Fruit Quality and Antioxidant Content in Durian (Durio zibethinus Murr.) cv. ‘Monthong’ in Different Maturity Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Determination of Fruit Growth
2.3. Determination of Color Change, Pulp Firmness, Dry Matter, TSS, and TA
2.4. Determination of Vitamin C Content
2.5. Determination of Total Phenolic Content
2.6. Determination of Total Flavonoid Content
2.7. Determination of Beta-Carotene Content
2.8. Determination of Lycopene Content
2.9. Determination of DPPH Radical-Scavenging Activity
2.10. Determination of ABTS Radical-Scavenging Activity
2.11. Statistical Analysis
3. Results
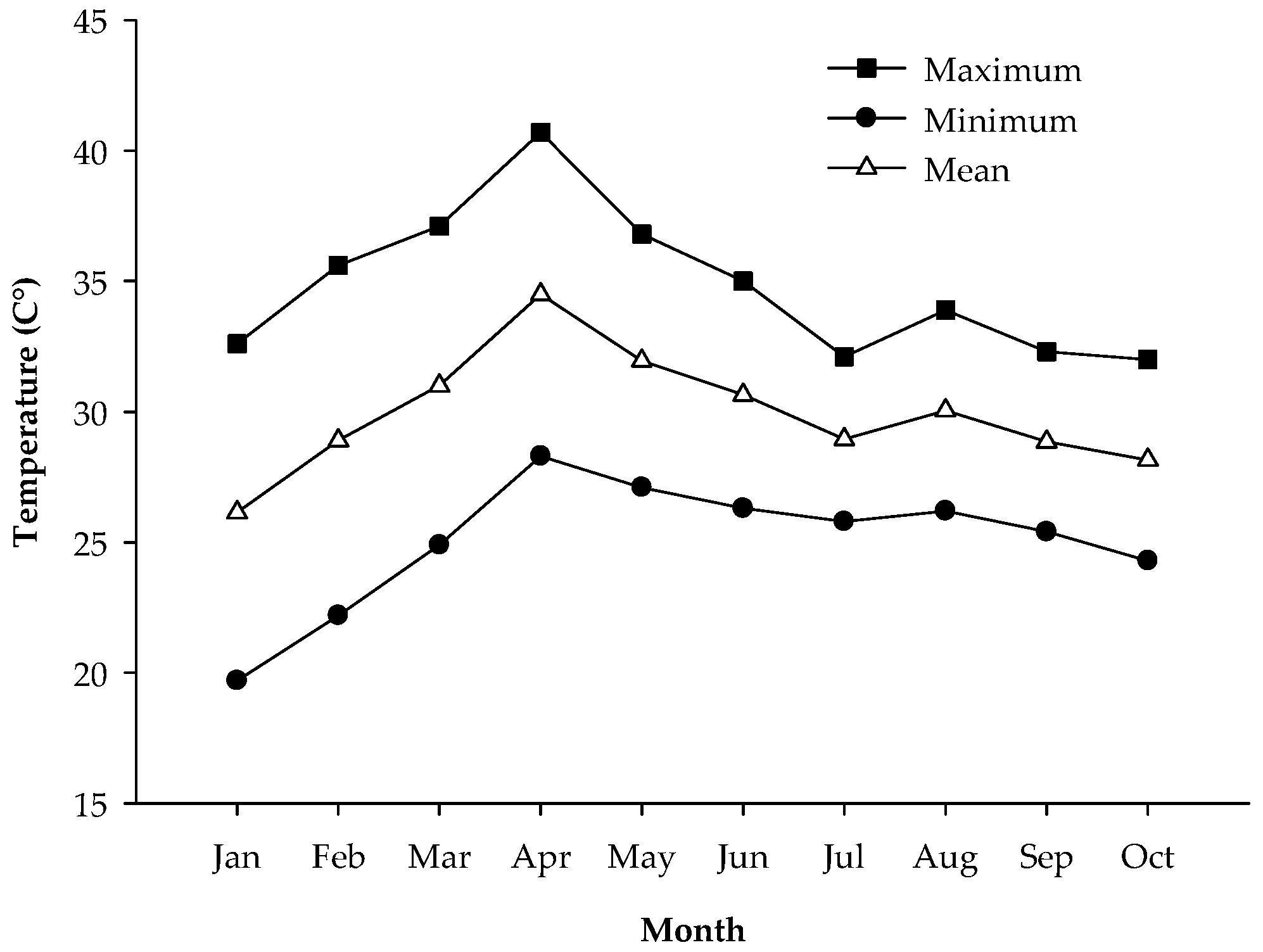
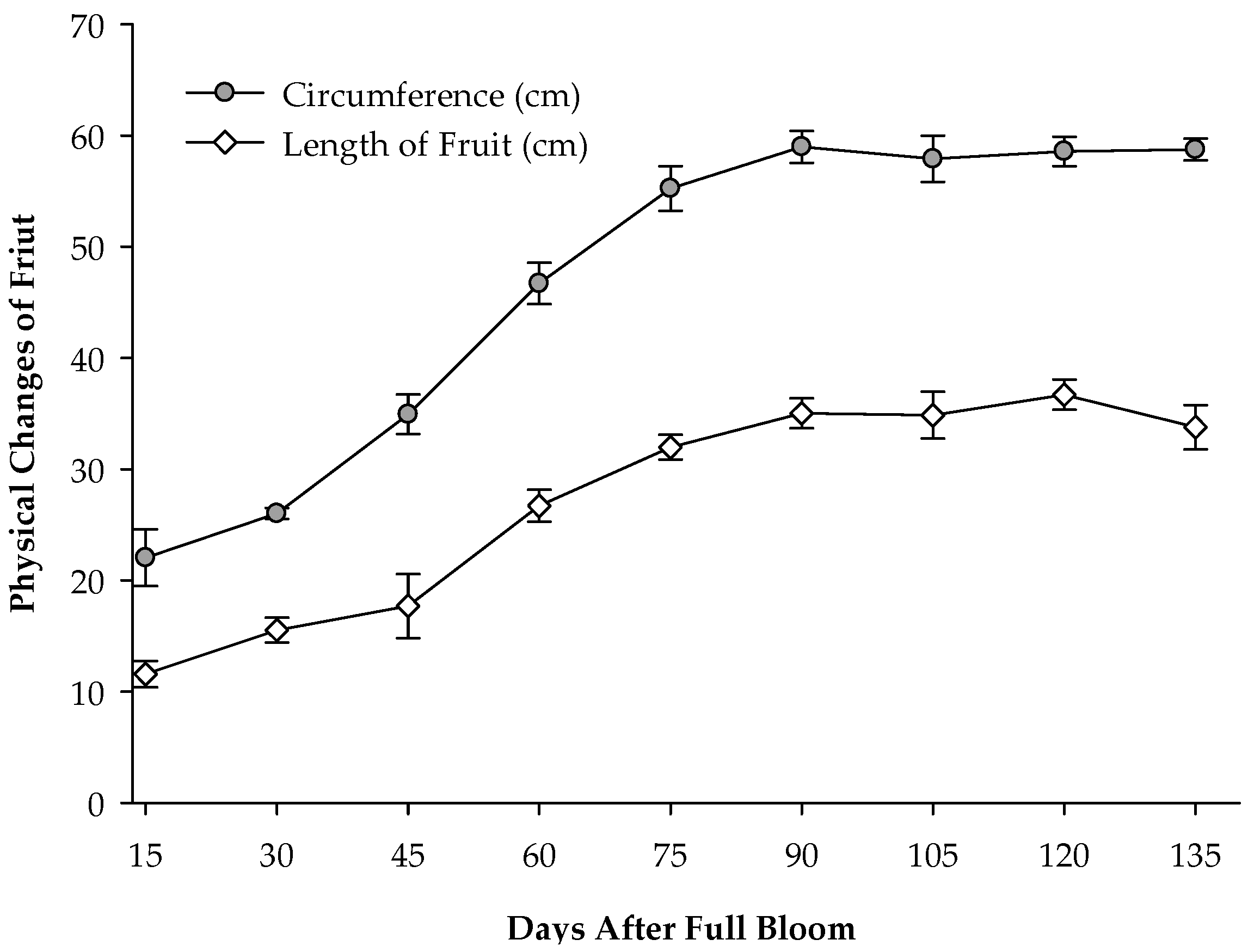
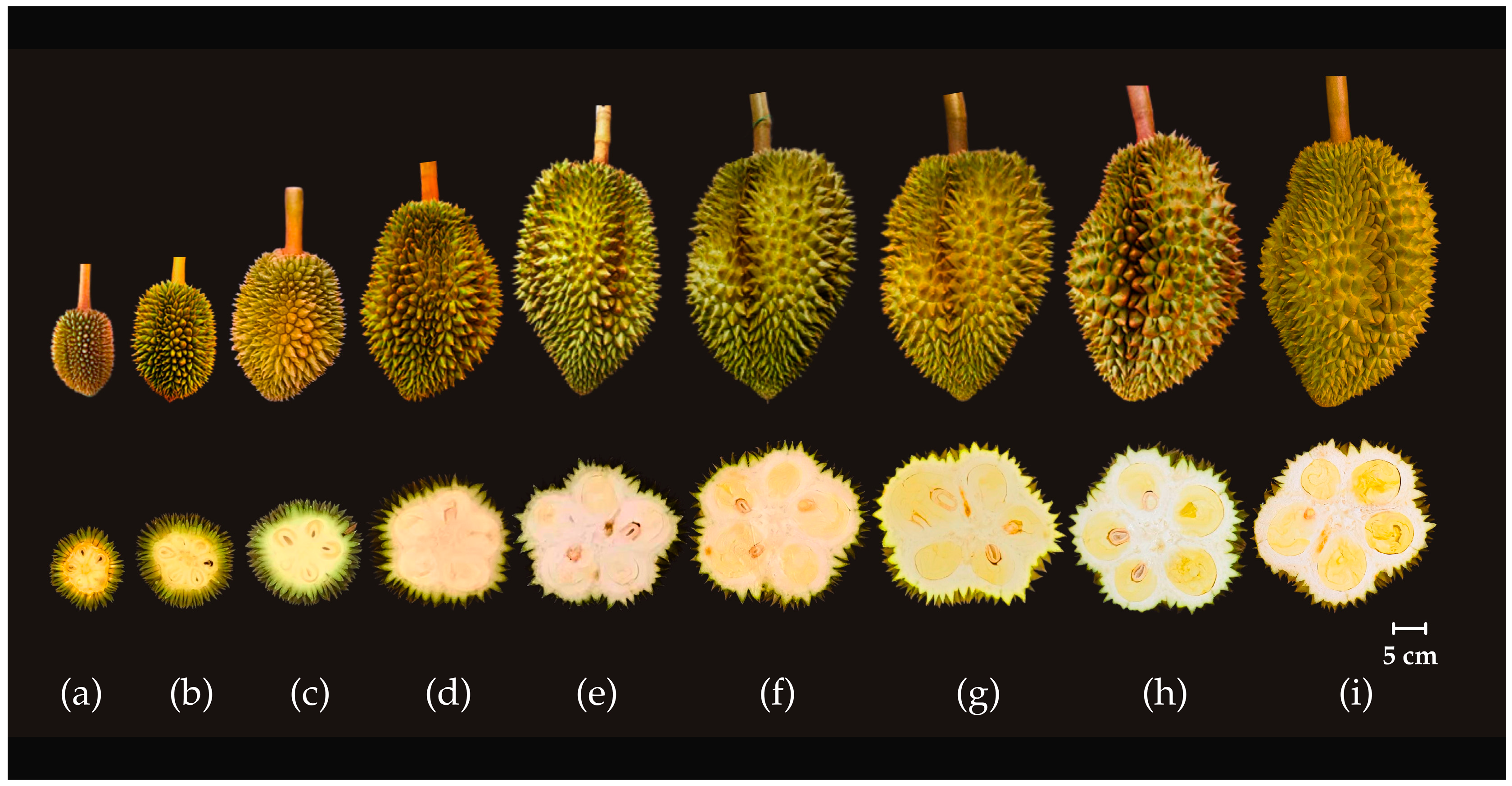
3.1. Growth and Development of ‘Monthong’ Durian Fruit from 15 to 135 Days After Full Bloom
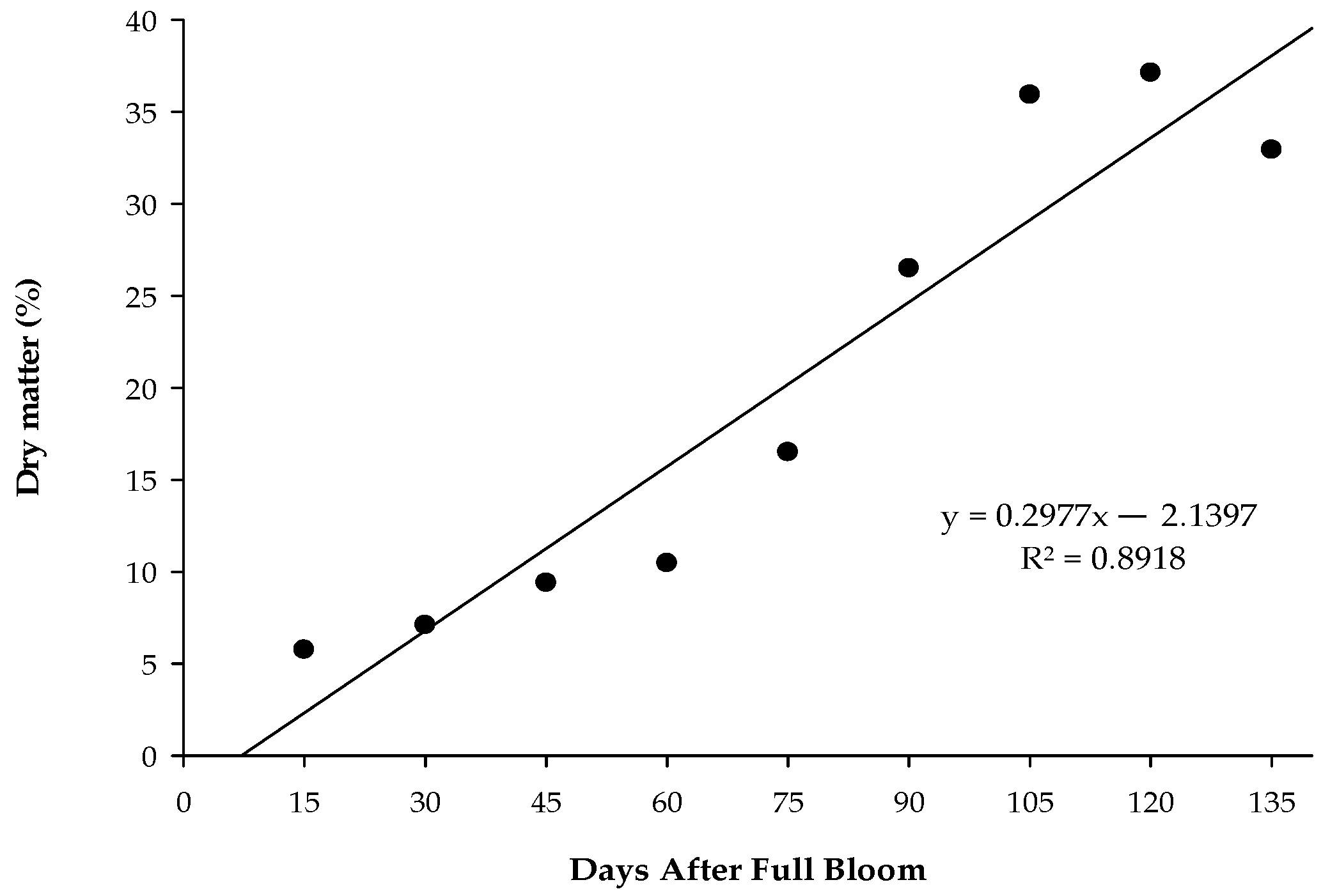
3.2. Dry Matter Accumulation, Fruit Weight, and Firmness Dynamics in ‘Monthong’ Durian Across Different Maturity Stages
3.3. Color Changes in ‘Monthong’ Durian Peel and Pulp at Different Harvest Maturity Stages
3.4. Fruit Quality Dynamics in ‘Monthong’ Durian Across Different Maturity Stages
3.5. Antioxidant Profiles and Dynamics in ‘Monthong’ Durian Across Different Maturity Stages
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaiprasart, P.; Ronnarit, R.; Puttapong, S. Quality Determination of Durian fruits cv. Monthong by Near Infrared Spectroscopy (NIR) at Uttaradit Province for Export; Research Report; DRI Naresuan University: Phitsanuloke, Thailand, 2015; p. 72. [Google Scholar]
- Ministry of Culture Thailand. Durian Situation. 2024. Available online: https://regional.moc.go.th/th/file/get/file/20240429416b720fc48c23e1e246c63221feda3f094402.pdf (accessed on 12 March 2025).
- Department of Intellectual Property. The Registration of Geographical Indication Sisaket Lava Durian No. 61100112 (in Thai). 2018. Available online: https://ipthailand.go.th/images/2284/GI61100112.pdf (accessed on 30 July 2024).
