Abstract
Banana is a commercially important crop widely cultivated in tropical and subtropical regions, but its cultivation in the canal command basins is challenged due to the development of waterlogged sodic soils. The present study aimed to induce sodicity tolerance through the integration of secondary metabolites in the plants during the tissue culture organogenesis phase. Secondary-metabolite-treated plants were assessed for their performance in the waterlogged sodic soil areas of Samesee block in Lucknow district of Uttar Pradesh, India. Metabolite-treated (MT) plants exhibited significantly better growth and yield compared to untreated control (UTC) plants. Key physiological enhancements in MT plants included increased activities of defense-related enzymes superoxide dismutase (SOD), phenylalanine ammonia lyase (PAL), peroxidase (POD), and proline along with a reduced Na+/K+ ratio. The metabolic profile of MT plants showed higher expression of antioxidants, phenolic compounds, and flavonoids. MT plants exhibited production of the metabolites such as trihydroxy methylene–di-oxyflavone, rush flavanone, rutin, anthocyanins, neodiosmin, arachidonic acid, and trigalloyl-HHDP-glucose, which belongs to the subclasses of flavonoids, anthocyanins, and sugar alcohols. Consequently, MT plants produced a significantly higher yield (20.85 kg per plant) compared to UTC plants (8.35 kg) and greater biomass. These results suggest that treatments using secondary metabolite extracted from salt-tolerant bacteria can be used as an effective strategy for enhancing sodicity tolerance in banana plants, contributing to sustainable production and economic viability in waterlogged sodic soil conditions.
1. Introduction
The world is facing a huge range of climatic changes due to modernization, industrialization, urbanization, and unorganized human activities, which drastically affect different environmental parameters, including soil properties and plant growth. Soil degradation due to abiotic stresses like drought, flooding, salinity, and sodicity has become a global concern, with approximately 20% of the world’s irrigated agricultural land being salt-affected [1]. Restoring commercial agriculture in salt-affected land can contribute to achieving at least three SDG goals: poverty, hunger, and life on lands. It is estimated that 96.40 mha (million hectares) of land (29 per cent of the total geographical area) is under the process of land degradation. Notably, 60% of salt-affected soils are sodic in nature [2]. In India, 6.7 million hectares of land isaffected by salinity and sodicity with 3.77 million ha of land being specifically sodicity-affected [3]. Sodic soils disrupt plant growth by increasing sodium levels and soil pH, impairing soil permeability, and limiting plant root development [4].
The global rise in sodicity and salinity necessitates developing effective reclamation technologies to ensure sustainable crop production. Introduction of canal irrigation increased agricultural productivity markedly in the command area. However, inadequate drainage and continuous seepage from the canal resulted in the rise of the water table, and subsequently upward movement of salts accumulating on the surface soil. Currently, soil salinization has intensified, accounting for 20% of agricultural land in the world, and over 50% of arable land is predicted to become saline and/or alkaline by 2050 [5]. While chemical amendments like gypsum have been widely used to ameliorate sodic soils, they are only effective at shallow depths (0–15 cm) [6]. Such amendments reclaimed only the topsoil layer of 0–15 cm depth but did not show any improvement in the pH and physical and biological properties of the sub-surface soil which makes them unsuitable for sustainable commercial agriculture [7]. Therefore, managing sodicity below 15 cm and improving soil bio-physical properties are critical for sustainable agriculture. Long-duration crops with extensive root systems may offer a sustainable alternative by enhancing sub-surface conditions over time. There is also a need for biological amendments to enhance the biological activities in the rhizospheric zone and also to reclaim the sub-surface [8]. Moreover, plants can retain memories of past threats, which enable them to respond more effectively to similar stresses subsequently encountered. This kind of stress memory may arise from hypersensitive immune responses, epigenetic modifications, or alterations in hormonal signaling [9]. Priming with beneficial microorganisms/bio-control agents and their internal metabolic changes inducestress memoryand reprogram the plant defense system to retain the imprints of a stress incident for future reference and respond accordingly, similar to the memory-based immune responses in the animal defense system [9]. Priming is reported to generate bothsomaticandtransgenerationalmemory which helps plants to adapt more efficiently to the changing environment by altering the genome-scale expression levels [10]. A huge improvement in the next-generation technologies associated with modern crop production would be one aimed at producing plantlets/seeds/planting materials with pre-triggered immunity, which can easily sustain crops in all environmental episodes.
Another approach to mitigating soil sodicity stress is to develop plants that can enhance their tolerance systems by producing secondary metabolites and strengthening memory cells, preparing them to combat adverse conditions before they occur. Previous research has shown that tissue-culture-raised plantlets can be bio-hardened using endophytic bacterial or plant growth-promoting rhizobacterial (PGPR) strains. This has proven effective in reducing BBTV infections in Robusta bananas [11] and in protecting cucumber plants against CMV virus using Bacillus sp. [12]. Numerous studies have focused on using bio-hardening techniques in banana and other crops to address biotic stresses, while a few have explored bio-priming to combat abiotic stresses [13]. For example, pre-incubated plants consistently performed better than non-primed plants when exposed to adverse environmental conditions [14]. Additionally, PGPR strains have been shown to enhance tomato resistance to salinity and sodicity [15,16]. However, no research has specifically focused on addressing abiotic stresses (especially sodicity) developed inwaterlogged command areas in banana plants. To fill this gap, bio-engineered bacterial molecules were introduced into the growth media during the organogenesis phase of tissue-culture-raised banana plantlets [16,17].
Banana (Musa spp.) is one of the world’s major fruit crops belonging to the Musaceae family which is native to the tropical region of Southeast Asia. India ranks at first position in banana production, with 31.5 million tons accounting for 26.43% of global output [18]. It has been cultivated for years in the basins of perennial rivers, but due to the development of a canal system in the command areas, the cultivable lands were changed to sodic soils which limited the cultivation opportunities of this crop [19]. This requires the development and implementation of efficient, inexpensive, and environmentally acceptable mitigation strategies to enhance the productivity of crop plants.
Chemical-metabolite-based induction of immune responses against biotic and abiotic stresses through acquired or induced resistance in plants is a new alternative area of research in terms of environmental safety. In this context, secondary metabolite engineering technology, which primes plants for stress resistance by triggering tolerance responses, offers a promising solution. Previous studies demonstrated the effectiveness of bio-hardening using beneficial bacterial strains in protecting crops like banana and cucumber from biotic stresses [11,12]. However, little research has been conducted on using secondary metabolites obtained from halo-tolerant bacteria to combat abiotic stresses, particularly sodicity, in banana crops. This study aimed to investigate the potential of halo-tolerant bacterial secondary metabolites in mitigating sodicity stress in banana (Musa spp.), one of the world’s major fruit crops. India, the leading global producer of bananas, faces challenges in banana cultivation due to the transformation of fertile lands of canal command regions into waterlogged sodic soils. Here, we report the effects of metabolite-treated (MT) banana plants on sodicity stress and compare their growth and yield performance with untreated control (UTC) plants.
2. Materials and Methods
2.1. Materials and Location of the Experiment
MT-immunized tissue-culture-raised Grand Naine plantlets (Patent no. 202111003761) were used as genetic material for the present investigation. Metabolite treatment is a process of using a lipo-polypeptide-based, engineered MTmolecule called “MT-immune” into in vitro tissue culture banana plantlets (var. Grand Naine) during the in vitro organogenesis phase for the production of metabolite-based vaccinated plantlets as described by Damodaran et al. [17]. The MT-immunized plants were primary- and secondary-hardened using standard protocols of hardening [17] in the mist chamber and net house at ICAR- Central Soil Salinity Research Institute, Regional Research Station, Lucknow, Uttar Pradesh, India. Finally, the secondary-hardened plants were distributed to the farmers for planting in sodicity-affected fields.
This investigation was carried out at Samesee, Lalai Kheda, Lucknow district, Uttar Pradesh, India (GPS: Lat 26.613309° N; Long 81.115563° E) in the farmers’ field which was a typical sodicity-affected area (Table 1). The plants were planted in the first week of June, 2020–2021 and 2021–2022, and all of the scientific package ofpractices of banana cultivation were followed. The experiment was designed by following randomized block design (RBD) with three replications, with a total of 50 plants per replication. Untreated tissue-culture-raised Grand Naine plants were used as a control for the experiment (Figure 1).

Table 1.
Soil physico-chemical parameters of the experimental field.

Figure 1.
Successful cultivation of MT banana plants of variety Grand Naine banana in waterlogged sodic soils. This figure shows a banana plantation located in Sameesee, Uttar Pradesh, India (coordinates: 26.613309° N, 81.115563° E) and the images were captured on 29 July 2022, at 3:33 PM. The top-left image provides a geographic reference with a map overlay, pinpointing the location of the plantation. The top-right and bottom-left images show healthy MT banana trees bearing large bunches of bananas, with some plants visibly thriving in well-maintained rows. The bottom-right image depicts banana trees with protective blue netting around the fruit bunches, used to prevent damage from pests and environmental factors.
The plant growth attributes such as plant height, plant girth, third leaf length, third leaf breadth, number of leaves/plant and yield attributes such as bunch weight, number of hands per bunch, and number of fingers per hand were recorded for both the MT-immunized (MT) plants as well as the untreated control (UTC) plants.
2.2. Soil Analysis of Micronutrients at the Experimental Location
Soil samples were collected from the fields through the agar block method, air-dried, and grounded to pass through a 2 mm sieve and saturated extracts were prepared. Various physico-chemical parameters were determined from these extracts by adopting standard methods. The pH of the soil extract was determined potentiometrically by the ORION ion analyzer (5 star series, Thermo Fisher Scientific India Pvt. Ltd., Powai, Maharashtra, India) using a pH electrode calibrated with a pH buffer of 7.0 and 10.0. Carbonate (CO3) and bicarbonate (HCO3) were determined by the titrimetric or acid–base titration method [20]. Calcium (Ca) and magnesium (Mg) contents were determined by the versenate method (EDTA titration). Physico-chemical properties of the soil determine soil characteristic properties and availability of nutrients as tabulated in Table 1.
