Valorization of Wasted Plant Parts: Mineral Bioavailability, Antioxidant, and Antimicrobial Properties of Wasted Aerial Parts of Selected Root Vegetables
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Phenolics and Antioxidant Extraction
2.3. Phenolic Determination
2.4. Flavonoids Determination
2.5. Antioxidant Activity
2.6. Determination of Anthocyanin Content
2.7. Determination of γ-Aminobutyric Acid Content
2.8. Vitamin C Measurement
2.9. HPLC Analysis of Phenolic Compounds
2.10. Antimicrobial Activity
2.11. Total and Available Mineral Determination
2.12. Statistical Analysis
3. Results and Discussion
3.1. Free, Bound, and Total Phenolic and Flavonoid Contents of Aerial Parts of Selected Root Vegetables
3.2. Antioxidant Activity and Vitamin C, GABA, and Anthocyanin Contents of Aerial Parts of Selected Root Vegetables
3.3. Phenolic Compounds of Aerial Parts of Selected Root Vegetables
3.4. Antimicrobial Activities of Aerial Part of Root Vegetables Extracts Against Different Bacterial Species
3.5. Mineral Content and Availability of Aerial Parts of Root Vegetables
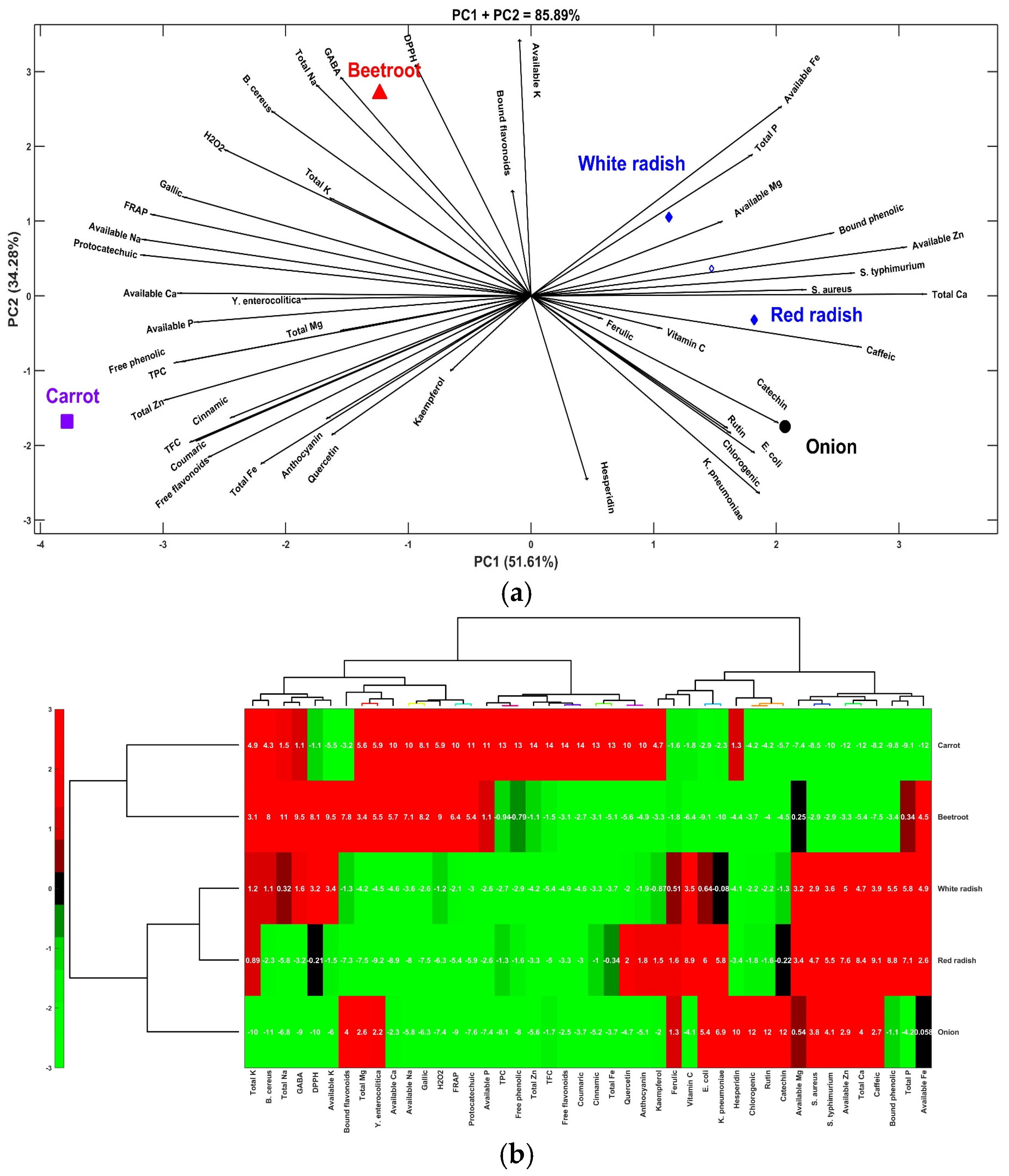
3.6. Multivariate Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 14 October 2024).
- Zhou, Y.; Tian, Y.; Yang, B. Root vegetable side streams as sources of functional ingredients for food, nutraceutical and pharmaceutical applications: The current status and future prospects. Trends Food Sci. Technol. 2023, 137, 1–16. [Google Scholar]
- Jiménez-Moreno, N.; Esparza, I.; Bimbela, F.; Gandía, L.M.; Ancín-Azpilicueta, C. Valorization of selected fruit and vegetable wastes as bioactive compounds: Opportunities and challenges. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2061–2108. [Google Scholar]
- Javed, A.; Ahmad, A.; Tahir, A.; Shabbir, U.; Nouman, M.; Hameed, A. Potato peel waste—Its nutraceutical, industrial and biotechnological applications. AIMS Agric. Food 2019, 4, 807–823. [Google Scholar]
- Mohammed, E.A.; Abdalla, I.G.; Alfawaz, M.A.; Mohammed, M.A.; Al Maiman, S.A.; Osman, M.A.; Yagoub, A.E.A.; Hassan, A.B. Effects of Extraction Solvents on the Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activity in the Aerial Part of Root Vegetables. Agriculture 2022, 12, 1820. [Google Scholar] [CrossRef]
- Asadi, S.Z.; Khan, M.A. The effect of beetroot (Beta vulgaris L.) leaves powder on nutritional, textural, sensorial and antioxidant properties of cookies. J. Culin. Sci. Technol. 2021, 19, 424–438. [Google Scholar] [CrossRef]
