface of untreated (T) leaves—150×; (e). Abaxial surface of dehaired untreated (T)—600×; (f). Adaxial surface of untreated (T) leaves—magnification factor 150×; (g). Abaxial surface of leaves treated with NZ (natural zeolite)—magnification factor 150×); (h). Abaxial surface of dehaired leaves treated with NZ (natural zeolite)—600×; (i). Adaxial surface of leaves treated with NZ (natural zeolite)—magnification factor 150×.
Regarding to stomatal characteristics (such as stomatal density, stomatal width, stomatal length) they provide information about the ecology of the place where the plant grows and the physiological state of the tree as reported in Quercus robur by Batos et al. [42].
It has been reported that zeolite can adsorb carbon dioxide molecules and release them slowly into environment, and it has also been suggested that when zeolite is spread on plant leaves, it can increase CO2 near the stomata simultaneously increasing photosynthesis [18].
It is important to underline that the differences in anatomical characteristics were more evident at the last date of sampling when treatment dust accumulated during the entire growing season, suggesting that these variations may be anatomical adaptations in response to the presence of dust film. The decrease in photosynthesis and transpiration rates observed at the end of annual applications in kaolin leaves cannot be only ascribed to the abundant accumulation of kaolin on leaf surface [19] but also to the decrease in stomatal number and size observed in kaolin leaves.
Observing the upper leaf surfaces at the first sampling date (fruit set stage), no differences in trichome density were observed, whereas at the end of the study (beginning of fruit colouring stage) the leaf surfaces subjected to NZ and K applications showed statistically lower values in trichome density of 23.72 and 23.04, respectively, compared to the density of T leaf surfaces. No differences in the size of the peltate trichomes were observed, in fact they showed the same total area and maximum diameter in all treatments and in both samplings as observed on the lower leaf side (Table 3)

Table 3.
Trichome density and size measured on the upper (adaxial) side of leaves collected at stage 69 and 81 in Bologna site. Different treatments: natural zeolite (NZ-B), kaolin (K-B), control (T-B).
Leaves covered with the two different geomaterials (NZ and K) had less need for protection and, therefore, developed a lower density of trichomes. Lower trichome density was also reported in kaolin-treated plants; this response also revealed a lower need for protection, as plants, in general, increase leaf pubescence to limit radiation interception and water vapour diffusion [43,44].
During leaf maturation, a decrease in the density of peltate trichomes was observed only in the upper surface of the treated leaves, as also observed by Roka et al. [45] in Koroneiky olive leaves and by Fernandez et al. [46] in Arbequina cv where as leaves become older some of the adaxial trichomes are shed or become buried in the epicuticolar waxes. Comparing the results reported by Razouk et al. [47] where 32 different olive varieties were studied for their drought tolerance and considering the higher genetic similarity between Frantoio and Correggiolo, the higher trichome density observed in Frantoio respect to result of the present study was ascribed to a phenotypic variability due to the different environmental conditions of the two different experimental sites.
3.1.2. Ecophysiological Measurements, Chlorophyll’s Content and Colour Leaf Measurements
The foliar gas exchanges of kaolin, natural zeolite and no treated trees measured at N.69 and N.81 growth stage of the BBCH scale are shown in Table 4. Data reported are the mean of three years of measurements, to include variability due to seasonality. At the fruit set stage, photosynthetic rate (A), transpiration rate (E), stomatal conductance (g) and intercellular CO2 concentration (Ci) did not statistically differ between treatments. Results reported on woody species for kaolin are conflicting: Glenn et al. [48] and Jifon and Syvertsen [49] found higher photosynthetic rate (A) in kaolin-treated plants in apple and grapefruit while other works [50,51] found a reduction in the same parameter (A) in kaolin-coated leaves. At the last stage of growth, there is an equal rate of photosynthesis of both kaolin and zeolite-treated plants compared with the untreated ones, but in K-B leaves a significant reduction in g, Ci and T was recorded. These results are in agreement with Gindaba and Wand [52] that hypothesized a blocking effect of kaolin coating on stomatal openings.

Table 4.
Ecophysiological parameters measured in Bologna site after foliar applications of kaolin (K-B), natural zeolite (NZ-B) and no treatment (T-B).
The reduction in photosynthetic rate has been attributed to the particle film, which has been observed to reduce the availability of usable light in photosynthesis [15,51,53]. This effect has been demonstrated for kaolin, while zeolite has been found to have no effect on light reflection [19]. The hypothesis formulated is that kaolin reduces photosynthesis under optimal conditions, whereas under conditions of thermal stress, the protection that provides is greater than the reduction caused by less light penetrating the leaf [48,50].
Treatments with kaolin and natural zeolite did not influence the chlorophyll content of leaves in both data samplings, as shown in Table 5. On the other hand, colorimetric measurements demonstrated a significant impact of leaf treatments, specifically, all CIELAB colour space values were found to be statistically significant, except for the a* coordinate in last sampling. The L* coordinate is indicative of brightness (L* = 0 indicates black and L* = 100 indicates white): higher values were recorded for kaolin at fruit set stage, and a similar value was recorded for kaolin and natural zeolite in beginning of fruit colouring stage, both of which were higher than the test, suggesting an accumulation of kaolin and zeolite on the leaves, in agreement with previous reports [19]. This behaviour can be explained by the different conformation and reflective power of these materials (kaolin is a phyllosilicate, while natural zeolite is a tectosilicate). The a* coordinates (where negative values indicate green and positive values indicate red) demonstrated significant disparities in the first sampling, when the test leaves exhibited higher green coordinates than those of kaolin and natural zeolite, at the N. 81 phase no differences were recorded anymore; this levelling of the a* coordinates values observed in October in T-B leaves is to be attributed to physiological mechanisms related to the senescence of the leaf.

Table 5.
Content of chlorophyll A and B, their ratio, and intensities of chromaticity coordinate (L*, a*) in leaves subjected to different foliar treatments: kaolin (K-B), no treatment (T-B) and natural zeolite (NZ-B).