2.3. Micronutrient Analysis of the Plant Tissues
Sodium (Na), potassium (K), calcium (Ca), and magnesium (Mg) were analyzed in the leaf, pseudostem, petiole, peduncle, sucker, and fruit samples of MT-immunized plants along with the samples collected from the untreated control. The collected samples were kept for complete drying in the shade for 15 days. Around 1 g of tissue was grounded and digested by nitric acid (HNO3) and perchloric acid (HClO4). Completely digested samples were then diluted and filtered. Further, the diluted samples were processed for sodium (Na) and potassium (K) estimation by flame photometer, adopting the protocol described by [20]. The same samples were analyzed for calcium (Ca) and magnesium (Mg) contents using the versenate method (EDTA titration) [18], as described for soil analysis.
2.4. Estimation of Stress Enzymes in MT-Immunized vs. Control Banana Leaves in Sodic Soils
Leaf samples of 2 g (three samples from each replicate) were homogenized with 2 mL of 0.1 M sodium citrate buffer (pH 5.0) at 48 °C and centrifuged for 20 min at 10,000 rpm. Enzyme extracted in 0.1 M sodium phosphate buffer (pH 7.0) was used for the estimation of peroxidase (PO) [21], polyphenol oxidase (PPO) [22,23], super oxide dismutase (SOD) [24], and phenylalanine ammonia lyase (PAL) [25]. The assay was carried out for MT-immunized plants (MT) and untreated control (UTC) plants. Proline was determined using the protocol as described by Bates et al. [26].
2.5. LC-MS Analysis of MT-Immunized vs. Control Banana Leaves in Sodic Soils
Liquid chromatography coupled with mass spectrophotometry (LC-MS) analysis was performed in the sodicity-stressed MT-immunized plant samples along with the untreated control in order to identify important organic compounds and their role in inducing tolerance against sodic conditions of the soil. Leaf samples were collected from the third leaf of the banana plants, washed with sterile distilled water, and then shade-dried for 30 days. The standard protocol for sample preparation was followed as prescribed by CSIR-Central Drug Research institute, Sophisticated Analytical Instrumentation Facility (SAIF), Lucknow, Uttar Pradesh, India.
A total of 250 g of completely dried leaf was crushed into a fine powder, extracted using 70% ethanol, and further purified with ethyl acetate. A small fraction of the solvent-free plant extract was analyzed using ESI-LC-MS (Micromass Quattro II triple quadrupole mass spectrometer with a JASCO PU-980 HPLC Pump, SpectraLab Scientific Inc., Markham, ON, Canada) using ESI/MS mode. The water absorption ODS (250_4.6mm_5I) column was used with acetonitrile: water + 0.1% formic acid solvent system; gradient elution was performed at 1.0 mL/min. The photodiode array was monitored at 200–650 nm and recorded at 220 nm. The mass spectra were scanned in the range 80–1000 DA in 2.5 S. The ESO capillary was set at 3.5 kv and the cone voltage at 40 V. The m/z spectral chromatographs were analyzed, key metabolites were predicted based on already published reports and m/z database, and these were presented for comparative analysis of the status of secondary metabolites in the banana leaf samples collected from MT-immunized and untreated “Grand Naine” plants grown in the waterlogged sodic soil.
2.6. Statistical Analysis
The experiment was conducted in RBD as described earlier with three replicates and the generated data were statistically analyzed by using the software IBM-SPSS statistics V.30 with default parameters for histograms/bar charts.
3. Results
3.1. Field Performance of MT Banana Plants Compared toUTC Plants
Field performance of tissue-culture-raised MT “Grand Naine” banana plants was evaluated for two consecutive years (2020–2021 and 2021–2022) in the sodic soils which had anaverage soil pH of 8.83, EC 0.326 dSm−1, sodium 13.21 meq L−1, and potassium 0.42 meq L−1 at Lalaikheda, Samesee, Uttar Pradesh (Table 1). It was observed that the presence of sodicity in soil reduced the growth and yield of the plants in the untreated control significantly (p ≤ 0.05) compared to the MT-immunized plants in both the years. Overall, plant growth frequencies were recorded to be higher in the MT banana plants compared to the UTC plants. In the first year of the trial, maximum plant height was recorded as higher in the MT plants (209.8 cm) than the UTC plants (119.6 cm). A similar trend was recorded in the second year of the trial, where the MT plants exhibited higher plant height (211.9 cm) than the UTC plants (123.2 cm). The highest number of leaves was also observed in the MT plants (9.35 nos.) compared tothe UTC plants (4.70 nos.) in two consecutive years of field trials. In the first year, the highest yield/plant was recorded in the MT plants (21.1 kg/plant) compared withthe UTC plants (8.3 kg). Similarly, during the second consecutive year, the MT plants also showed the highest yield/plant (20.6 kg) in comparison with the UTC (9.0 kg). The average data of two consecutive years of field trials showed that MT conditionshad a significant effect on the yield per plant (p ≤ 0.05). The pooled data of two consecutive years also evinced that the yield/plant was significantly higher in the MT plants compared with the UTC plants (20.85 and 8.65 kg/plant) (Table 2).

Table 2.
Efficiency of metabolites treatment on morphological characteristics of Grand Naine banana variety planted in sodicity-affected soils at Lalaikheda, Samesee, Uttar Pradesh.
3.2. Assessment of Role of MT Plants in Na/K Uptake Based on Soil and Ca Homeostasis
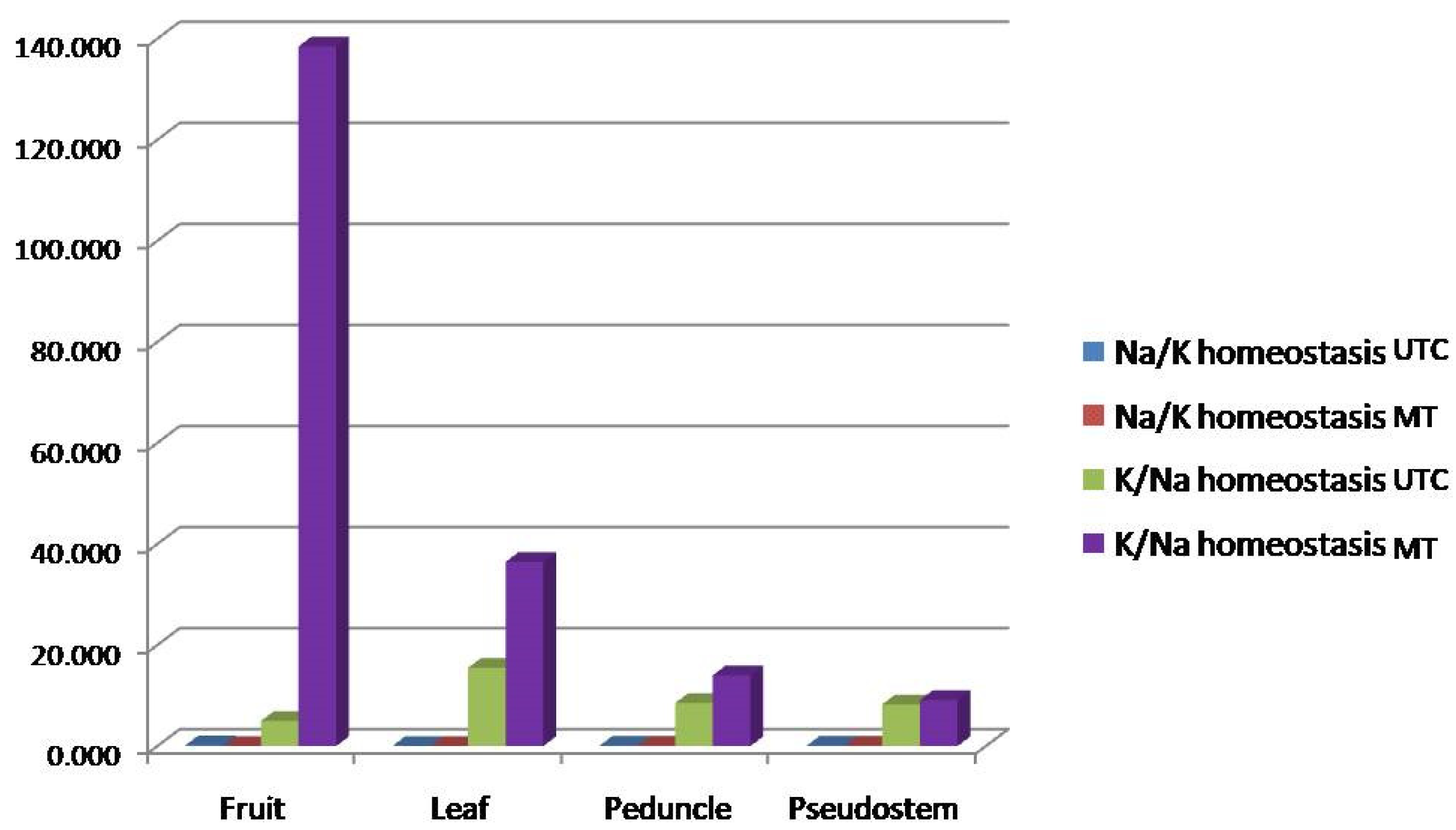
The investigation into the nutrient uptake and translocation patterns in banana plants grown in sodic soils reveals significant improvements in the MT-immunized (MT) plants compared to the untreated control (UTC) plants. The study observed that MT plants enhanced the soil’s physico-chemical properties, leading to a notable decrease in sodium (Na+) uptake and a corresponding increase in potassium (K+) uptake across various plant tissues. Specifically, while Na+ deposition was highest in the pseudostem (2.21) of MT plants, K+ levels peaked in the fruits (3.17), which effectively reduced the Na+/K+ ratio from the pseudostem to the fruit, indicating improved nutrient balance and management. In contrast, UTC plants exhibited higher Na+ levels across all tissues and a significantly elevated Na+/K+ ratio (pseudostem: 3.80), correlating with increased sodicity stress.