- Leite, C.W.; Boroski, M.; Boeing, J.S.; Aguiar, A.C.; Franca, P.B.; De Sousa, N.E.; Visentainer, J.V. Chemical characterization of leaves of organically grown carrot (Dacus carota L.) in various stages of development for use as food. Ciênc. Tecnol. Aliment. Camp. 2011, 31, 735–738. [Google Scholar]
- Tamayo Tenorio, A.; Gieteling, J.; De Jong, G.A.H.; Boom, R.M.; Van Der Goot, A.J. Recovery of protein from green leaves: Overview of crucial steps for utilization. Food Chem. 2016, 203, 402–408. [Google Scholar]
- Song, R.; Ismail, M.; Baroutian, S.; Farid, M. Effect of subcritical water on the extraction of bioactive compounds from carrot leaves. Food Bioprocess Technol. 2018, 11, 1895–1903. [Google Scholar]
- Beevi, S.S.; Narasu, M.L.; Gowda, B.B. Polyphenolics profile, antioxidant and radical scavenging activity of leaves and stem of Raphanus sativus L. Plant Foods Hum. Nutr. 2010, 65, 8–17. [Google Scholar]
- Kurnia, D.; Ajiati, D.; Heliawati, L.; Sumiarsa, D. Antioxidant Properties and Structure-Antioxidant Activity Relationship of Allium Species Leaves. Molecules 2021, 26, 7175. [Google Scholar] [CrossRef]
- Melse-Boonstra, A. Bioavailability of Micronutrients from Nutrient-Dense Whole Foods: Zooming in on Dairy, Vegetables, and Fruits. Front. Nutr. 2020, 7, 101. [Google Scholar]
- Finocchiaro, F.; Ferrari, B.; Gianinetti, A.; Dall’asta, C.; Galaverna, G.; Scazzina, F.; Pellegrini, N. Characterization of antioxidant compounds of red and white rice and changes in total antioxidant capacity during processing. Mol. Nutr. Food Res. 2007, 51, 1006–1019. [Google Scholar]
- Waterhouse, A.L. Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar]
- Chang, S.T.; Wu, J.H.; Wang, S.Y.; Kang, P.L.; Yang, N.S.; Shyura, L.F. Antioxidant activity of extracts from Acacia confusa bark and heart wood. J. Agric. Food Chem. 2001, 49, 3420–3424. [Google Scholar] [CrossRef]
- Gulcin, I.; Oktay, M.; Kufre, I.; Vioglu, O.; Aslan, A. Determination of antioxidant activity of lichen Cetraria islandica (L) Ach. J. Ethnopharmacol. 2002, 79, 325–329. [Google Scholar] [PubMed]
- Jayaprakasha, G.K.; Jaganmohan, R.L.; Sakariah, K.K. Antioxidant activities of flavidin in different in vitro model systems. Bioorg. Med. Chem. 2004, 12, 5141–5146. [Google Scholar]
- Egbuna, C.; Ifemeje, J.C.; Maduaka, M.C.; Tijjani, H.; Udedi, S.C.; Nwaka, A.C.; Ifemeje, M.O. Phytochemical test methods: Qualitative, Quantitative, and proximate analysis. In Phytochemistry: Fundamentals, Modern Techniques, and Application; Egbuna, C., Ifemeje, J.C., Udedi, S.C., Kumar, S., Eds.; CRC and Apple Academic Press, Inc.: Oakville, ON, Canada, 2018; pp. 381–425. [Google Scholar]