3.1.3. Fruit Oil Quantity, Chemical and Sensory Analysis of Extra Virgin Olive Oil (EVOO)
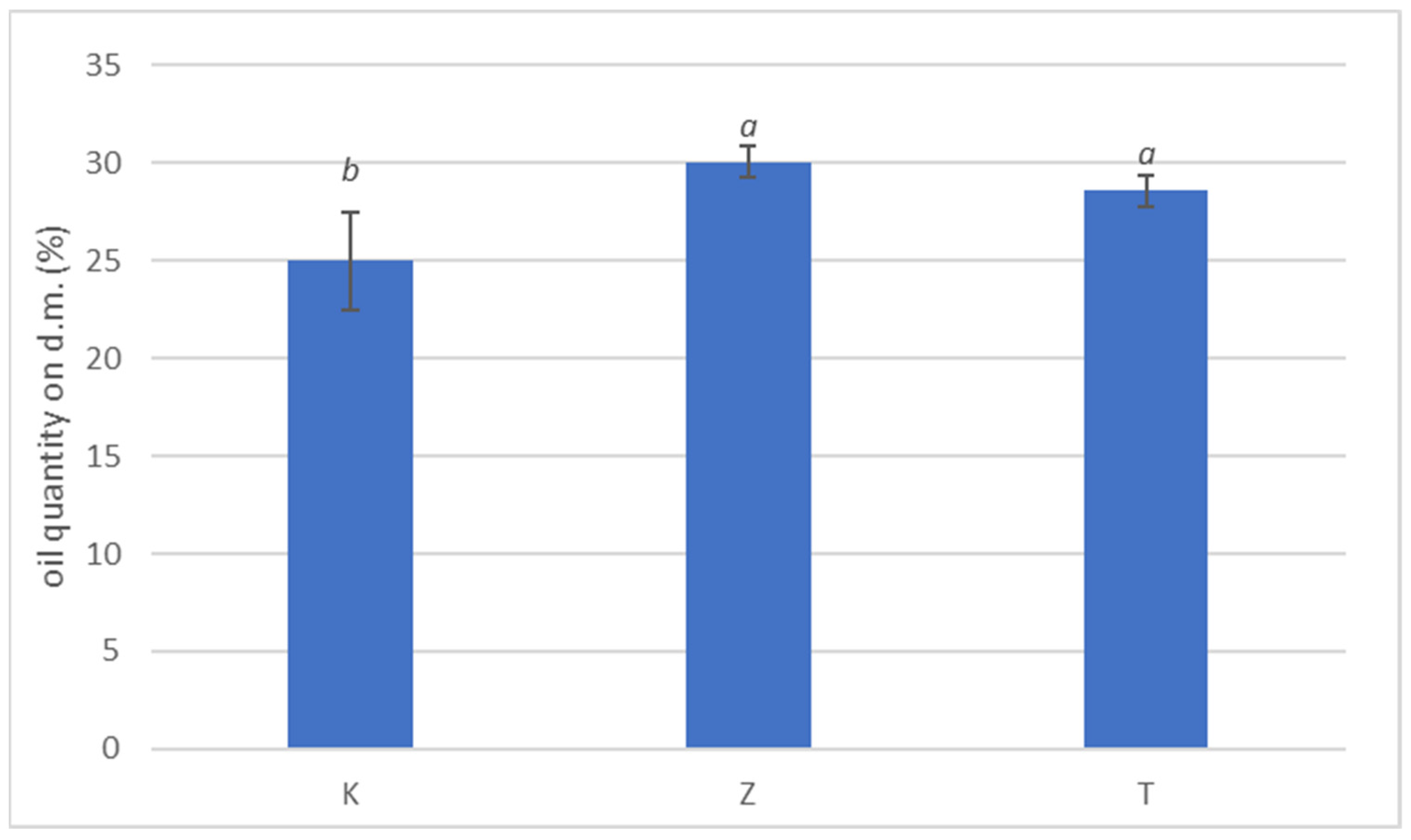
The amount of oil chemically extracted from the drupes at beginning of fruit colouring stage is shown in Figure 4. No significant variations were observed in the amount of oil contained in drupes subjected to natural zeolite treatments compared to test, while kaolin treatment showed significant lower value. The reduction in oil content in K-treated plants is probably because of different ecophysiological parameters such as stomatal conductance and intracellular CO2 concentration (Table 4); adequate stomatal conductance rate is essential to maintain good concentrations of CO2 within the cells, thereby promoting effective photosynthesis that contributes to oil production in the drupe. Actually, few works reported the effect of zeolite foliar film technology on oil content (Rotondi et al. [3]) and, in general, data on the foliar application of this geomaterial are scarce while available data on kaolin foliar application are more abundant on olive (Brito at al. [12]) and Saour and Makee [54]. It has been reported that zeolite-based product influenced positively plant production on different species in terms of growth and productivity [55,56] and in terms of fruit characteristics [57,58,59].
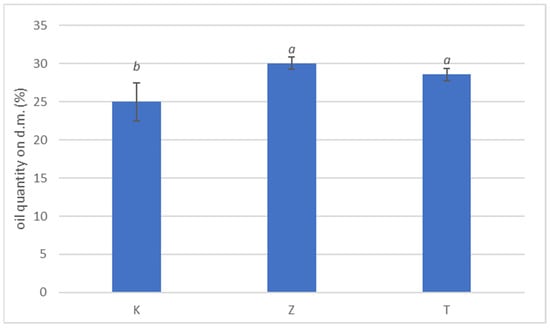
Figure 4.
Percentage of oil content on olive dry weight. Data are expressed as mean of three replicates ± standard deviation. Kaolin (K), no treatment (T) and natural zeolite (NZ). Different letters (a, b) indicate significant differences according to ANOVA and Tukey HSD test (p < 0.05) at each application date.
Regarding the chemical and sensory characteristics of olive oil, no differences were found between the treated and untreated oils, except for the total phenolic content (Table 6). This result is in agreement with several reports that found different quality profile in berries [56,57,58]. The qualitative characteristics of all the olive oils tested were found to be below the legal limits, thus classifying them as extra virgin oils. The sensory profiles of all the oils showed the typical characteristics of the Correggiolo cultivar, with an average intensity of bitterness, fruitiness and pungency, and slight herbaceous hints (Table A2—Appendix A).

Table 6.
Chemical parameters determined on olive oils obtained by the transformation of olive fruits produced under different treatments: Kaolin (K-B), no treatment (T-B) and natural Zeolite (NZ-B).
3.2. San Lazzaro Site
3.2.1. Anatomical Analyses
Leaf Morphology Analyses
The area of leaves treated with both geomaterials, natural zeolite (NZ-SL) and NH4+-enriched zeolite (EZ-SL), showed greater areas, 632.93 mm2 and 606.06 mm2, respectively, compared to untreated leaves’ (T-SL) area of 573.64 mm2. In NZ-SL leaves, the increase is more pronounced and statistically significant (see Table A3—Appendix A). It is generally accepted that an increase in leaf area is a common adaptation to low irradiance in order to capture more light [60]. However, in this case, the geomaterial leaf film cannot be considered responsible for low irradiance, so the increased leaf area development could be attributed to a general ‘well-being’ of the plants treated with the two types of zeolites (natural and enriched).
Light Microscopy
Anatomical analysis showed a statistical difference only in cell wall thickness; in this case, the leaves of EZ-SL-treated plants showed less thick xylem vessel cell wall (Table A4—Appendix A). This result could be related to the administration of ammonium ion. Indeed, high nitrogen levels can lead to a decrease in lignin content. For example, in rice, higher nitrogen availability reduces lignin deposition in the secondary cell walls, thereby weakening the mechanical tissue structure [61]. Conversely, in hybrid poplar, elevated nitrogen levels result in thinner secondary cell walls and altered xylem structure, which improves water transport efficiency but increases vulnerability to drought [62]. Similarly, Peñuelas et al. [63] demonstrated the significant impact of nitrogen fertilization on plants, reporting reduced cell wall elasticity, likely due to a decrease in cell wall thickness.
3.2.2. Ecophysiological Measurements, Chlorophyll’s Content and Colour Leaf Measurements
The ecophysiological parameters indicated that during the summer period (first three surveys), the photosynthesis rate was higher in the EZ-SL plants than in the other treatments as well as stomatal conductance and transpiration rate (Table 7). The biostimulant action of zeolite in association with N as reported by Quezada and Bragazza [64], suggest a better adaptation of EZ-SL leaves to higher temperature; on these dates, the photosynthesis values were very low due to the high summer temperatures that characterized 2021. This trend only changed at the end of September, when the photosynthesis values became similar between the different treatments (Table 7). These findings are consistent with those reported by Morrone et al. [8] in earlier studies on foliar techniques involving enriched zeolites. Transpiration rate was significant higher in EZ-SL plant, probably correlated to the less thick xylem vessels that induced a higher xylem lumen.

