Abstract
Loquat (Eriobotrya japonica Lindl.) is one of the most important subtropical evergreen fruit trees. However, due to the lack of widely applicable genetic transformation platforms, the research about gene functional characterization and molecular mechanisms is largely confined. In this study, the efficient protocol of protoplast isolation (the enzyme solution composed of 2.4% macerozyme R-10, 4.8% cellulase RS, dissolved in a 0.6 M mannitol solution) and the method of protoplast purification (CPW solution containing 5% sucrose and 11% mannitol) have been achieved with protoplast yields of 12.6 × 106/g·FW, reaching a viability rate of up to 91%. A protoplast transient gene expression system has been established with an efficiency of approximately 40% using GFP reporter gene. Using this reliable and efficient system, the protein localization characteristics of transcription factor EjDELLA, EjbHLH79, and marker gene OsPHT4 were also utilized for further analysis. To our knowledge, this is the first report on establishing an efficient system for protoplast isolation, purification, and transformation of loquat mesophyll. The system reported here will definitely promote rapid progress in breeding, genetic transformation, and molecular research.
1. Introduction
Loquat, belonging to the family Rosaceae, is one of the most popular subtropical evergreen fruit trees in the world [1]. In recent years, fundamental studies of loquat have made great progress in the genomic study [2,3], gene function identification [4], and molecular mechanisms involved in fruit or plant growth and development [5], fruit quality [6], resistance [7], and physiology [8]. However, due to its long juvenile period and large plant size, loquat exhibits recalcitrant to regeneration, resulting in the existing transformation syst still lacking widespread applicability [9]. Thus, a transformation system specific to loquat needs to be established urgently to improve fundamental studies into gene functional characterization including subcellular localization, protein–protein interaction, and transgenic studies.
Protoplasts are plant cells from which the cell wall has been enzymatically or mechanically removed, and they possess totipotency, sensitivity, and versatileness [10]. Protoplasts are a distinctive single-cell system that represent excellent materials for basic research and crop enhancement [11]. In recent years, protoplasts have gradually offered valuable insights into plant gene function and crop breeding via protoplast manipulation, such as transient transformation [12], single-cell sequencing [13], and somatic hybridization [14]. Although protoplasts have been successfully isolated in a number of crop plants such as wheat [15], rice [16], maize [17], citrus [18], and grapevine [19], it is still challenging for numerous horticultural woody plants [20].
Transient gene expression, due to its rapid and convenient process, has emerged as one of the most effective techniques in studying subcellular localization [21,22], protein complexes [23], gene promoter activity analysis [7], signal transduction pathway identification, and gene silencing [24]. Meanwhile, transient transformation exhibits the vast perspectives in DNA-free CRISPR technology in vivo for gene editing breeding [25,26,27]. DNA-free gene editing plants were successfully obtained in citrus through PEG-mediated transient transformation, which paved the way for the application of protoplast transient expression strategy for gene editing plant in woody plants [28]. Enabling a more direct targeting of individual cells, polyethylene glycol (PEG)-mediated protoplast transformation is the most common method for transient transformation. However, although there are numerous efficient protoplast isolation protocols for model plants and many crops are established, it is still challenging for most horticultural plants, which requires optimization of the efficient isolation and transfection methods.
In this study, young leaves of loquat ‘Huabai 1’ were used as the plant materials to develop the efficient protoplast isolation, purification, and transformation protocols. We established a protoplast isolation method and transformation system by optimizing every step specifically. The protoplast purification system was also optimized using the sugar-alcohol solution method. Then, the fluorescent marker and subcellular localization of EjDELLA, EjbHLH79, and OsPHT4 proteins were carried out to verify this system successfully. Our research reported here paves the way for the future biotechnology breeding and gene functional identification in the loquat.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
The white-fleshed loquat cultivar of ‘Huabai 1’ was selected as experimental material in this study. The mature seeds of ‘Huabai 1’ were sowed in 200 mL plastic pots filled with steam-sterilized artificial soil mixture, which was suitable for plant growth (black peat supplemented with washed perlite and vermiculite blended in a 1:1:1 volumetric ratio) in the greenhouse (25 °C, 3000 lx light intensity, 16 h/8 h (light/dark) photocycle). After approximately one-month of the light culturing, the young leaves sprouting from the seedlings (the young leaves’ sizes were 2 cm × 5 cm, approximately) were optimally suitable for mesophyll protoplast operation. For the reliability test experiments of the protoplast isolation system, the hypocotyls of the seedling (10–15 cm length) were available after three to four weeks following sowing in the dark, and the callus induced though anther culture [29] was sub-cultured monthly in solid culture medium (B5 + 1 mg·L−1 2,4-D + 0.5 mg·L−1 6-BA + 0.1 mg·L−1 NAA + 30 g·L−1 Sucrose + 8 g·L−1 Agar, pH 5.8) stored at 25 °C in the dark. Prior to digestion, the callus that had been cultured for 10 days in the suspension medium (MS + 30 g·L−1 Sucrose, without Agar, pH 5.8) was collected for protoplast operation. The blooming flowers were collected from donor trees planted in Center of Teaching and Research, College of Horticulture and Landscape Architecture, Southwest University (SWU), Chongqing, China.
2.2. Plasmid Construction
To optimize the transient expression procedure and demonstrate the applicability of the transient expression system, the transient expression of loquat mesophyll protoplast was performed by using pCAMBIA2300-35S vector with GFP tagging. In addition, the vectors of pCAMBIA1300-35s-EjDELLA-GFP, pCAMBIA1300-35s-EjbHLH79-GFP, and OsPH4-GFP [30] were transformed to verify the subcellular localization of the corresponding genes.
2.3. Protoplast Isolation
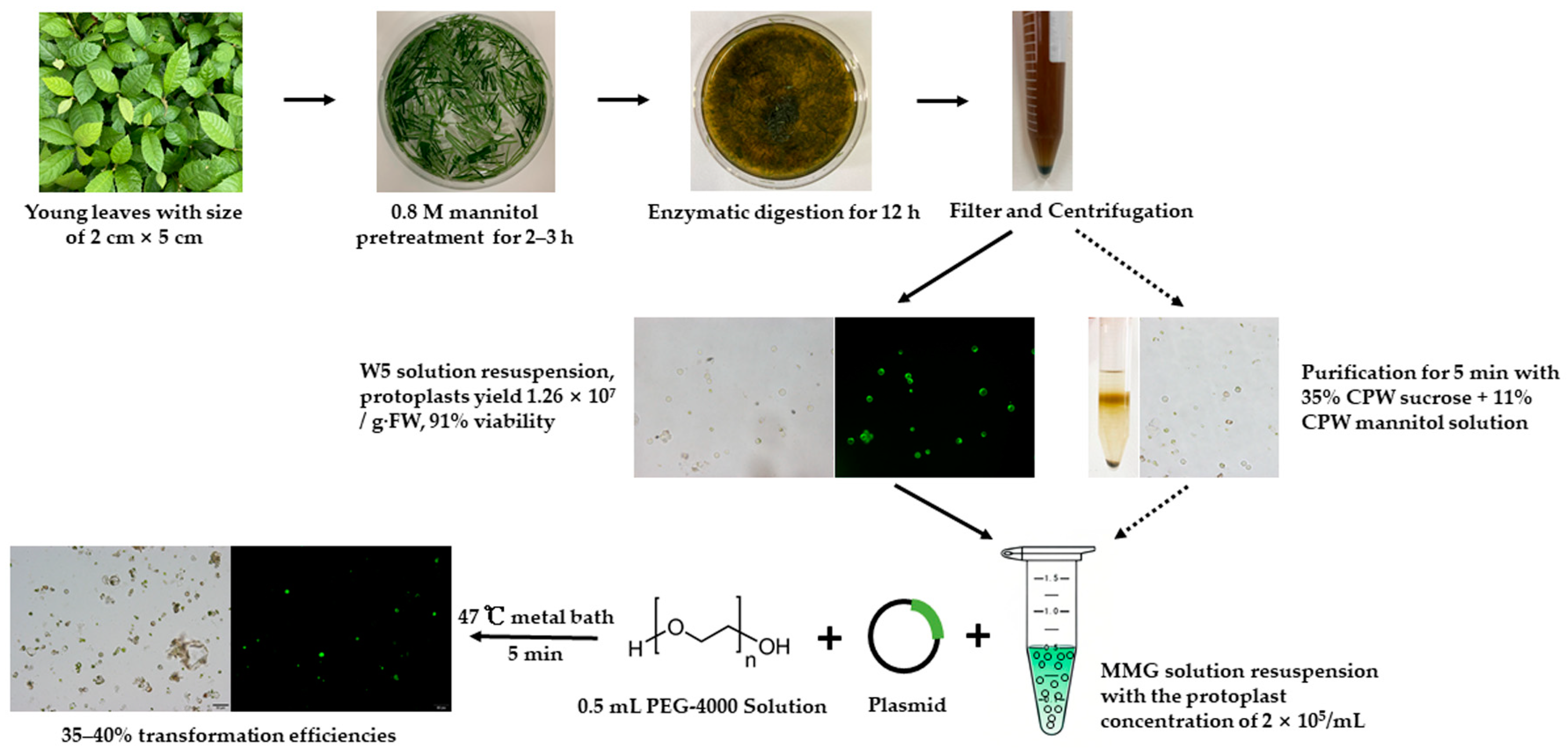
Five young leaves (about 1 g) were cut into thin shreds with a blade (Figure 1a,b). These thin shreds were first pre-treated by 0.8 M mannitol solution for 2–3 h and then directly transferred into the enzyme solution containing Cellulase RS, Macerozyme, mannitol, 10 mM MES (pH 5.8), 0.1% BSA, and 1 mM CaCl2 with horizontal shaking (40 rpm) at 27 °C in the dark overnight using an oscillating incubator (Zhichu, Shanghai, China). After digestion, the solution was diluted with 10 mL of W5 solution (154 mM NaCl, 125 mM CaCl2, 5 mM KCl, 2 mM MES (pH 5.8)) and the mixture continued to release the protoplast for additional 10 min with horizontal shaking at a speed of 40 rpm (Figure 1c). After that, the mixture was filtered through a 100 μm nylon mess screen to remove the undigested callus cells and debris. The resulting filtrate was transferred into a 15 mL centrifuge tube and then centrifuged at 100× g for 9 min using a table centrifuge (Eppendorf, Hamburg, Germany) (Figure 1d). The supernatant was carefully discarded and the protoplasts were re-suspended in the W5 solution to wash off the enzyme solution. After purification through mannitol-sucrose gradient centrifugation method, the protoplasts were transferred into a new tube and suspended by adding 10 mL of W5 solution. After centrifuge, the suspension solution was removed, and the amount of protoplast was calculated using a hemacytometer (Hausser Scientific, Horsham, PA, USA). Finally, the protoplasts were diluted to a density of 105–106/mL in the MMG solution [0.6 M Mannitol, 15 mM MgCl2, 4 mM MES (pH 5.8)].