The data indicated notable variations in the concentrations of key ions Na+, K+, calcium (Ca2+), and magnesium (Mg2+) within the different tissues under UTC and MT conditions. Under the MT treatment, Na+ levels generally decreased across most tissues, although a slight increase in fruit was noted, suggesting a specific adaptive mechanism. In contrast, K+ levels consistently declined, particularly in the fruit, indicating potential limitations in K+ uptake or retention. Interestingly, Ca2+ levels increased significantly in all tissues, especially in fruits, which may imply an adaptive response or alteration in calcium homeostasis to counteract the sodicity stress. Mg2+ levels displayed variable responses, decreasing in fruit and leaf tissues, while remaining stable or slightly increased in the peduncle and pseudostem (Figure 2).
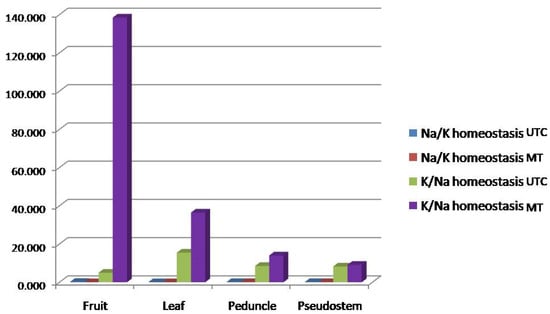
Figure 2.
Effect of MT on the Na/K ratio and differential deposition of Na, K, Ca, and Mg in different parts of a banana plant. The image depicts a bar graph showing Na/K and K/Na homeostasis in various parts of the banana plant under two conditions: untreated control (UTC) and metabolite treatment (MT). The parts of the banana plant assessed were the fruit, leaf, peduncle, andpseudostem. The X-axis represents the different parts of the banana plant viz., the fruit, leaf, peduncle, and pseudostem, while the Y-axis represents the concentration or homeostasis levels (values expressed as fold changes). K/Na homeostasis under MT (purple bars) is significantly higher in all parts of the banana plant compared to the control (UTC). The leaf shows an especially high increase in K/Na under MT treatment. Na/K homeostasis under MT (red bars) shows only minor increases in comparison to K/Na, suggesting that potassium absorption or availability greatly dominate that ofsodium in themetabolites treatment. The fruit shows the least variation in all cases, indicating that the primary impact of MT is observed in the leaf and peduncle parts.
Furthermore, the Na+/K+ influx ratio demonstrated a significant reduction under MT treatment, particularly in fruit tissue, where it dropped from 0.2006 in UTC to 0.0072 in MT. This suggests a strong inhibition of Na+ influx or enhanced K+ influx due to MT-immunization. Conversely, the K+/Na+ translocation ratio showed a substantial increase in fruit tissue under MT, rising from 4.98 (UTC) to 138.48, indicating a marked shift in ion dynamics favoring potassium retention. However, in leaf, peduncle, and pseudostem tissues, the K+/Na+ translocation ratio decreased, reflecting differential tissue responses to MTimmunization. Overall, these findings highlight the effectiveness of metabolite treatment technology in improving nutrient uptake and translocation in banana plants, enhancing their resilience to sodicity stress.
3.3. Metabolic Profiling of Sodicity-Stressed MT Plants in Comparison with UTC
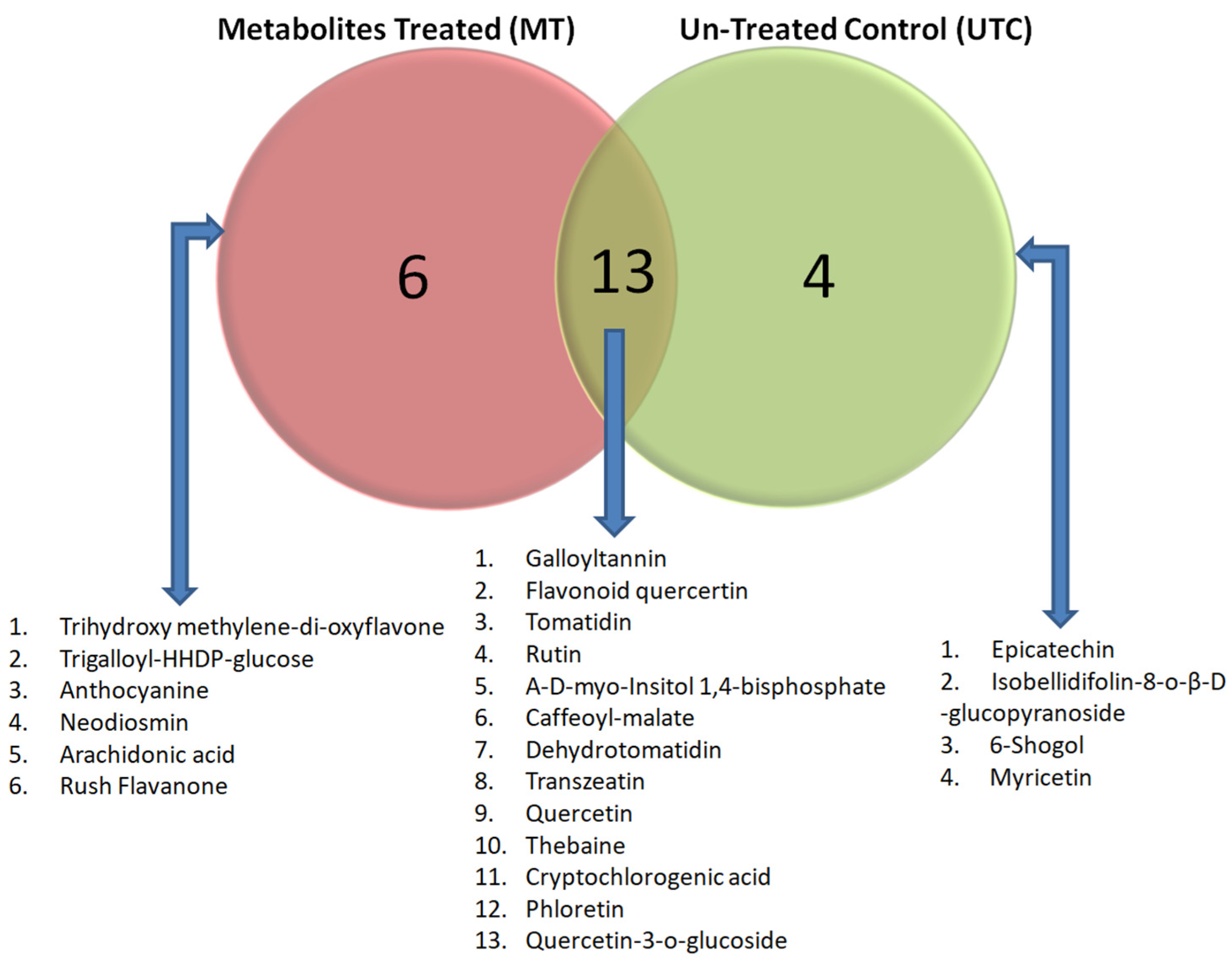
The crude metabolites of MT plants and UTC Grand Naine banana plants were analyzed by LC-MS to detect the unique secondary metabolites expression during the period of sodicity stress. LC-MS spectra profiles with different retention times and relative intensity (%) facilitated the identification of the compound when their distinct peak values were compared with similar molecular mass-by-charge (m/z) values reported for distinct compounds. The compound identified through LCMS were broadly categorized as phenolic esters, antioxidants, and fatty acids. The MT plants expressed six numbers of unique compounds viz., trihydroxy methylene–di-oxyflavone, trigalloyl-HHDP-glucose, anthocyanins, neodiosmin, arachidonic acid, rush flavanone, etc. In contrast, UTC plants showed the expression of four unique compounds like epicatechin, isobellidifolin-8-O-β-D-glucopyranoside, 6-shogaol, and myricetin. However, 13 metabolites were expressed in both MT and UTC plants, which includedgalloyltannin, flavonoid quercertin, tomatidin, rutin, α-D-myo-inositol 1,4-bissphosp, caffeoyl-malate, dehydrotomatidin, transzeatin, quercetin, thebaine, cryptochlorogenic acid, phloretin, and quercetin-3-O-glucoside (Table 3; Figure 3). In MT plants, compounds in the classes of antioxidants, phenolic compounds, and bio-flavonoids were mainly expressed with higher intensity and their corresponding retention time ranged from 6.3 to 28.25 min, which helped the MT-immunized plants to combat sodicity stress.

Table 3.
Metabolic profiling of metabolite-treated plants (MT) versus untreated control (UTC) plants grown insodic soils at Sameese, Lucknow, Uttar Pradesh.
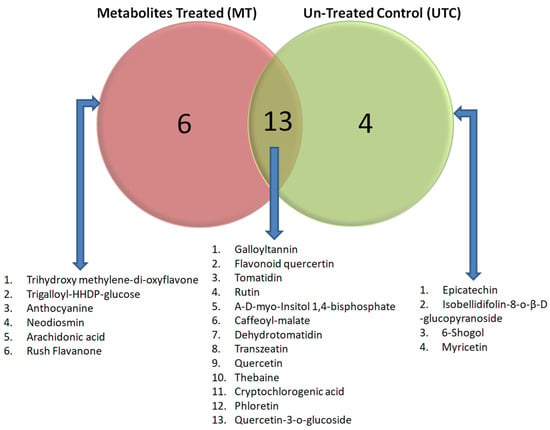
Figure 3.