Table 7.
Ecophysiological parameters measured after each foliar applications of natural zeolite (NZ-SL), NH4+-enriched zeolite (EZ-SL) and control (T-SL).
It has been reported that zeolites can adsorb carbon dioxide molecules and release them slowly into the environment; also it has been supposed that when zeolites are spread on plant leaves, they may increase the amounts of CO2 near the stomata concomitantly increasing the photosynthesis rate [18]. In these experiments we have observed significant differences on photosynthesis rate and intercellular CO2 concentration only at the third date on EZ-SL plants (see Table 7).
3.2.3. Fruit Weight, Oil Quantity, Chemical and Sensory Analysis of Extra Virgin Olive Oil (EVOO)
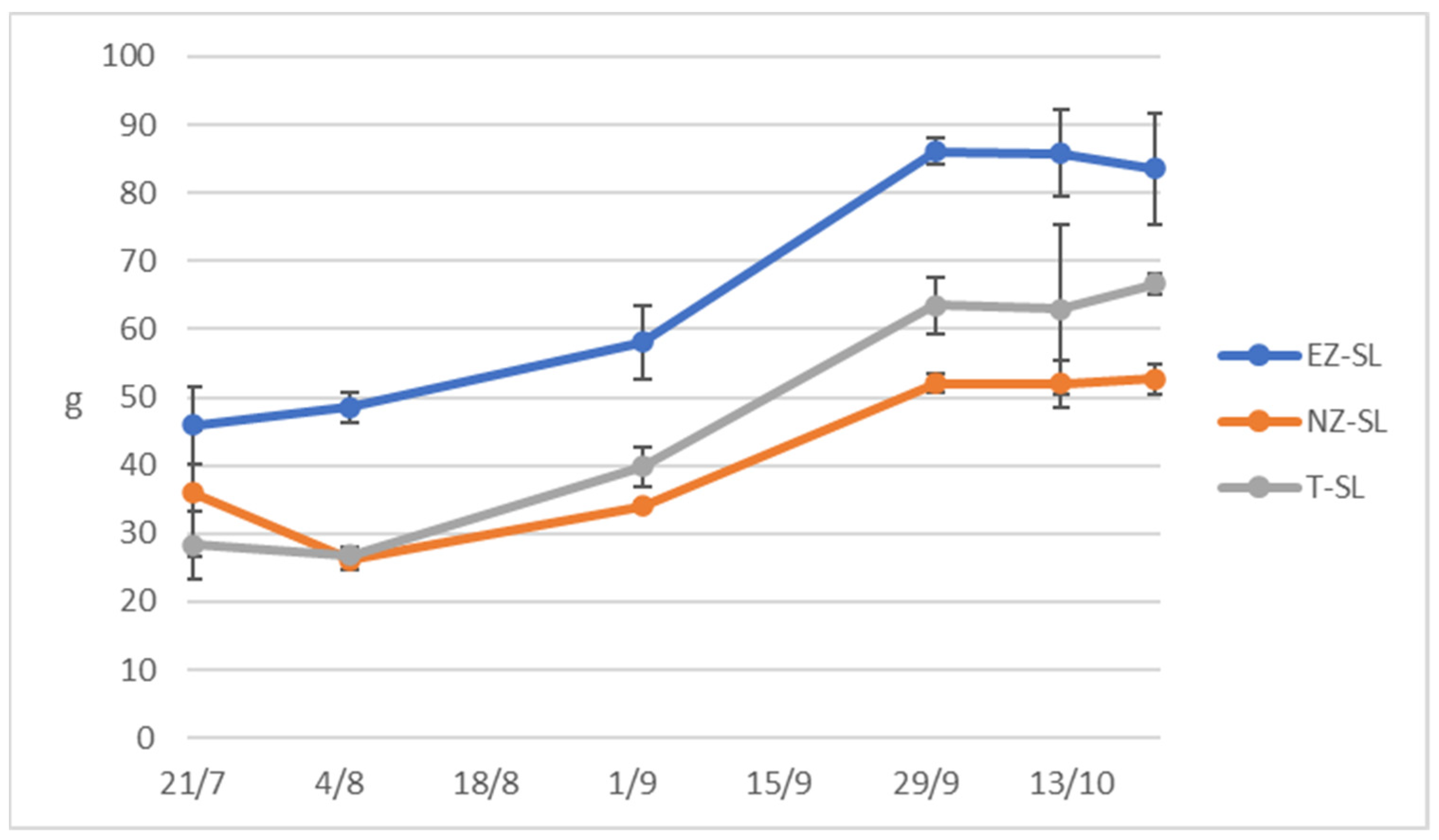
Olive fresh weight reported in Figure 5 exhibited higher trend in EZ-SL olive fruit compared to the other treatments, NZ-SL olive fresh weight presents a similar trend with control, this is disagreement with previous studies on Cucumis sativus plants sprayed with natural zeolite that increased of fruit length [9]. Another study on an annual crop found the improvement of zeolite foliar application in fruit number and weight [55], while [65] studying grape performance under kaolin and zeolite treatments did not find statistical differences on yield per vine and weight.
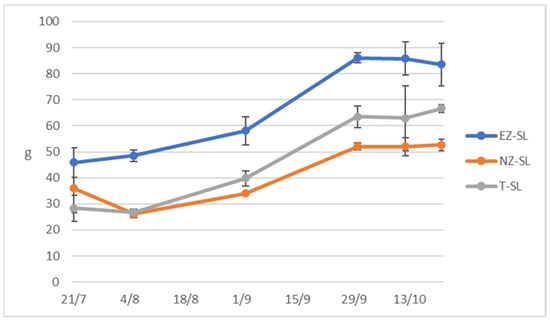
Figure 5.
Fresh weigh of 50 olives collected form trees subjected to different foliar treatments in San Lazzaro site: ammonium-enriched zeolite (EZ-SL), no treatment (T-SL) and natural zeolite (NZ-B).
Regni and Proietti [66] evaluated the effect on the vegetative and productive performance of nitrogen foliar fertilization on olive plant, reporting that it does not induce significant effects while our study indicates that use zeolite as NH4 carriers helps tree nutritional status. The same authors asserted that the first stages of fruit growth are the ones where the trees require N and this agrees with the higher olive weight of EZ-SL treated trees.
