Abstract
In this study, the effects of different modifiers on rhizosphere soil microorganisms, their functions, and the soil properties of continuous tomato cropping were investigated. Nine amendments were selected to treat the soil from a 14-year continuous tomato cropping system. Tomato yield, soluble solids, soil physical and chemical properties, and soil enzyme activities were measured. Changes in soil microbial community structure and function were determined by metagenomic sequencing, and their correlation with environmental factors was analyzed. The results showed that among the nine amendments, the combination of farmyard manure + Bacillus subtilis + Trichoderma harzianum (T2) and plant-derived straw decomposed soil + Bacillus subtilis + Trichoderma harzianum (T3) had the most significant effects. The tomato yield, soil hydrolyzable nitrogen, available phosphorus, available potassium, organic matter, and total nitrogen contents and soil phosphatase activities were significantly increased under the T2 and T3 treatments. Compared with the CK treated with T2, the contents of yield, alkali-hydrolyzed nitrogen, available phosphorus, available potassium, organic matter, and total nitrogen were significantly increased by 34.46%, 41.84%, 52.44%, 45.01%, 24.5%, and 41.18%, respectively. The soil microbial community structure also changed significantly. The relative abundance of Proteobacteria, Chloroflexi, and Bacteroidota increased significantly. The relative abundance of Hyphomicrobium, Rhodomicrobium, and Rhodoplanes increased significantly compared with the control. The soil microbial function was mainly enriched in two pathways of amino acid metabolism and carbohydrate metabolism. Among them, T2 significantly enriched six community functions, such as bacterial chemotaxis. T3 significantly enriched three community functions, such as glutathione metabolism. A correlation analysis showed that soil hydrolyzable nitrogen, available phosphorus, pH, phosphatase, and catalase were the key factors affecting microbial community changes. The treatment of farm manure/plant-derived straw decomposed soil + Bacillus subtilis + Trichoderma harziensis improved the soil environment, increased crop yield, clarified the effects of different modifiers on the functional mechanisms of the soil microbial community, and provided a practical solution to the problem of soil degradation in agriculture monoculture.
1. Introduction
The black soil in Northeast China is rich in organic matter and fertility and has excellent properties, make it ideal for plant growth and production potential. Tomatoes are a major dominant vegetable in China, and they are also one of the vegetables with a wide area of facility cultivation in Northeast China. In recent years, to meet growing demands, the tomato planting area has expanded. However, continuous planting and improper fertilizer use have led to soil acidification and nutrient depletion. A long-term continuous cropping study found that soil rhizosphere microbial flora changed significantly, the number of pathogenic bacteria increased, and the number of beneficial microorganisms decreased. Studies have shown that long-term continuous cropping affects soil microecological communities, leading to nutrient imbalances and thus reducing crop yields. In recent years, plant growth retardation, yield reduction, quality deterioration, and disease aggravation caused by continuous cropping occurred frequently, and the organic matter content of black soil decreased significantly, which posed a serious threat to the sustainable development of agriculture in Northeast China and even in our country [1,2].
Soil microorganisms are sensitive indicators of changes in soil environment and quality, which can drive the process of nutrient cycling and material transformation in soil [3]. Soil microorganisms maintain soil health, fertility, and ecosystem stability by promoting nutrient cycling, organic matter formation, disease control, and pollutant degradation [4]. Long-term continuous cropping alters the soil microbial community, affecting diversity, richness, and structure. For instance, continuous cropping of soybean and corn can reduce the fungal abundance and fungal species diversity in soil microorganisms [5,6]. Some studies have shown that Bacillus and Trichoderma harzia can promote plant growth, induce plant resistance mechanisms, and promote healthy plant growth. Studying soil microbial diversity, function, and interactions with environmental factors can optimize soil management and improve agricultural productivity [7].
Advances in molecular biology have made metagenomic sequencing a powerful tool for studying soil microorganisms, offering high throughput, large sample analysis, and accuracy [8]. Metagenomic analysis enhances the resolution of microbial community analysis and also has a variety of advantages. It provides species, gene, and functional information down to the strain level, allowing for a more accurate analysis of microbial community structure and function [9]. Soil metagenomics offers comprehensive information on functional genes through sequence assembly and bioinformatics analysis. It allows for a more intuitive study of the dynamic changes in microbial diversity and community structure and the response of microorganisms to the environment [10,11].
To solve a series of related problems brought by continuous cropping to the soil environment, compound microbial modifiers to improve the soil nutrient environment have gradually emerged. A microbial improver has a good effect on improving leaf quality, fruit quality, and soil fertility [12,13]. Few studies have investigated the effects of the combination of organic matter and amendments on soil microbial structure and function. This experiment used metagenomic sequencing to analyze soil samples from continuously cropped tomatoes, studying changes in the rhizosphere environment, microbial community diversity, composition, and function after treatment with different microbial modifiers and clarifying the microbial community’s response to soil environment changes. It provides a theoretical basis for exploring the improvement mechanism of continuous cropping obstacles from a soil microbiology perspective, laying a foundation for alleviating tomato cropping issues and supporting the development of the tomato industry in northern China.
2. Materials and Methods
2.1. Study Area Overview
The experimental site is located in Xiangfang District, Harbin City, Heilongjiang Province (126°66′ E, 45°70′ N). It is a temperate continental monsoon climate. It is cold and dry in winter, with less precipitation. In summer, it is warm and humid, with concentrated precipitation and sufficient light. The test tomato variety was ‘Yafen Classic’, and the test soil type was black soil of tomato continuously cultivated for 14 years. It was planted in March 2024 and applied with 20 kg/mu nitrogen, phosphorus, and potassium compound fertilizer (12:18:15) and 40 kg/mu phosphoric acid diamine as the base fertilizer. During the growth of tomatoes, water-soluble fertilizers were applied for topdressing.
2.2. Experimental Setup
The experiment was carried out in April 2024 and used a randomized block design. Soil treatment was carried out three days before planting, and a total of 10 treatments were set up. Each treatment was repeated three times, and one pool was repeated each time. The pool size was 120 cm × 65 cm, the plant spacing was 35 cm, and there were 7 seedlings in one pool. The specific treatment was set as follows (Table 1), and the tomato plant height and stem diameter were measured every 20 days for a total of three times. Starting from the maturity of the first ear fruit, each ear fruit was harvested, and the yield was counted. The yield of 4 tomato plants was measured in each replicate. Dosage was administered according to the amendment instructions. The topographic map of experimental processing is shown in Table S1.