Venn diagram showing the comparative distribution of metabolites among the metabolite-treated plants (MT) and untreated control (UTC) planted at Lalaikheda, Samesee, Lucknow district, Uttar Pradesh, India. This figure highlights the distinct and shared biochemical profiles of the MT and UTC plants, indicating potential differences in stress tolerance and defense mechanisms due to the treatment. The left circle (red) represents metabolites unique to MT with six specific compounds viz., trihydroxy methylene-di-oxyflavone, trigalloyl-HHDP-glucose, anthocyanine, neodiosmin, arachidonic acid, and rush flavanone. The right circle (green) represents metabolites unique to UTC plants, with four specific compounds viz., epicatechin, isobellidifolin-8-O-β-D-glucopyranoside, 6-Shogaol, and myricetin. The overlapping section (yellow) represents 13 metabolites common to both MT and UTC plants.
3.4. Profiling of Stress-Related Enzymes in MT vs. UTC
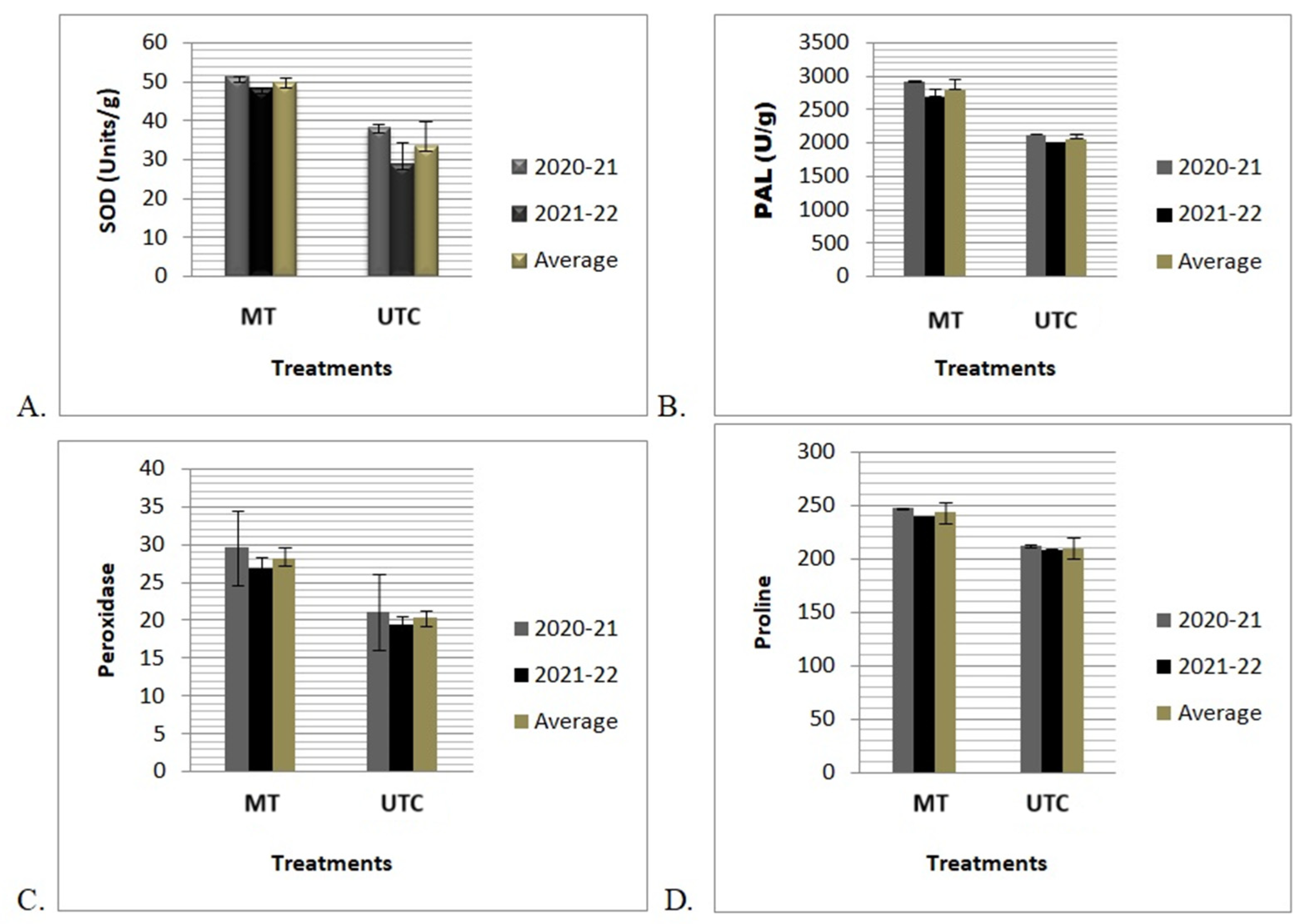
The antioxidant activities of the stress-related enzymes SOD, PAL, POD, and proline were measured in the MT and UTC plant leaf samples. The quantitative assay of stress-related enzymes varied significantly (p ≤ 0.05) between MT and UTC plants grown in sodicity-stressed soils. MT plants grown in sodic soil showed a higher level of stress-related enzyme activity: SOD (33.45 ± 6.3); PAL (2444.16 ± 142.02); proline (243.4 ± 4.87); and peroxidase (28.16 ± 1.57), whereas in the case of UTC plants a lower level of stress enzyme activity was found: SOD (41.50 ± 9.5); PAL (2074.39 ± 66.85);proline (209.7 ± 2.16); and peroxidase (20.31 ± 0.98). The results of the antioxidant enzyme test revealed that MT-immunized techniques had a significant impact on antioxidant activities in MT banana plants compared to untreated control in sodicity-affected soils (Figure 4).
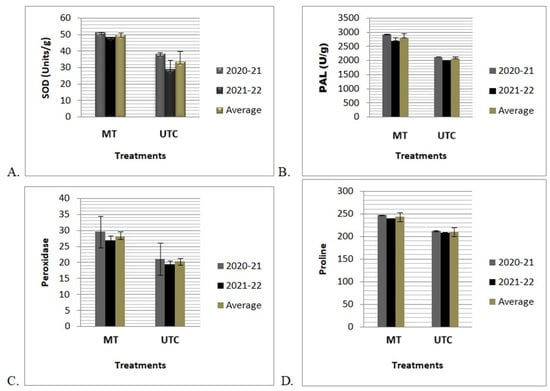
Figure 4.
Effects of MT on stress enzymes under sodicity conditions. (A) Superoxide dismutase (SOD) (Units g−1 fresh wt.), (B) PAL (U/g), (C) peroxidase (POD) (Unit g−1 fresh wt.), and (D) proline of tissue-culture-raised “Grand Naine” bananas grown in the sodicity-affected soils at Samesee, Lucknow district, Uttar Pradesh, India. Values are the mean of three replicates of two consecutive years (2020–2021 and 2021–2022) and average of two consecutive years (2020–2022). Values are the mean ± SD of three replications. F values are significant at alpha p ≤ 0.05.
3.5. Performance of MT Plants in Sodic Soils in Terms of Biomass
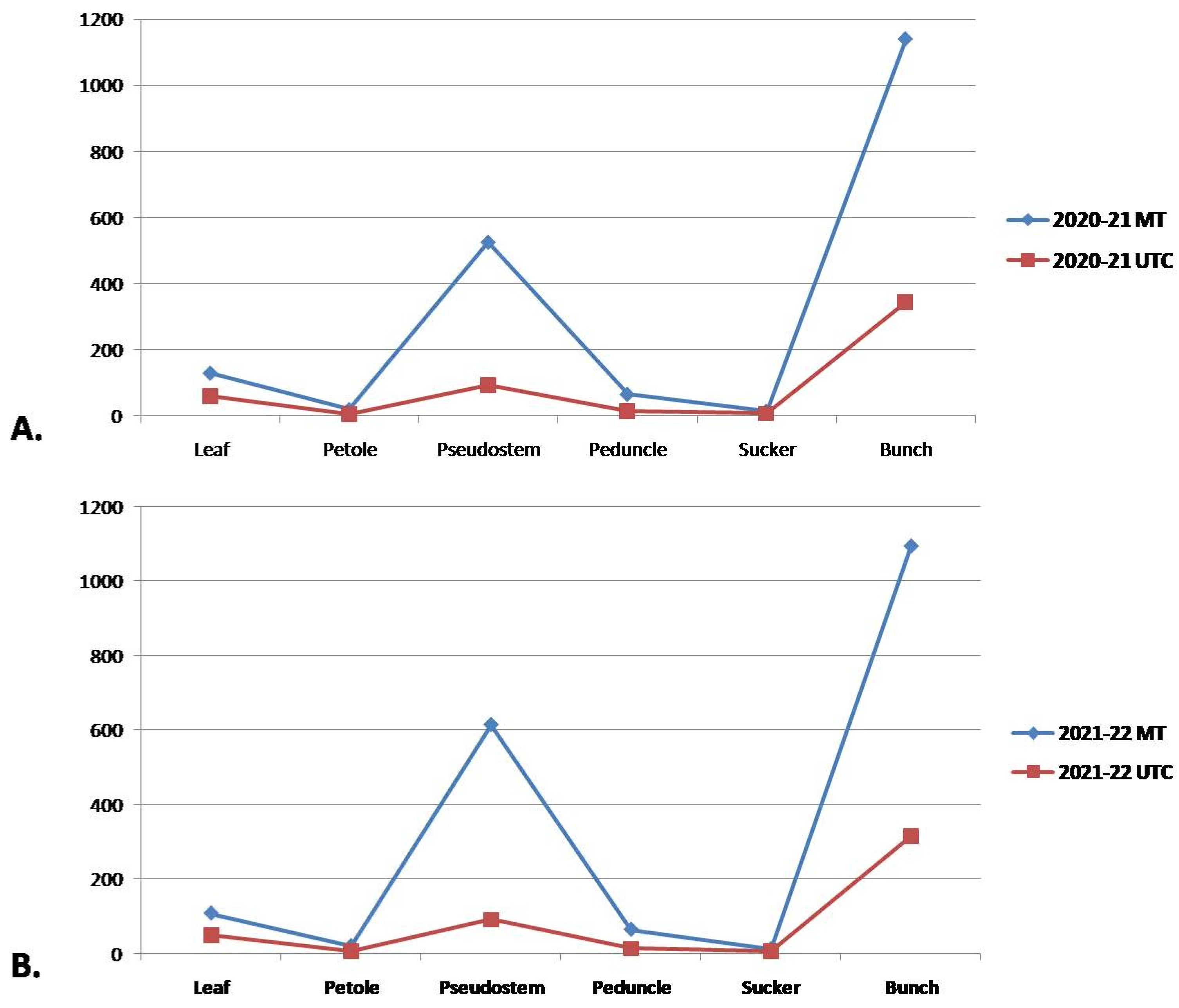
In the first year of the trial, the leaf biomass was found to be higher in the MT plants (129 kg), whereas UTC plants produced 59.01 kg of leaf biomass. Also, MT plants showed higher pseudostem biomass (525 kg) and fruit bunch biomass (1140 kg) than the UTC (93.75 kg and 345 kg, respectively). In the second year of the trial, MT plants again recorded higher biomass production in the case of the leaf (108 kg), petiole (20.25 kg), pseudostem (615 kg), bunch (1095 kg), sucker (12.75 kg), peduncle (64.35), etc., compared to UTC plants (Figure 5).