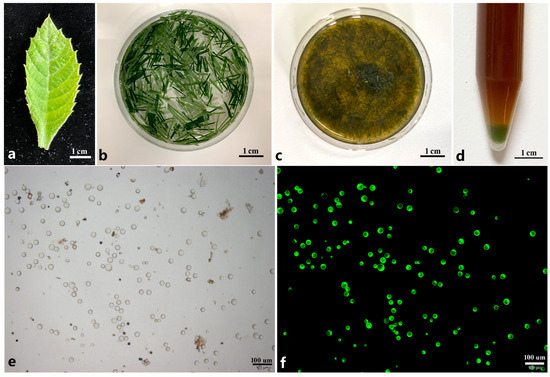
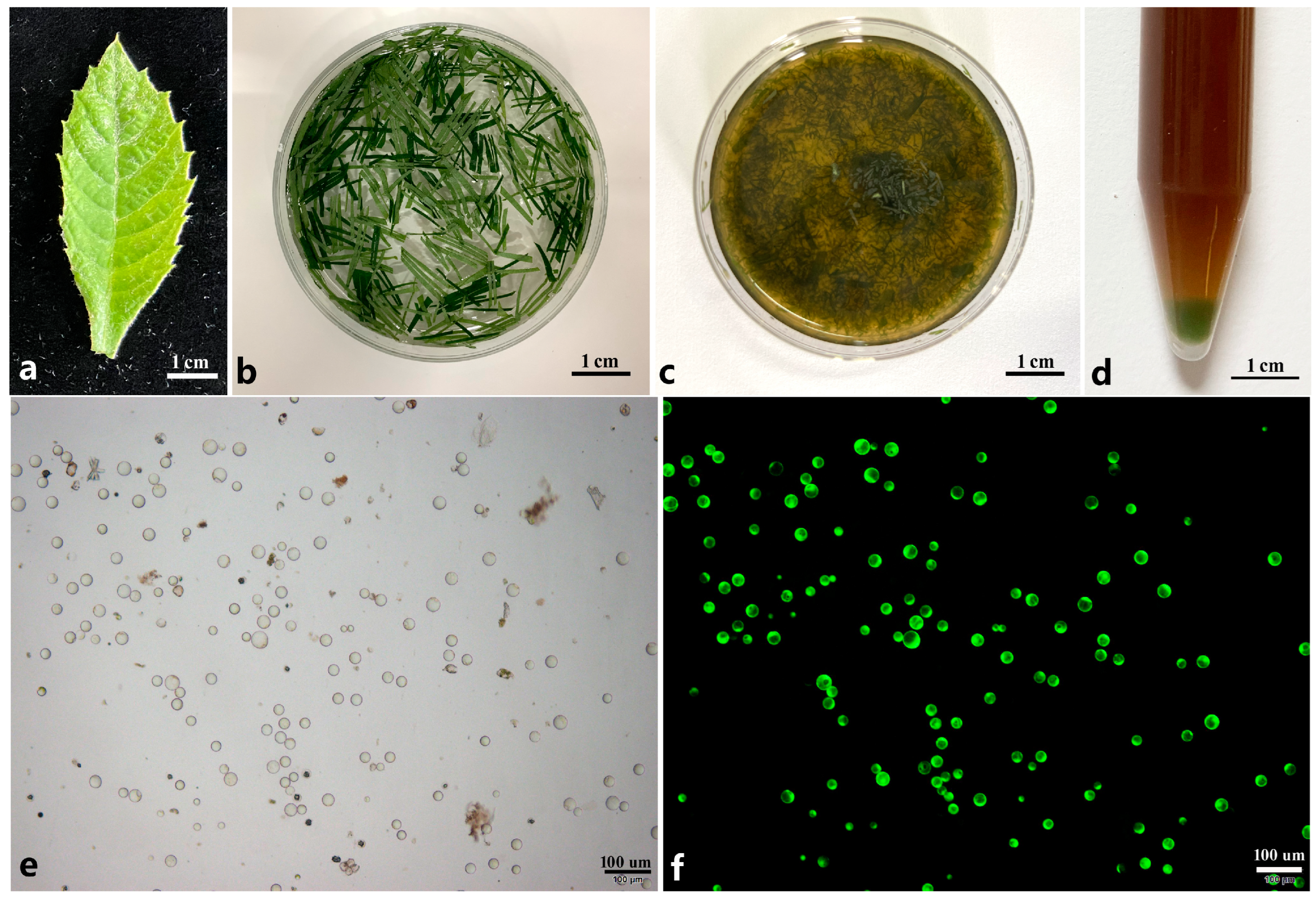
Figure 1.
Isolation of protoplasts from young leaf of loquat. (a) One-month-old young leaf used for protoplast isolation; (b) young leaves cut into thin shreds using a blade were pre-treated by mannitol solution; (c) young leaves were digested by enzyme solution; (d) protoplasts were collected at the tube bottom through centrifuge; (e) isolated protoplasts; (f) protoplasts were dyed by FDA solution under dark filed.
2.4. Protoplast Purification
The isolated protoplasts were suspended in 5 mL of CPW solution containing sucrose (21% w/v, 25% w/v, 29% w/v, 31% w/v, 33% w/v, 35% w/v, 37% w/v, 39% w/v). The CPW salt solution was formulated as follows: 0.2 mM KH2PO4, 1 mM KNO3, 1.35 mM CaCl2, 2.08 mM MgSO4, 0.0064 mM FeSO4·6H2O, 0.00096 mM KI, 0.00016 mM CuSO4. Subsequently, 2 mL of the CPW solution containing mannitol (11% w/v, 13% w/v, 15% w/v, 17% w/v, 19% w/v, 21% w/v) was added gently along the inner wall of the tube directly on the top of the sucrose layer to keep these two layers unmixed. Different combinations of sucrose and mannitol concentration gradients were used for exploring the optimal protoplast purification method (Table S1). After centrifuge for 5 min at 100× g, a ring of purified protoplasts would appear at the interface between the two layers.
2.5. Protoplast Transformation
The protoplast transformation was carried out using the polyethylene glycol (PEG)-mediated method as described previously [31] with modifications. Prior to the transformation, fresh PEG solution and the plasmid should be prepared. Briefly, the PEG solution consisted of 0.4 M mannitol, 5–80% PEG solution, and 0.1 mg/mL CaCl2. A total of 0.5 mL of protoplast suspension, with a cell concentration of 2 × 105/mL suspended in the MMG solution, was placed and retained in a 15 mL round-bottomed falcon tube and then mixed with the plasmid. After adding 0.5 mL of PEG solution, the mixed solution was shaken gently and thoroughly and then incubated at 47 °C for 5–30 min. Next, add 2 mL of W5 solution to dilute the mixture solution and shake it gently to stop the transformation reaction. Five minutes later, add an additional 2 mL of W5 solution further dilute the transformation solution and shake it gently once more. Finally, the mixture was centrifuged at 150× g to collect the protoplasts. These collected protoplasts were then re-suspended in 1.5 mL of WI solution (0.6 M mannitol, 4 mM KCl, 4 mM MES (pH 5.8)) and kept in the dark at room temperature for the subsequent direct analysis.
2.6. Data Analysis
After staining with FDA (fluorescein diacetate), the viability of the protoplasts was calculated under the ultraviolet light as (the number of viable protoplasts/the total number of protoplasts) × 100%. For each parameter, the experiment was repeated three times at least, and in each replicate, the number of protoplasts was calculated in at least ten microscope fields. Data and statistics analysis was performed using Microsoft Excel 2010 and the statistically significant difference was determined at the 5% level by using one-way analysis of variance (ANOVA) with SPSS Statistics 25.0 software (SPSS Inc., Chicago, IL, USA). Finally, the results of statistical analysis were presented using Prism 8 software (GraphPad Software, La Jolla, CA, USA).
3. Results
3.1. Exploration of an Optimal Enzyme Combination for Protoplast Isolation from Loquat Mesophyll
In this study, one-month-old leaves of loquat were used for protoplast isolation (Figure 1a). These leaves were cut into strips and then immersed in the enzyme solution for digestion (Figure 1b). Owing to the distinct compositions of cell walls in different plants, the digestion enzymes were optimized at first. The Cellulase (including Cellulase R-10 and Cellulase RS) was combined with the Pectolase Y-23 and Macerozyme R-10, respectively, at gradient concentrations under different osmotic pressures (0.6 M, 0.7 M, 0.8 M) (Table S2). The results showed that when using the enzyme combination of Cellulase RS and Pectolase Y-23 under different concentrations and osmotic pressures, clustering cells (Figure S1a), bursting cells (Figure S1b), or no cells (Figure S1c) were detected under the microscope. Moreover, no intact protoplasts were found. When Pectolase Y-23 in the enzyme combination was replaced by Macerozyme R-10, intact spherical cells were detected under the largest concentration of enzyme (Figure S1d). However, when the enzyme combination of Cellulase R-10 and Macerozyme R-10 was employed for protoplast isolation, impact protoplasts could only be successfully isolated in the enzyme solution at a high concentration. The results suggest that, for the isolation of loquat mesophyll protoplasts, the most efficient digestion solution is the enzyme combination of Cellulase RS and Macerozyme R-10.
3.2. Protoplast Isolation of Loquat Leaf via Optimizing Key Isolating Factors
To establish an efficient and stable method for protoplast isolation from loquat leaf, the key factors of digestion progress including pretreatment time, osmotic pressure, enzyme concentration, and digestion time were specifically optimized.