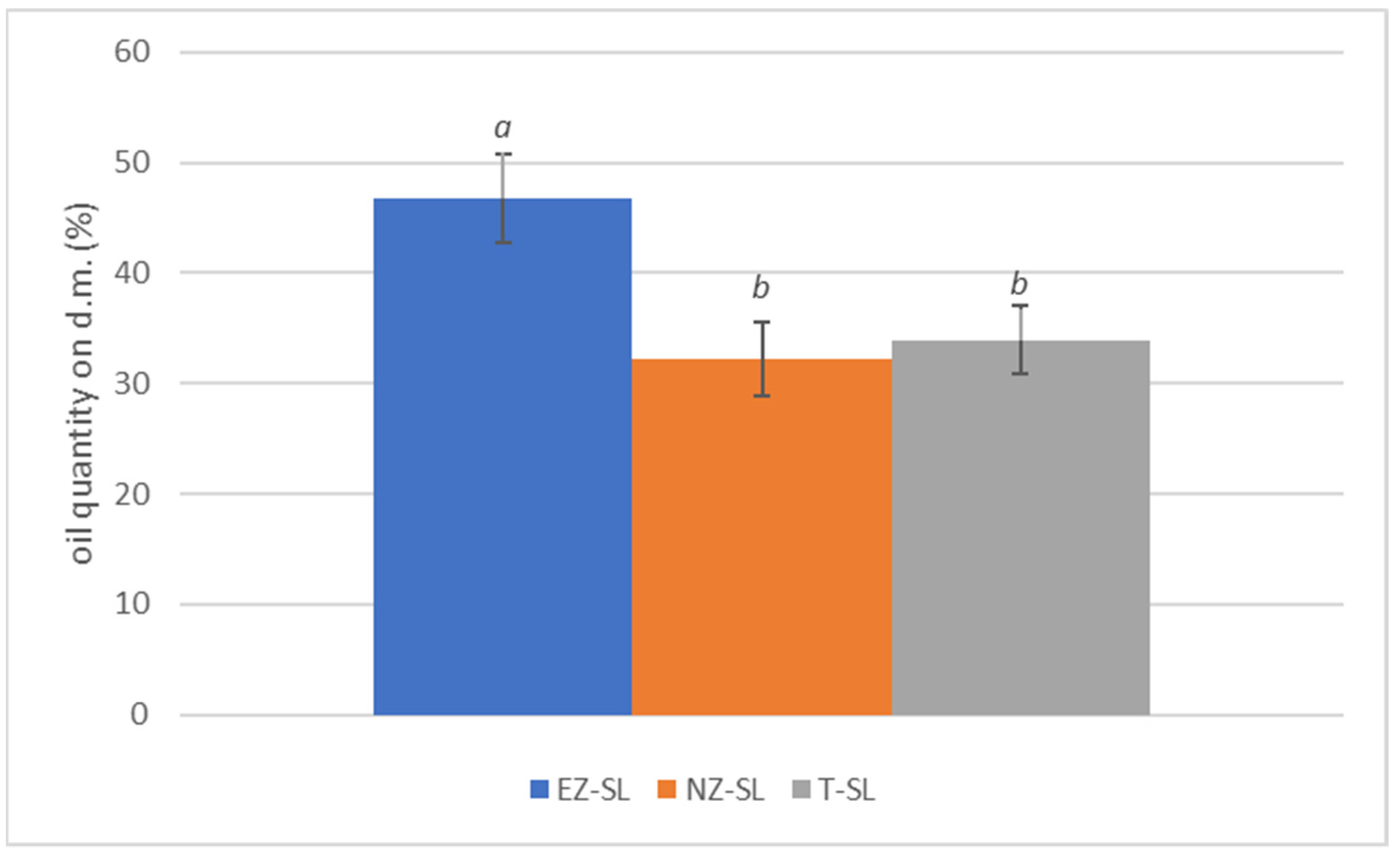
Olive fruits developed under EZ treatment exhibited a statistically significant higher of oil content (Figure 6). This result is in agreement whit the conclusions of Quezada and Bragazza [64] that state the beneficial effects of foliar zeolite treatments as a biostimulants on maize and wheat. In olive cultivation foliar application with zeolite-base products have positive influenced oil content in olive fruit [3,8].
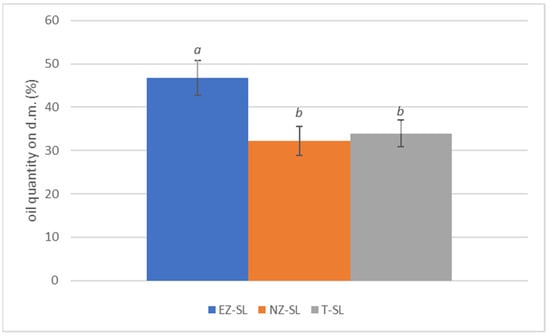
Figure 6.
Percentage of oil content on olive dry weight in plants subjected to different foliar treatments San Lazzaro site: ammonium-enriched zeolite (EZ-SL), no treatment (T-SL) and natural zeolite (NZ-B). Data are expressed as mean of three replicates ± standard deviation. Different letters (a, b) indicate significant differences according to ANOVA and Tukey HSD test (p < 0.05).
A comparison of the chemical parameters of the oils produced with different foliar treatments revealed that all samples can be classified in the product class of extra virgin olive oils.
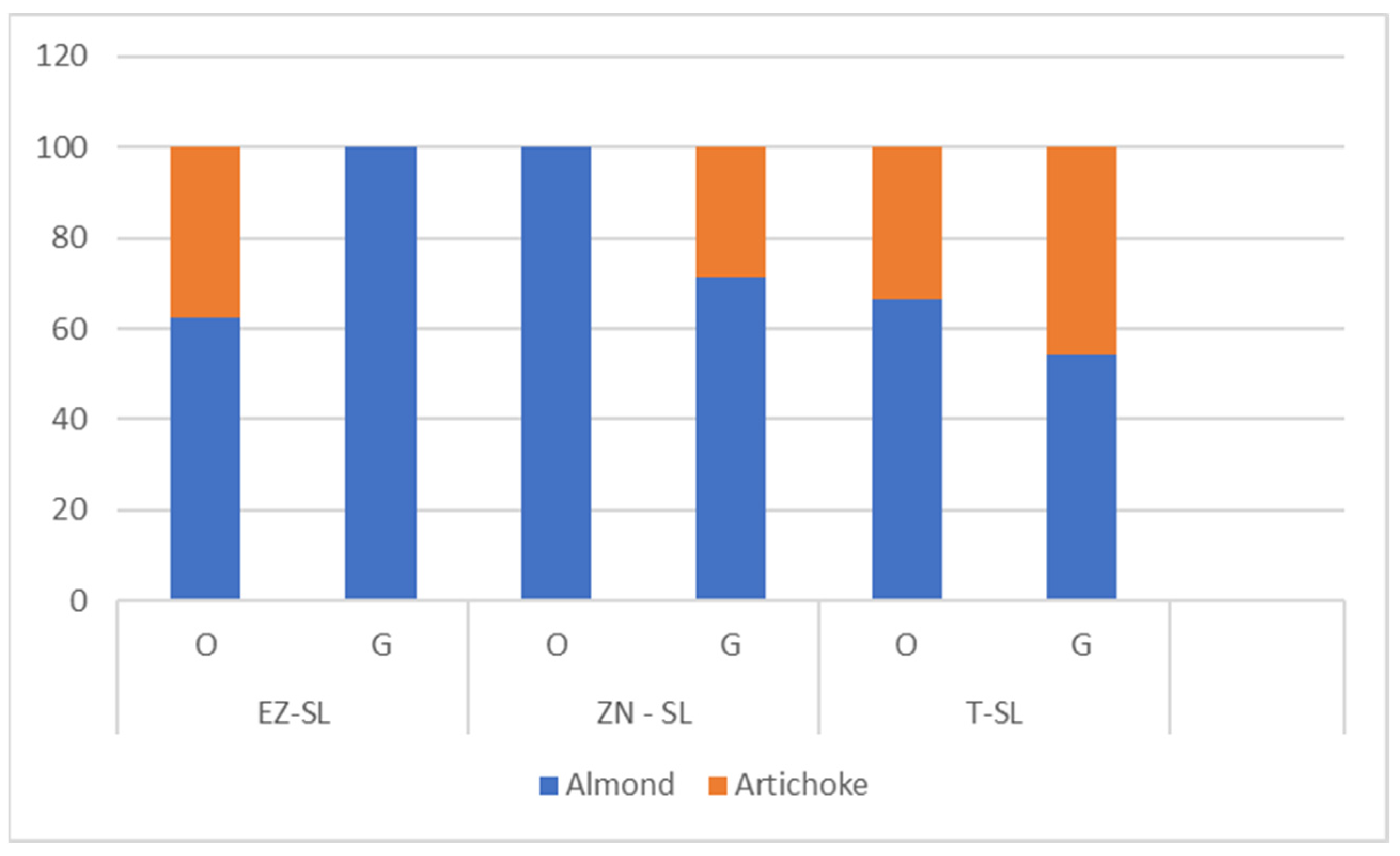
Sensory analysis did not show statistical differences in the intensity of sensory attributes (Table A5—Appendix A). Regarding the pleasant flavours of oils produced from trees undergone different foliar treatments (Figure 7) we observed how at an olfactory level NZ-SL oil differs in the absence of artichoke hint. At a gustatory aroma level artichoke hint missed in EZ-SL oil. Pleasant flavours of olive oils are relevant in the characterization since differentiate monovarietal oils and impact on the overall sensory experience. These results are consistent with those previously reported on oils from Correggiolo cv, where oils produced from plants treated with natural zeolite exhibited enhanced sensory profiles compared to oils obtained from plants treated with kaolin and ammonium-enriched zeolite [8].