Table 1.
Treatment and dosage of different amendments.
2.3. Tomato Fruit Yield and Quality Determination
The average yield of each tomato was calculated by using the average yield of 4 plants per plant. The tomato fruits with uniform maturity and fruit size were selected for quality parameter determination. The vitamin C content, organic acid, and soluble solids of the fruit were measured, and the results were averaged. The content of vitamin C was determined by 2,6-dichloroindole phenol titration, the content of organic acid was determined by the acid–base neutralization method, and the content of total soluble solids was determined by a hand-held refractometer [14].
2.4. Soil Sample Collection and Pretreatment
Soil samples were collected in September 2024. When collecting, the plant is uprooted, the larger soil blocks and other impurities attached to the root are gently shaken off, and then the soil attached to the root is carefully collected. All the soil samples were mixed and passed through a 2 mm mesh. The sieved soil was divided into three portions for preservation. A part of the air-dried soil samples was used to determine soil physical and chemical indicators and organic carbon components, including soil organic matter and soil available nutrients. Part of the fresh soil was stored in a refrigerator at −4 °C to determine soil enzyme activity, and the other part of the soil was placed in a 10 mL sterile tube, quickly placed in a liquid nitrogen tank, and brought back to the laboratory for DNA extraction at −80 °C for metagenomic analysis.
2.5. Determination of Soil Physical and Chemical Indexes and Soil Enzyme Activity
Soil physical and chemical properties were determined as follows: pH was measured with a pH meter (BSISL, Beijing, China), conductivity (EC) with a conductivity meter (Shanghai Precision Scientific Instrument Co., Ltd., Shanghai, China), and soil organic matter (OM) by the potassium dichromate volumetric method. Soil hydrolyzable nitrogen (HN) and total nitrogen (TN) were quantified by the alkaline dissolution diffusion method and automatic Kjedahl device method, respectively. The soil available potassium (AK) was determined by the neutral ammonium acetate solution leaching method combined with flame spectrophotometry. The molybdenum antimony colorimetric method was used to measure soil available phosphorus (AP) [15].
Soil enzyme activity was determined for urease (SUE), phosphatase (ALP), catalase (CAT), sucrase (SUC), cellulase (CEL) and aminotransferase (AST). The activities of soil enzymes were determined by an activity detection kit produced by Solarbio (No. 16, Huanke Middle Road, Beijing Economic-Technological Development Area, Beijing, China) biotechnology. The kit models were BC0125, BC0105, BC0245, BC0155, and BC0285.
2.6. Soil DNA Extraction and Microbial Community Analysis
A Power Soil Kit was used to extract the total DNA from the soil microbiome. The purity and concentration of the extracted DNA were detected by a NanoDrop 2000 ultra-micro spectrophotometer (Semmerfeld Technology, Auburn, MA, USA), and the integrity was detected by 1% agarose gel electrophoresis. Based on the Illumina NovaSeq high-throughput sequencing platform, the genome shotgun (WGS) strategy was used to randomly break the total genomic DNA of the extracted bacterial colonies into short fragments, and a library of insert fragments with appropriate length was constructed. These libraries were sequenced by paired-end (PE) sequencing, and a library was constructed for each sample. The original data generated by sequencing were collated and counted, and the quality of the original data was comprehensively evaluated using FastQC. Cutadapt (v1.2.1) and FastQC (v0.20.0) were used to screen and filter the original data to obtain a clean data set. Kraken2 was used for species annotation, and the species annotation information and the number of non-splicing sequences annotated to the species were counted to obtain the species abundance table of each sample at each classification level. MMseqs2 compared sequences with the KOBAS protein database, and MinPath was used to infer and calculate the abundance of the KEGG metabolic pathway.
2.7. Statistical Analysis
Data were processed using Microsoft Excel 2019, with a histogram of microbial community structure and KEGG function composition plotted, and SPSS 25.0 software was used for statistical analysis. The α diversity index was calculated using R (4.2.1), and the composition of the dominant microbial community was plotted using Prism10 software. A redundancy analysis (RDA) of soil microbial community structure and soil environmental parameters was performed using RDA in Canoco 5.0 software, and the correlation between soil microbial community and functional composition and soil environmental parameters was tested by obtaining correlation coefficients.