- Zhang, Q.; Xiang, J.; Zhang, L.; Zhu, X.; Evers, J.; van der Werf, W.; Duan, L. Optimizing soaking and germination conditions to improve gamma-aminobutyric acid content in japonica and indica germinated brown rice. J. Funct. Foods 2014, 10, 283–291. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association Office: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Brandt, A.L.; Castillo, A.; Harris, K.B.; Keeton, J.T.; Hardin, M.D.; Taylor, T.M. Inhibition of Listeria monocytogenes by food antimicrobials applied singly and in combination. J. Food Sci. 2010, 75, M557–M563. [Google Scholar]
- Chapman, H.D.; Pratt, P.F. Method for the Analysis of Soil, Plant and Water, 2nd ed.; California University Agricultural Division; Priced Publication; Oakland, CA, USA, 1982; p. 170. [Google Scholar]
- Chauhan, B.M.; Mahjan, L. Effect of natural fermentation on the extractability of minerals from pearl millet flour. J. Food Sci. 1988, 53, 1576–1577. [Google Scholar]
- Khan, P.S.; Khan, S.S.; Siddique, R. Radish (Raphanus Sativus): Potential Antioxidant role of Bioactive Compounds extracted from radish leaves—A review. Pakistan J. Med. Health Sci. 2022, 16, 2–4. [Google Scholar]
- Gawlik-Dziki, U.; Dziki, L.; Anisiewicz, J.; Habza-Kowalska, E.; Sikora, M.; Dziki, D. Leaves of White Beetroot As a New Source of Antioxidant and Anti-Inflammatory Compounds. Plants 2020, 9, 944. [Google Scholar] [CrossRef]
- Bardakçi, M.S.; Özçelik, A.; Karacabey, E. Does Daucus carota L. leaf provide a high potential as a source of bioactive constituents: A case study about the influences of process/storage conditions. Food Sci. Nutr. 2024, 12, 5882–5889. [Google Scholar]
- Yuasa, M.; Kawabeta, K.; Morikawa, M.; Iwami, M.; Tominaga, M. Antioxidant and taste properties of fresh onion (Allium cepa L.). leaves. J. Food Measur. Character 2021, 15, 1083–1091. [Google Scholar]
- Rubóczki, T.; Hájos, M.T. Leaf and root evaluation of bioactive compounds of different beetroot varieties. Acta Agrar. Debreceniensis 2018, 74, 135–139. [Google Scholar]
- Khazail, M.H.H.; Asadi-Gharneh, H.A. Evaluation of nitrate accumulation, bioactive compounds and antioxidant activity in leaves of radish cultivars. Inter. J. Veget. Sci. 2023, 29, 481–491. [Google Scholar]
- Goyeneche, R.; Roura, S.; Ponce, A.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Uribe, E.; Di Scala, K. Chemical characterization and antioxidant capacity of red radish (Raphanus sativus L.) leaves and roots. J. Funct. Foods 2015, 16, 256–264. [Google Scholar]