- Harnsuek, P.; Lekjarn, S.; Tangthin, S.; Saiyut, P. Factors related to consumers’ purchasing decisions of Lava Durian Sisaket in northeast Thailand. Khon Kaen Agric. J. 2019, 47 (Suppl. S1), 895–902. [Google Scholar]
- Siriphanich, J. Durian (Durio zibethinus Merr.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits, Vol 3: Cocona to Mango; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 80–116. [Google Scholar] [CrossRef]
- Youryon, P.; Supapvanich, S. Quality comparison of naturally and artificially ripened ‘Monthong’ durian (Durio zibethinus) fruits harvested at various maturity stages. Curr. Appl. Sci. Technol. 2022, 22, 1–11. [Google Scholar] [CrossRef]
- Onsiri, Y.; Kunprom, C.; Romphophak, T. Effect of the harvesting maturity of durian fruit cv. Monthong on their postharvest quality relationship. Agric. Sci. J. 2018, 49 (Suppl. S4), 30–34. [Google Scholar]
- Arsa, S.; Wipatanawin, A.; Suwapanich, R.; Makkerdchoo, O.; Chatsuwan, N.; Kaewthong, P.; Pinsirodom, P.; Taprap, R.; Haruenkit, R.; Poovarodom, S.; et al. Properties of different varieties of durian. Appl. Sci. 2021, 11, 5653. [Google Scholar] [CrossRef]
- Razali, N.A.; Rosly, N.K.; Hamzah, F.A.; Abdullah, S.A.; Hussin, W. Optimum storage temperature of mature drop durian (Durio zibethinus cv. Musang King). Int. J. Agric. For. Plant. 2020, 10, 267–273. [Google Scholar]
- Subhadrabandhu, S.; Ketsa, S. Durian: King of Tropical Fruit; CABI: Egham, UK, 2023. [Google Scholar] [CrossRef]
- Kalra, S.K.; Tandon, D.K.; Singh, B.P. Mango. In Handbook of Fruit Science and Technology-Production, Composition, Storage and Processing; Salunkhe, D.K., Kadam, S.S., Eds.; Mercel Dekker, Inc.: New York, NY, USA, 1995; pp. 123–169. [Google Scholar]
- Bouzayen, M.; Latché, A.; Nath, P.; Pech, J.C. Mechanism of Fruit Ripening. In Plant Developmental Biology-Biotechnological Perspectives; Springer: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Sulaiman, S.F.; Yousoff, N.A.M.; Eldeen, I.M.; Seow, E.M.; Sajak, A.A.B.; Ooi, K.L. Correlation between total Phenolic and mineral contents with antioxidant activity of eight Malaysian banana (Musa sp.). J. Food Compos. Anal. 2011, 24, 1–10. [Google Scholar] [CrossRef]
- Mouhannad, A.H.; Baqir, S.J.; Aowda, S.A.; Hussein, F.A.; Alasedi, M.K. Determination of Vitamin C (Ascorbic Acid) Concentration in Some of Commercial Products, by Redox Titration; Ministry of Health: Hilla, Babylon, 2020; p. 15. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Volker, B.; Puspitasari, N.L.; Ferruzzi, M.G.; Schwartz, S.J. Trolox equivalent antioxidant capacity of different geometrical isomers of α-carotene, β-carotene, lycopene, and zeaxanthin. J. Agric. Food Chem. 2002, 50, 221–226. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 2008, 110, 881–890. [Google Scholar] [CrossRef]
- Montelongo, R.G.; Lobo, M.G.; González, M. Antioxidant activity in banana peel extracts: Testing extraction conditions and related bioactive compounds. Food Chem. 2010, 119, 1030–1039. [Google Scholar] [CrossRef]
- Wisutiamonkul, A.; Dwamena, C.A.; Allan, A.C.; Ketsa, S. Carotenoid accumulation and gene expression during durian (Durio zibethinus) fruit growth and ripening. Sci. Hortic. 2017, 220, 233–242. [Google Scholar] [CrossRef]
- Ketsa, S.; Wisutiamonkul, A.; Palapol, Y.; Paull, R.E. The durian: Botany, horticulture, and utilization. Hortic. Rev. 2020, 47, 125–211. [Google Scholar] [CrossRef]
- Lipka, E.; Herrmann, A.; Mueller, S. Mechanisms of plant cell division. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef]
- Ishida, K.; Yokoyama, R. Reconsidering the function of the xyloglucan endotransglucosylase/hydrolase family. J. Plant Res. 2022, 135, 145–156. [Google Scholar] [CrossRef]
- Techawongstien, S. Post-harvest Technology of Horticultural Crops; Department of Horticulture, Faculty of Agriculture, Khon Kaen University: Khon Kean, Thailand, 1992; p. 158. [Google Scholar] [CrossRef]
- Barbosa, N.C.S.; Dornelas, M.C. The roles of gibberellins and cytokinins in plant phase transitions. Trop. Plant Biol. 2021, 14, 11–21. [Google Scholar] [CrossRef]
- Gupta, K.; Wani, S.H.; Razzaq, A.; Skalicky, M.; Samantara, K.; Gupta, S.; Deepu Pandita, D.; Sonia Goe, S.; Sapna Grewa, S.; Hejnak, V.; et al. Abscisic acid: Role in fruit development and ripening. Front. Plant Sci. 2022, 13, 817500. [Google Scholar] [CrossRef]
- Chaisrichonlathan, P.; Chavapradit, C. Durian maturity meter by dry weight. Acta Hortic. 2017, 1186, 165–170. [Google Scholar] [CrossRef]
- Pokhrel, D.R.; Sirisomboon, P.; Khurnpoon, L.; Posom, J.; Saechua, W. Comparing machine learning and PLSDA algorithms for durian pulp classification using inline NIR spectra. Sensors 2023, 23, 5327. [Google Scholar] [CrossRef]
- Kalayanamitra, K.; Sornsrivichai, J.; Yantarasri, T. Model for evaluation of maturity index of durian fruit (Durio zibethinus Murray ‘Monthong’). Acta Hortic. 2005, 682, 587–592. [Google Scholar] [CrossRef]
- Ngoenchai, P.; Suwonsichon, S.; Ritthiruangdej, P.; Jirapakkul, W.; Sangwanangkul, P. Volatile compound analysis of “Monthong” durian harvested at different maturity stages using flash gas chromatography electronic nose. J. Food Sci. Agric. Technol. 2022, 6, 145–150. [Google Scholar]
- Thai Agricultural Standard TAS3-2013; Durian [Internet]. National Bureau of Agricultural Commodity and Food Standards: Bangkok, Thailand, 2022. Available online: http://patricklepetit.jalbum.net/RAYONG/LIBRARY/durian-TIS.pdf (accessed on 7 May 2022).