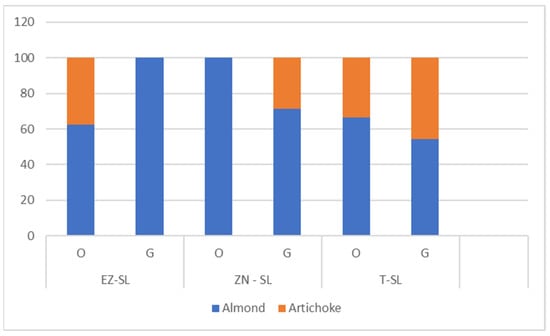
Figure 7.
Pleasant flavours olfactive (O) and gustative (G) of olive oils produced by plant treated with ammonium-enriched zeolite (EZ-SL), no treatment (T-SL) and natural zeolite (NZ-B).
4. Conclusions
This research was created from the need to evaluate the internal and external adaptations of the olive leaves that underwent three years of treatments based on kaolin and zeolite for the control of olive fruit fly.
In general, the specific minerals present in kaolin and zeolite can influence their interaction with the leaf surface, their ability to absorb or release water, and their potential to deliver nutrients: the presence of specific clay minerals in kaolin might explain its effect on reducing transpiration, while the presence of certain exchangeable cations in zeolite could contribute to its nutrient delivery capacity. In our study, kaolin negatively impacted transpiration and stomatal conductance, while zeolite, particularly when enriched with ammonium, showed promise in enhancing photosynthetic rate and fruit size.
The research was not limited to the properties of the leaf tissues but also to their eventual changes in their functions; that is, the physiological parameters linked to gas exchanges were evaluated; therefore, the effect of oil accumulation in the fruit and its extra virgin oil quality could not be overlooked.
The most significant innovation in this research concerned the use of zeolite as a carrier of nutritional substances: the NH₄⁺-enriched zeolite could represent a promising multifunctional product combining protective and nutritional benefits. Further studies will be focused on the investigation of seasonal variations in leaf responses and their impact on oil accumulation and on the analysis of nutrient transport pathways in geo-materials treated leaves and their influence on fruit development. The findings of this study have demonstrated the potential for the utilization of geomaterials; however, the limitations associated with the frequency of application, in relation to rainfall and subsequent washing of the treatment, persist. These further studies will be carried out in different environmental conditions and on different olive varieties.
Author Contributions
Conceptualization, A.R. and L.M.; methodology, A.R. and T.G.; investigation, A.C., M.R., R.D. and M.R.; data curation, L.M., T.G., A.C. and M.R.; writing—original draft preparation, A.R.; writing—review and editing, A.C. and T.G.; supervision, A.R.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors gratefully thank Matteo Mari for his technical support, Barbara Alfei and the panel of AMAP-Marche for sensory analysis, Franco Corticelli for ESEM-EDX analysis support and Osvaldo Facini for statistical support.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Morphological parameters of leaves treated with different geomaterial: natural zeolite (NZ-B), kaolin (K-B), control (T-B) in Bologna site.
Table A1.
Morphological parameters of leaves treated with different geomaterial: natural zeolite (NZ-B), kaolin (K-B), control (T-B) in Bologna site.
| Treatments | Leaf Area (mm2) |
|---|---|
| NZ-B | 696.45 a |
| T-B | 707.69 a |
| K-B | 669.33 a |
Different letters in the same column indicate significant differences (p < 0.05) according to Tukey’s HSD test.

Table A2.
Sensory profiles of olive oils produced by plants treated under different treatments: Kaolin (K-B), no treatment (T-B) and natural zeolite (NZ-B).
Table A2.
Sensory profiles of olive oils produced by plants treated under different treatments: Kaolin (K-B), no treatment (T-B) and natural zeolite (NZ-B).
| Treatment | Olfactory Olive Fruity | Olfactory Pleasant Flavours | Gustatory Olive Fruity | Bitter | Pungent | Grass | Gustatory Pleasant Flavours |
|---|---|---|---|---|---|---|---|
| NZ-B | 4.55 | 2.15 | 4.20 | 3.55 | 3.55 | 1.25 | 1.95 |
| K-B | 4.95 | 2.50 | 4.90 | 3.95 | 3.30 | 1.15 | 2.15 |
| T-B | 5.10 | 2.90 | 4.60 | 3.30 | 4.00 | 1.80 | 3.05 |
| Pr > F | 0.929 | 0.494 | 0.774 | 0.630 | 0.911 | 0.774 | 0.978 |

Table A3.
Morphological parameters of leaves treated with different geomaterial: NH4+-enriched zeolite (EZ-SL), natural zeolite (NZ-SL) and control (T-SL) in San Lazzaro site.
Table A3.
Morphological parameters of leaves treated with different geomaterial: NH4+-enriched zeolite (EZ-SL), natural zeolite (NZ-SL) and control (T-SL) in San Lazzaro site.
| Treatments | Leaf Area (mm2) |
|---|---|
| EZ-SL | 606.03 ab |
| T-SL | 573.64 b |
| NZ-SL | 632.93 a |
Different letters (a, b) indicate significant differences according to ANOVA and Tukey HSD test (p < 0.05) at each application date.

Table A4.
Anatomical parameters of leaf derived by trees treated with natural zeolite (NZ-SL), control (T-SL) and NH4+-enriched zeolite (EZ-SL).
Table A4.