- Chihoub, W.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Harzallah-Skhiri, F.; Ferreira, I.C.F.R. Valorization of the green waste parts from turnip, radish and wild cardoon: Nutritional value, phenolic profile and bioactivity evaluation. Food Res. Inter. 2019, 126, 108651. [Google Scholar]
- Eugenio, M.H.A.; Pereira, R.G.F.A.; Abreu, W.C.D.; Pereira, M.C.D.A. Phenolic compounds and antioxidant activity of tuberous root leaves. Int. J. Food Proper. 2017, 20, 2966–2973. [Google Scholar]
- Battistella Lasta, H.F.; Lentz, L.; Gonçalves Rodrigues, L.G.; Mezzomo, N.; Vitali, L.; Salvador Ferreira, S.R. Pressurized liquid extraction applied for the recovery of phenolic compounds from beetroot waste. Biocatal. Agric. Biotechnol. 2019, 21, 101353. [Google Scholar]
- Burri, S.C.M.; Ekholm, A.; kansson, Å.; Tornberg, E.; Rumpunen, K. Antioxidant capacity and major phenol compounds of horticultural plant materials not usually used. J. Funct. Foods 2017, 38, 119–127. [Google Scholar] [PubMed]
- Chihoub, W.; Sassi, A.; Amina, C.M.; Souilem, F.; Ayeb, A.E.; Djlassi, B.; Ascrizzi, R.; Flamini, G.; Harzallah-Skhiri, F. Valorization of the Green Waste from Two Varieties of Fennel and Carrot Cultivated in Tunisia by Identification of the Phytochemical Profile and Evaluation of the Antimicrobial Activities of Their Essentials Oils. Chem. Biodivers. 2019, 16, e1800546. [Google Scholar]
- Thampi, N.; Shalini, J.V. In vitro antibacterial studies of fresh green onion leaves and the characterization of its bioactive compounds using Fourier transform infrared spectroscopy (FTIR). J. Chem. Pharmaceut. Res. 2015, 7, 1757–1766. [Google Scholar]
- Ahmadi, S.; Soleimanian-Zad, S.; Zaeim, D. Antibacterial and Antifungal Activity of the Aqueous and Methanolic Extracts and Essential Oils of Red Beets Beta vulgaris Leaves. Zahedan J. Res. Med. Sci. 2020, 22, e83725. [Google Scholar]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Encinar, J.A.; Rodríguez-Díaz, J.C.; Micol, V. Antimicrobial capacity of plant polyphenols against Gram-positive bacteria: A comprehensive review. Curr. Med. Chem. 2020, 27, 2576–2606. [Google Scholar] [PubMed]
- Mandal, M.K.; Domb, A.J. Antimicrobial Activities of Natural Bioactive Polyphenols. Pharmaceutics 2024, 16, 718. [Google Scholar] [CrossRef]