- Sangwanangkul, P.; Siriphanich, J. Growth and maturation of durian fruit cv. Monthong. Thai J. Agric. Sci. 2000, 33, 75–82. [Google Scholar]
- Wongchan, B.; Chayanuch, T.; Suppaluck, A. Growth and seasonal change in nutrient of neck orange (Citrus reticulata Blanco.). Khon Kaen Agric. J. 2014, 42 (Suppl. S3), 99–104. [Google Scholar]
- Goulao, L.F.; Oliveira, C.M. Cell wall modifications during fruit ripening: When a fruit is not the fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef]
- Timkhum, P.; Terdwongworakul, A. Non-destructive classification of durian maturity of ‘Monthong’cultivar by visible spectroscopy of the husk. Thai Soc. Agric. Eng. J. 2013, 19, 1–6. [Google Scholar] [CrossRef]
- Siriphanich, J.; Khurnpoon, L. Dry matter as a possible maturity index of ‘Monthong’ durians. Thai J. Agric. Sci. 2003, 37, 365–372. [Google Scholar]
- Siriphanich, J. Durian: Post-harvest Management; Postharvest Technology Innovation Center, Chiang Mai University: Chiang Mai, Thailand, 2024; p. 430. [Google Scholar]
- de Jesus, O.N.; Lima, L.K.S.; dos Santos, I.S.; dos Santos, M.A.; Rosa, R.C.C. Bright red passion fruit-evaluation of colorimetry and physicochemical quality for the fresh fruit market. Sci. Hortic. 2023, 317, 112016. [Google Scholar] [CrossRef]
- Gupta, A.K.; Medhi, M.; Chakraborty, S.; Monica Yumnam, M.; Mishra, P. Development of rapid and non-destructive technique for the determination of maturity indices of pomelo fruit (Citrus grandis). J. Food Meas. Charact. 2021, 15, 1463–1474. [Google Scholar] [CrossRef]
- Mathiazhagan, M.; Chinnaiyan, V.; Venkata Sudhakar Rao, D.; Seetharamaiah Shivashankara, K.; Venkataramana Ravishankar, K. Estimation of Pulp Pigment Content Using Color Space Values in Guava. Appl. Fruit Sci. 2024, 66, 1997–2005. [Google Scholar] [CrossRef]
- Srivastav, M.; Radadiya, N.; Ramachandra, S.; Jayaswal, P.K.; Singh, N.; Singh, S.; Mahato, A.K.; Tandon, G.; Gupta, A.; Devi, R.; et al. High resolution mapping of QTLs for fruit color and firmness in Amrapali/Sensation mango hybrids. Front. Plant Sci. 2023, 14, 1135285. [Google Scholar] [CrossRef]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar signaling during fruit ripening. Front. Plant Sci. 2020, 11, 564917. [Google Scholar] [CrossRef] [PubMed]
- Krishna, H.; Parashar, A. Phytochemical constituents and antioxidant activities of some indian jujube (Ziziphus mauritiana Lamk.) cultivars. J. Food Biochem. 2013, 37, 571–577. [Google Scholar] [CrossRef]
- Flores-Fernández, C.N.; Cárdenas-Fernández, M.; Lye, G.J.; Ward, J.M. Synergistic action of thermophilic pectinases for pectin bioconversion into D-galacturonic acid. Enzym. Microb. Technol. 2022, 160, 110071. [Google Scholar] [CrossRef] [PubMed]
- Haryanto, B.; Royaningsih, S. Relationship between durian maturity of Sunan and its physical properties. Agritech 2003, 23, 33–36. [Google Scholar]
- Siriphanich, J. Physiology and Postharvest Technology of Vegetable and Fruits; Department of Horticulture, Faculty of Agriculture, Kamphaeng Saen, Kasetsart University: Bangkok, Thailand, 2006; p. 396. [Google Scholar]
