Study on Decomposition Characteristics of Early Spring Ephemeral Plant Litter in Various Forest Types
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Design and Data Collection
2.3. Statistical Analysis
3. Results
3.1. Soil
3.2. Litter Nutrients
3.3. Decomposition of ESPL
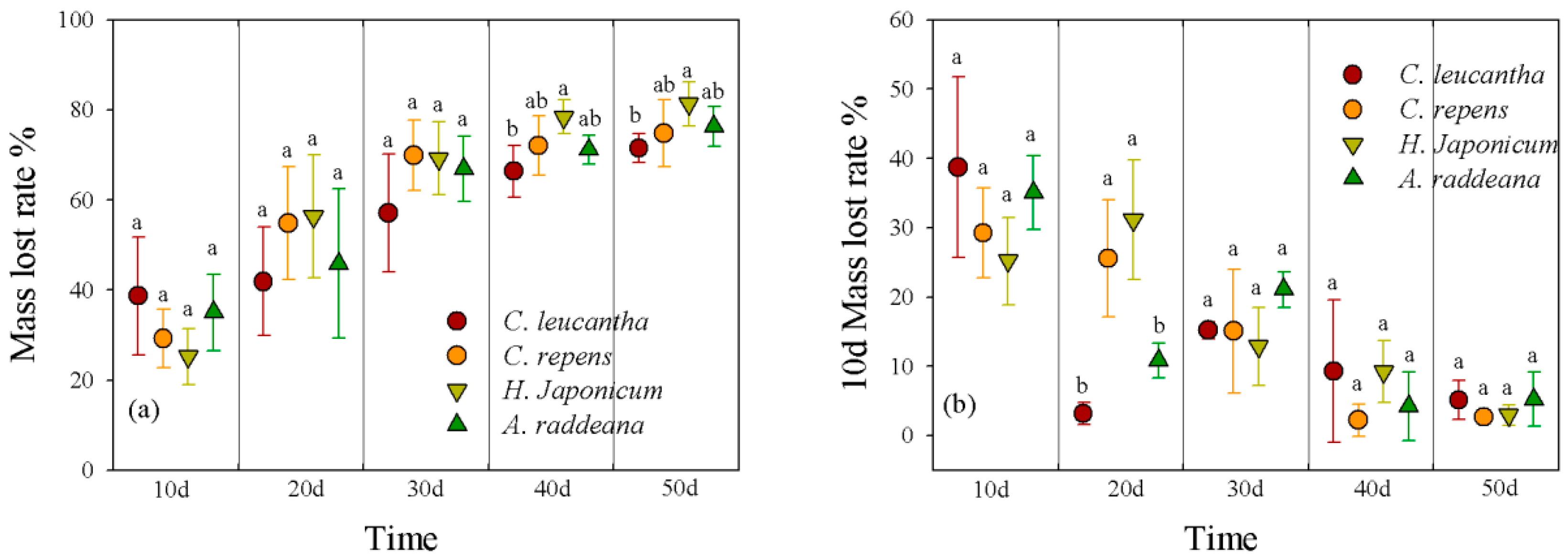
3.3.1. Mass Loss
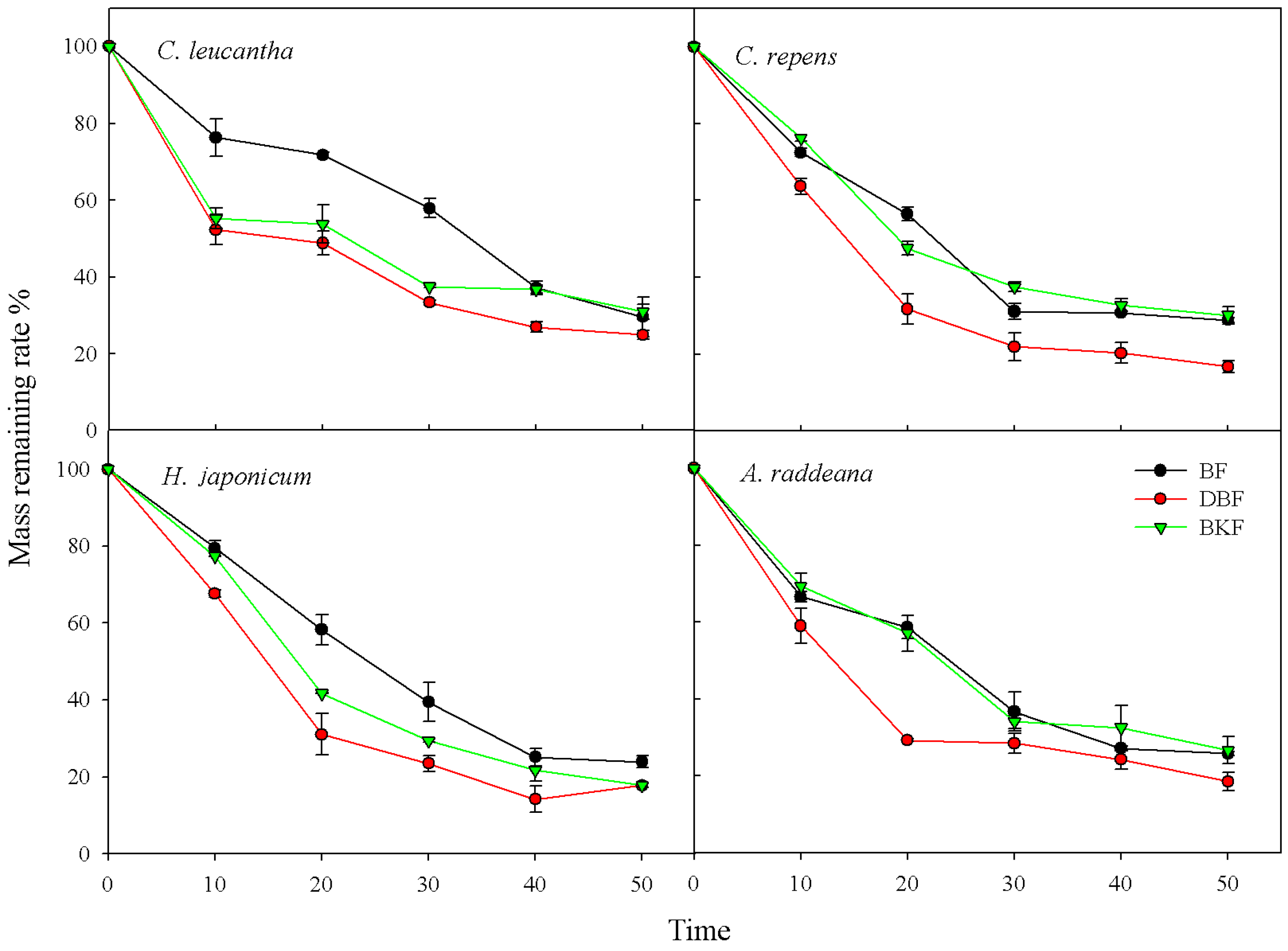
3.3.2. Litter Decomposition of Different Stands
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berg, B.; Mcclaugherty, C. Chemical Constituents as Rate Regulating: Initial Variation and Changes during Decomposition. New Tradit. Anal. Tech. 2014, 2014, 109–142. [Google Scholar]
- Ibrahima, A.; Joffre, R.; Gillon, D. Changes in litter during the initial leaching phase: An experiment on the leaf litter of Mediterranean species. Soil Biol. Biochem. 1995, 27, 931–939. [Google Scholar] [CrossRef]
- Coûteaux, M.M.; Bottner, P.; Berg, B. Litter decomposition, climate and liter quality. Trends Ecol. Evol. 1995, 10, 63–66. [Google Scholar]
- Meentemeyer, V. Macroclimate and lignin control of litter decomposition rates. Ecology 1978, 59, 465–472. [Google Scholar] [CrossRef]
- Bradford, M.A.; Berg, B.; Maynard, D.S.; Wieder, W.R.; Wood, S.A. Understanding the dominant controls on litter decomposition. J. Ecol. 2016, 104, 229–238. [Google Scholar] [CrossRef]
- Parton, W.; Silver, W.L.; Burke, I.C.; Grassens, L.; Harmon, M.E.; Currie, W.S.; King, J.Y.; Adair, E.C.; Brandt, L.A.; Hart, S.C. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 2007, 315, 361–364. [Google Scholar] [CrossRef]