- Biondo, P.B.F.; Boeing, J.S.; Barizao, É.O.; de Souza, N.E.; Matsushita, M.; de Oliveira, C.C.; Baroski, M.; Visentainer, J.V. Evaluation of beetroot (Beta vulgaris L.) leaves during its developmental stages: A chemical composition study. Food Sci. Technol. Camp. 2014, 34, 94–101. [Google Scholar]
- Mella, C.; Rojas, N.; Calderon-Bravo, H.; Munoz, L.A. Evaluating Biocompounds in Discarded Beetroot (Beta vulgaris) Leaves and Stems for Sustainable Food Processing Solutions. Foods 2024, 13, 2603. [Google Scholar] [CrossRef]
- Székely, D.; Furulyás, D.; Seteger-mate, M. Investigation of Mineral and Vitamin C Contents in Different Parts of Beetroots (Beta vulgaris L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 615–620. [Google Scholar]
- Golubev, F.V.; Golubkina, N.A.; Gorbunov, Y.N. Mineral Composition of Wild Onions and Their Nutritional Value. Appl. Biochem. Microbiol. 2003, 39, 532–535. [Google Scholar] [CrossRef]
- Nemtinov, V.; Kostanchuk, Y.; Motyleva, S.; Katskaya, A.; Timasheva, L.; Pekhova, O.; Pashtetskiy, V.; Kulikov, I.; Medvedev, S.; Bokhan, A. Mineral composition of Allium cepa L. leaves of Southern subspecies. Potravinarstvo Slovak J. Food Sci. 2020, 14, 216–223. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Root Vegetables | Free Phenolic | Bound Phenolic | Total Phenolic |
| Onion | 16.9 ± 0.65 e | 2.7 ± 0.16 b | 19.6 ± 0.41 d |
| White radish | 29.5 ± 0.36 d | 3.8 ± 0.25 a | 33.3 ± 0.30 c |
| Red radish | 37.1 ± 0.36 b | 3.8 ± 0.40 a | 40.9 ± 0.38 b |
| Beetroot | 31.7 ± 0.95 c | 2.6 ± 0.33 b | 34.3 ± 0.64 c |
| Carrot | 66.3 ± 1.49 a | 2.2 ± 0.28 b | 68.8 ± 0.89 a |
| Root Vegetables | Free flavonoids | Bound flavonoids | Total flavonoids |
| Onion | 30.9 ± 0.78 b | 7.4 ± 0.23 b | 38.3 ± 0.51 b |
| White radish | 26.3 ± 0.72 b | 5.3 ± 0.68 c | 31.6 ± 0.71 c |
| Red radish | 27.8 ± 0.93 b | 1.1 ± 0.36 e | 28.9 ± 0.65 c |
| Beetroot | 24.3 ± 1.05 b | 8.6 ± 0.88 a | 32.9 ± 0.97 c |
| Carrot | 59.8 ± 0.72 a | 3.7 ± 0.79 d | 63.5 ± 0.76 a |
| Root Vegetables | DPPH (mg Trolox/g) | FRAP (mg AAE/g) | H2O2(%) |
| Onion | 4.4 ± 0.337 c | 0.5 ± 0.08 c | 76.1 ± 0.20 b |
| White radish | 5.3 ± 0.250 ab | 2.7 ± 0.33 bc | 80.6 ± 1.04 a |
| Red radish | 5.2 ± 0.083 b | 4.0 ± 0.11 b | 75.3 ± 0.94 b |
| Beetroot | 5.7 ± 0.087 a | 9.7 ± 0.09 a | 82.5 ± 1.10 a |
| Carrot | 4.98 ± 0.105 b | 9.9 ± 0.11 a | 81.1 ± 0.19 a |
| Root Vegetables | Vitamin C (mg/g) | GABA (mg/g) | Anthocyanin (mg/g) |
| Onion | 2.9 ± 0.69 c | 2.0 ± 0.040 c | 14.4 ± 0.06 d |
| White radish | 9.7 ± 0.23 b | 13.6 ± 0.09 b | 29.5 ± 0.00 b |
| Red radish | 17.6 ± 0.70 a | 13.6 ± 0.09 b | 23.7 ± 0.11 c |