Anatomical parameters of leaf derived by trees treated with natural zeolite (NZ-SL), control (T-SL) and NH4+-enriched zeolite (EZ-SL).
| Treatment | Transverse Diameter of Epidermal Cell | Longitudinal Diameter of Epidermal Cell | Palisade Thickness | Spongy Thickness | Maximum Diameter of Leaf Xylem Vessels | Cell wall Thickness of Xylem Vessels |
|---|---|---|---|---|---|---|
| NZ-SL | 14.125 a | 14.295 a | 167.141 a | 227.325 a | 12.314 a | 2.187 a |
| T-SL | 14.001 a | 14.407 a | 168.176 a | 225.309 a | 12.001 a | 2.155 a |
| EZ-SL | 14.083 a | 14.362 a | 164.270 a | 226.642 a | 11.581 a | 1.901 b |
| Pr > F | 0.587 | 0.844 | 0.160 | 0.480 | 0.297 | 0.005 |
Different letters (a, b) above the bars for each group of histograms indicate significant differences according to ANOVA and Tukey’s HSD test (p < 0.05).

Table A5.
Sensory profiles of olive oils produced by plants treated under different treatments in San Lazzaro site: natural zeolite (NZ-SL), NH4+-enriched zeolite (EZ-SL) and control (T-SL).
Table A5.
Sensory profiles of olive oils produced by plants treated under different treatments in San Lazzaro site: natural zeolite (NZ-SL), NH4+-enriched zeolite (EZ-SL) and control (T-SL).
| Treatment | Olfactory Olive Fruity | Olfactory Pleasant Flavours | Gustatory Olive Fruity | Bitter | Pungent | Grass | Gustatory Pleasant Flavours |
|---|---|---|---|---|---|---|---|
| NZ-SL | 3.05 | 1.0 | 3.70 | 5.55 | 4.45 | 1.50 | 1.85 |
| T-SL | 3.85 | 2.0 | 3.85 | 5.70 | 4.50 | 2.10 | 2.30 |
| EZ-SL | 2.70 | 1.5 | 3.15 | 3.35 | 3.55 | 1.15 | 0.50 |
| Pr > F | 0.594 | 0.600 | 0.781 | 0.075 | 0.134 | 0.901 | 0.939 |
References
- Malheiro, R.; Casal, S.; Baptista, P.; Pereira, J.A. A Review of Bactrocera Oleae (Rossi) Impact in Olive Products: From the Tree to the Table. Trends Food Sci. Technol. 2015, 44, 226–242. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Cunha, S.C.; Baptista, P.; Pereira, J.A. Identification of Leaf Volatiles from Olive (Olea Europaea) and Their Possible Role in the Ovipositional Preferences of Olive Fly, Bactrocera Oleae (Rossi) (Diptera: Tephritidae). Phytochemistry 2016, 121, 11–19. [Google Scholar] [CrossRef]
- Rotondi, A.; Bertazza, G.; Faccini, B.; Ferretti, G.; Morrone, L. Effect of Different Foliar Particle Films (Kaolin and Zeolitite) on Chemical and Sensory Properties of Olive Oil. Agronomy 2022, 12, 3088. [Google Scholar] [CrossRef]
- European Union. Commission Implementing Regulation (EU) 2019/1090-of 26 June 2019-Concerning the Non-Renewal of Approval of the Active Substance Dimethoate, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending the Annex to Commission Implementing Regulation (EU) No 540/2011. OJ L 2019, 173, 39–41. [Google Scholar]
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K.; et al. Summary for Policymakers. In Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; The Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar]
- Pascual, S.; Cobos, G.; Seris, E.; González-Núñez, M. Effects of Processed Kaolin on Pests and Non-Target Arthropods in a Spanish Olive Grove. J. Pest Sci. 2010, 83, 121–133. [Google Scholar] [CrossRef]
- Gharbi, N.; Abdallah, S.B. Effectiveness of Kaolin Treatment for the Control of the Olive Fruit Fly Bactrocera Oleae in Tunisian Olive Groves. Tunis. J. Plant Prot. 2016, 11, 73–81. [Google Scholar]
- Morrone, L.; Neri, L.; Facini, O.; Galamini, G.; Ferretti, G.; Rotondi, A. Influence of Chabazite Zeolite Foliar Applications Used for Olive Fruit Fly Control on Volatile Organic Compound Emission, Photosynthesis, and Quality of Extra Virgin Olive Oil. Plants 2024, 13, 698. [Google Scholar] [CrossRef] [PubMed]
- Bozorgi, H.R. Effects of Foliar Spraying with Marine Plant Ascophyllum Nodosum Extract and Nano Iron Chelate Fertilizer on Fruit Yield and Several Attributes of Eggplant (Solanum melongena L.). J. Agric. Sci. Technol. 2012, 7, 357–362. [Google Scholar]
- Shellie, K.C.; King, B.A. Kaolin Particle Film and Water Deficit Influence Malbec Leaf and Berry Temperature, Pigments, and Photosynthesis. Am. J. Enol. Vitic. 2013, 64, 223–230. [Google Scholar] [CrossRef]
- Denaxa, N.K.; Roussos, P.A.; Damvakaris, T.; Stournaras, V. Comparative Effects of Exogenous Glycine Betaine, Kaolin Clay Particles and Ambiol on Photosynthesis, Leaf Sclerophylly Indexes and Heat Load of Olive Cv. Chondrolia Chalkidikis under Drought. Sci. Hortic. 2012, 137, 87–94. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Ferreira, H.; Rocha, L.; Pavia, I.; Moutinho-Pereira, J.; Correia, C.M. Kaolin Particle Film Modulates Morphological, Physiological and Biochemical Olive Tree Responses to Drought and Rewatering. Plant Physiol. Biochem. 2018, 133, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Dinis, L.-T.; Moutinho-Pereira, J.; Correia, C.M. Drought Stress Effects and Olive Tree Acclimation under a Changing Climate. Plants 2019, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Dinis, L.-T.; Silva, E.; Gonçalves, A.; Matos, C.; Rodrigues, M.A.; Moutinho-Pereira, J.; Barros, A.; Correia, C. Kaolin and Salicylic Acid Foliar Application Modulate Yield, Quality and Phytochemical Composition of Olive Pulp and Oil from Rainfed Trees. Sci. Hortic. 2018, 237, 176–183. [Google Scholar] [CrossRef]
- Rosati, A.; Metcalf, S.G.; Buchner, R.P.; Fulton, A.E.; Lampinen, B.D. Effects of Kaolin Application on Light Absorption and Distribution, Radiation Use Efficiency and Photosynthesis of Almond and Walnut Canopies. Ann. Bot. 2007, 99, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Shellie, K.; Glenn, D.M. Wine Grape Response to Foliar Particle Film under Differing Levels of Preveraison Water Stress. HortScience 2008, 43, 1392–1397. [Google Scholar] [CrossRef]
- Glenn, D.M. Effect of Highly Processed Calcined Kaolin Residues on Apple Productivity and Quality. Sci. Hortic. 2016, 201, 101–108. [Google Scholar] [CrossRef]
- De Smedt, C.; Someus, E.; Spanoghe, P. Potential and Actual Uses of Zeolites in Crop Protection. Pest Manag. Sci. 2015, 71, 1355–1367. [Google Scholar] [CrossRef]