- Keiser, A.D.; Bradford, M.A. Climate masks decomposer influence in a cross-site litter decomposition study. Soil Biol. Biochem. 2017, 107, 180–187. [Google Scholar] [CrossRef]
- Cruz, Y.A.; Garcia-Franco, J.G.; Zotz, G. Microsites and early litter decomposition patterns in the soil and forest canopy at regional scale. Biogeochemistry 2020, 151, 15–30. [Google Scholar] [CrossRef]
- Zhang, D.; Hui, D.; Luo, Y.; Zhou, G. Rates of litter decomposition in terrestrial ecosystems: Global patterns and controlling factors. J. Plant Ecol. 2008, 1, 85–93. [Google Scholar] [CrossRef]
- Djukic, I.; Kepfer-Rojas, S.; Schmidt, I.K.; Larsen, K.S.; Beier, C.; Berg, B.; Verheyen, K. Early stage litter decomposition across biomes. Sci. Total Environ. 2018, 628–629, 1369–1394. [Google Scholar] [CrossRef]
- Manzoni, S.; Piñeiro, G.; Jackson, R.B.; Jobbágy, E.G.; Kim, J.H. Analytical models of soil and litter decomposition: Solutions for mass loss and time-dependent decay rates. Soil Biol. Biochem. 2012, 50, 66–76. [Google Scholar]
- Eickmeier, W.G.; Schussler, E.E. Responses of the spring ephemeral claytonia virginica l. to light and nutrient manipulations and implications for the vernal-dam hypothesis. Bull. Torrey Bot. Club 1993, 120, 157–165. [Google Scholar]
- Anderson, W.B.; Eickmeier, W.G. Nutrient resorption in Claytonia virginica L.: Implications for deciduous forest nutrient cycling. Can. J. Bot. 2000, 78, 832–839. [Google Scholar]