| Beetroot | 2.4 ± 0.11 c | 30.1 ± 0.05 a | 9.35 ± 0.09 e |
| Carrot | 7.1 ± 0.23 bc | 13.8 ± 0.24 b | 40.9 ± 0.11 a |
| Phenolic Compound | Onion | White Radish | Red Radish | Beetroot | Carrot |
|---|---|---|---|---|---|
| Phenolic acids | |||||
| Gallic acid | 0.2 ± 0.02 c | 0.5 ± 0.02 b | 0.1 ± 0.00 d | 0.7 ± 0.08 a | 0.6 ± 0.05 a |
| Protocatechuic acid | 0.6 ± 0.04 c | 0.6 ± 0.05 c | 0.8 ± 0.01 b | 1.1 ± 0.10 a | 1.2 ± 0.03 a |
| Chlorogenic acid | 0.1 ± 0.03 a | Nd | Nd | Nd | Nd |
| Caffeic acid | 0.1 ± 0.01 b | 0.1 ± 0.01 b | 0.3 ± 0.01 a | Nd | 0.3 ± 0.00 a |
| Coumaric acid | 0.1 ± 0.00 b | 0.1 ± 0.00 b | 0.1 ± 0.00 b | 0.1 ± 0.01 b | 0.2 ± 0.01 a |
| Ferulic acid | 1.2 ± 0.14 b | 2.7 ± 0.08 a | 0.2 ± 0.06 c | 0.2 ± 0.04 c | 1.1 ± 0.09 b |
| Cinnamic acid | 1.0 ± 0.04 d | 4.0 ± 0.09 b | 2.4 ± 0.17 c | 0.4 ± 0.03 e | 9.9 ± 0.07 a |
| Flavonoids | |||||
| Catechin | 5.6 ± 0.17 a | 1.9 ± 0.08 b | 0.9 ± 0.06 c | 0.4 ± 0.05 d | 0.6 ± 0.07 d |
| Rutin | 16.5±0.03 a | 1.0 ± 0.07 b | 0.9 ± 0.04 c | 0.4 ± 0.04 d | 0.9 ± 0.03 c |
| Hesperidin | 3.7 ± 0.58 a | 0.5 ± 0.03 d | 0.8 ± 0.04 c | 0.8 ± 0.07 c | 1.9 ± 0.04 b |
| Quercetin | 7.7 ± 0.27 c | 15.9 ± 1.23 b | 14.0 ± 1.17 b | 3.4 ± 0.28 d | 24.7 ± 1.67 a |
| Kaempferol | 6.2 ± 0.95 c | 0.1 ± 0.01 d | 13.8 ± 0.89 a | 7.7 ± 0.62 c | 10.1 ± 0.21 b |
| Root Vegetables | Inhibition Zone (mm) | |||||
|---|---|---|---|---|---|---|
| Klebsiella pneumoniae | Yersinia enterocolitica | E. coli | S. typhimurium | S. aureus | B. cereus | |
| Onion | 12.7 ± 0.58 a | 11.0 ± 1.00 a | 12.3 ± 0.58 b | 13.0 ± 0.00 b | 9.3 ± 0.58 b | 8.0 ± 0.00 d |
| White radish | 11.7 ± 0.50 ab | 9.0 ± 0.00 b | 10.3 ± 0.58 d | 15.7 ± 1.015 a | 8.0 ± 0.00 c | 11.0 ± 1.00 b |
| Red radish | 12.0 ± 0.00 ab | 9.0 ± 0.00 b | 13.3 ± 0.58 a | 12.0 ± 0.50 c | 10.3 ± 0.29 a | 10.0 ± 0.00 c |
| Beet | 8.0 ± 0.00 c | 11.5 ± 0.50 a | 10.0 ± 0.00 d | 10.0 ± 1.00 d | 9.0 ± 0.50 b | 12.0 ± 0.00 a |
| Carrot | 11.0 ± 0.00 b | 11.0 ± 0.00 a | 11.0 ± 0.00 c | 9.0 ± 0.00 e | 7.7 ± 0.58 c | 11.0 ± 1.00 b |
| Vegetable | Macro elements | |||||||
| Ca | Mg | Na | K | |||||
| Total | Available | Total | Available | Total | Available | Total | Available | |
| Onion | 37.3 ± 1.16 ab | 37.1 ± 1.14 bc | 11.1 ± 0.48 a | 66.3 ± 0.72 c | 33.0 ± 1.37 c | 13.6 ± 2.15 b | 133.7 ± 2.53 e | 41.5 ± 1.75 c |
| White radish | 38.0 ± 2.00 ab | 31.4 ± 1.29 c | 11.0 ± 0.75 a | 61.2 ± 1.88 d | 77.7 ± 1.63 b | 12.4 ± 2.16 bc | 221.3 ± 1.86 a | 46.3 ± 0.28 ab |