- Khaksar, G.; Kasemcholathan, S.; Sirikantaramas, S. Durian (Durio zibethinus L.): Nutritional composition, pharmacological implications, value-added products, and omics-based investigations. Horticulturae 2024, 10, 342. [Google Scholar] [CrossRef]
- Wiangsamut, B.; Wiangsamut, M.E.L.; Wattana, K.; Bamrung, P.; Charroenmoon, K. Effect of ethephon on fruit ripening and fruit components of durian cv.‘monthong’ after harvest. Int. J. Agric. Technol. 2021, 17, 2021–2034. [Google Scholar]
- Bastos, V.J.; Neves, L.C.; Silva, P.M.C.; Shahab, M.; Colombo, R.C.; Roberto, S.R. Harvest point determination of indian jujube fruit (Ziziphus mauritiana L.) based on physicochemical and functional parameters. Sci. Hortic. 2016, 213, 392–402. [Google Scholar] [CrossRef]
- Haruenkit, R.; Poovarodom, S.; Vearasilp, S.; Namiesnik, J.; Kaszynska, M.S.; Park, Y.S.; Gorinstein, S. Comparison of bioactive compounds, antioxidant and antiproliferative activities of Mon Thong durian during ripening. Food Chem. 2010, 118, 540–547. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Effects of maturity status on biochemical content, polyphenol composition and antioxidant capacity of pomegranate fruit arils (cv.‘Bhagwa’). S. Afr. J. Bot. 2013, 85, 23–31. [Google Scholar] [CrossRef]
- Boonyaritthongchai, P.; Apiradee, U.; Sailom, S.; Pongphen, J. Effect of Fresh Cut and Freezing Process on Bioactive Compound of Durian cv. Monthong; Research Report; The Thailand Research Fund (TRF): Bangkok, Thailand, 2016; pp. 118–124. [Google Scholar]
- Cheng, W.; Li, S.; Bao, F.; Xu, Z.; Li, T. The changes of antioxidant contents and antioxidant activities of the tomato cultivar Fentailang during the maturity stages. J. Oleo Sci. 2023, 72, 577–583. [Google Scholar] [CrossRef]

| Days After Full Bloom (Day) | Fruit Weight (g) | Firmness (N/cm2) | Dry Matter (%) |
|---|---|---|---|
| 15 | 137.35 e | 30.97 c | 5.75 f |
| 30 | 288.94 de | 34.67 c | 7.10 ef |
| 45 | 614.87 d | 36.97 c | 9.40 ef |
| 60 | 990.43 c | 45.38 bc | 10.46 e |
| 75 | 2385.00 b | 56.70 b | 16.50 d |
| 90 | 2472.00 b | 75.64 a | 26.50 c |
| 105 | 3316.70 a | 76.08 a | 35.94 ab |
| 120 | 3652.30 a | 42.08 bc | 37.13 a |
| 135 | 3352.30 a | 1.62 d | 32.94 b |
| F-test | ** | ** | ** |
| CV (%) | 8.22 | 17.80 | 7.99 |
| Days After Full Bloom (Day) | Peel | Pulp | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |
| 15 | 26.82 a | 1.42 ab | 5.02 de | 74.31 c | 6.41 a | 27.27 c |
| 30 | 27.38 a | 1.01 abc | 4.07 e | 76.80 bc | 5.55 a | 26.15 cd |
| 45 | 29.36 a | 0.89 bc | 3.60 e | 80.18 abc | 0.83 b | 21.33 def |
| 60 | 28.11 a | 0.41 cd | 3.34 e | 82.13 ab | 0.26 b | 19.38 ef |
| 75 | 14.57 b | 1.22 abc | 12.50 bc | 80.35 abc | 0.49 b | 17.94 f |
| 90 | 16.22 b | 1.26 abc | 9.64 cd | 79.96 abc | 0.84 b | 38.92 b |
| 105 | 19.63 ab | 1.49 ab | 12.36 bc | 82.78 ab | 1.68 b | 24.00 cde |
| 120 | 15.10 b | −0.12 d | 18.37 a | 83.22 a | 0.56 b | 47.47 a |