- Muller, R.N.; Bormann, F.H. Role of Erythronium americanum Ker. in Energy Flow and Nutrient Dynamics of a Northern Hardwood Forest Ecosystem. Science 1976, 193, 1126–1128. [Google Scholar]
- Jiang, J.; Zhang, C.; Zhao, X. Relationship between plant species and area in 42 hm2 mixed forest plot in Jiaohe, Jilin Province. Chin. J. Plant Ecol. 2012, 36, 30–38. [Google Scholar]
- Xia, F.; Zhang, C.; Zhao, X.; Pan, C. Community structure and cluster analysis of early spring herbaceous plants. J. Northeast Norm. Univ. Nat. Sci. Ed. 2008, 40, 109–114. [Google Scholar]
- LY/T 1237-1999; Determination of Organic Matter in Forest Soils and Calculation of Carbon-to-Nitrogen Ratio. Ministry of Forestry of the People’s Republic of China: Beijing, China, 1999.
- LY/T 1271-1999; Forest Plants and Forest Litter Layer Total Nitrogen, Phosphorus, Potassium, Sodium, Calcium, Magnesium Determination. Ministry of Forestry of the People’s Republic of China: Beijing, China, 1999.
- LY/T 1228-2015; Method for Determination of Nitrogen in Forest Soils. Ministry of Forestry of the People’s Republic of China: Beijing, China, 2015.
- LY/T 1232-2015; Method for Determination of Phosphorus in Forest Soils. Ministry of Forestry of the People’s Republic of China: Beijing, China, 2015.
- LY/T 1234-2015; Method for Determination of Potassium in Forest Soils. Ministry of Forestry of the People’s Republic of China: Beijing, China, 2015.