| Red radish | 38.7 ± 1.55 a | 31.5 ± 1.41 c | 9.8 ± 0.48 a | 74.3 ± 1.62 a | 31.6 ± 2.34 c | 8.8 ± 0.76 c | 175.8 ± 2.81 d | 44.3 ± 2.40 bc |
| Beetroot | 35.3 ± 1.55 ab | 43.4 ± 1.09 ab | 10.9 ± 0.52 a | 70.3 ± 0.87 b | 124.1 ± 3.85 a | 45.9 ± 3.39 a | 184.7 ± 2.31 c | 50.2 ± 3.61 a |
| Carrot | 34.2 ± 1.68 b | 44.4 ± 0.80 a | 11.2 ± 0.35 a | 60.1 ± 1.53 d | 66.8 ± 2.54 b | 46.7 ± 1.65 a | 205.1 ± 2.55 b | 41.7 ± 3.08 c |
| Vegetable | Micro elements | |||||||
| P | Fe | Zn | ||||||
| Total | Available | Total | Available | Total | Available | |||
| Onion | 61.2 ± 3.29 c | 20.7 ± 2.79 b | 11.3 ± 0.58 b | 11.6 ± 2.63 c | 0.5 ± 0.00 c | 18.1 ± 1.39 b | ||
| White radish | 154.3 ± 4.04 a | 19.9 ± 2.20 b | 11.0 ± 1.73 b | 18.9 ± 2.42 a | 1.2 ± 0.06 b | 24.0 ± 1.90 a | ||
| Red radish | 135.3 ± 4.62 a | 29.7 ± 2.95 ab | 14.7 ± 2.08 b | 12.0 ± 1.38 c | 0.9 ± 0.06 bc | 21.4 ± 1.52 ab | ||
| Beetroot | 97.0 ± 3.46 b | 29.8 ± 1.28 ab | 9.0 ± 1.73 b | 15.6 ± 0.00 b | 1.0 ± 0.06 bc | 13.3 ± 1.84 c | ||
| Carrot | 50.7 ± 2.31 c | 36.4 ± 3.41 a | 22.7 ± 2.89 a | 3.9 ± 0.86 d | 5.1 ± 0.98 a | 7.9 ± 0.52 d | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, A.B.; Ahmed, I.A.M.; Alkaltham, M.S.; Qasem, A.A.; Mohammed, M.A.; Saleh, A.; Mohammed, B.M. Valorization of Wasted Plant Parts: Mineral Bioavailability, Antioxidant, and Antimicrobial Properties of Wasted Aerial Parts of Selected Root Vegetables. Horticulturae 2025, 11, 361. https://doi.org/10.3390/horticulturae11040361
Hassan AB, Ahmed IAM, Alkaltham MS, Qasem AA, Mohammed MA, Saleh A, Mohammed BM. Valorization of Wasted Plant Parts: Mineral Bioavailability, Antioxidant, and Antimicrobial Properties of Wasted Aerial Parts of Selected Root Vegetables. Horticulturae. 2025; 11(4):361. https://doi.org/10.3390/horticulturae11040361
Chicago/Turabian StyleHassan, Amro B., Isam A. Mohamed Ahmed, Mohammed Saeed Alkaltham, Akram A. Qasem, Mohammed A. Mohammed, Ali Saleh, and Belal M. Mohammed. 2025. "Valorization of Wasted Plant Parts: Mineral Bioavailability, Antioxidant, and Antimicrobial Properties of Wasted Aerial Parts of Selected Root Vegetables" Horticulturae 11, no. 4: 361. https://doi.org/10.3390/horticulturae11040361
APA StyleHassan, A. B., Ahmed, I. A. M., Alkaltham, M. S., Qasem, A. A., Mohammed, M. A., Saleh, A., & Mohammed, B. M. (2025). Valorization of Wasted Plant Parts: Mineral Bioavailability, Antioxidant, and Antimicrobial Properties of Wasted Aerial Parts of Selected Root Vegetables. Horticulturae, 11(4), 361. https://doi.org/10.3390/horticulturae11040361