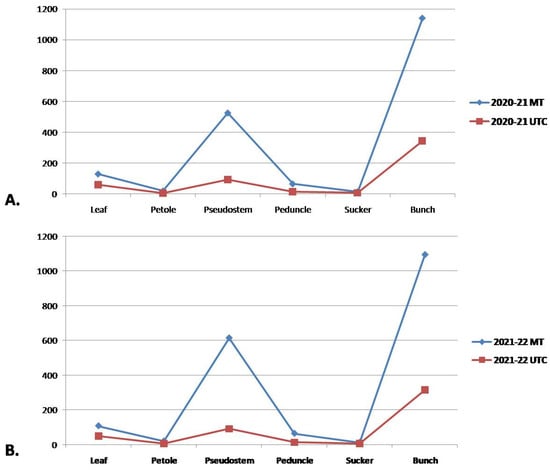
Figure 5.
Graphical representation of effect of MT on total biomass production (leaf, petiole, peduncle, sucker, and bunch) at Lalaikheda, Samesee, Uttar Pradesh for two consecutive years ((A) 2021 and (B) 2022).
3.6. Mechanism of Sodicity Tolerance
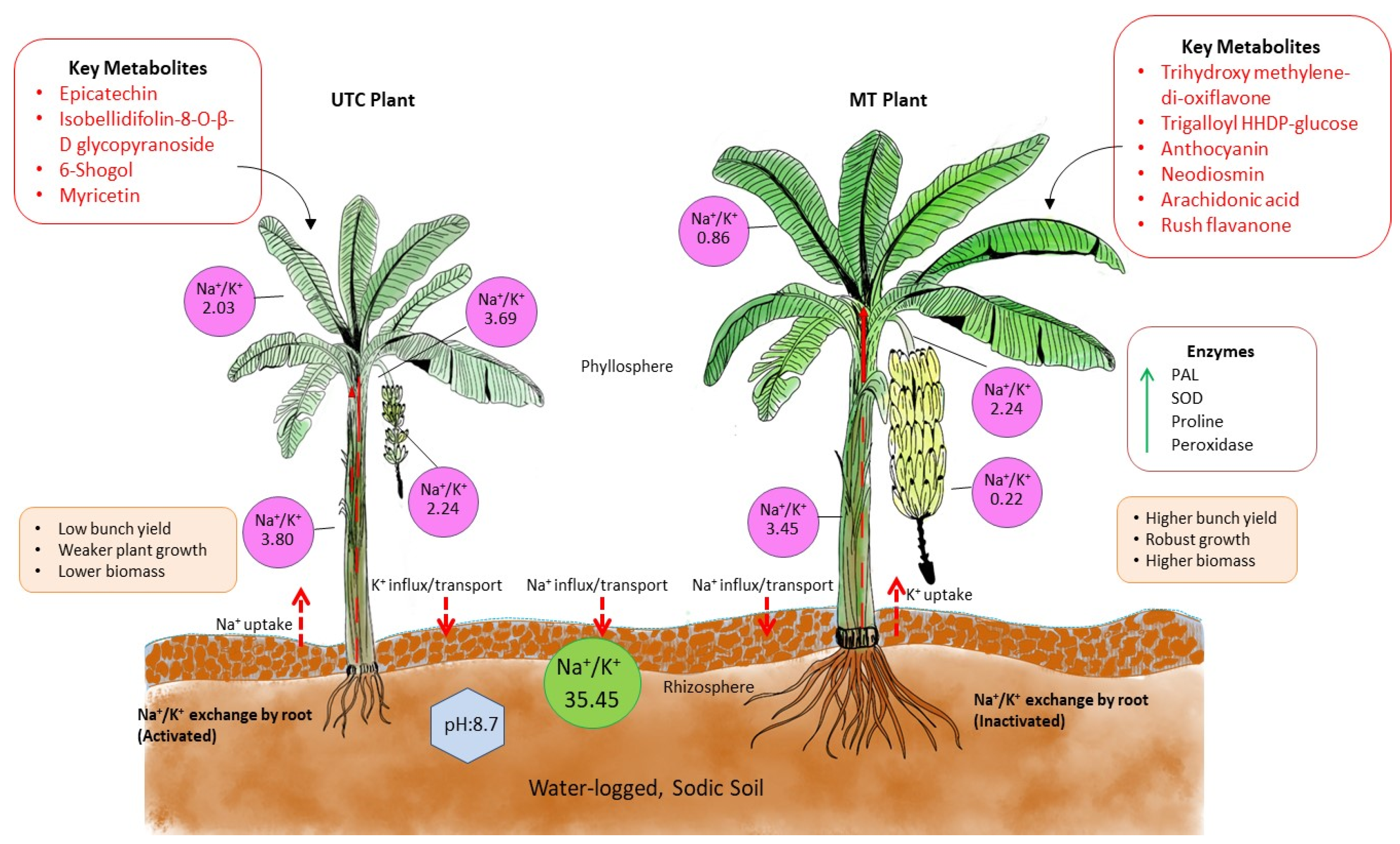
The mechanism of tolerance in MT plants was elucidated from the comparativeanalysis between MT and UTC banana plants grown in sodic soils, which illustrates the differences in metabolite production, Na+/K+ ratios, enzymatic activity, and plant performance (Figure 6). In the UTC plant, higher Na+/K+ ratios across the rhizosphere (3.80), leaves (2.03), and fruit (3.69) indicate greater sodium stress, leading to reduced growth, lower biomass, and lower bunch yields. These plants also produced metabolites such as epicatechin, myricetin, and 6-shogaol, which appear insufficient to mitigate sodicity stress effectively. In contrast, the MT plants demonstrated significantly lower Na+/K+ ratios, particularly in the rhizosphere (0.22) and leaves (0.86), which suggest enhanced sodium exclusion and potassium retention. Additionally, MT plants produced key metabolites like anthocyanin, neodiosmin, and arachidonic acid, which are associated with increased osmo-protection and antioxidant defense. Elevated enzymatic activity of PAL, SOD, proline, and peroxidase further indicates an enhanced stress response system in the MT plants. As a result, these plants showed stronger growth, higher biomass, and a substantial increase in bunch yield compared to the UTC plants. The mechanism postulated in the figure illustrates the effectivebenefits of metabolite treatment in improving banana plant resilience and productivity under sodicity stress.
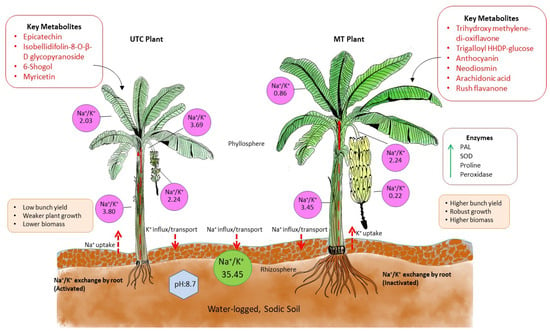
Figure 6.
The mechanism of sodicity tolerance in metabolite-treated banana plants. The figure presents the comparison of the performance of metabolite-treated (MT) banana plants with untreated control (UTC) plants in waterlogged sodic soil conditions, highlighting differences in key metabolites, enzyme activities, Na+/K+ ratios, and overall plant health. The blue dotted line denotes waterlogging conditions and the red dotted arrow represents the movement of sodium and potassium ions, the green solid arrow line indicates the upregulation of enzymes, and the red-bordered box represents key metabolites. The peach-colored box denotes the physiological characteristics of in vitro selected banana plants.