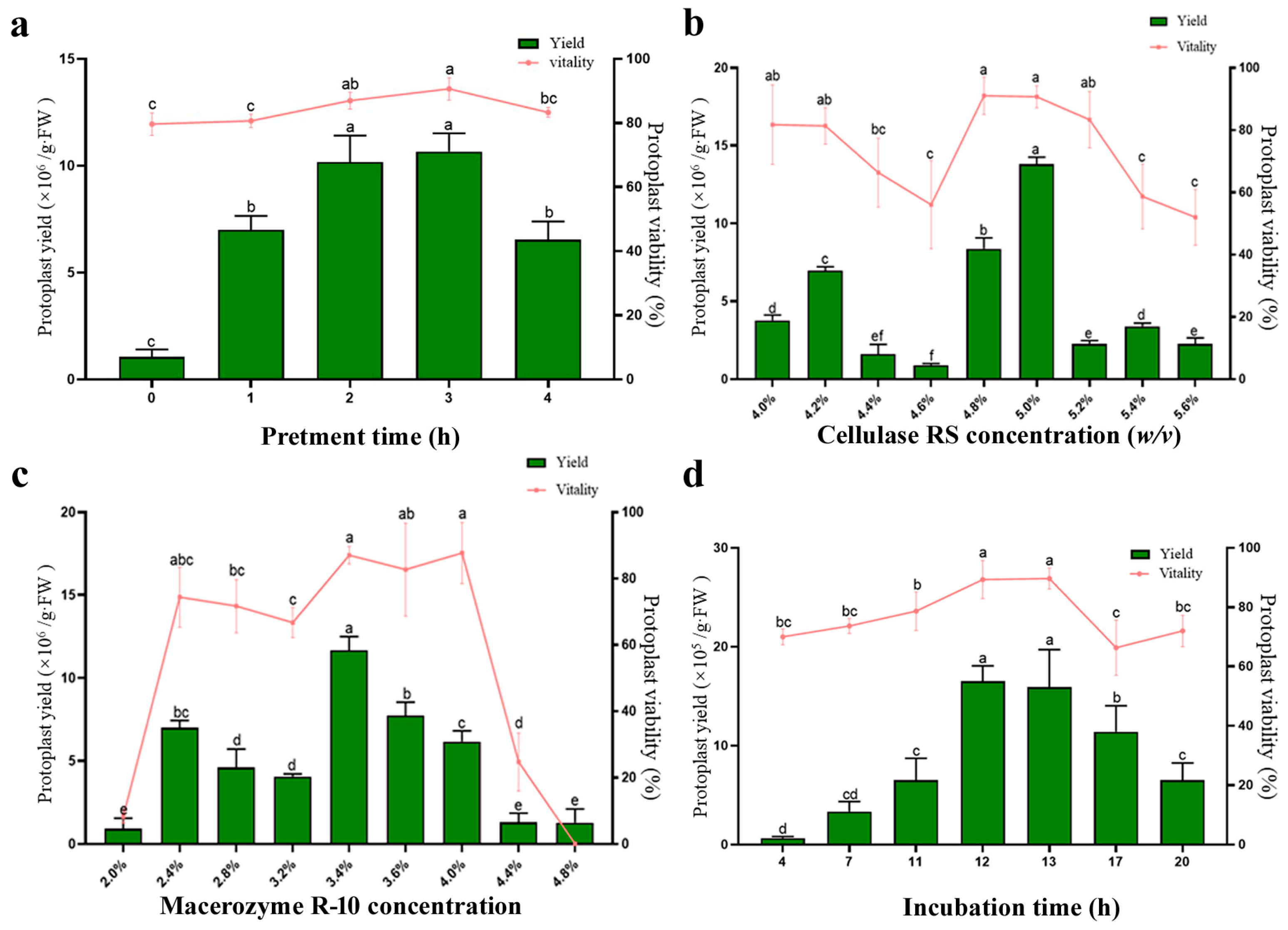
The effect of pretreatment of the experimental material prior to digestion was evaluated first. We found that, when the osmotic pressure of the pretreatment solution was 0.8 M mannitol, the loquat cell underwent incipient plasmolysis. Therefore, a 0.8 M mannitol pretreatment solution was chosen as optimal pretreatment solution for protoplast isolation. We tested different pretreatment durations (0 h, 1 h, 2 h, 3 h, 4 h), and the results indicated that when the osmotic pressure of pretreatment solution was 0.8 M mannitol, the isolation efficiency could be increased significantly, and a pretreatment time of 2–3 h yielded the highest protoplast output (Figure 2a).
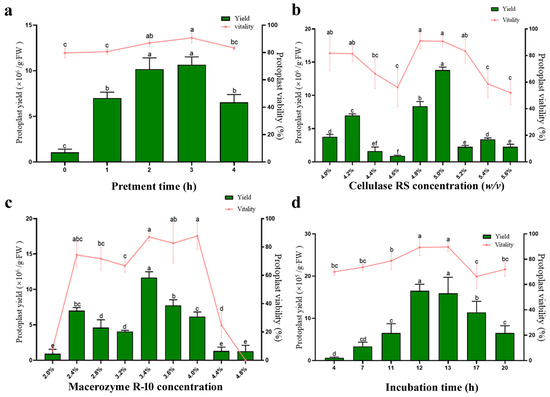
Figure 2.
Optimization of loquat protoplast isolation conditions. (a) Pretreatment time; (b) Cellulase RS concentration; (c) Macerozyme R-10 concentration; (d) incubation time. Different letters indicate a statistically significant difference at p ≤ 0.05.
Osmotic pressure is a critical factor for protoplast isolation. We tested the effects of different osmotic pressures (0.5 M, 0.6 M, 0.7 M, 0.75 M, 0.8 M, 0.9 M, 1.0 M mannitol) (Table 1). Both excessively high and low osmotic pressure could cause protoplast rupture, resulting in no detectable protoplasts after digestion. When the osmotic pressure of the digestion solution ranged from 0.7 M mannitol to 0.8 M mannitol, deformed protoplasts could be isolated from the leaves (Figure S2b,c). However, when the osmotic pressure was 0.6 M mannitol, a large number of intact and spherical protoplasts were observed (Figure S2a). The results indicated that the osmotic pressure of 0.6 M mannitol is optimal in the progress of protoplast isolation.

Table 1.
Mannitol concentration gradient for protoplast isolation.
To investigate the effect of enzyme concentration on the quantity and quality of protoplasts, different concentrations of Cellulase RS (ranging from 4.0% w/v to 5.6% w/v at 0.2% intervals) and Macerozyme R-10 (ranging from 2.0% w/v to 4.8% w/v at 0.4% intervals) were respectively employed for protoplast isolation (Figure 2b,c). A similar concentration-dependent trend was observed in both enzymes: protoplast yield initially increased with rising enzyme concentration. However, an excess enzyme concentration led to a decrease in both the yield and viability of protoplasts. The highest yield and viability were achieved by combining 5.0% w/v Cellulase RS with 3.4% w/v Macerozyme R-10.
To determine the optimal incubation time during the digestion process, the leaves were digested using the optimal enzyme concentration described above. Incubation periods ranging from 4 h to 20 h were evaluated (Figure 2d). The yield and viability of isolated intact protoplasts increased gradually with incubated time, reaching an optimal level after 12 h. However, a significant reduction in intact protoplast yield was observed longer than 17 h of incubation, presumably due to protoplast rupture caused by prolonged enzymatic digestion.
In summary, the yield and viability of loquat leaf protoplasts reached their highest levels under the following conditions: pretreatment with the 0.8 M mannitol solution for 2 h, followed by digestion with an enzyme solution consisting of 5.0% Cellulase RS + 3.4% Macerozyme R-10 + 0.6 M mannitol + 10 mM MES + 0.1% BSA + 1 mM CaCl2 for 12 h. Under this optimized protocol, the yield of protoplasts was approximately 1.26 × 10⁷/g·FW, and the viability was 91% (Figure 1e,f).
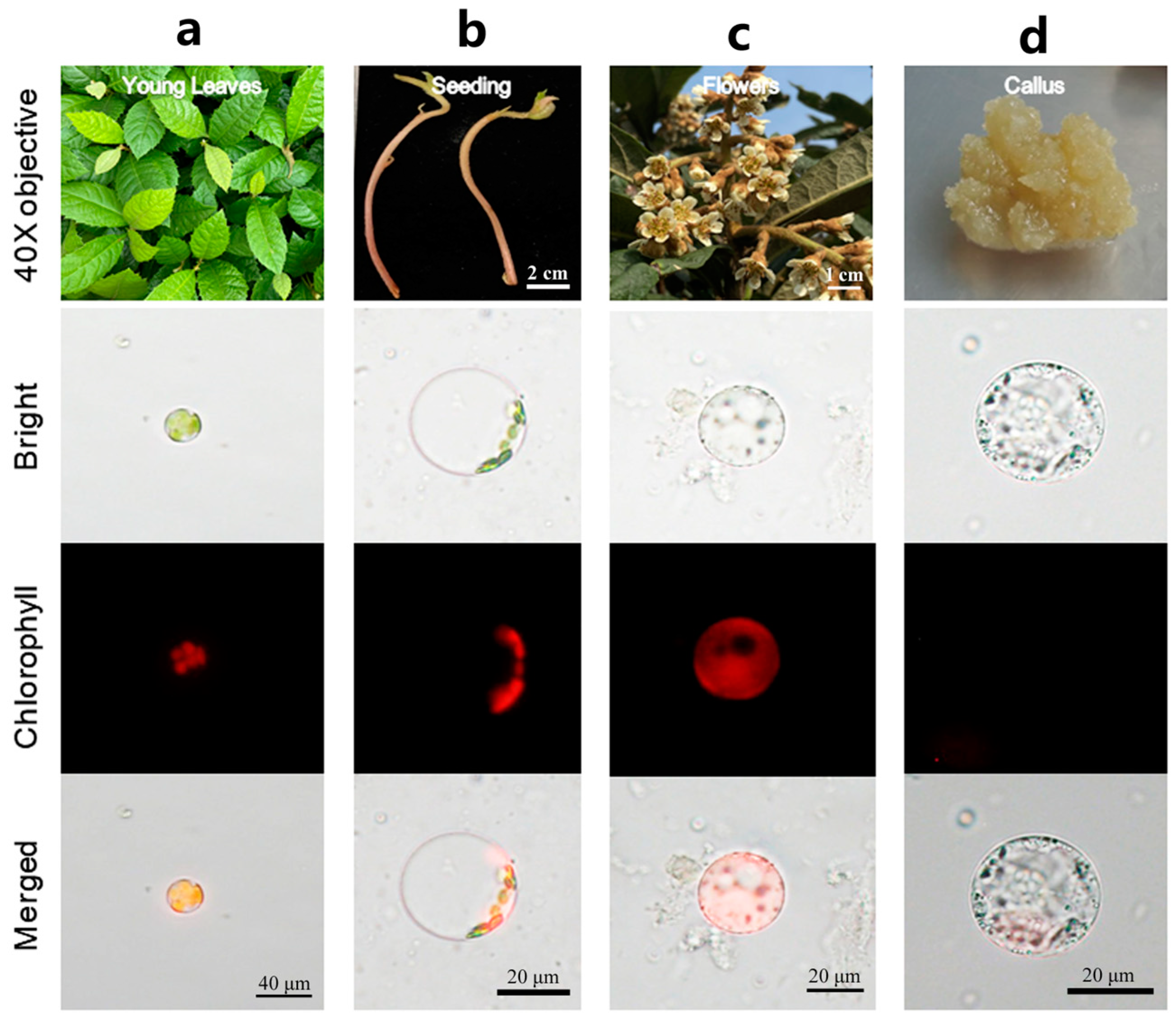
To extend the application of this method to different tissues in loquat, samples were collected from etiolated seedling, flowers, and callus and evaluated. The results showed that this method was also highly effective in facilitating the protoplast isolation from these tissues (Figure 3a–d). Among them, young leaves yielded the highest number of isolated protoplasts (Figure S3). Nevertheless, the quantity of protoplasts isolated from various tissues using the isolation method optimized here has met the quantitative requirements for subsequent experimental applications. In conclusion, the protocol detailed herein exhibits broad applicability for isolation high-quality protoplasts with maximal yield and viability from various loquat tissues.