- Rotondi, A.; Morrone, L.; Facini, O.; Faccini, B.; Ferretti, G.; Coltorti, M. Distinct Particle Films Impacts on Olive Leaf Optical Properties and Plant Physiology. Foods 2021, 10, 1291. [Google Scholar] [CrossRef]
- Tekaya, M.; Mechri, B.; Cheheb, H.; Attia, F.; Chraief, I.; Ayachi, M.; Boujneh, D.; Hammami, M. Changes in the Profiles of Mineral Elements, Phenols, Tocopherols and Soluble Carbohydrates of Olive Fruit Following Foliar Nutrient Fertilization. LWT-Food Sci. Technol. 2014, 59, 1047–1053. [Google Scholar] [CrossRef]
- Colella, C. Recent Advances in Natural Zeolite Applications Based on External Surface Interaction with Cations and Molecules. Stud. Surf. Sci. Catal. 2007, 170, 2063–2073. [Google Scholar] [CrossRef]
- Cerrato, A.; Giovanardi, D.; Coltorti, M.; Stefani, E. LIFE MICROFIGHTERS: An EU funded project for the implementation and use of innovative Zeo-biopesticides, based on beneficial microorganisms, as an alternative to the use of copper-based products Abstracts of Presentations at the XXVII Congress of the Italian Phytopathological Society (SIPaV). J. Plant Pathol. 2022, 104, 1207–1280. [Google Scholar] [CrossRef]
- Modica, F.; Fagioli, L.; Coltorti, M.; Giovanardi, D.; Reyes, F.; Stefani, E. Reduction of copper inputs in the management of key diseases of grapevine, olive and tomato by an innovative Zeo-biopesticide. Abstracts of Presentations at the XXVIII Congress of the Italian Phytopathological Society (SIPaV). J. Plant Pathol. 2023, 105, 1237–1323. [Google Scholar] [CrossRef]
- Calabrese, J.; Pacini, C.; Vazzana, C.; Nikolla, M. Sustainability Comparison Between Organic and Conventional Systems at Farm and Field Scale: A Case Study in Olive Production Systems in Apulia Region. Eur. J. Sustain. Dev. 2013, 2, 19. [Google Scholar] [CrossRef]
- Galamini, G.; Ferretti, G.; Rosinger, C.; Huber, S.; Medoro, V.; Mentler, A.; Díaz-Pinés, E.; Gorfer, M.; Faccini, B.; Keiblinger, K.M. Recycling Nitrogen from Liquid Digestate via Novel Reactive Struvite and Zeolite Minerals to Mitigate Agricultural Pollution. Chemosphere 2023, 317, 137881. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Cortés, F.; Martinez-Calvo, J.; Badenes, M.L.; Bleiholder, H.; Hack, H.; Llacer, G.; Meier, U. Phenological Growth Stages of Olive Trees (Olea Europaea). Ann. Appl. Biol. 2002, 140, 151–157. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 1999; Volume 198, ISBN 0-19-508956-1. [Google Scholar]
- Donald, A.M. The Use of Environmental Scanning Electron Microscopy for Imaging Wet and Insulating Materials. Nat. Mater. 2003, 2, 511–516. [Google Scholar] [CrossRef]
- Danilatos, G.D. Foundations of Environmental Scanning Electron Microscopy. Adv. Electron. Electron Phys. 1988, 71, 109–250. [Google Scholar] [CrossRef]
- Jifon, J.L.; Syvertsen, J.P. Moderate Shade Can Increase Net Gas Exchange and Reduce Photoinhibition in Citrus Leaves. Tree Physiol. 2003, 23, 119–127. [Google Scholar] [CrossRef]
- Larbi, A.; Vázquez, S.; El-Jendoubi, H.; Msallem, M.; Abadía, J.; Abadía, A.; Morales, F. Canopy Light Heterogeneity Drives Leaf Anatomical, Eco-Physiological, and Photosynthetic Changes in Olive Trees Grown in a High-Density Plantation. Photosynth. Res. 2015, 123, 141–155. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–55. [Google Scholar] [CrossRef]
- Randall, E.L. Improved Method for Fat and Oil Analysis by a New Process of Extraction. J. AOAC Int. 1974, 57, 1165–1168. [Google Scholar] [CrossRef]
- European Union. Commission Delegated Regulation (EU) 2022/2104 of 29 July 2022 Supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as Regards Marketing Standards for Olive Oil, and Repealing Commission Regulation (EEC) No 2568/91 and Commission Implementing Regulation (EU) No 29/2012. OJ L 2022, 284, 1–22. [Google Scholar]
- Morrone, L.; Pupillo, S.; Neri, L.; Bertazza, G.; Magli, M.; Rotondi, A. Influence of Olive Ripening Degree and Crusher Typology on Chemical and Sensory Characteristics of Correggiolo Virgin Olive Oil. J. Sci. food Agric. 2017, 97, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Cerretani, L.; Bendini, A.; Biguzzi, B.; Lercker, G.; Toschi, T.G. Evaluation of the Oxidative Stability of Extra-Virgin Olive Oils, Obtained by Different Technological Plants, with Respect to Some Qualitative Parameters[Stabilità Ossidativa Di Oli Extravergini Di Oliva Ottenuti Con Diversi Impianti Tecnologici]. Ind. Aliment. 2003, 42, 706–711. [Google Scholar]
- Rotondi, A.; Bendini, A.; Cerretani, L.; Mari, M.; Lercker, G.; Toschi, T.G. Effect of Olive Ripening Degree on the Oxidative Stability and Organoleptic Properties of Cv. Nostrana Di Brisighella Extra Virgin Olive Oil. J. Agric. Food Chem. 2004, 52, 3649–3654. [Google Scholar] [CrossRef]
- Martin-Benito, D.; Anchukaitis, K.J.; Evans, M.N.; Del Río, M.; Beeckman, H.; Cañellas, I. Effects of Drought on Xylem Anatomy and Water-Use Efficiency of Two Co-Occurring Pine Species. Forests 2017, 8, 332. [Google Scholar] [CrossRef]
- Dinis, L.T.; Bernardo, S.; Luzio, A.; Pinto, G.; Meijón, M.; Pintó-Marijuan, M.; Cotado, A.; Correia, C.; Moutinho-Pereira, J. Kaolin Modulates ABA and IAA Dynamics and Physiology of Grapevine under Mediterranean Summer Stress. J. Plant Physiol. 2018, 220, 181–192. [Google Scholar] [CrossRef]
- do Forno, B.C.B. Influence of Kaolin Application on Physiological Behavior of Olive Trees (Olea europaea L.) Submitted to Water Deficit. Master’s Thesis, Universidade De Trás-Os-Montes E Alto Douro, Vila Real, Portugal, 2017. [Google Scholar]
- Liakopoulos, G.; Stavrianakou, S.; Karabourniotis, G. Trichome Layers versus Dehaired Lamina of Olea Europaea Leaves: Differences in Flavonoid Distribution, UV-Absorbing Capacity, and Wax Yield. Environ. Exp. Bot. 2006, 55, 294–304. [Google Scholar] [CrossRef]