4. Discussion
Abiotic stresses such as drought, salinity, and sodicity adversely affect plant growth and yield, thereby reducing the productivity of crop plants as reported in tomatoes and bananas [44,45,46,47,48]. The global impact of climate change over the past two decades has raised ahuge concern for soil salinization and sodification, which require soil reclamation [49]. Banana is seriously affected by soil- and water-based salinity and sodicity problems which primarily affect plant rhizosphere and later affect every stage of plant growth, development, and reproduction, and eventually results in the death of plants [19]. Salinity and sodicity conditions have resulted in a reduction in growth parameters such as height, pseudostem girth, number of leaves, and bunch weight due to the ion toxicity, osmoticimbalance, nutrient deficiency, and oxidative damage induced by the high salt concentrations [50,51]. The probable means of chemical reclamation of sodic- and salinity-affected soils is the application of gypsum, pyrite, and organic amendments, but for limitations in sodic soils which do not allowchanges in the soil physical and biological properties [8]. Alternatively, a vast array of microbial-derived bio-products involving microbes have resulted in partial successin indirect soil reclamation experiments with restricted function at the pH range of 7.0–8.5. However, a biological approach using PGPR tolerant to high pH (>8.5) proved to induce salinity tolerance in which endophytic bacteria were associated withan increase in the growth of plants in neutral soils [52,53,54] and saline soils inpot experiments with tomato and cucumber [55]. Most recently, a patented PGPR of bacterial origin engineered MT molecules which were used to immunize tissue-culture-raised/in vitro selected plantlets during organogenesis, which were able to combat the fungal pathogen Fusarium oxysporum f. sp. cubense Foc-TR4 [17]. Its potential in conferring abiotic stress tolerance was assessed by evaluating the MT plants in the sodic soils. It was found that initially plant growth was reduced under sodicity stress, which might have been due to water congestion in the surface soil and also the effect of sodium (Na+) and carbonates in the rhizosphere. The MT plants grown in the sodic soils not only exhibited better plant growth parameters but also resulted in higher bunch yield/plant and biomass compared toUTC plants. In contrast, there was a significant reduction in plant growth parameters in the control (UTC) which supports the earlier reports of Soussi et al. [56] and Abrar et al. [57], wherein they have recorded salinity and sodicity conditions affecting plant growth conditions. This reduction in the plant yield and biomass under stress may be due to the inhibition or hydrolysis of reserved food and its translocation to the growing shoot portions [55,56]. In the present study, it was noted that MT treatment substantially altered the balance of sodium and potassium ions in plant tissues, particularly affecting the fruit tissue. It was also observed in the current experiment that the yield of banana (bunch weight) increased with the decrease in the Na/K ratio, proving that the metabolite-treated technology seems to alter the Na+ and K+ uptake of the plants as well as their translocation. The highest accumulation of K in the fruits and pseudostem due to K/Na homeostasis from root cells to edible portions shows the source–sink relationship in MT plants [57,58]. Contrarily, in UTC plants, Na uptake and translocation and itspresence recorded in the edible tissues indicates the sodicity susceptibility.
The salt-induced adverse effects on the plants are primarily due to specific ion toxicity, production of oxidants, and so on [59,60]. A higher concentration of Na+ ions in the root surface under saline and sodic conditions has adversely affected the K+ ions and their uptake patterns [61,62]. In our study, similar results were also recorded but MT plants showed a lower Na+/K+ ratio. However, in the current study, a considerable amount of Ca deposition was also observed in the plant parts (Figures S1 and S2). Ca2+ ions are also known for ameliorating the adverse effects of salinity in plants by way of an increased K+/Na+ ratio [61]. A similar mode of action to Ca2+ ions serving as a catalyst to maintain the balance between the K+/Na+ ratio for plant survival must have occurred in sodic soils, owing to the higher Na+ absorption rate [63,64,65].
Plants produce low-molecular-weight organic solutes such as proline or enzymes like SOD, PAL, and PO to tolerate the effects of salt stress [66]. However, most plant species do not produce sufficient levels of antioxidants to fulfill their growth requirements under salt stress, which warrants additional alternate mechanisms for survival. But the present study has shown significant levels of increase in the antioxidant enzymes as well as osmolytes in MT banana plants grown in sodic soils. Previously, bio-inoculation induced salt tolerance by increasing the activity of antioxidant enzymes (SOD, PO, and PAL) and organic solutes like proline [67]. Such mechanisms have been extensively reported wherein increased SOD, PO, and PAL were recorded under sodic stress conditions [68,69]. Previously, exo-polysaccharide-producing sodicity-tolerant bacteria with plantgrowth-promoting activities were found to induce osmolyte accumulation under sodic stress conditions in rice [70]. In the current study, it was observed that MT plants triggered proline production in the cell compared to the UTC plants. Salt-stress-induced defense-system-based enzyme expression was found to be higher in the MT plants than the UTC plants in our study. The elevation of plant stress enzyme activities in the MT plant systems is indicative of the induction of the plant defense system in counteracting the sodicity stress and this could be one of the possible mechanisms of adaptively induced host tolerance.
In the present study, the major class of metabolites identified in the MT plants through LC-MS analysis were phenolic esters, antioxidants, and fatty acids, which corroborates with the earlier reports, thus establishing their role in conferring salt tolerance. MT plants exhibited production of metabolites such as trihydroxy methylene–di-oxyflavone, rush flavanone, anthocyanins, neodiosmin, arachidonic acid, and trigalloyl-HHDP-glucose, which belong to the subclasses of flavonoids, anthocyanins, and sugar alcohols (Figure S2). All these unique metabolites reported in MT plants in the present study have implications on the sodicity tolerance which isdiscussed here. Rutin (3,3′,4′,5,7-pentahydroxyflavone-3-rhamnoglucoside) is a flavanol glycoside found in buckwheat, tobacco, tea, apple, and citrus that is reported to have antibacterial and antifungal properties [71]. It has also been reported to improveplant salinity tolerance byregulating K+ retention and Na+ exclusion from leaf mesophyll in quinoa and broad beans [72]. Arachidonic acid has implications in conferring salt tolerance and its external application in rice resulted in the alteration of the fatty acid composition in the leaves and panicles of salinity-stressed plants [73]. The changes in lipid composition in response to salt stress have been notedfor causing an interaction with the intracellular Na+/K+ ratio in sunflowers [74]. Cryptochlorogenic acid is a natural product found in Withania somnifera and Coffea arabica which has been reported to be induced by salinity stress [75]. Tomatidine and de-hydrotomatidin are steroidal alkaloidsfound in tomatoes, potatoes, and eggplants and are reported to be synthesized and accumulated as noxious molecules in response to environmental modulations along with biotic and abiotic stresses [76,77]. Transzeatinis is a natural cytokinin that induces cell division and shoot formation in maize (Zea mays L.) and it has also been reported to confer tolerance in plants to manage environmental stresses [78]. Caffeoylmalic acid, or caffeoylmalate, belongs to the class of coumaric acids and its derivatives which have been reported to be involved in the conversion of lipophilic fatty alcohols into antioxidants [79]. Further, Gao et al. [80] have reported that proline, betaine, and p-coumaryl alcohol are candidates for screening saline-alkali tolerance in oat cultivars.
These metabolites are responsible for the antioxidant defense systems of plants. Excessive production of reactive oxygen species (ROS) causes oxidative stress leading to cellular damage and ultimately leading to senescence during sodicity stress. To prevent or alleviate the ROS-induced damage, plants have evolved an intriguing antioxidant defense system which produces ROS scavengers or enzymes such as SOD, PO, etc. which function to keep levels of reactive or active oxygen species under control. This is also evident from the enzyme assays as well as LC-MS data. Antioxidant defense systems comprise both enzymatic as well as non-enzymatic components. Phytohormones and osmolytes are low-molecular-weight metabolites (amino acids, polyamines, and sugars) that act as regulators of homeostasis and also help to protect cells from abiotic stress [81]. Osmolytes mitigate the adverse effects of abiotic stresses by protecting the molecular membrane structure and scavenging ROS. Proline is considered as the most important osmolyte to protect the plant against abiotic stress. The comparative metabolic profile of MT and UTC plants showed anelevated expression of defense-related compounds in the MT plants rather than UTC plants. The probable cause behind the elevated expression is that the application of biologically engineered molecules in the plant system during organogenesis may have triggered the innate immune system of plants well in advance and subsequently memory cells were also produced in the plant system, as it was observed previously that bio-inoculation also enhanced the defense mechanism of plants due to the elevated level of enzyme activity in the host plants [9]. It could be speculated that in the MT plants, the innate immune system was triggered under sodicity stress, which increased the host tolerance. Metabolite treatment during organogenesis of MT plants triggered the host defense system by programming the plant memory and activating it upon exposure to external stress. Such a phenomenon of adaptive host tolerance to sodicity conditions through osmo-protection and antioxidant defense have been established in the current study which is illustrated in the Figure 6.
The MT plant demonstrates better sodium management (lower Na+/K+ ratios), higher enzyme activity, and superior growth characteristics (higher biomass and bunch yield) compared to the UTC plant, which shows weaker growth and lower yield. These differences could be attributed to the enhanced production of beneficial metabolites and enzyme activation in the MT plant, leading to improved stress tolerance and nutrient uptake efficiency. Metabolite-treated technology significantly enhances banana plants’ tolerance to sodicity by improving sodium regulation, activating defense enzymes, and boosting the production of protective metabolites. This results in stronger plant growth, higher yields, and greater biomass, making it a promising strategy for sustainable banana cultivation in waterlogged sodic soils.
5. Conclusions
The current investigation confirms that metabolite treatment technology (patent no. 202111003761) plays a crucial role in promoting sustainable banana production in sodic soils. While metabolite-treated plants using engineered bio-molecules have previously shown success in combating Fusarium wilt caused by Foc TR4, this study extends the application to abiotic stress tolerance, particularly salinity and sodicity. The findings reveal that MT banana plants exhibit significant increases in yield and biomass, demonstrating the technology’s effectiveness in enhancing crop performance under stressful conditions. This innovation holds promise for farmers in sodicity-affected regions, offering a viable solution for cultivating banana in challenging soils. The success of this study could pave the way for expanding banana cultivation into new sodicity-affected areas, contributing to sustainable and profitable banana farming.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11040416/s1. Figure S1: Effect of MT on Na/K ratio and differential deposition of Na, K, Ca and Mg in different parts of a banana plants. Figure S2: LC-MS profiles of MT (A. Metabolites Treated) and UTC (B. Untreated control) independent files (Original data files).