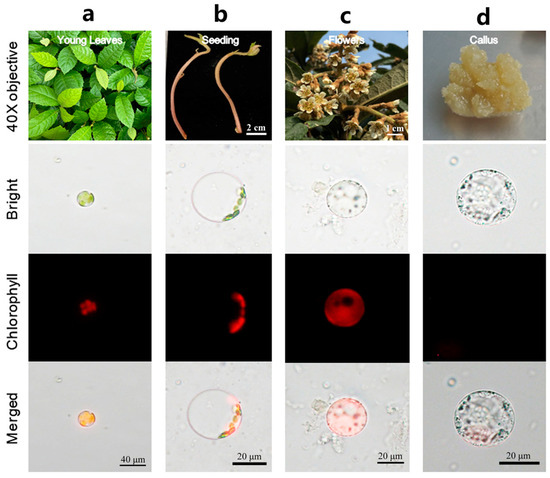
Figure 3.
Protoplast isolation using different tissues of loquat. (a) Young leaf; (b) stem of seeding; (c) petal; (d) callus.
3.3. Purification Protoplast Through Optimizing CPW Solution
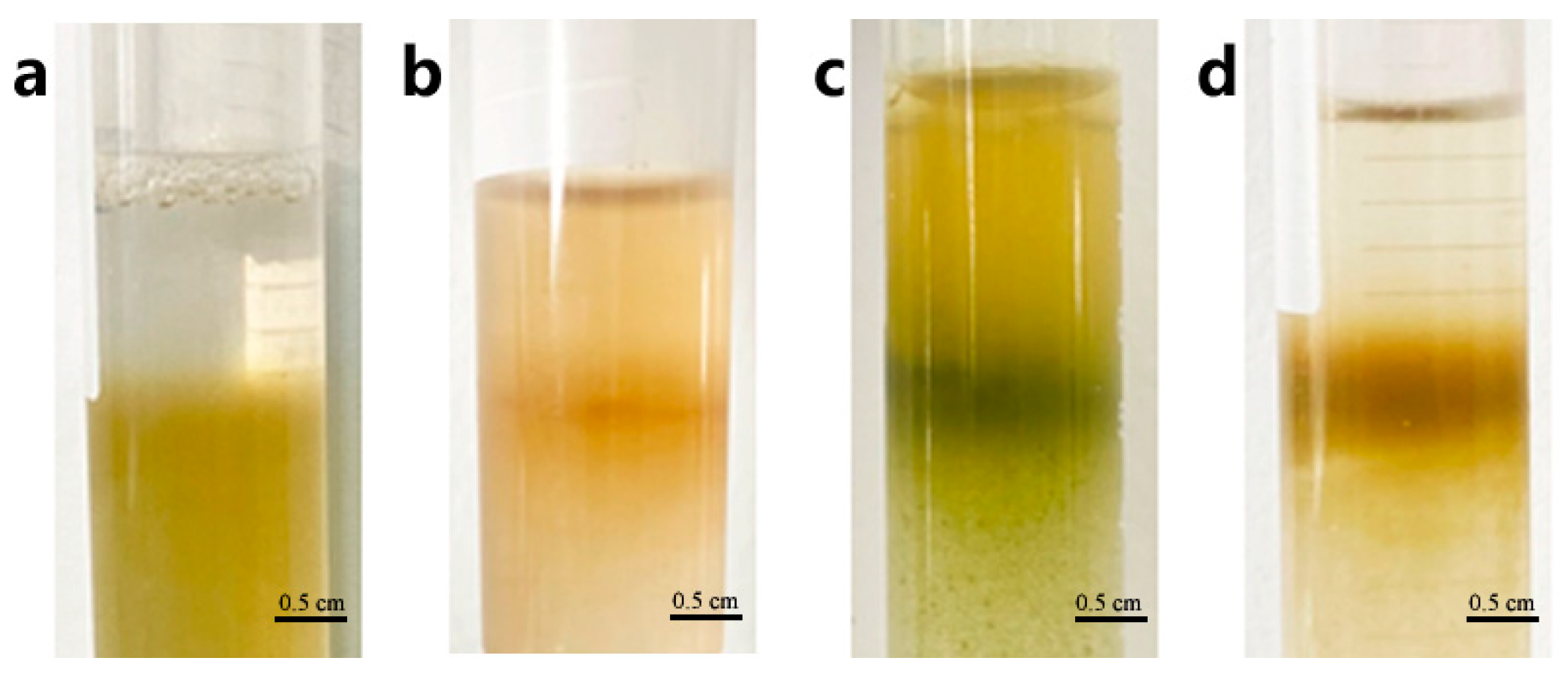
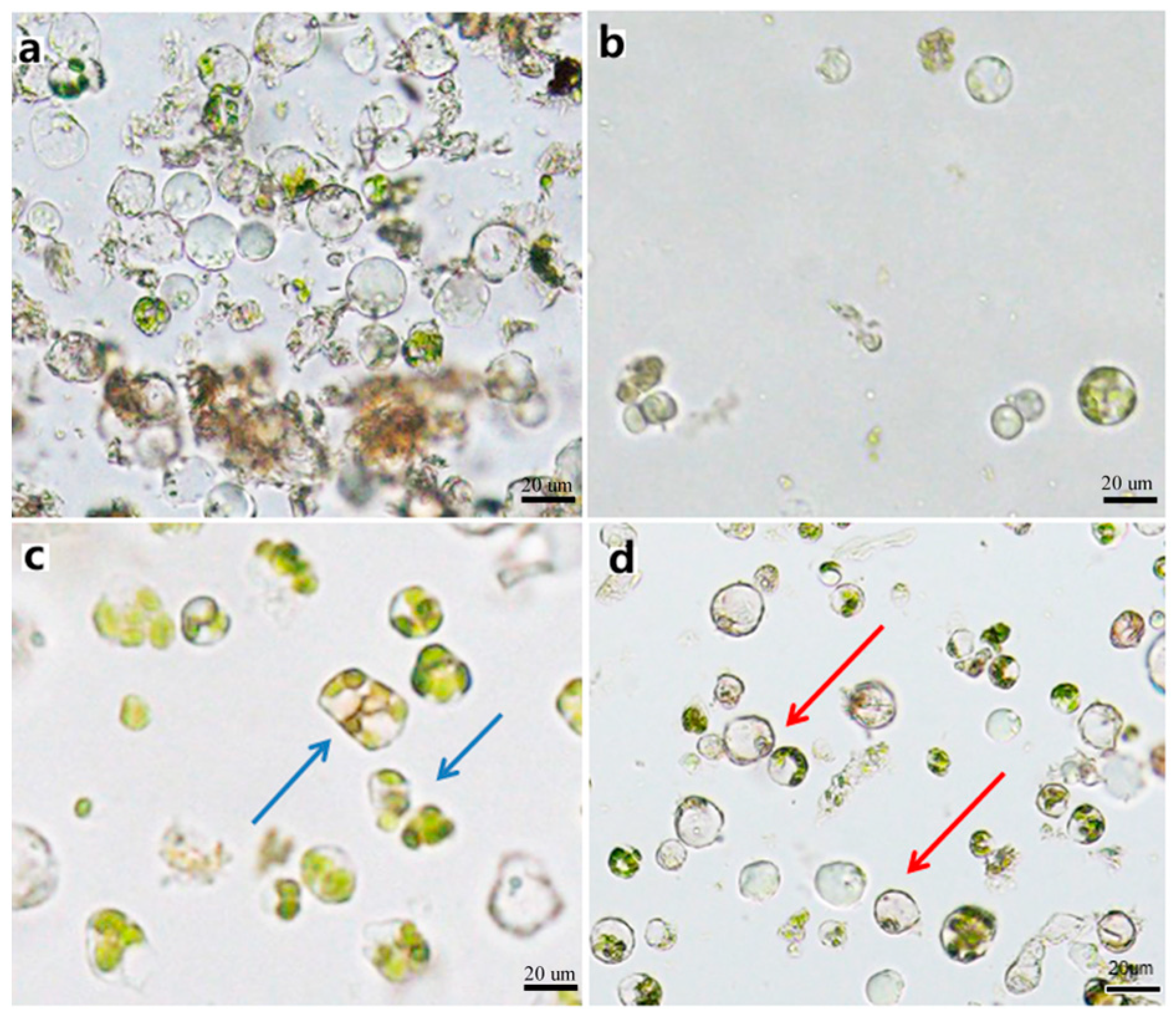
The protoplast purification process enables the separation of pure protoplasts from impurities in precipitation obtained by centrifuging the isolating solution. We investigated the protoplast purification method using the interface method by adjusting the concentration of sucrose and mannitol in the CPW solution. The precipitated protoplasts were suspended in a CPW sucrose solution (the concentrations of sucrose were 39%, 37%, 35%, 33%, 31%, 29%, 25%, and 21% w/v, respectively), and then the different concentration CPW mannitol solutions (the concentrations of mannitol were ranging from 5% w/v to 15% w/v at 2% intervals) were added gently at a ratio of 3:1, respectively (Table S1). A distinct stratification would form between the two liquids. Following centrifugation, the protoplasts accumulated as a ring at the interface. When the sucrose concentration of CPW sucrose solution was below 29% w/v, and any mannitol concentration of CPW solutions sucrose was added, the interface between the two solutions disappeared and only a small number of protoplasts could be observed under the microscope (Figure 4a,b and Figure 5b). However, when the sucrose concentration of CPW sucrose solution exceeded 35% w/v, the deformed protoplasts were able to accumulate at the interface, forming a distinct ring. (Figure 4c and Figure 5c). The optimal interface method for the purification of loquat protoplasts involved using a CPW sucrose solution with sucrose concentration of 35% w/v in combined with a CPW mannitol solution with mannitol concentration of 11% w/v. Under these conditions, a distinct ring formed at the interface of the two CPW solution, and it contained abundant intact protoplasts with high purity (Figure 4d and Figure 5d).