3. Results and Analysis
3.1. Effects of Different Amendments on Tomato Yield and Quality
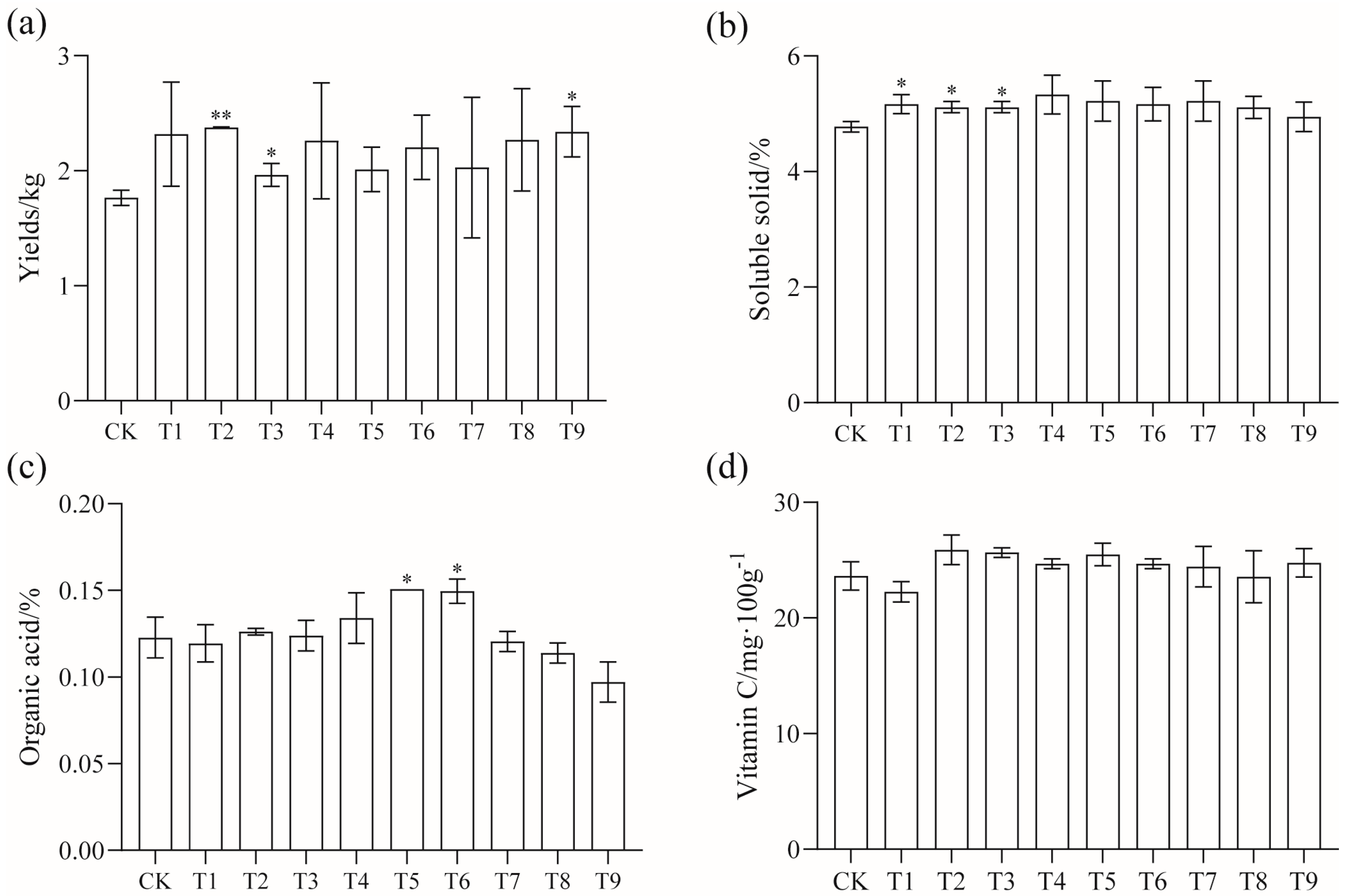
The yield, quality, plant height, and stem diameter of tomatoes were measured after the application of modifiers. The results are shown in Figure 1. The amendments had significant effects on yield, soluble solids, and organic acids. The yield of the T2, T3, and T9 treatments was significantly higher than that of the CK, with an increase of 34.46%, 10.73%, and 32.2%. The soluble solids content of the T1, T2, and T3 treatments was also significantly higher than that of the CK, and the organic acid content of the T5 and T6 treatments was significantly higher than that of the other treatments (p < 0.05).
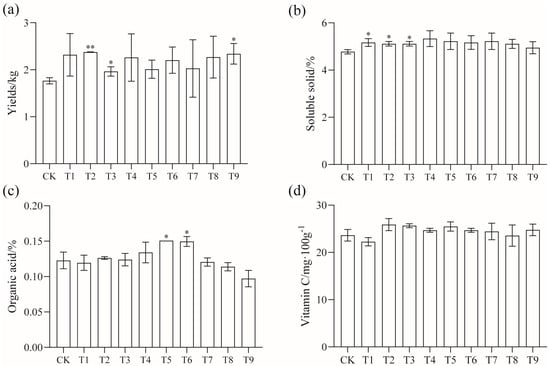
Figure 1.
Effects of different amendments on tomato (a) yield, (b) soluble solids, (c) organic acids, and (d) vitamin C; * shows a significant difference compared with CK treatment (p < 0.05); ** shows a very significant difference (p < 0.01).
From Table 2, it can be seen that, compared with the CK, the plant height and stem diameter of the T3 and T8 treatments increased significantly when tomato seedlings were planted for 20 days. At 40 days after planting, the plant height and stem diameter of the T2 treatment increased significantly, and the plant height of the T3, T4, T6, T7, T8, and T9 treatments increased significantly. At 60 days of colonization, the plant height of the T2, T5, and T7 treatments increased significantly (p < 0.05).

Table 2.
Effects of different amendments on plant height and stem diameter of tomato plants.
3.2. Effects of Different Amendments on Physical and Chemical Properties of Tomato Continuous Cropping Rhizosphere Soil
Different amendment treatments had significant effects on the physical and chemical properties of the rhizosphere soil of the continuous cropping tomato. It can be seen from Table 3 that the physical and chemical properties of the tomato rhizosphere soil treated with different amendments were significantly different from the CK (p < 0.05). The pH value, EC value, alkali-hydrolyzable nitrogen, available phosphorus, available potassium, organic matter, and total nitrogen content of the rhizosphere soil treated with T2 increased significantly, with an increase of 1.96%, 36.26%, 41.84%, 52.44%, 45.01%, 24.5%, and 41.18%, respectively. The contents of available nitrogen, available potassium, organic matter, and total nitrogen in the rhizosphere soil of the T3 treatment also increased significantly, with an increase of 35.48%, 34.69%, 47.98%, and 52.94%, respectively. Compared with the CK treatment, the pH value of the T1 treatment increased significantly. The pH value, EC value, and total nitrogen content of the T4 treatment increased significantly. The EC value of the T6 treatment increased significantly. The available potassium content of T5, T7, and T9 was also significantly higher than that of the CK (p < 0.05).

Table 3.
Effects of different amendments on soil physical and chemical properties.
3.3. Effects of Different Amendments on Enzyme Activities in Rhizosphere Soil of Tomato Continuous Cropping
It can be seen from Table 4 that the rhizosphere soil enzyme activities of the continuous cropping tomato were significantly different under different modifier treatments. Compared with the CK, the phosphatase activity of the T2 and T3 treatments increased significantly by 91.85% and 17.87%, respectively. The catalase activity was significantly decreased by 29.18% and 45.15%. The content of urease was the highest in the T5 treatment and the lowest in the T3 treatment. Compared with the CK, the activities of catalase and transaminase in the T1 treatment were significantly decreased. The catalase activity of the T4 treatment was significantly reduced; the phosphatase activity of the T7 treatment was significantly increased, and the cellulase activity was significantly decreased. These results indicate that the amendments caused changes in the physical and chemical properties and enzyme activities of the tomato rhizosphere soil, which may affect the composition and characteristics of its rhizosphere microbial community structure (p < 0.05).

Table 4.
Effects of different amendments on soil enzyme activities.