| 135 | 15.55 b | 1.92 a | 15.61 ab | 82.83 ab | 1.09 b | 49.90 a |
| F-test | ** | ** | ** | ** | ** | ** |
| CV (%) | 20.93 | 39.08 | 21.89 | 3.28 | 66.33 | 8.21 |
| Days After Full Bloom (Day) | Total Soluble Solid (°Brix) | Titratable Acidity (%) | TSS/TA | Vitamin C (mg/100 g FW) |
|---|---|---|---|---|
| 15 | 2.80 f | 0.69 c | 0.46 | 37.79 d |
| 30 | 7.47 e | 1.54 b | 4.85 | 41.01 d |
| 45 | 8.67 de | 1.55 b | 5.59 | 41.35 d |
| 60 | 9.53 cde | 1.59 b | 5.99 | 43.89 d |
| 75 | 10.47 cd | 1.64 b | 6.38 | 47.45 d |
| 90 | 11.47 c | 1.68 b | 6.83 | 72.50 d |
| 105 | 20.50 b | 2.67 a | 7.68 | 512.33 a |
| 120 | 23.36 a | 1.02 c | 22.90 | 283.00 b |
| 135 | 25.00 a | 0.78 c | 32.05 | 166.17 c |
| F-test | ** | ** | ** | |
| CV (%) | 7.11 | 11.50 | 20.10 |
| Days After Full Bloom (Day) | Total Phenolic (mg/100 g FW) | Total Flavonoid Content (mg catechin/ 100 g FW) | β-Carotene Content (mg/100 g FW) | Lycopene Content (mg/100 g FW) | ABTS (mg TE/100 g FW) | DPPH (mg TE/100 g FW) |
|---|---|---|---|---|---|---|
| 15 | - | - | - | - | - | - |
| 30 | 9.17 d | 3.51 b | 0.44 d | 8.88 d | 2.22 cd | 2.22 b |
| 45 | 12.14 d | 3.42 b | 0.53 d | 9.28 d | 3.33 bc | 1.19 b |
| 60 | 12.35 d | 3.84 b | 0.51 d | 10.61 d | 4.21 ab | 1.58 b |
| 75 | 12.65 d | 5.54 ab | 0.5 d | 11.58 d | 5.17 a | 3.53 ab |
| 90 | 25.79 c | 5.83 ab | 0.69 cd | 25.79 c | 5.89 a | 4.00 ab |
| 105 | 39.12 b | 6.01 ab | 1.05 ab | 37.97 b | 1.02 d | 4.09 ab |
| 120 | 43.98 a | 8.33 a | 1.35 a | 53.98 a | 1.09 d | 6.32 a |
| 135 | 39 b | 4.22 b | 0.89 bc | 48.80 ab | 1.01 d | 4.06 ab |
| F-test | ** | ** | ** | ** | ** | ** |
| CV (%) | 6.58 | 13.03 | 17.79 | 18.10 | 17.49 | 20.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yongyut, N.; Baopa, P.; Meetha, S.; Isarangkool Na Ayutthaya, S.; Chiu, C.-I.; Sripontan, Y.; Posom, J.; Nampila, S. Fruit Quality and Antioxidant Content in Durian (Durio zibethinus Murr.) cv. ‘Monthong’ in Different Maturity Stages. Horticulturae 2025, 11, 432. https://doi.org/10.3390/horticulturae11040432
Yongyut N, Baopa P, Meetha S, Isarangkool Na Ayutthaya S, Chiu C-I, Sripontan Y, Posom J, Nampila S. Fruit Quality and Antioxidant Content in Durian (Durio zibethinus Murr.) cv. ‘Monthong’ in Different Maturity Stages. Horticulturae. 2025; 11(4):432. https://doi.org/10.3390/horticulturae11040432
Chicago/Turabian StyleYongyut, Naruemon, Phormporn Baopa, Somyot Meetha, Supat Isarangkool Na Ayutthaya, Chun-I Chiu, Yuwatida Sripontan, Jetsada Posom, and Supatchaya Nampila. 2025. "Fruit Quality and Antioxidant Content in Durian (Durio zibethinus Murr.) cv. ‘Monthong’ in Different Maturity Stages" Horticulturae 11, no. 4: 432. https://doi.org/10.3390/horticulturae11040432
APA StyleYongyut, N., Baopa, P., Meetha, S., Isarangkool Na Ayutthaya, S., Chiu, C.-I., Sripontan, Y., Posom, J., & Nampila, S. (2025). Fruit Quality and Antioxidant Content in Durian (Durio zibethinus Murr.) cv. ‘Monthong’ in Different Maturity Stages. Horticulturae, 11(4), 432. https://doi.org/10.3390/horticulturae11040432