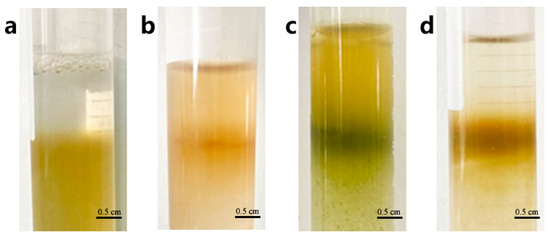
Figure 4.
Protoplast purification though the sugar-alcohol method. (a) No ring appeared between sugar and alcohol solution after centrifuge. (b) The boundary was not clear between sugar and alcohol solution after centrifuge. (c) The protoplasts did not gather into a ring. (d) The protoplasts gather into a clear ring between sugar and alcohol solution.
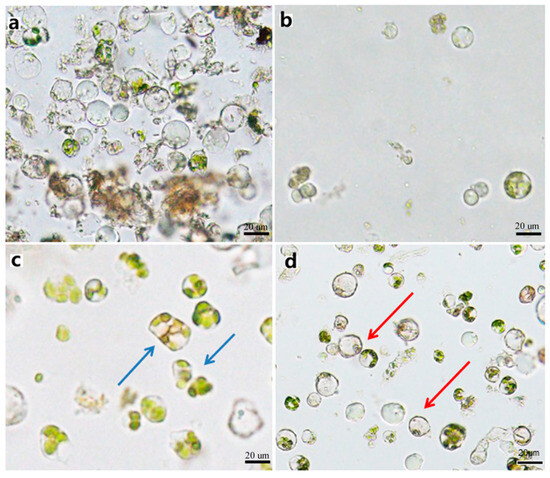
Figure 5.
Purified protoplast. (a) Precipitated protoplasts with impurities before purification. (b) A few protoplasts obtained after purification. (c) Deformed protoplasts obtained after purification. (d) Intact spherical protoplasts without impurities obtained after purification. Note: Blue arrows denote the malformed protoplasts, red arrows denote the out normal protoplasts.
3.4. Optimizing the Procedure for an Efficient PEG—Mediated Transient Transformation System in Loquat Mesophyll Protoplasts
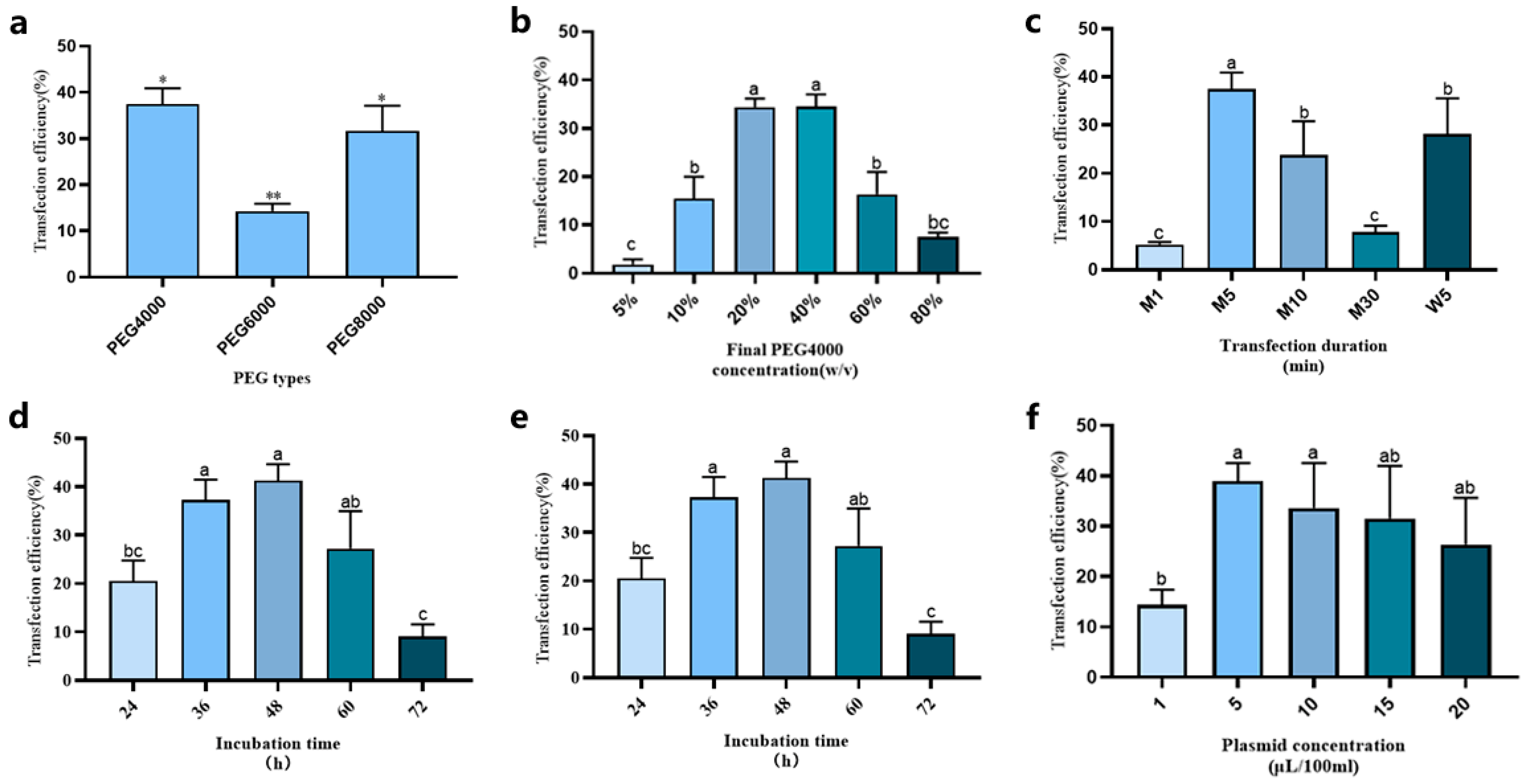
In this study, we also systematically optimized multiple parameters to improve protoplast transformation efficiency using the pCAMBIA2300-35S-GFP vector. These parameters included different types of PEG, PEG concentration, mannitol concentration, plasmid concentration in transfection solution, transfection duration, and transfection time. Notably, after optimizing each individual factor, the identified optimal condition was integrated into the subsequent experimental procedures.
To observe the effect of PEG molecular weights on transformation frequency, experiments were conducted with constant protoplast density (2 × 105/mL) and PEG concentration (40% w/v) of the transfection solution. The highest transformation efficiency (approximately 38%) was achieved using PEG-4000, which was consequently selected as the optimal PEG type for subsequent loquat protoplast transformation experiments (Figure 6a).
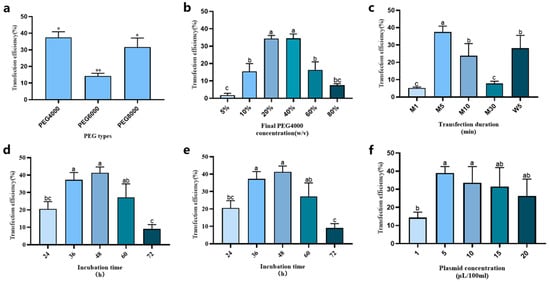
Figure 6.
Optimization protoplast transient expression system of loquat. (a) PEG types; (b) PEG-4000 concentration; (c) transfection duration; (d) mannitol concentration; (e) plasmid amount; (f) incubation time. Different letters and * indicate a statistically significant difference at p ≤ 0.05; ** indicate a statistically significant difference at p ≤ 0.01.
The PEG-4000 concentration of the transfection solution was tested at 5% w/v, 10% w/v, 20% w/v, 40% w/v, 60% w/v, and 80% w/v. As shown in Figure 6b, with the increase in the PEG-4000 concentration of transfection solution, the transformation efficiency first climbed up and then declined. Specifically, when the final PEG-4000 concentrations were 20% w/v and 40% w/v, the transformation efficiency peaked, reaching 35–40%. Based on these results, we selected 20% w/v as the final PEG-4000 concentration of transfection solution for subsequent experimental steps.
To investigate the effects of heating temperatures and durations on transformation efficiency during transformation process, both metal bath heating (1, 5, 10, and 30 min; designated as M1, M5, M10, and M30, respectively) and water bath heating (5 min; designated as W5) were tested at 47 °C. The results showed that during transformation, when using the 5-min metal bath heating (M5), transformation efficiency achieved its highest value (Figure 6c).
During the transformation process, the mannitol concentration of transfection solution can also influence the transformation efficiency. We analyzed different mannitol concentrations (0.1, 0.2, 0.3, 0.4, 0.5, and 0.6 M). The results indicated that when the mannitol concentration in the transfection solution ranged from 0.2 M to 0.4 M, there was no significant difference in the transformation efficiency (Figure 6d). However, when the mannitol concentration of the transfection solution fell below 0.2 M, the transfected protoplast would burst. Therefore, we selected 0.4 M as the final mannitol concentration for the transfection solution in the process of transient transformation.
The influence of plasmid concentration on transformation efficiency was evaluated across a range of 1–20 μg/100 μL (Figure 6e). The results revealed that the transformation efficiency demonstrated an initial increase with plasmid concentration, peaking at 5 μg/100 μL, followed by a progressive decline at higher concentrations. Therefore, 5 μg/100 μL (0.005% w/v) was identified as the optimal plasmid concentration for achieving the highest transformation efficiency.
Furthermore, we discovered that the incubation time in the WI medium after transfection affected the detection of transfected protoplasts. Therefore, we also measured different incubation times (24, 36, 48, 60, and 72 h) after transfection. The results showed that when the protoplasts were detected at 48 h after transfection, the transformation efficiency reached its peak (higher than 40%). Moreover, when the incubation time after transformation exceeded 72 h, the transformation efficiency dropped to less than 10% (Figure 6f). This finding suggests that the optimal time for detecting transformed protoplasts is 48 h post-transformation.