3.4. Microbial Community Diversity in Rhizosphere Soil
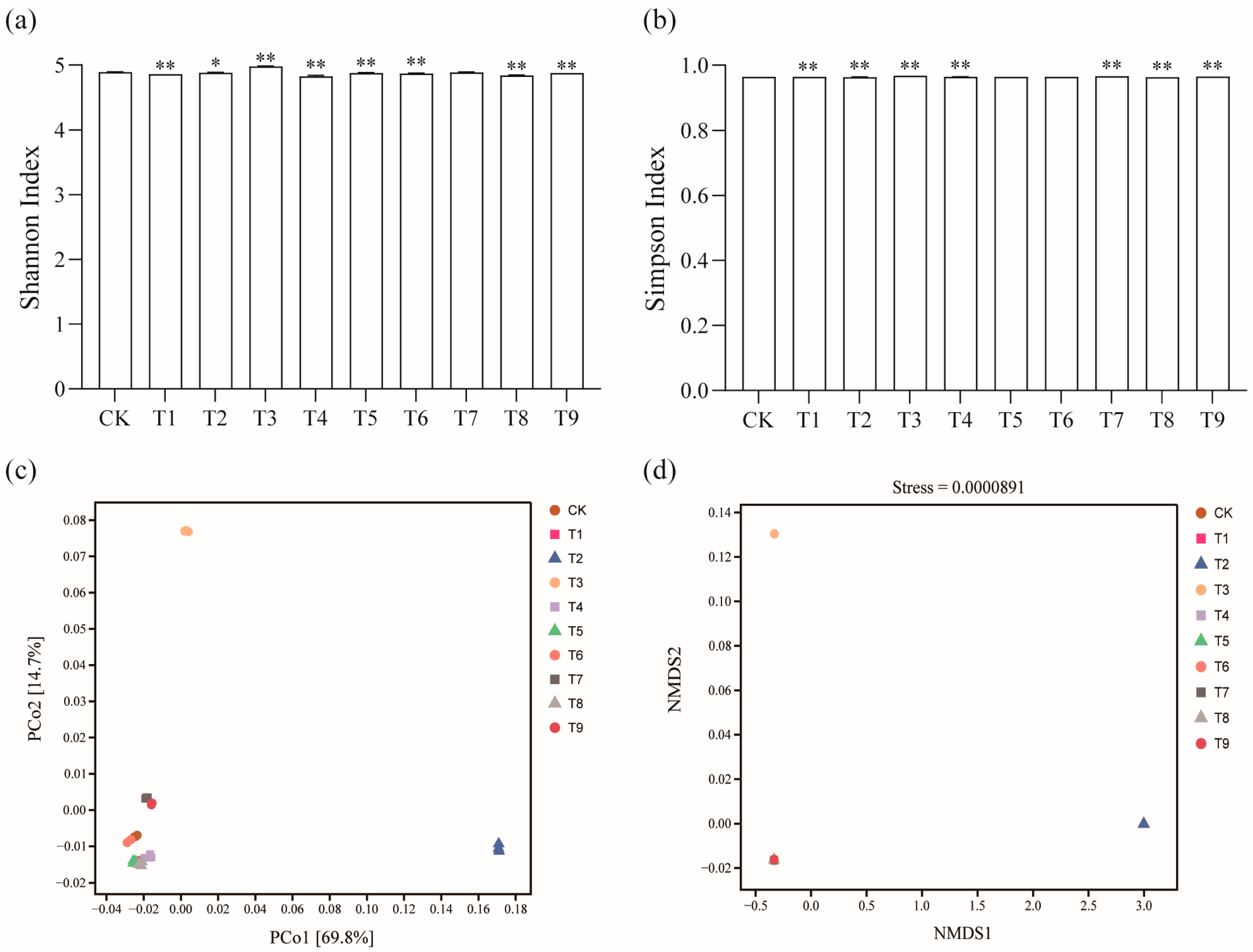
According to metagenomic analysis, a total of 25,319 microorganisms were obtained, including archaea, bacteria, fungi, viruses, and unknown species. The relative abundance of bacteria was the highest in all the soil samples, with an average relative abundance of about 98%. The Shannon Index and Simpson Index are indicators of microbial community diversity. The alpha diversity analysis of the soil microbial community showed that the Shannon Index and Simpson Index of the T3 treatment increased significantly compared with the CK treatment. The Shannon Index and Simpson Index of T1, T2, T4, and T8 were significantly reduced; the Shannon Index of T5, T6, and T9 decreased significantly. The Simpson Index of T7 and T9 increased significantly (Figure 2a,b). This shows that the microbial community diversity in the soil changed after the application of different modifiers (p < 0.05).
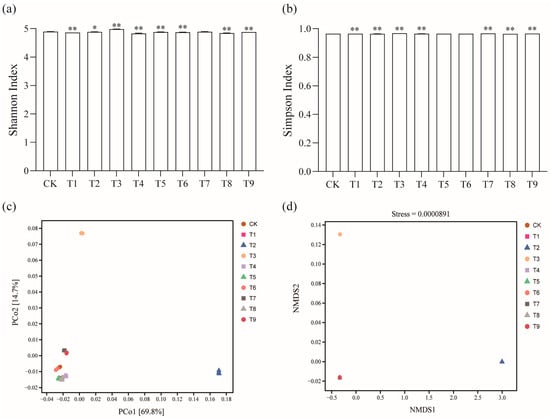
Figure 2.
Effects of different amendments on tomato rhizosphere soil diversity: (a,b) α diversity analysis; (c) PCoA analysis; (d) NMDS analysis. * shows a significant difference compared with CK treatment (p < 0.05); ** shows a very significant difference (p < 0.01).
The PCoA and NMDS analysis explained the difference in microbial community composition diversity between treatments. The results of the PCoA showed that the T2 and T3 treatments were far away from the other treatments of tomato rhizosphere bacteria, and the separation degree was good. Each sample in the group gathered one place and had good repeatability (Figure 2c) (p < 0.05). In the NMDS analysis, the stress function value = 0.0000891 (<0.05). The ordination model is reasonable and can accurately reflect the real existence of bacterial community structure in tomato soil with different amendments. Among them, the bacterial communities of the T2 and T3 treatments were far away from other treatments, the similarity was small, and the difference was large (Figure 2d) (p < 0.05).