- Bingrui, J. Litter decomposition and its underlying mechanisms. Chin. J. Plant Ecol. 2019, 43, 648–657. [Google Scholar]
- Rothstein, D.E. Spring Ephemeral Herbs and Nitrogen Cycling in a Northern Hardwood Forest: An Experimental Test of the Vernal Dam Hypothesis. Oecologia 2000, 124, 446–453. [Google Scholar]
- Warren, M.; Zou, X. Seasonal nitrogen retention in temperate hardwood forests. Acta Phytoecol. Sin. 2003, 27, 11–15. [Google Scholar]
- Berg, B.; Mcclaugherty, C. Plant Litter. Decomposition, Humus Formation, Carbon Sequestration; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Giweta, M. Role of litter production and its decomposition, and factors affecting the processes in a tropical forest ecosystem: A review. J. Ecol. Environ. 2020, 44, 9. [Google Scholar]
- Cornwell, W.K.; Cornelissen, J.H.C.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [PubMed]
- Tomlinson, K.W.; Poorter, L.; Sterck, F.J.; Borghetti, F.; Ward, D.; de Bie, S.; van Langevelde, F. Leaf adaptations of evergreen and deciduous trees of semi-arid and humid savannas on three continents. J. Ecol. 2013, 101, 430–440. [Google Scholar]
- Sun, T.; Hobbie, S.E.; Berg, B.; Zhang, H.; Httenschwiler, S. Contrasting dynamics and trait controls in first-order root compared with leaf litter decomposition. Proc. Natl. Acad. Sci. USA 2018, 115, 201716595. [Google Scholar]
- Mayer, P.M. Ecosystem and decomposer effects on litter dynamics along an old field to old-growth forest successional gradient. Acta Oecol. 2008, 33, 222–230. [Google Scholar]
- Muller, R.N. Nutrient Relations of the Herbaceous Layer in Deciduous Forest Ecosystems. In The Herbaceous Layer in Forests of Eastern North America; Oxford University Press: New York, NY, USA, 2014; Available online: https://www.semanticscholar.org/paper/Nutrient-Relations-of-the-Herbaceous-Layer-in-Muller/a8dbc5d7dbea3a7cc6f7ccad47e78652a04277a8 (accessed on 5 April 2024).
- Freschet, G.T.; Aerts, R.; Cornelissen, J.H.C. A plant economics spectrum of litter decomposability. Funct. Ecol. 2012, 26, 56–65. [Google Scholar]
- Roumet, C.; Birouste, M.; Picon-Cochard, C.; Ghestem, M.; Osman, N.; Vrignon-Brenas, S.; Cao, K.; Stokes, A. Root structure-function relationships in 74 species: Evidence of a root economics spectrum related to carbon economy. New Phytol. 2016, 210, 815–826. [Google Scholar]
- Santiago, L.S. Extending the leaf economics spectrum to decomposition: Evidence from a tropical forest. Ecology 2007, 88, 1126–1131. [Google Scholar] [PubMed]
- Berg, B.; Ekbohm, G. Litter mass-loss rates and decomposition patterns in some needle and leaf litter types. Long-term decomposition in a Scots pine forest. VII. Can. J. Bot. 1991, 69, 113–119. [Google Scholar]
- Keiser, A.D.; Warren, R.; Filley, T.; Bradford, M.A. Signatures of an abiotic decomposition pathway in temperate forest leaf litter. Biogeochemistry 2021, 153, 177–190. [Google Scholar]
- Rawlik, K.; Kasprowicz, M.; Mirosaw, N.; Andrzej, M.J. The afterlife of herbaceous plant species: A litter decomposition experiment in a temperate oak-hornbeam forest. For. Ecol. Manag. 2022, 507, 120008. [Google Scholar]