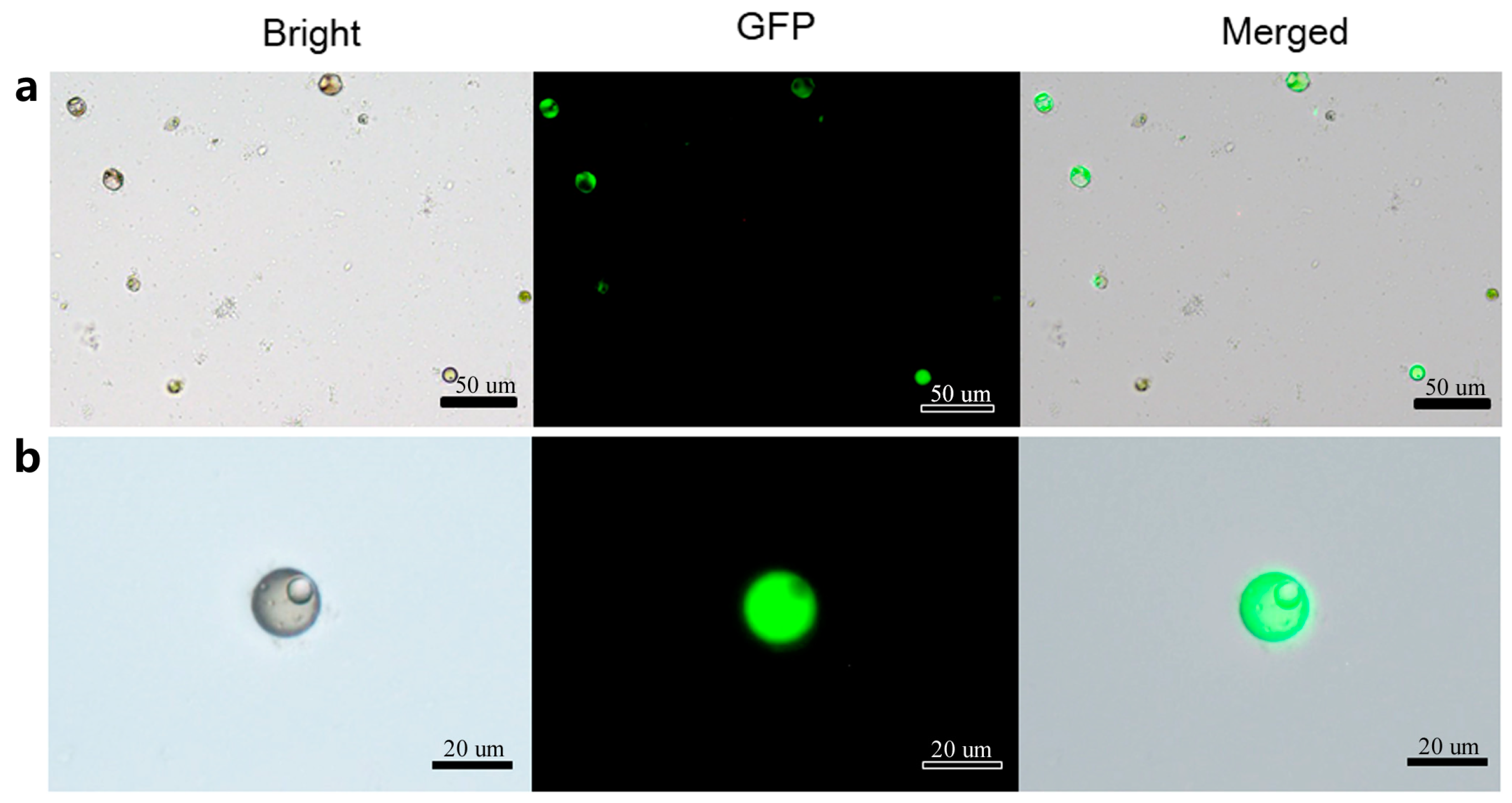
Based on the acquired data, the optimal protocol for loquat protoplast transformation was established to be incubated in the transfection solution with 20% PEG-4000, 5 µg plasmid, 0.4 M mannitol concentration in metal bath for 5 min. Utilizing this optimal system, a maximum transformation efficiency of approximately 35–40% can be stably achieved. Microscopic analysis conducted 48 h after transformation revealed distinct GFP fluorescence signals in transformed protoplasts (Figure 7). Under bright-field illumination, the transformed protoplasts exhibited intact cellular morphology and regular shape, which demonstrated the reliability of this transformation system.
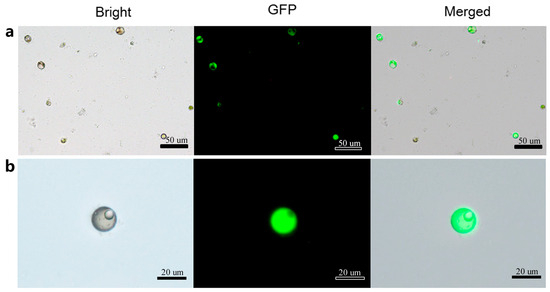
Figure 7.
Transformed protoplasts with green fluorescent protein (GFP) fluorescence. (a) GFP fluorescence signals in transformed protoplasts observed under bright field, dark field and merged field respectively; (b) GFP fluorescence signals in single transformed protoplast observed under bright field, dark field and merged field respectively.
3.5. Protein Subcellular Localization in Loquat Protoplasts Expanding the Application of Transient Transformation
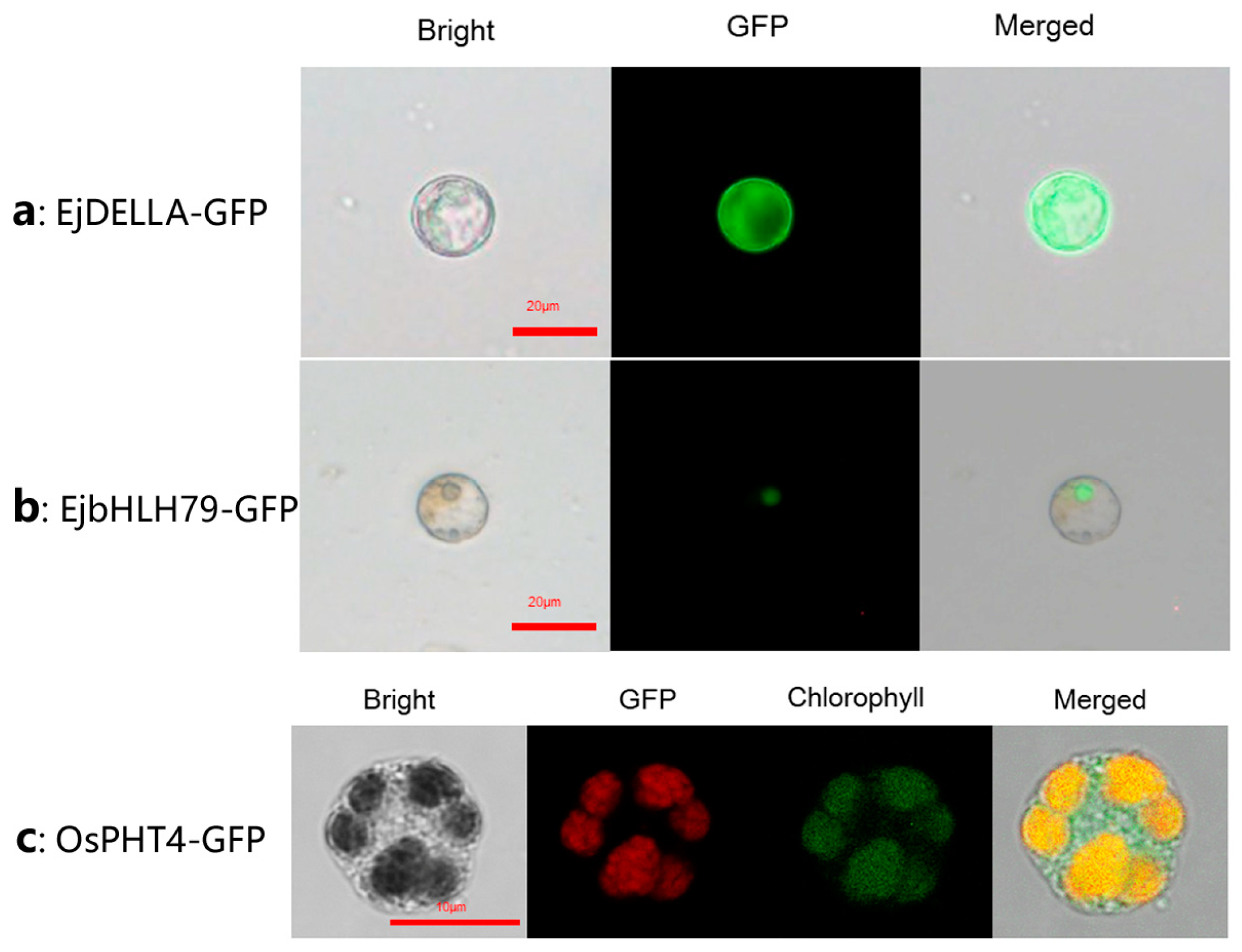
To validate the application of the transient expression system in subcellular localization studies, we transiently conducted expression assays of EjDELLA, EjbHLH79, and OsPHT4 proteins in loquat mesophyll protoplasts. DELLA-mediated gibberellin (GA) signal pathway genes are known to influence flowering in Loquat [32]. However, the subcellular localization of EjDELLA remained unclear. Our analysis revealed that, within this homologous expression system, the expressed EjDELLA proteins exhibited fluorescence throughout the cytoplasm (Figure 8a). The nuclear localization of EjbHLH79, whose function and subcellular localization were identified by Hu et al. [33] though heterologous expression, was confirmed by our protoplast system (Figure 8b), corroborating prior findings. Additionally, the OsPH4-GFP organelle markers, developed in the rice chloroplast system [30], successfully detected chloroplast-specific fluorescence signals using our protoplast transient transformation system of loquat mesophyll (Figure 8c). Collectively, these results indicate that the transient expression system in loquat we reported here can be effectively applied to determine the subcellular localization of proteins in the cell nucleus, cytoplasm, and organelles.
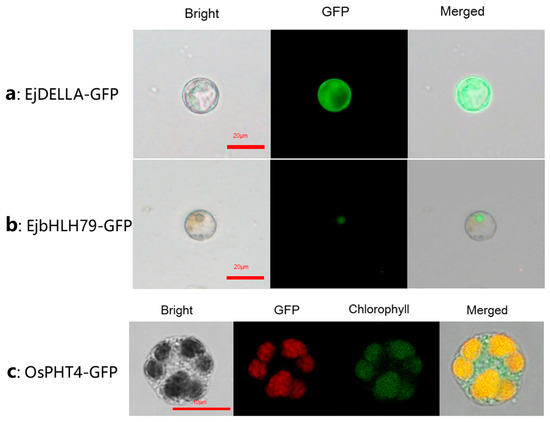
Figure 8.