3.5. Composition of Microbial Community Structure in Rhizosphere Soil
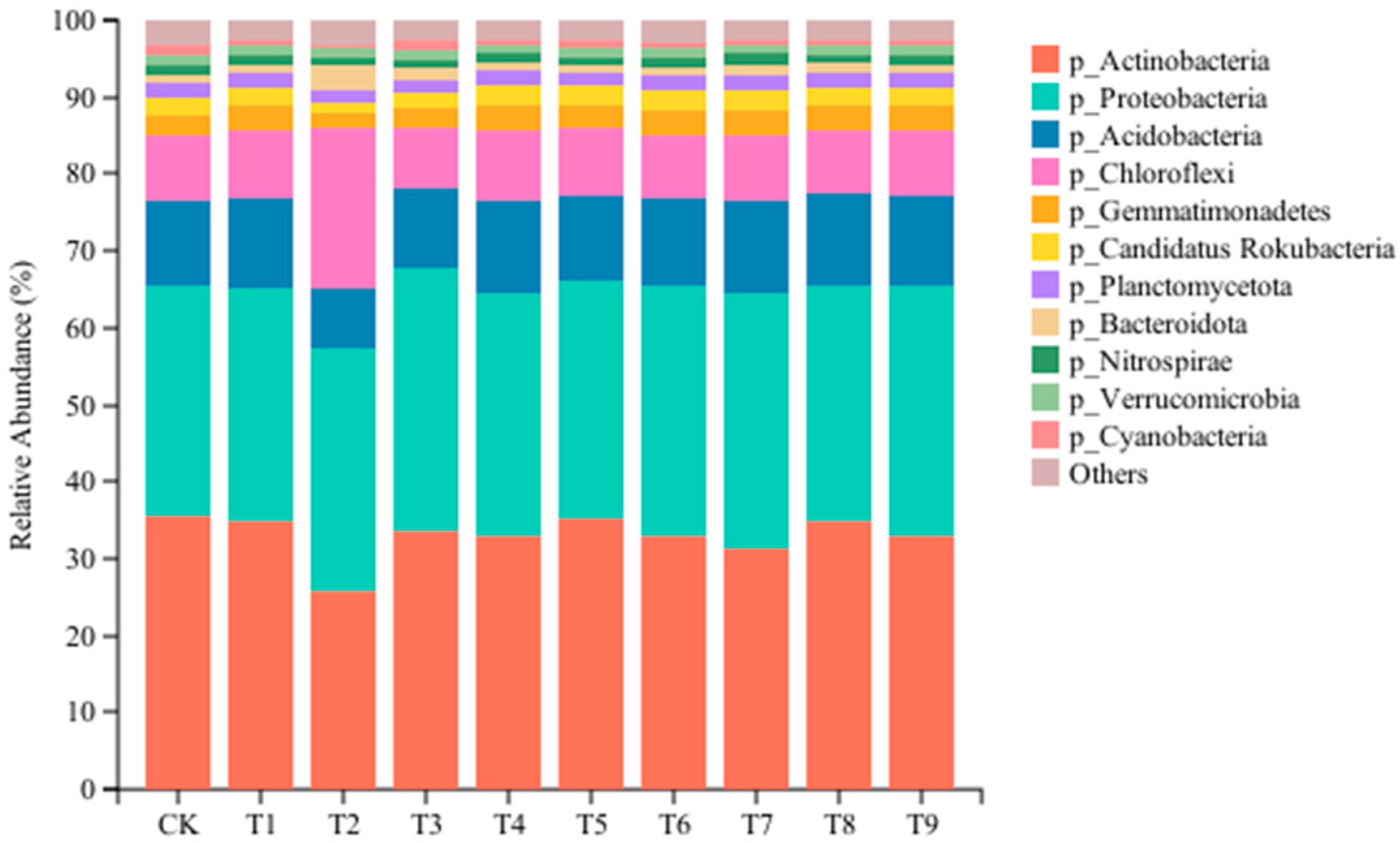
In order to clarify the effects of different amendments on the microbial community of tomato continuous cropping soil, the species with a relative abundance less than 1% in 30 samples were combined at the level of phylum and genus, and the relative abundance of the community was obtained by averaging the three repeated samples in the group. As shown in Figure 3, the composition of the dominant gates under different treatments is the same, but the proportion of each gate is affected by different treatments. At the phylum level, 11 bacterial communities with relative abundance >1% were detected, and 4 dominant communities with relative abundance >5% were detected: Actinobacteria (25.62–35.72%), Proteobacteria (29.95–34.28%), Acidobacteria (7.88–12.35%), and Chloroflexi (7.67–20.95%) (p < 0.05). Compared with the CK, the relative abundance of Proteobacteria increased significantly in the nine amendment treatments, and the maximum increase was 14% in the T3 treatment. The relative abundance of Actinobacteria decreased significantly, among which the T2 treatment decreased the most by 27.26%. The relative abundance of Chloroflexi increased significantly in the T1, T2, T4, T5, and T7 treatments and decreased significantly in the T3, T6, and T8 treatments. Compared with the other treatments, the relative abundance of Acidobacteria, Gemmatimonadetes, Candidatus Rokubacteria, Planctomycetota, and Verrucomicrobia in the T2 and T3 treatments decreased significantly, and the relative abundance of Bacteroidota increased significantly. The relative abundance of Nitrospirae was significantly lower than that of the CK in the T2, T3, T4, T5, T6, T8, and T9 treatments. The relative abundance of Cyanobacteria in the T3 treatment was significantly higher than that in other treatments (p < 0.05).
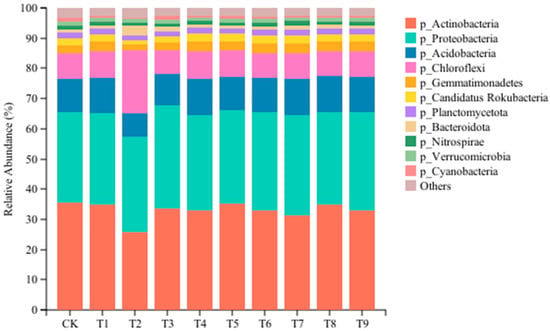
Figure 3.
Effects of different amendments on the dominant microbial phylum in tomato rhizosphere soil.