- Batos, B.; Vilotić, D.; Orlović, S.; Miljković, D. Inter and Intra-Population Variation of Leaf Stomatal Traits of Quercus Robur L. in Northern Serbia. Arch. Biol. Sci. 2010, 62, 1125–1136. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.M.; Diaz-Espejo, A.; Morales-Sillero, A.; Martín-Palomo, M.J.; Mayr, S.; Beikircher, B.; Fernández, J.E. Shoot Hydraulic Characteristics, Plant Water Status and Stomatal Response in Olive Trees under Different Soil Water Conditions. Plant Soil 2013, 373, 77–87. [Google Scholar] [CrossRef]
- Segura-Monroy, S.; Uribe-Vallejo, A.; Ramirez-Godoy, A.; Restrepo-Diaz, H. Effect of Kaolin Application on Growth, Water Use Efficiency, and Leaf Epidermis Characteristics of Physallis Peruviana Seedlings under Two Irrigation Regimes. J. Agric. Sci. Technol. 2015, 17, 1585–1596. [Google Scholar]
- Roka, L.; Koudounas, K.; Daras, G.; Zoidakis, J.; Vlahou, A.; Kalaitzis, P.; Hatzopoulos, P. Proteome of Olive Non-Glandular Trichomes Reveals Protective Protein Network against (a)Biotic Challenge. J. Plant Physiol. 2018, 231, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.; Almonte, L.; Bahamonde, H.A.; Galindo-Bernabeu, A.; Sáenz-Arce, G.; Colchero, J. Chemical and Structural Heterogeneity of Olive Leaves and Their Trichomes. Commun. Biol. 2024, 7, 352. [Google Scholar] [CrossRef]
- Razouk, R.; Hssaini, L.; Alghoum, M.; Adiba, A.; Hamdani, A. Phenotyping Olive Cultivars for Drought Tolerance Using Leaf Macro-Characteristics. Horticulturae 2022, 8, 939. [Google Scholar] [CrossRef]
- Glenn, D.M.; Prado, E.; Erez, A.; McFerson, J.; Puterka, G.J. A Reflective, Processed-Kaolin Particle Film Affects Fruit Temperature, Radiation Reflection, and Solar Injury in Apple. J. Am. Soc. Hortic. Sci. 2002, 127, 188–193. [Google Scholar] [CrossRef]
- Jifon, J.L.; Syvertsen, J.P. Kaolin Particle Film Applications Can Increase Photosynthesis and Water Use Efficiency of `Ruby Red’ Grapefruit Leaves. J. Am. Soc. Hortic. Sci. 2003, 128, 107–112. [Google Scholar] [CrossRef]
- Le Grange, M.; Wand, S.J.E.; Theron, K.I. Effect of Kaolin Applications on Apple Fruit Quality and Gas Exchange of Apple Leaves. Acta Hortic. 2004, 636, 545–550. [Google Scholar] [CrossRef]
- Rosati, A.; Metcalf, S.G.; Buchner, R.P.; Fulton, A.E.; Lampinen, B.D. Physiological Effects of Kaolin Applications in Well-Irrigated and Water-Stressed Walnut and Almond Trees. Ann. Bot. 2006, 98, 267–275. [Google Scholar] [CrossRef]
- Gindaba, J.; Wand, S.J.E. Comparative Effects of Evaporative Cooling, Kaolin Particle Film, and Shade Net on Sunburn and Fruit Quality in Apples. HortScience 2005, 40, 592–596. [Google Scholar] [CrossRef]
- Wünsche, J.N.; Lombardini, L. “Surround” Particle Film Applications—Effects on Whole Canopy Physiology of Apple. Acta Hortic. 2004, 636, 565–571. [Google Scholar] [CrossRef]
- Saour, G.; Makee, H. Effects of Kaolin Particle Film on Olive Fruit Yeld, Oil Content and Quality. Adv. Hortic. Sci. 2003, 17, 1000–1003. [Google Scholar] [CrossRef]
- Conversa, G.; Pacifico, S.; La Rotonda, P.; Lazzizera, C.; Bonasia, A.; Elia, A. Foliar Application of Natural Zeolites Affects the Growth and Productivity of Processing Tomato. Eur. J. Agron. 2024, 154, 127100. [Google Scholar] [CrossRef]
- Petoumenou, D.G. Enhancing Yield and Physiological Performance by Foliar Applications of Chemically Inert Mineral Particles in a Rainfed Vineyard under Mediterranean Conditions. Plants 2023, 12, 1444. [Google Scholar] [CrossRef] [PubMed]
- Calzarano, F.; Seghetti, L.; Pagnani, G.; Di Marco, S. Italian Zeolitites in the Control of Grey Mould and Sour Rot and Their Effect on Leaf Reflectance, Grape and Wine. Agriculture 2020, 10, 580. [Google Scholar] [CrossRef]
- Valentini, G.; Pastore, C.; Allegro, G.; Mazzoleni, R.; Colucci, E.; Filippetti, I. Foliar Application of Kaolin and Zeolites to Adapt the Adverse Effects of Climate Change in Vitis Vinifera L. Cv. Sangiovese. BIO Web Conf. 2022, 44, 1003. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Valentini, G.; Pastore, C.; Allegro, G.; Gottardi, D.; Patrignani, F.; Spinelli, F.; Filippetti, I. A Comprehensive Study on the Effect of Foliar Mineral Treatments on Grapevine Epiphytic Microorganisms, Flavonoid Gene Expression, and Berry Composition. Oeno One 2024, 58. [Google Scholar] [CrossRef]
- Yang, X.; Tang, J.; Mustard, J.F. Beyond Leaf Color: Comparing Camera-Based Phenological Metrics with Leaf Biochemical, Biophysical, and Spectral Properties throughout the Growing Season of a Temperate Deciduous Forest. J. Geophys. Res. Biogeosci. 2014, 119, 181–191. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, L.; Ding, Y.; Yao, X.; Wu, X.; Weng, F.; Li, G.; Liu, Z.; Tang, S.; Ding, C.; et al. Nitrogen Fertilizer Application Affects Lodging Resistance by Altering Secondary Cell Wall Synthesis in Japonica Rice (Oryza sativa). J. Plant Res. 2017, 130, 859–871. [Google Scholar] [CrossRef]
- Plavcová, L.; Hacke, U.G.; Almeida-Rodriguez, A.M.; Li, E.; Douglas, C.J. Gene Expression Patterns Underlying Changes in Xylem Structure and Function in Response to Increased Nitrogen Availability in Hybrid Poplar. Plant Cell Environ. 2013, 36, 186–199. [Google Scholar] [CrossRef]
- Penuelas, J.; Filella, I.; Serrano, L.; Savé, R. Cell Wall Elasticity and Water Index (R970 Nm/R900 Nm) in Wheat under Different Nitrogen Availabilities. Int. J. Remote Sens. 1996, 17, 373–382. [Google Scholar] [CrossRef]
- Quezada, J.C.; Bragazza, L. Foliar Applications of a Zeolite-Based Biostimulant Affect Soil Enzyme Activity and N Uptake in Maize and Wheat under Different Levels of Nitrogen Fertilization. J. Plant Nutr. 2024, 47, 501–513. [Google Scholar] [CrossRef]
- Valentini, G.; Pastore, C.; Allegro, G.; Muzzi, E.; Seghetti, L.; Filippetti, I. Application of Kaolin and Italian Natural Chabasite-Rich Zeolitite to Mitigate the Effect of Global Warming in Vitis Vinifera l. Cv. Sangiovese. Agronomy 2021, 11, 1035. [Google Scholar] [CrossRef]
- Regni, L.; Proietti, P. Effects of Nitrogen Foliar Fertilization on the Vegetative and Productive Performance of the Olive Tree and on Oil Quality. Agriculture 2019, 9, 252. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).