Protein subcellular localization of loquat. (a) EjDELLA is localized in the cytoplasm of protoplasts, green color is fluorescent patterns of GFP; (b) EjbHLH79 was expressed in the cell nucleus, green color is fluorescent patterns of GFP; (c) The organelle marker OsPH4-GFP was expressed in chloroplast of loquat, red color is fluorescent patterns of GFP, green color is protoplast chloroplast autofluorescence.
4. Discussion
4.1. The Transcient Transformation of Loquat Mesophyll Protoplasts Will Have Great Potential in Basic Research
Loquat is one of the most popular economic subtropical evergreen fruit trees in the world. The loquat industry has been experiencing rapid expansion and development, emerging as one of the main featuring characteristic fruits of China [6]. Due to the absence of a robust and widely applicable plant regeneration and genetic transformation system [9,29], the establishment of a PEG-mediated protoplast transient gene expression system holds great potential for wide-ranging applications in protein subcellular localization, protein–protein reaction, gene promoter activity, gene editing, biotechnology and so on [34]. In this study, we successfully established an optimized system for the isolation, purification, and transformation of mesophyll protoplasts derived from in vitro-grown loquat seedlings.
4.2. Critical Factors in the Loquat Mesophyll Protoplast Isolation
Since protoplast transient expression syst are dependent on the high yield of protoplast isolation with high viability, an efficiency isolation system of protoplast represents a vital step. Plant protoplast isolation has been reported using a diverse range of tissues as materials, such as root tip, mesophyll cells [20], fruits [35], stem [36], seed [37], anthers [38], and calluses [39]. In loquat, research on protoplast manipulation has been delayed. Lin and Chen [40] managed to isolate protoplasts from callus using a combination of pectinase and cellulase as digestion enzymes, and the isolated protoplasts were successfully induced to regenerate into plants. However, following this pioneering research, there have been no further reports on loquat protoplast-related research. In this study, the young leaf, due to its most convenient sources and containing a large number of uniform cells, was selected as material for establishing the protoplast isolation system. Considering that the osmotic pressure of the maintaining solution has a direct impact on the protoplast stability, the osmotic gradient was optimized first [41]. Therefore, in the present study, we determined that the mannitol concentration in the digestion solution for loquat protoplast isolation was 0.6 M. An optimal enzyme combination of Cellulase RS and Macerozyme R-10 was employed for digestion in this efficiency system. This system was then applied to isolate high-quality protoplasts from other organs of loquat, such as callus, etiolated seeding and petal with high quality in loquat (Figure 4). This indicates that the protoplast isolation protocol detailed in this study is applicable to various organs of loquat.
4.3. An Optimized Protoplast Transient Transformation System via PEG-Mediated DNA Delivery
The protoplasts transient expression system enables the stable expression of target protein or gene efficiently and rapidly. In the realm of protoplast transient expression, the methods of agrobacterium-mediated transformation, particle bombardment, electro-transformation, and PEG-mediated transformation stand as the primary methods [34]. PEG-mediated transformation is the most common method because of its high efficiency, ease of operation, rapid processing time, and independence of specific equipment. While onion epidermal cells and tobacco leaves are frequently utilized for subcellular protein localization or protein–protein interaction research in numerous plant species, heterologous syst may give rise to divergent expression patterns [16]. However, there is currently no published protocol for protoplast transient transfection in loquat. In this study, to establish an efficient transformation system, we comprehensively optimized multiple transformation-related factors, which included the plasmid amount, the type and concentration of PEG, the duration time and incubation temperature of the transformation process. PEG interacts with protoplasts and enables the entry of target DNA, which is regarded as the most critical factor for protoplast transfection. We examined the effect of different PEG types on transformation efficiency, and the results demonstrated that PEG-4000 exhibited the highest efficacy for loquat protoplast transfection, while this finding is consistent with previous reports in Arabidopsis [42], Magnolia [21], grape [19], citrus [18], apple [43], and perennial ryegrass [44]. Building on established protocols for transient expression syst [21,45], we determined 20% (w/v) as the optimal PEG-4000 concentration for transient transformation, achieving transformation efficiencies of 35–40% in our loquat system. This parameter range has been validated across multiple plant species for efficient DNA delivery while maintaining protoplast viability (Figure 9).
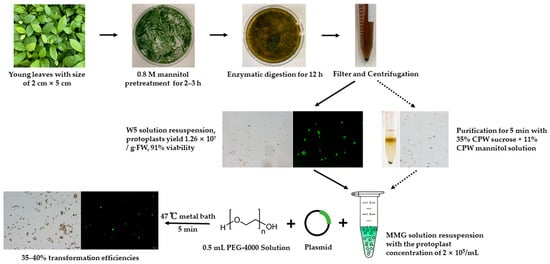
Figure 9.
Flow chart of mesophyll protoplast isolation, purification, and transformation in Loquat.
We explored the efficiency of transformation by using fluorescent markers and analyzing the subcellular localization of genes EjHLH79 and EjDELLA. The results achieved here were consistent with previous reports [32,33]. Therefore, the system we have developed in this study is rapid and convenient for gene transformation studies with high efficiency. It is bound to accelerate significant advancements in somatic cell fusion for breeding, genetic transformation, and molecular studies in the loquat.
5. Conclusions
In summary, an efficient system for the isolation, purification, and PEG-mediated transient transformation of loquat mesophyll was established in this study, and it was successfully applied in fluorescent marker and subcellular localization. To our knowledge, this is the first report on developing the method of PEG-mediated protoplast transfection in the loquat. This efficient and convenient protocol will greatly contribute to future biotechnology studies including gene editing, subcellular localization, protein complexes, gene promoter activity analysis, signal transduction pathway identification, and gene silencing.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11040391/s1, Figure S1: Four shapes of protoplast under different isolated conditions; Figure S2: Protoplast morphology in different osmotic pressure of protoplast isolation solution; Figure S3: Comparison of protoplast isolated from young leaves and seedlings; Table S1: Different sucrose and mannitol concentration gradient combinations in CPW solution for protoplast purification; Table S2: Enzyme species and osmotic pressure screening for protoplast isolation.
Author Contributions
S.W. and L.W. performed protoplast isolation, purification, and transformation experiments, analyzed the data and wrote the manuscript. Y.X. performed vector construction experiments. Z.L. performed protoplast isolation experiments. D.J. and Q.G. analyzed the data and revised the manuscript. G.L. and Q.H. conceived, supervised the research, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Key R&D Program of China (No. 2019YFD1000900), National NSF of China (No. 32171820), Chongqing Science and Technology Commission (cstc2020jcyj-msxmX0429) and the Open Fund of Key laboratory of Loquat Germplasm Innovation and Utilization (Putian University), Fujian Province University (2019003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank Shoukai Lin (Key laboratory of Loquat Germplasm Innovation and Utilization (Putian University), Fujian Province University, Putian, 351100) for his efforts to this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jing, D.; Liu, X.; He, Q.; Dang, J.; Hu, R.; Xia, Y.; Wu, D.; Wang, S.; Zhang, Y.; Xia, Q.; et al. Genome assembly of wild loquat (Eriobotrya japonica) and resequencing provide new insights into the genomic evolution and fruit domestication in loquat. Hortic. Res. 2023, 10, uhac265. [Google Scholar] [PubMed]
- Jiang, S.; An, H.; Xu, F.; Zhang, X. Chromosome-level genome assembly and annotation of the loquat (Eriobotrya japonica) genome. GigaScience 2020, 9, giaa015. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Jing, Y.; Lin, S.; Yue, Z.; Yang, X.; Xu, J.; Wu, J.; Zhang, Z.; Xia, R.; Zhu, J.; et al. Polyploidy underlies co-option and diversification of biosynthetic triterpene pathways in the apple tribe. Proc. Natl. Acad. Sci. USA 2021, 118, e2101767118. [Google Scholar] [PubMed]
- Yu, Y.; Yang, M.; Liu, X.; Xia, Y.; Hu, R.; Xia, Q.; Jing, D.; Guo, Q. Genome-wide analysis of the WOX gene family and the role of EjWUSa in regulating flowering in loquat (Eriobotrya japonica). Front. Plant Sci. 2022, 13, 1024515. [Google Scholar] [CrossRef]
- Xu, H.; Meng, D.; Yang, Q.; Chen, T.; Qi, M.; Li, X.; Ge, H.; Chen, J. Sorbitol induces flower bud formation via the MADS-box transcription factor EjCAL in loquat. J. Integr. Plant Biol. 2023, 65, 1241–1261. [Google Scholar]
- Peng, Z.; Zhao, C.; Li, S.; Guo, Y.; Xu, H.; Hu, G.; Liu, Z.; Chen, X.; Chen, J.; Lin, S.; et al. Integration of genomics, transcriptomics and metabolomics identifies candidate loci underlying fruit weight in loquat. Hortic. Res. 2022, 9, uhac037. [Google Scholar]