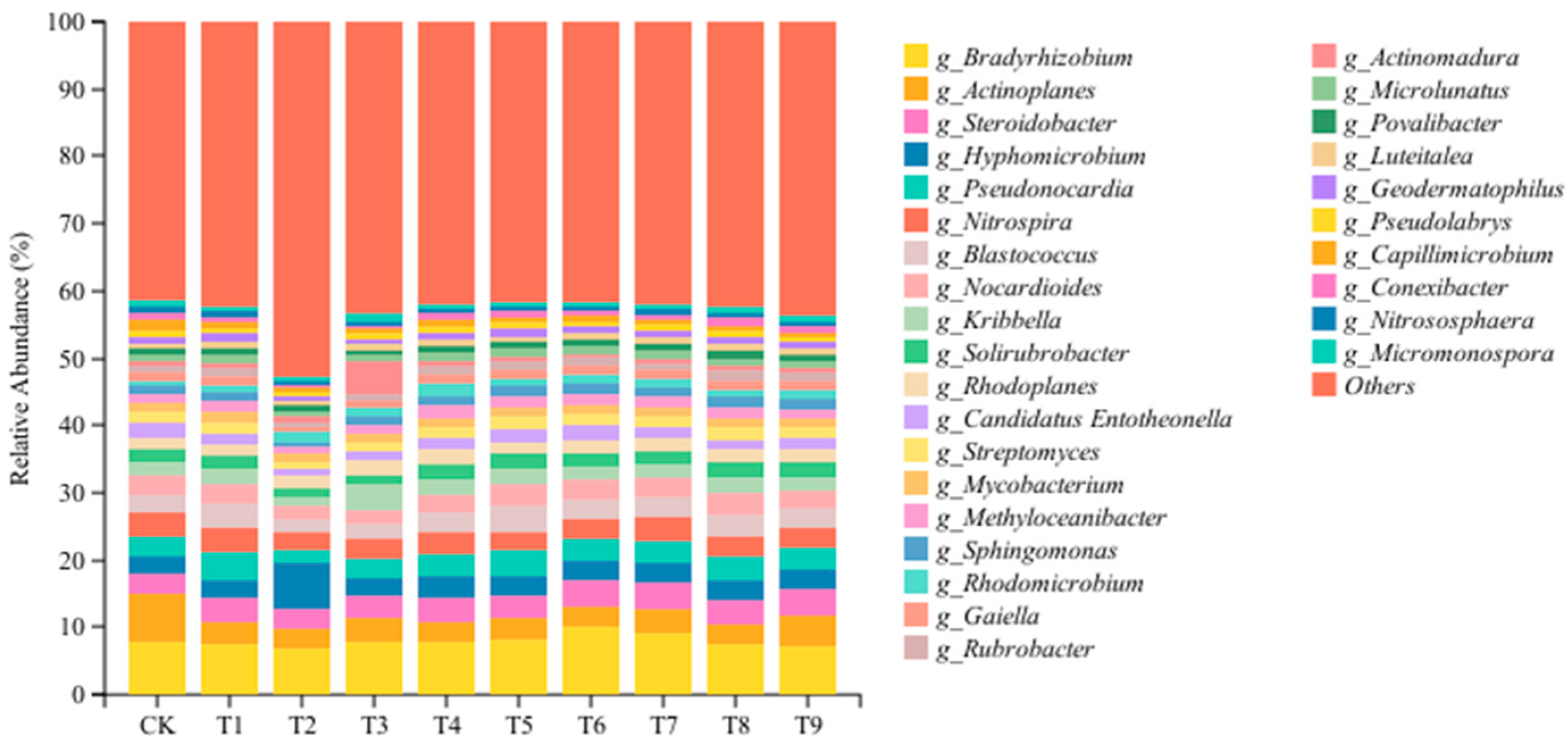
In order to further understand the changes in the abundance and composition of soil microbial communities, we analyzed soil bacterial communities at the genus level. As shown in Figure 4, the bacterial communities treated with different amendments were significantly different at the genus level. At the genus level, 29 bacterial communities with relative abundance >1% were detected, and the dominant genera with relative abundance >5% were Bradyrhizobium and Actinoplanes. Compared with the CK, the relative abundance of Bradyrhizobium increased significantly in the T5, T6, and T7 treatments and decreased significantly in the T1, T2, T8, and T9 treatments. The relative abundance of Hyphomicrobium and Rhodomicrobium increased significantly after treatment with the nine modifiers, and the relative abundance of Actinoplanes decreased significantly. The relative abundance of Pseudonocardia, Blastococcus, Solirubrobacter, and Rubrobacter increased significantly in the T1, T5, and T8 treatments and decreased significantly in the T2 and T3 treatments. The relative abundance of Rhodoplanes increased significantly in the T2, T3, T4, T5, T7, T8, and T9 treatments, while the relative abundance of Candidatus Entotheonella decreased significantly. The relative abundance of Nocardioides, Methyloceanibacter, Sphingomonas, and Gaiella decreased significantly in the T2 and T3 treatments but increased significantly in other improved treatments (p < 0.05).
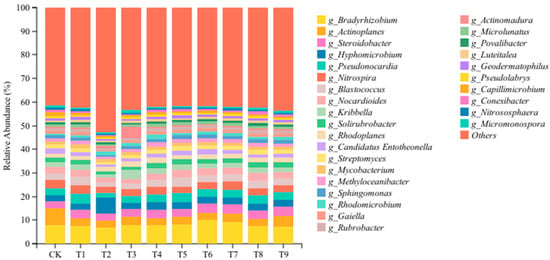
Figure 4.
Effects of different amendments on the microbial dominant genera in the tomato rhizosphere soil.