Author Contributions
D.T. conceived and designed the experiments and drafted the manuscript, M.M. (Muthukumar Manoharan) analyzed the LC-MS data and drafted and revised the manuscript, P.D. conducted the field experiment, analyzed the data, and helped in drafting and revising the manuscript, S.K. (Sangeeta Kumari) performed the bio-chemical assays, M.M. (Maneesh Mishra) analyzed the data sets of field experiments, S.K. (Sandeep Kumar) prepared the images and helped in the revision of the manuscript, and S.K.J. and C.L.V. performed the soil analysis and data analysis of the field trails. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors thank the Director of ICAR-Central Soil Salinity Research Institute, Regional Research Station, Lucknow and the Director of ICAR-Central Institute for Subtropical Horticulture, Lucknow for their support and guidance. The authors acknowledge the use of AI tools like Chatgpt 4 version that have facilitated the improvement of the overall quality of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PGPR | Plant growth-promoting rhizobacterial |
| MT | Metabolite-treated |
| UTC | Untreated control |
| RBD | Randomized block design |
| EC | Electrical conductivity |
| PO | Peroxidase |
| PPO | Polyphenol oxidase |
| SOD | Super oxide dismutase |
| PAL | Phenylalanine ammonia lyase |
| LC-MS | Liquid chromatography coupled with mass spectrophotometry |
References
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 2, 123–131. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, P.K. Soil salinity and food security in India. Front. Sustain. Food Syst. 2020, 4, 533781. [Google Scholar] [CrossRef]
- Mohanavelu, A.; Naganna, S.R.; Al-Ansari, N. Irrigation induced salinity and sodicity hazards on soil and groundwater: An overview of its causes, impacts and mitigation strategies. Agriculture 2021, 11, 983. [Google Scholar] [CrossRef]
- Mehrotra, N.K.; Agarwala, S.C. Micronutrients disorders associated with helomorphic soils of Uttar Pradesh. Indian J. Plant Nutr. 1987, 6, 51–81. [Google Scholar]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotechnol. 2005, 16, 123–132. [Google Scholar] [CrossRef]
- Roy, S.; Chowdhury, N. Salt Stress in plants and amelioration strategies: A critical review. In Abiotic Stress in Plants; Fahad, S., Saud, S., Chen, Y., Wu, C., Wang, D., Eds.; Intech Open: London, UK, 2021; Volume 1, pp. 391–422. [Google Scholar]
- Damodaran, T.; Rai, R.B.; Mishra, V.K.; Sharma, D.K.; Ram, R.A.; Rai, S.; Kumar, H. Integrated Farming System and Livelihood Security: An Approach; Central Soil Salinity Research Institute (CSSRI): Karnal, India, 2011; pp. 1–108. [Google Scholar]
- Qadir, M.; Oster, J.D. Vegetative bioremediation of calcareous sodic soils: History, mechanisms and evaluation. J. Irrig. Sci. 2002, 21, 91–101. [Google Scholar]
- Kashyap, S.; Agarwala, N.; Sunkar, R. Understanding plant stress memory traits can provide a way for sustainable agriculture. Plant Sci. 2024, 340, 111954. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Priming crops for future: Reviewing stress memory. Trends Plant Sci. 2022, 27, 699–716. [Google Scholar] [CrossRef]
- Harish, S.; Kavino, M.; Kumar, N.; Samiyappan, R. Bio-priming banana with plant growth-promoting Endophytic bacteria induces systemic resistance against banana bunchy top virus. Acta Hortic. 2009, 829, 295–302. [Google Scholar] [CrossRef]
- Murphy, J.F.; Reddy, M.S.; Ryu, C.M.; Kloepper, J.W.; Li, R. Rhizobacteria mediated growth promotion of tomato leads to protection against cucumber mosaic virus. Phytopathology 2003, 93, 1301–1307. [Google Scholar] [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C.M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Goellner, K.; Conrath, U. Priming: It’s all the world to induced disease resistance. Eur. J. Plant Pathol. 2008, 121, 233–242. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, B.R. Plant growth promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, T.; Jha, S.K.; Kumari, S.; Gupta, G.; Mishra, V.K.; Sharma, P.C.; Gopal, R.; Singh, A.; Jat, H.S. Development of halotolerant microbial consortia for salt stress mitigation and sustainable tomato production in sodic soils: An enzyme mechanism approach. Sustainability 2023, 15, 5186. [Google Scholar] [CrossRef]
- Damodaran, T.; Mishra, M.; Muthukumar, M.; Rajan, S.; Yadav, K.; Kumar, A.; Debnath, P.; Kumari, S.; Bora, P.; Gopal, R.; et al. Secondary metabolite induced tolerance to Fusarium oxysporum f. sp. cubense TR4 in banana cv. Grand Naine through in vitro bio-immunization: A prospective research translation from induction to field tolerance. Front. Microbiol. 2023, 14, 1233469. [Google Scholar] [CrossRef]
- Hinge, V.R.; Shaikh, I.M.; Chavhan, R.L.; Deshmukh, A.S.; Shelake, R.M.; Ghunge, S.A.; Dethe, A.M.; Suprasanna, P.; Kadam, U.S. Assessment of genetic diversity and volatile content of commercially grown banana (Musa spp.) cultivars. Sci. Rep. 2022, 12, 7979. [Google Scholar] [CrossRef]
- Jeyabaskaran, K.J. Studies on fixing critical limits of K, Na and K/Na ratio for bananas in saline sodic soil conditions. InfoMusa 2000, 9, 34. [Google Scholar]
- Chang, K.L.; Bray, R.H. Determination of calcium and magnesium in soil and plant material. Soil Sci. 1951, 72, 449–458. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and improvement of saline and alkali Soils. In USDA Handbook; Forgotten Books: London, UK, 1954; Volume 60. [Google Scholar]
- Hammerschmidt, R.; Nuckles, E.M.; Kuc, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Mayer, A.M.; Harel, E. Polyphenol oxidases in plants. Phytochemistry 1979, 18, 193–215. [Google Scholar] [CrossRef]
- Constantine, N.G.; Stanley, K.R. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar]
- Şirin, S.; Aydaş, S.B.; Aslım, B. Biochemical evaluation of phenylalanine ammonia lyase from endemic plant Cyathobasis fruticulosa (Bunge) Aellen. for the dietary treatment of Phenylketonuria. Food Technol. Biotechnol. 2016, 54, 296–303. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Brahmachari, G.; Jash, S.K.; Gangopadhyay, A.; Sarkar, S.; Laskar, S.; Gorai, D. Chemical constitution of Limnophila indica. Indian J. Chem. 2008, 47, 1898–1902. [Google Scholar]
- Lachowicz, S.; Oszmiański, J.; Rapak, A.; Ochmian, I. Profile and Content of Phenolic Compounds in Leaves, Flowers, Roots, and Stalks of Sanguisorba officinalis L. determined with the LC-DAD-ESI-QTOF-MS/MS analysis and their in vitro antioxidant, antidiabetic, antiproliferative potency. Pharmaceuticals 2020, 13, 191. [Google Scholar] [CrossRef]
- Damodaran, T.; Rajan, S.; Muthukumar, M.; Gopal, R.; Yadav, K.; Kumar, S.; Ahmad, I.; Kumari, N.; Mishra, V.K.; Jha, S.K. Biological Management of Banana Fusarium Wilt Caused by Fusarium oxysporum f. sp. Cubense Tropical Race 4 Using Antagonistic Fungal Isolate CSR-T-3 (Trichoderma reesei). Front. Microbiol 2020, 11, 595845. [Google Scholar]
- Mz Cloud. Advanced Mass Spectral Database. Available online: https://www.mzcloud.org/ (accessed on 24 February 2025).