- Wang, Y.; Liu, X.; Wang, Z.; Shuai, H.; Jin, B.; Zhao, X. Litterfall decomposition and turnover of two Reaumuria soongorica communities in the Sangong River basin. Ecol. Sci. 2023, 42, 92–97. [Google Scholar]
- Mcclaugherty, C.A.; Pastor, J.; Aber, J.D.; Melillo, J.M. Forest Litter Decomposition in Relation to Soil Nitrogen Dynamics and Litter Quality. Ecology 1985, 66, 266–275. [Google Scholar]
- Ge, X.; Zeng, L.; Xiao, W.; Huang, Z.; Geng, X.; Tan, B. Effect of litter substrate quality and soil nutrients on forest litter decomposition: A review. Acta Ecol. Sin. 2013, 33, 102–108. [Google Scholar]
- Park, B.B.; Han, S.H.; Hernandez, J.O.; An, J.Y.; Youn, W.B.; Choi, H.S.; Jung, S. Leaf litter decomposition of deciduous Quercus acutissima Carruth. and evergreen Quercus glauca Thunb. in an inter-site experiment in three contrasting temperate forest stands in South Korea. Ann. For. Sci. 2021, 78, 34. [Google Scholar]
- Yang, K.; Zhu, J.; Zhang, W.; Zhang, Q.; Lu, D.; Zhang, Y.; Zheng, X.; Xu, S.; Wang, G. Litter decomposition and nutrient release from monospecific and mixed litters: Comparisons of litter quality, fauna and decomposition site effects. J. Ecol. 2022, 110, 1673–1686. [Google Scholar]
- Bourget, M.Y.; Fanin, N.; Fromin, N.; Hättenschwiler, S.; Roumet, C.; Shihan, A.; Huys, R.; Sauvadet, M.; Freschet, G.T. Plant litter chemistry drives long-lasting changes in the catabolic capacities of soil microbial communities. Funct. Ecol. 2023, 37, 2014–2028. [Google Scholar]
- Bai, S.H.; Gallart, M.; Singh, K.; Hannet, G.; Komolong, B.; Yinil, D.; Field, D.J.; Muqaddas, B.; Wallace, H.M. Leaf litter species affects decomposition rate and nutrient release in a cocoa plantation. Agric. Ecosyst. Environ. 2022, 324, 107705. [Google Scholar]
- Magill, A.H.; Aber, J.D.; Berntson, G.M.; Mcdowell, W.H.; Nadelhoffer, K.J.; Melillo, J.M.; Steudler, P. Long-term nitrogen additions and nitrogen saturation in two temperate forests. Ecosystems 2000, 3, 238–253. [Google Scholar]
- Hessen, D.O.; Ågren, G.I.; Anderson, T.R.; Elser, J.J.; De Ruiter, P.C. Carbon sequestration in ecosystems: The role of stoichiometry. Ecology 2004, 85, 1179–1192. [Google Scholar]
- Gosz, J.R.; Likens, G.E.; Bormann, F.H. Nutrient release from decomposing leaf and branch litter in the Hubbard Brook Forest, New Hampshire. Ecol. Monogr. 1973, 43, 173–191. [Google Scholar]
- Staaf, H.; Berg, B. Accumulation and release of plant nutrients in decomposing Scots pine needle litter. Long-term decomposition in a Scots pine forest II. Can. J. Bot. 1982, 60, 1561–1568. [Google Scholar]
- Aber, J.D.; Melillo, J.M. Nitrogen immobilization in decaying hardwood leaf litter as a function of initial nitrogen and lignin content. Can. J. Bot. 1982, 60, 2263–2269. [Google Scholar] [CrossRef]
- Taylor, B.R.; Parkinson, D.; Parsons, W.F.J. Nitrogen and Lignin Content as Predictors of Litter Decay Rates: A Microcosm Test. Ecology 1989, 70, 97–104. [Google Scholar] [CrossRef]