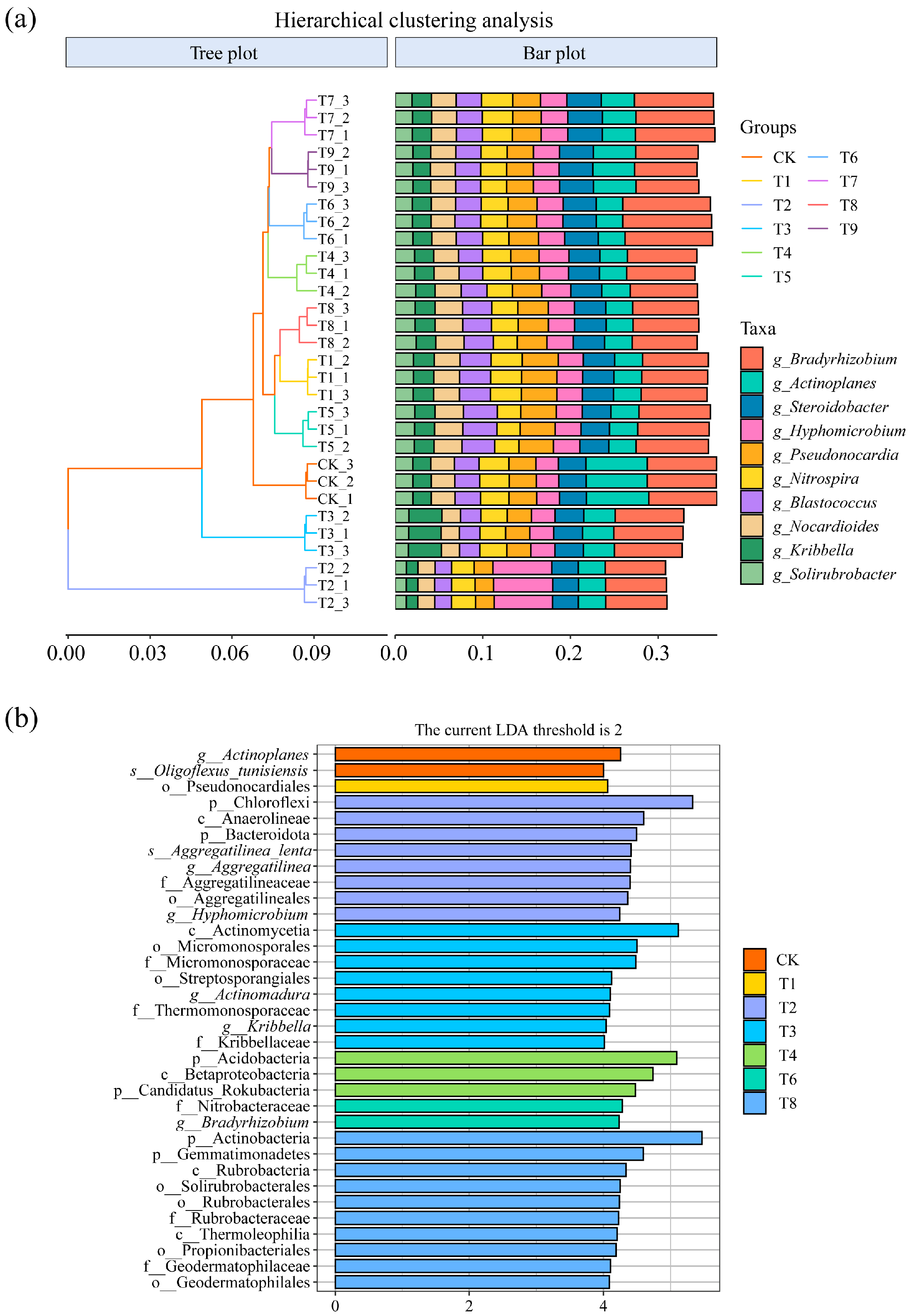
Hierarchical clustering analysis was performed on different treatments based on the top 10 dominant genera, and the results are shown in Figure 5a. Samples from the same process can be clustered into a separate group, and all processes are grouped separately. It indicated that there were differences in soil microbial community structure among the different soil amendment treatments.
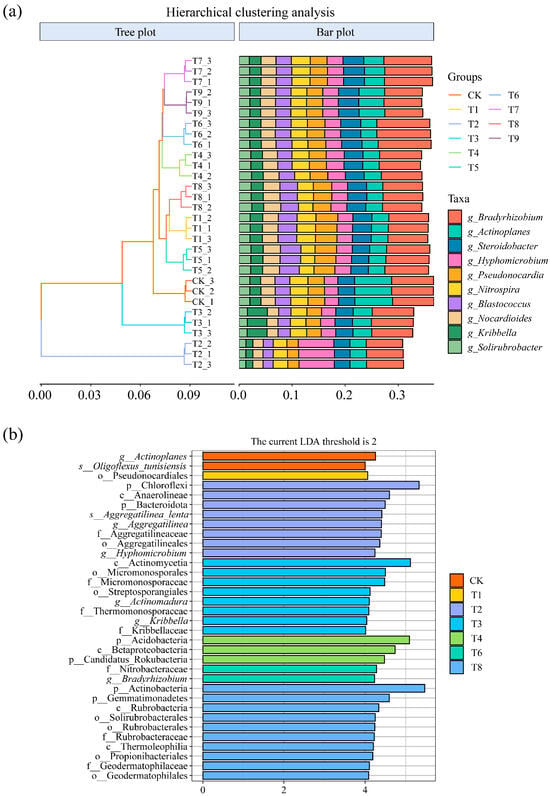
Figure 5.
(a) Hierarchical clustering analysis of different treatments at the genus level. (b) Significantly different taxa detected by LEfSe in microbial communities under different treatments.
The LEfSe method was used to further analyze the differentiated taxa in the microbial communities of the different treatments, and the results are shown in Figure 5b. Compared with other treatments, in the CK treatment, the communities with significant differences were Actinoplanes and Oligoflexus_tunisiensis; pseudonocardiales had significant differences in the T1 treatment. There were significant differences in the Chloroflexi, Anaerolineae, Bacteroidota, Aggregatilinea_lenta, Aggregatilinea, Hyphomicrobium, and Aocregaulineaceae communities under the T2 treatment. There were significant differences in the Actinomycetia, Micromonosporales, Micromonosporaceae, Streptosporangiales, Actinomadura, Thermomonosporaceae, Kribbella, and Kribbellaceae communities under the T3 treatment. There were significant differences in Acidobacteria, Betaproteobacteria, and Candidatus_Rokubacteria in the T4 treatment and the Nitrobacteraceae and Bradyrhizobium communities in the T6 treatment. The communities with significant differences in the T8 treatment were Actinobacteria, Gemmatimonadetes, Rubrobacteria, Solirubrobacterales, Rubrobacterales, Rubrobacteraceae, Thermoleophilia, Propionibacteriales, Geodermatophilaceae, and Geodermatophilales.