- Wang, D.; Chen, Q.; Chen, W.; Guo, Q.; Xia, Y.; Wang, S.; Jing, D.; Liang, G. Physiological and transcription analyses reveal the regulatory mechanism of melatonin in inducing drought resistance in loquat (Eriobotrya japonica Lindl.) seedlings. Environ. Exp. Bot. 2021, 181, 104291. [Google Scholar]
- Liu, Y.; Liu, Q.; Xu, X.; Xiao, Y.; Liao, M.; Deng, Q.; Zhang, H.; Lin, L. Effects of intercropping with Solanum photeinocarpum and its post-grafting generations on cadmium accumulation in loquat (Eriobotrya japonica). Int. J. Phytoremediat. 2022, 24, 753–762. [Google Scholar]
- Lin, L.H.; Li, J.Q.; Zong, H.; Wang, Y.Q. Effect of antibiotics on Agrobacterium-mediated transformation from anther derived embryos of Eriobotrya japonica (Thunb.) Lindl. cv.‘Dawuxing’. J. Hortic. Sci. Biotechnol. 2021, 96, 172–182. [Google Scholar]
- Eeckhaut, T.; Lakshmanan, P.S.; Deryckere, D.; Van Bockstaele, E.; Van Huylenbroeck, J. Progress in plant protoplast research. Planta 2013, 238, 991–1003. [Google Scholar]
- Du, J.; Zhang, H.; Li, W.; Li, X.; Wang, Z.; Zhang, Y.; Xiong, A.; Li, M. Optimization of Protoplast Preparation System from Leaves and Establishment of a Transient Transformation System in Apium graveolens. Agronomy 2023, 13, 2154. [Google Scholar] [CrossRef]
- Wang, X.; Xu, L.; Liu, X.; Xin, L.; Wu, S.; Chen, X. Development of potent promoters that drive the efficient expression of genes in apple protoplasts. Hortic. Res. 2021, 8, 211. [Google Scholar] [PubMed]
- Sun, X.X.; Feng, D.L.; Liu, M.Y.; Qin, R.X.; Li, Y.; Lu, Y.; Zhang, X.M.; Wang, Y.H.; Shen, S.X.; Ma, W.; et al. Single-cell transcriptome reveals dominant subgenome expression and transcriptional response to heat stress in Chinese cabbage. Genome Biol. 2022, 23, 262. [Google Scholar]
- Xiao, S.X.; Biswas, M.K.; Li, M.Y.; Deng, X.X.; Xu, Q.; Guo, W.W. Production and molecular characterization of diploid and tetraploid somatic cybrid plants between male sterile Satsuma mandarin and seedy sweet orange cultivars. Plant Cell Tissue Organ Cult. 2014, 116, 81–88. [Google Scholar]
- Han, S.; Qu, G.; Li, X.; Zhang, F. Highly efficient endosperm and pericarp protoplast preparation system for transient transformation of endosperm-related genes in wheat. Plant Cell Tissue Organ Cult. 2023, 155, 165–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, J.; Duan, S.; Ao, Y.; Dai, J.; Liu, J.; Wang, P.; Li, Y.; Liu, B.; Feng, D.; et al. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 2011, 7, 30. [Google Scholar]
- Cao, J.; Yao, D.; Lin, F.; Jiang, M. PEG-mediated transient gene expression and silencing system in maize mesophyll protoplasts: A valuable tool for signal transduction study in maize. Acta Physiol. Plant. 2014, 36, 1271–1281. [Google Scholar] [CrossRef]
- Yang, W.; Ren, J.; Liu, W.; Liu, D.; Xie, K.; Zhang, F.; Wang, P.; Guo, W.; Wu, X. An efficient transient gene expression system for protein subcellular localization assay and genome editing in citrus protoplasts. Hortic. Plant J. 2023, 9, 425–436. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Zhan, J.; Huang, W.; Xu, H. An efficient PEG-mediated transient gene expression system in grape protoplasts and its application in subcellular localization studies of flavonoids biosynthesis enzymes. Sci. Hortic. 2015, 191, 82–89. [Google Scholar] [CrossRef]
- Li, S.; Zhao, R.; Ye, T.; Guan, R.; Xu, L.; Ma, X.; Zhang, J.; Xiao, S.; Yuan, D. Isolation, purification and PEG-mediated transient expression of mesophyll protoplasts in Camellia oleifera. Plant Methods 2022, 18, 141. [Google Scholar]
- Shen, Y.; Meng, D.; McGrouther, K.; Zhang, J.; Cheng, L. Efficient isolation of Magnolia protoplasts and the application to subcellular localization of MdeHSF1. Plant Methods 2017, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, S.; Yi, F.; Gao, Y.; Zhu, D.; Wang, Y.; Cai, Y.; Hou, D.; Lin, X.; Shen, J. Organelle Visualization With Multicolored Fluorescent Markers in Bamboo. Front. Plant Sci. 2021, 12, 658836. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Stone, J.M.; Heard, J.E.; Kovtun, Y.; Yorgey, P.; Sheen, J.; Ausubel, F.M. Fumonisin B1-Induced Cell Death in Arabidopsis Protoplasts Requires Jasmonate-, Ethylene-, and Salicylate-Dependent Signaling Pathways. Plant Cell 2000, 12, 1823–1835. [Google Scholar]
- Ren, R.; Gao, J.; Yin, D.; Li, K.; Lu, C.; Ahmad, S.; Wei, Y.; Jin, J.; Zhu, G.; Yang, F. Highly Efficient Leaf Base Protoplast Isolation and Transient Expression Syst for Orchids and Other Important Monocot Crops. Front. Plant Sci. 2021, 12, 626015. [Google Scholar] [CrossRef]
- Woo, J.W.; Kim, J.; Kwon, S.I.; Corvalán, C.; Cho, S.W.; Kim, H.; Kim, S.G.; Kim, S.T.; Choe, S.; Kim, J.S. DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat. Biotechnol. 2015, 33, 1162–1164. [Google Scholar] [CrossRef]
- Osakabe, Y.; Liang, Z.; Ren, C.; Nishitani, C.; Osakabe, K.; Wada, M.; Komori, S.; Malnoy, M.; Velasco, R.; Poli, M.; et al. CRISPR-Cas9-mediated genome editing in apple and grapevine. Nat. Protoc. 2018, 13, 2844–2863. [Google Scholar] [CrossRef]
- Lin, C.S.; Hsu, C.T.; Yuan, Y.H.; Zheng, P.X.; Wu, F.H.; Cheng, Q.W.; Wu, Y.L.; Wu, T.L.; Lin, S.; Yue, J.J.; et al. DNA-free CRISPR-Cas9 gene editing of wild tetraploid tomato Solanum peruvianum using protoplast regeneration. Plant Physiol. 2022, 188, 1917–1930. [Google Scholar] [CrossRef]
- Su, H.; Wang, Y.; Xu, J.; Omar, A.A.; Grosser, J.W.; Calovic, M.; Zhang, L.; Feng, Y.; Vakulskas, C.A.; Wang, N. Generation of the transgene-free canker-resistant Citrus sinensis using Cas12a/crRNA ribonucleoprotein in the T0 generation. Nat. Commun. 2023, 14, 3957. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Lin, L.; Zhou, L.; Luo, N.; Deng, Q.; Xian, J.; Hou, C.; Qiu, Y. Embryogenesis and plant regeneration from anther culture in loquat (Eriobotrya japonica L.). Sci. Hortic. 2008, 115, 329–336. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.L.; Xu, L.; Sun, M.; Yi, K.; Zhao, H. Functional Analysis of Phosphate Transporter OsPHT4 Family Members in Rice. Rice Sci. 2020, 27, 493–503. [Google Scholar]
- Fang, Y.; Xie, K.; Xiong, L. Conserved miR164-targeted NAC genes negatively regulate drought resistance in rice. J. Exp. Bot. 2014, 65, 2119–2135. [Google Scholar] [PubMed]
- Jiang, Y.; Liu, Y.; Gao, Y.; Peng, J.; Su, W.; Yuan, Y.; Yang, X.; Zhao, C.; Wang, M.; Lin, S.; et al. Gibberellin Induced Transcriptome Profiles Reveal Gene Regulation of Loquat Flowering. Front. Genet. 2021, 12, 703688. [Google Scholar]
- Hu, R.; Jing, D.; Guo, Q.; Liang, G. Analyses of the sequence and expression of flowering-related EjbHLH79 gene in triploid loquat. J. Plant Physiol. 2021, 57, 1300–1310. [Google Scholar]
- Chen, K.B.; Chen, J.L.; Pi, X.; Huang, L.J.; Li, N. Isolation, Purification, and Application of Protoplasts and Transient Expression Syst in Plants. Int. J. Mol. Sci. 2023, 24, 16892. [Google Scholar] [PubMed]
- Honda, C.; Moriguchi, T. High GUS expression in protoplasts isolated from immature peach fruits. Sci. Hortic. 2006, 109, 244–247. [Google Scholar]
- Chen, S.; Tao, L.; Zeng, L.; Vega-Sanchez, M.E.; Umemura, K.; Wang, G. A highly efficient transient protoplast system for analyzing defence gene expression and protein-protein interactions in rice. Mol. Plant Pathol. 2006, 7, 417–427. [Google Scholar] [CrossRef]
- Duijn, B.V.; Flikweert, M.T.; Heidekamp, F.; Wang, M. Different properties of the inward rectifying potassium conductance of aleurone protoplasts from dormant and non-dormant barley grains. Plant Growth Regul. 1996, 18, 107–113. [Google Scholar] [CrossRef]
- Abreu-Tarazi, M.F.; Leite, L.R.; Dantas, A.C.M.; Guerra, M.P. Isolation and culture of protoplasts from anthers of apple (Malus pumila Mill.) rootstock ‘M9’. J. Hortic. Sci. Biotechnol. 2009, 84, 513–518. [Google Scholar] [CrossRef]
- Hong, K.; Chen, Z.; Radani, Y.; Zheng, R.; Zheng, X.; Li, Y.; Chen, J.; Yang, L. Establishment of PEG-Mediated Transient Gene Expression in Protoplasts Isolated from the Callus of Cunninghamia lanceolata. Forests 2023, 14, 1168. [Google Scholar] [CrossRef]
- Lin, S.; Chen, Z. Plant regeneration from protoplast in Loquat. Acta Hortic. Sci. 1996, 23, 313–318. [Google Scholar]
- Priyadarshani, S.V.G.N.; Hu, B.; Li, W.; Ali, H.; Jia, H.; Zhao, L.; Ojolo, S.P.; Azam, S.M.; Xiong, J.; Yan, M.; et al. Simple protoplast isolation system for gene expression and protein interaction studies in pineapple (Ananas comosus L.). Plant Methods 2018, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Mathur, J.; Koncz, C.; Szabados, L. A simple method for isolation, liquid culture, transformation and regeneration of Arabidopsis thaliana protoplasts. Plant Cell Rep. 1995, 14, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Malnoy, M.; Viola, R.; Jung, M.; Koo, O.; Kim, S.; Kim, J.; Velasco, R.; Kanchiswamy, C.N. DNA-Free genetically edited grapevine and apple protoplast using CRISPR/Cas9 ribonucleoproteins. Front. Plant Sci. 2016, 7, 1904. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Cheng, Q.; Xie, Z.; Xu, B.; Huang, B.; Zhao, B. An efficient protocol for perennial ryegrass mesophyll protoplast isolation and transformation, and its application on interaction study between LpNOL and LpNYC1. Plant Methods 2017, 13, 46. [Google Scholar]
- Lin, Z.; Huang, L.J.; Yu, P.; Chen, J.; Du, S.; Qin, G.; Zhang, L.; Li, N.; Yuan, D. Development of a protoplast isolation system for functional gene expression and characterization using petals of Camellia Oleifera. Plant Physiol. Biochem. 2023, 201, 107885. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).