- Aber, J.D.; Melillo, J.M.; Mcclaugherty, C.A. Predicting long-term patterns of mass loss, nitrogen dynamics, and soil organic matter formation from initial fine litter chemistry in temperate forest ecosystems. Botany 1990, 68, 2201–2208. [Google Scholar] [CrossRef]
- Martínez-Yrízar, A.; Núez, S.; Búrquez, A. Leaf litter decomposition in a southern Sonoran Desert ecosystem, northwestern Mexico: Effects of habitat and litter quality. Acta Oecol. 2007, 32, 291–300. [Google Scholar] [CrossRef]
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decomposition in terrestrial ecosystems. Stud. Ecol. 1979, 5, 2772–2774. [Google Scholar]
- Gartner, T.B.; Cardon, Z.G. Site of leaf origin affects how mixed litter decomposes. Soil Biol. Biochem. 2006, 38, 2307–2317. [Google Scholar] [CrossRef]
- Aerts, R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: A triangular relationship. Oikos 1997, 79, 439–449. [Google Scholar] [CrossRef]
- Ge, J.L.; Xie, Z.Q.; Xu, W.T.; Zhao, C.M. Controls over leaf litter decomposition in a mixed evergreen and deciduous broad-leaved forest, Central China. Plant Soil 2017, 412, 345–355. [Google Scholar] [CrossRef]
- Wang, Q.W.; Pieristè, M.; Liu, C.; Kenta, T.; Robson, T.M.; Kurokawa, H. The contribution of photodegradation to litter decomposition in a temperate forest gap and understorey. New Phytol. 2021, 229, 2625–2636. [Google Scholar] [CrossRef]
- Mcgroddy, M.E.; Silver, W.L.; Oliveira, R.C.D. The Effect of Phosphorus Availability on Decomposition Dynamics in a Seasonal Lowland Amazonian Forest. Ecosystems 2004, 7, 172–179. [Google Scholar]
- Güsewell, S.; Verhoeven, J.T. Litter N: P ratios indicate whether N or P limits the decomposability of graminoid leaf litter. Plant Soil 2006, 287, 131–143. [Google Scholar]
- Cleveland, C.C.; Townsend, A.R.; Taylor, P.; Alvarez-Clare, S.; Bustamante, M.M.C.; Chuyong, G.; Dobrowski, S.Z. L Grierson, P.; Harms, K.E.; Houlton, B.Z.; et al. Relationships among net primary productivity, nutrients and climate in tropical rain forest: A pan-tropical analysis. Ecol. Lett. 2011, 14, 939–947. [Google Scholar] [PubMed]
| Forest Type | pH | C (g/kg) | HN (µg/kg) | AP (µg/kg) | AK (µg/kg) | TN (g/kg) | TP (g/kg) | TK (g/kg) |
|---|---|---|---|---|---|---|---|---|
| BF | 4.47 | 459.42 | 1553.84 | 2.57 | 350.29 | 16.58 | 4.17 | 1.33 |
| DBF | 5.31 | 332.26 | 261.87 | 10.30 | 344.58 | 10.70 | 1.15 | 11.83 |
| BKF | 5.99 | 103.56 | 954.21 | 24.08 | 354.91 | 3.89 | 4.25 | 19.62 |
| Time (d) | Mean Temperature ± SD °C | ||
|---|---|---|---|
| Birch Forest | Deciduous Broad-Leaved Forest | Broad-Leaved Korean Pine Forest | |
| 0–10 | 11.68 ± 1.09 | 11.52 ± 1.03 | 10.79 ± 1.12 |
| 10–20 | 13.33 ± 0.38 | 13.24 ± 0.34 | 12.24 ± 0.42 |
| 20–30 | 14.37 ± 0.49 | 14.57 ± 0.61 | 13.98 ± 0.58 |
| 30–40 | 15.89 ± 0.47 | 16.01 ± 0.38 | 15.03 ± 0.39 |
| 40–50 | 17.51 ± 0.88 | 17.83 ± 1.13 | 17.11 ± 1.29 |
| Mean | 14.56 a | 14.62 a | 13.82 a |
| Species | C (g/kg) | N (g/kg) | P (g/kg) | K (g/kg) | C/N | C/P |
|---|---|---|---|---|---|---|
| C. leucantha | 456.39 a | 15.20 a | 0.72 c | 190.38 b | 30.02 b | 635.13 a |
| C. repens | 446.90 a | 15.56 a | 3.20 b | 199.22 b | 28.72 b | 139.68 b |