- Lu, C.; Jiang, Y.; Yue, Y.; Sui, Y.; Hao, M.; Kang, X.; Wang, Q.; Chen, D.; Liu, B.; Yin, Z.; et al. Glutathione and neodiosmin feedback sustain plant immunity. J. Exp. Bot. 2022, 74, 976–990. [Google Scholar] [CrossRef]
- Savchenko, T.; Walley, J.W.; Chehab, E.W.; Xiao, Y.; Kaspi, R.; Pye, M.F.; Mohamed, M.E.; Lazarus, C.M.; Bostock, R.M.; Dehesh, K. Arachidonic acid: An evolutionarily conserved signaling molecule modulates plant stress signaling networks. Plant Cell. 2010, 22, 3193–3205. [Google Scholar] [CrossRef]
- Malikov, V.M.; Yuldashev, M.P. Phenolic Compounds of Plants of the Scutellaria L. Genus. Distribution, Structure, and Properties. Chem. Net. Copd. 2002, 38, 358–406. [Google Scholar]
- Ling, H.; Yang, H.; Tan, S.H.; Chui, W.K.; Chew, E.H. 6-Shogaol, an active constituent of ginger, inhibits breast cancer cell invasion by reducing matrix metalloproteinase-9 expression via blockade of nuclear factor-κB activation. Br. J. Pharmacol. 2010, 161, 1763–1777. [Google Scholar] [CrossRef]
- Jose, J.; Dhanya, A.; Haridas, K.R.; Kumar, T.S.; Jayaraman, S.; Variyar, E.J.; Sudhakaran, S. Structural characterization of a novel derivative of myricetin from Mimosa pudica as an anti-proliferative agent for the treatment of cancer. Biomed. Pharmacother. 2016, 84, 1067–1077. [Google Scholar]
- Magalhaes, L.M.; Ramos, I.I.; Reis, S.; Segundo, M.A. Antioxidant profile of commercial oenological tannins determined by multiple chemical assays. Aust. J. Grape Wine Res. 2014, 20, 72–79. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Pl. Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef]
- Drapal, M.; Carvalho, E.B.D.; Rouard, M.; Amah, D.; Sardos, J.; Houwe, I.V.; Brown, A.; Roux, N.; Swennen, R.; Fraser, P.D. Metabolite profiling characterises chemotypes of Musa diploids and triploids at juvenile and pre-flowering growth stages. Sci. Rep. 2019, 9, 4657. [Google Scholar] [CrossRef] [PubMed]
- Kozukue, N.; Han, J.-S.; Lee, K.-R.; Friedman, M. Dehydrotomatine and α-tomatine content in tomato fruits and vegetative plant tissues. J. Agric. Food Chem. 2004, 52, 2079–2083. [Google Scholar] [CrossRef] [PubMed]
- Grobkinsky, D.K.; Edelsbrunner, K.; Pfeifhofer, H.; Graaff, E.V.; Roitsch, T. Cis- and trans-zeatin differentially modulate plant immunity. Plant Signal. Behav. 2013, 8, e24798. [Google Scholar] [CrossRef]
- Tok, T.T.; Gowder, S.J.T. Structural and pharmacological properties of alkaloids with special reference to thebaine type alkaloids. Biomed. J. Sci. Tech. Res. 2019, 17, 12767–12780. [Google Scholar]
- Pun, M.; Khazanov, N.; Galsurker, O.; Weitman, M.; Kerem, Z.; Senderowitz, H.; Yedidia, I. Phloretin, an Apple phytoalexin, affects the virulence and fitness of Pectobacterium brasiliense by interfering with Quorum-Sensing. Front. Pl. Sci. 2021, 12, 671807. [Google Scholar] [CrossRef]
- Nile, A.; Nile, S.H.; Shin, J.; Park, G.; Oh, J.W. Quercetin-3-Glucoside Extracted from Apple Pomace Induces Cell Cycle Arrest and Apoptosis by Increasing Intracellular ROS Levels. Int. J. Mol. Sci. 2021, 22, 10749. [Google Scholar] [CrossRef]
- Tiwari, H.S.; Agarwal, R.M.; Bhatt, R.K. Photosynthesis, stomatal resistance and related characteristics, as influenced by potassium under normal water supply and water stress conditions in rice (Oryza sativa L.). Indian J. Plant Physiol. 1998, 3, 314–316. [Google Scholar]
- Jatav, K.S.; Agarwal, R.M.; Tomar, N.S.; Tyagi, S.R. Nitrogen metabolism, growth and yield responses of wheat (Triticum aestivum L.) to restricted water supply and varying potassium treatments. J. Indian Bot. Sic. 2014, 93, 177–189. [Google Scholar]
- Ramegowda, V.; Senthil-kumar, M. The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 2015, 176, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Al-Karaki, G.N. Growth and mineral acquisition by mycorrhizal tomato under salt stress. Mycorrhiza 2000, 10, 51–54. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y. How do plants maintain pH and ion homeostasis under saline-alkali stress? Front. Plant Sci. 2023, 14, 1217193. [Google Scholar] [CrossRef]
- Fracasso, A.; Trindade, L.; Amaducci, S. Drought tolerance strategies highlighted by two Sorghum bicolor races in a dry-down experiment. J. Plant Physiol. 2016, 190, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hareem, M.; Danish, S.; Obaid, S.A.; Ansari, M.J.; Datta, R. Mitigation of drought stress in chili plants (Capsicum annuum L.) using mango fruit waste biochar, fulvic acid and cobalt. Sci. Rep. 2024, 14, 14270. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Maqbool, M.A.; Cengiz, R. Drought Stress in Maize (Zea mays L.): Effects, Resistance Mechanisms, Global Achievements and Biological Strategies for Improvement; Springer: Cham, Switzerland, 2015; Volume 1, pp. 1–58. [Google Scholar]
- Kloepper, J.W.; Beauchamp, C.J. A review of issues related to measuring colonization of plant roots by bacteria. Can. J. Microbiol. 1992, 38, 1219–1232. [Google Scholar] [CrossRef]
- Joseph, B.; Patra, R.; Lawrence, R. Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arieatinum L.). Int. J. Plant Prod. 2007, 2, 141–152. [Google Scholar]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Shurigin, V.; Gopalakrishnan, S.; Sharma, R. Growth and symbiotic performance of chickpea (Cicer arietinum) cultivars under saline soil conditions. J. Biol. Chem. Res. 2014, 56, 1–10. [Google Scholar]
- Soussi, M.; Ocana, A.; Lluch, C. Effects of salt stress on growth, photosynthesis and nitrogen fixation in chick-pea (Cicer arietinum L.). J. Exp. Bot. 1998, 49, 1329–1337. [Google Scholar] [CrossRef]
- Abrar, M.M.; Sohail, M.; Saqib, M.; Akhtar, J.; Abbas, G.; Wahab, H.A.; Mumtaz, M.Z.; Mehmood, K.; Memon, M.S.; Sun, N.; et al. Interactive salinity and water stress severely reduced the growth, stress tolerance, and physiological responses of guava (Psidium guajava L.). Sci. Rep. 2022, 12, 18952. [Google Scholar] [CrossRef] [PubMed]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S.; et al. Impacts of salinity stress on crop plants: Improving salt tolerance through genetic and molecular dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Akram, N.A. Improving salinity tolerance of plants through conventional breeding and genetic engineering: An analytical comparison. Biotechnol. Adv. 2009, 27, 744–752. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Maathuis, F.J.M.; Amtmann, A. K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Wakeel, A. Potassium-sodium interactions in soil and plant under saline-sodic conditions. J. Plant Nutr. Soil Sci. 2013, 176, 344–354. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na+ and K+ Transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, J.; Wu, X.; Dong, L. Na+ /K+ Balance and transport regulatory mechanisms in weedy and cultivated rice (Oryza sativa L.) under salt stress. BMC Plant Biol. 2018, 18, 375. [Google Scholar] [CrossRef]
- Li, J.; He, P.; Chen, J.; Hamad, A.A.A.; Dai, X.; Jin, Q.; Ding, S. Tomato performance and changes in soil chemistry in response to salinity and Na/Ca ratio of irrigation water. Agric. Water Manag. 2023, 285, 108363. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Dikilitas, M.; Tuna, A.L. Alleviation of salt stress-induced adverse effects on maize plants by exogenous application of indoleacetic acid (IAA) and inorganic nutrients—A field trial. Aust. J. Crop Sci. 2013, 7, 249–254. [Google Scholar]
- Mishra, P.; Sharma, P. Superoxide Dismutases (SODs) and their role in regulating abiotic stress induced oxidative stress in plants. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; Wiley: Hoboken, NJ, USA, 2019; Volume 1, pp. 53–88. [Google Scholar]
- Ali, N.; Rafiq, R.; Nisa, Z.; Wijaya, L.; Ahmad, A.; Kaushik, P. Exogenous citric acid improves growth and yield by concerted modulation of antioxidant defense system in brinjal (Solanum melongena L.) under salt-stress. J. King Saud Univ. Sci. 2024, 36, 103012. [Google Scholar] [CrossRef]
- Gunasekaran, Y.; Thiyageshwari, S.; Ariyan, M.; Roy-Choudhury, A.; Park, J.H.; Selvi, D.; Chithra, L.; Anandham, R. Alleviation of sodic stress in rice by exploring the exo-polysaccharide producing sodic-tolerant bacteria. Agriculture 2022, 12, 1451. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2016, 25, 149–164. [Google Scholar] [CrossRef]
- Ismail, H.; Dragisic-Maksimovic, J.; Maksimovic, V.; Shabala, L.; Zivanovic, B.D.; Tian, Y.; Jacobsen, S.E.; Shabala, S. Rutin, a flavonoid with antioxidant activity, improves plant salinity tolerance by regulating K+ retention and Na+ exclusion from leaf mesophyll in quinoa and broad beans. Funct. Plant Biol. 2015, 43, 75–86. [Google Scholar] [CrossRef]
- Wahab, P.A.A.; Ahmad, A. Effects of exogenous arachidonic acid on morphological traits and fatty acid profile of rice (Oryza sativa L.) grown on saline soil. Univ. Malays. Teren. J. Undergrad. Res. 2019, 1, 68–78. [Google Scholar] [CrossRef]
- Gogna, M.; Choudhary, A.; Mishra, G.; Kapoor, R.; Bhatla, S.C. Changes in lipid composition in response to salt stress and its possible interaction with intracellular Na+-K+ ratio in sunflower (Helianthus annuus L.). Environ. Exp. Bot. 2020, 178, 104147. [Google Scholar] [CrossRef]
- Yan, K.; Cui, M.; Zhao, S.; Chen, X.; Tang, X. Salinity stress is beneficial to the accumulation of chlorogenic acids in honeysuckle (Lonicera japonica Thunb.). Front. Plant Sci. 2016, 7, 1563. [Google Scholar] [CrossRef]
- Schaller, H. Sterol and steroid biosynthesis and metabolism in plants and microorganisms. In Comprehensive Natural Products II; Lew, M., Hung-Wen, L., Eds.; Elsevier: Oxford, UK, 2010; Volume 1, pp. 755–787. [Google Scholar]
- Dey, A.; Mukherjee, A. Plant derived alkaloids: A promising window for neuroprotective drug discovery. In Discovery and Development of Neuroprotective Agents from Natural Products; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 237–320. [Google Scholar]
- Murofushi, N.; Yamane, H.; Sakagami, Y.; Imaseki, H.; Kamiya, Y.; Iwamura, H.; Hirai, N.; Tsuji, H.; Yokota, T.; Ueda, J. 8.02—Plant Hormones. In Comprehensive Natural Products Chemistry: Plant Hormones; Barton, S.D., Nakanishi, K., Meth-Cohn, O., Eds.; Elsevier: Oxford, UK, 1999; Volume 8, pp. 19–136. [Google Scholar]
- Anankanbil, S.; Pérez, B.; Cheng, W.; Ambrosio, G.G.; Zheng, G. Caffeoyl maleic fatty alcohol monoesters: Synthesis, characterization and antioxidant assessment. J. Colloid Interface Sci. 2019, 536, 399–407. [Google Scholar] [CrossRef]
- Gao, Y.; Jin, Y.; Guo, W.; Xue, Y.; Yu, L. Metabolic and physiological changes in the roots of two oat cultivars in response to complex saline-alkali stress. Front. Plant Sci. 2022, 13, 835414. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Shahwar, D. Role of Heat Shock Proteins (HSPs) and Heat Stress Tolerance in Crop Plants. In Sustainable Agriculture in the Era of Climate Change; Mirza, H., Roychowdhury, R., Srivastava, S., Choudhury, S., Eds.; Springer: Cham, Switzerland, 2020; Volume 1, pp. 211–234. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).