| H. japonicum | 436.30 a | 16.34 a | 3.05 b | 347.12 a | 26.70 b | 143.26 b |
| A. raddeana | 447.12 a | 10.46 b | 4.34 a | 159.94 c | 42.74 a | 103.05 b |
| Source | Species | Time | Mass Lost | C | N | P | K | C/N | C/P |
|---|---|---|---|---|---|---|---|---|---|
| Mass lost rate | C. leucantha | 0.823 ** | 1 | −0.623 * | 0.091 | 0.481 | −0.618 * | −0.589 * | −0.570 * |
| C. repens | 0.838 ** | 1 | −0.270 | 0.723 ** | 0.788 ** | −0.579 * | −0.661 ** | −0.775 ** | |
| H. japonicum | 0.886 ** | 1 | 0.616 * | 0.770 ** | 0.586 * | −0.717 ** | −0.690 ** | −0.321 | |
| A. raddeana | 0.933 ** | 1 | −0.480 | 0.849 ** | −0.331 | −0.637 ** | −0.838 ** | 0.118 | |
| 10 d Mass lost rate | C. leucantha | −0.604 * | −0.324 | 0.716 ** | 0.457 | −0.396 | 0.253 | 0.146 | 0.667 ** |
| C. repens | −0.876 ** | −0.659 ** | 0.264 | −0.454 | −0.888 ** | 0.807 ** | 0.483 | 0.860 ** | |
| H. japonicum | −0.823 ** | −0.610 * | −0.452 | −0.611 * | −0.328 | 0.815 ** | 0.544 * | 0.007 | |
| A. raddeana | −0.780 ** | −0.656 ** | 0.387 | −0.796 ** | 0.471 | 0.392 | 0.802 ** | −0.150 |
| Species | Forest Type | Regression Equation | R2 | P | K (g·g−1·d−1) | t0.05 (d) | t0.95 (d) |
|---|---|---|---|---|---|---|---|
| C. leucantha | BF | y = 100.6054e−0.0220t | 0.9594 | 0.0006 | 0.0220 Bb | 32 | 136 |
| DBF | y = 92.3789e−0.0332t | 0.9588 | 0.0025 | 0.0332 Aa | 18 | 88 | |
| BKF | y = 90.8058e−0.0263t | 0.8814 | 0.0055 | 0.0263 ABa | 23 | 110 | |
| C. repens | BF | y = 98.9167−0.0301t | 0.9671 | 0.0004 | 0.0301 Bab | 23 | 99 |
| DBF | y = 98.9881e−0.0467t | 0.9747 | 0.0002 | 0.0467 Aa | 15 | 64 | |
| BKF | y = 98.5882e−0.0292t | 0.9662 | 0.0004 | 0.0292 Ba | 23 | 102 | |
| H. japonicum | BF | y = 102.6333e−0.0306t | 0.9876 | <0.0001 | 0.0306 Ba | 24 | 99 |
| DBF | y = 100.6109e−0.0475t | 0.9726 | 0.0003 | 0.0475 Aa | 15 | 63 | |
| BKF | y = 102.4126e−0.0388t | 0.9797 | 0.0002 | 0.0388 ABa | 18 | 78 | |
| A. raddeana | BF | y = 97.7255e−0.0301t | 0.9691 | 0.0002 | 0.0301 Ba | 22 | 99 |
| DBF | y = 96.0367e−0.0433t | 0.9451 | 0.0012 | 0.0433 Aa | 15 | 68 | |
| BKF | y = 97.8242e−0.0294t | 0.9775 | 0.0002 | 0.0294 Ba | 23 | 101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Zheng, J.; Fan, C. Study on Decomposition Characteristics of Early Spring Ephemeral Plant Litter in Various Forest Types. Horticulturae 2025, 11, 382. https://doi.org/10.3390/horticulturae11040382
Liu Q, Zheng J, Fan C. Study on Decomposition Characteristics of Early Spring Ephemeral Plant Litter in Various Forest Types. Horticulturae. 2025; 11(4):382. https://doi.org/10.3390/horticulturae11040382
Chicago/Turabian StyleLiu, Qiang, Jinping Zheng, and Chunnan Fan. 2025. "Study on Decomposition Characteristics of Early Spring Ephemeral Plant Litter in Various Forest Types" Horticulturae 11, no. 4: 382. https://doi.org/10.3390/horticulturae11040382
APA StyleLiu, Q., Zheng, J., & Fan, C. (2025). Study on Decomposition Characteristics of Early Spring Ephemeral Plant Litter in Various Forest Types. Horticulturae, 11(4), 382. https://doi.org/10.3390/horticulturae11040382