3.6. Functional Characteristics of Rhizosphere Soil Microbial Community
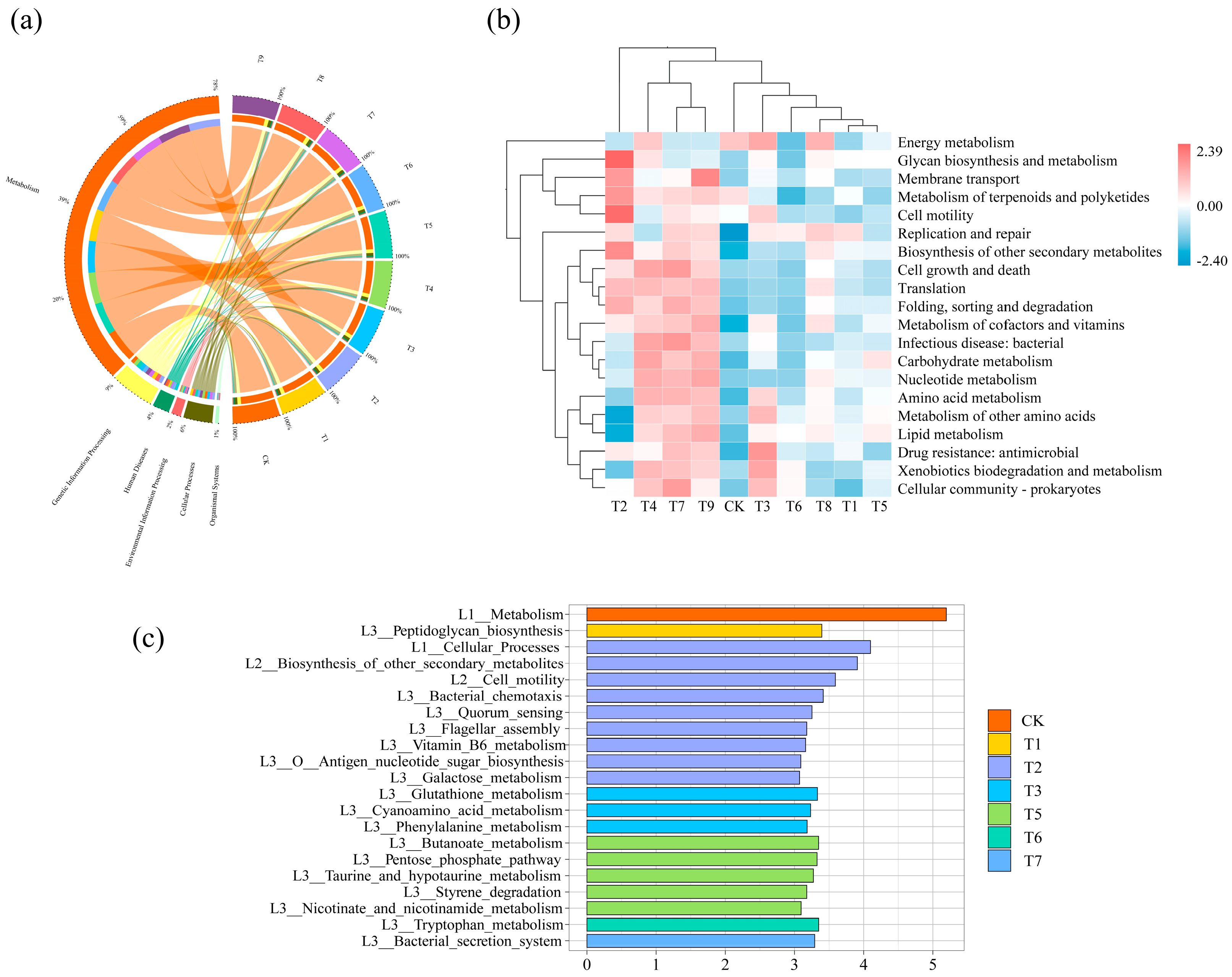
In order to explore the differences in functional metabolic pathways of tomato soil microbial communities after the application of different amendments, a KEGG analysis of microbial genes was carried out. The results showed that compared with the CK treatment, the function of cellular processes and human diseases in the T2 treatment was significantly improved, and the function of metabolism was significantly reduced. The human diseases function of the T6 treatment and the environmental information processing function of the T9 treatment were significantly higher than those of the CK treatment. The cellular processes function of the T1 and T8 treatments was significantly lower than that of the CK treatment (Figure 6a) (p < 0.05).
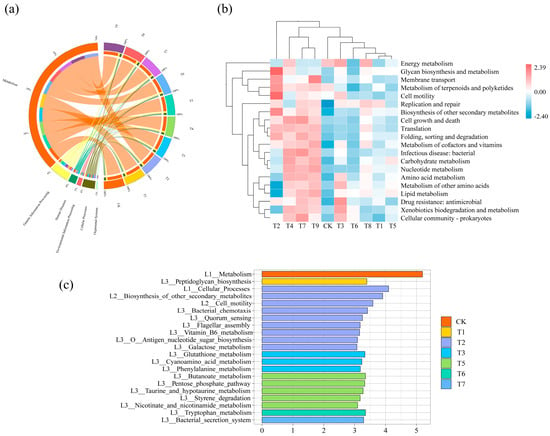
Figure 6.
Analysis of KEGG metabolic pathway level under different treatments: (a) relative abundance distribution of soil microbial community functional composition at KEGG 1 level; (b) differences in the functional pathways of soil microbial communities at KEGG 2 level under different treatments; (c) significantly enriched functional pathways within each treatment.
A total of 35 KEGG biological pathways were annotated at the KEGG level 2 classification level, of which 19 functional pathways had relative abundance >1%. The cluster heat map was used to show the differences in metabolic pathways between groups, and the similarity between treatment groups was revealed by cluster analysis. The results showed that the community functions of T2 were mainly clustered in glycan biosynthesis and metabolism, membrane transport, the metabolism of terpenoids and polyketides, cell motility, the biosynthesis of other secondary metabolites, translation, and folding, sorting, and degradation. T4, T7, and T9 clustered mainly in cell growth and death, translation, folding, sorting, and degradation, metabolism of cofactors and vitamins, infectious disease: bacterial, and carbohydrate, nucleotide, and amino acid metabolism. T3 mainly clustered in energy metabolism, metabolism of other amino acids, drug resistance: antimicrobial, xenobiotics biodegradation and metabolism, and cellular community—prokaryotes (Figure 6b).
LEfSe analysis was performed on all the treated samples of tomato rhizosphere soil. The results showed that there were statistically different functions in soil samples with LDA values greater than 3. A total of 21 functions with significant differences were detected in the experiment (Figure 6c). The significantly enriched community function of T1 was peptidoglycan biosynthesis. The community functions significantly enriched by T2 were bacterial chemotaxis, quorum sensing, flagellar assembly, vitamin B6 metabolism, antigen nucleotide sugar biosynthesis, and galactose metabolism. T3 significantly enriched glutathione metabolism, cyanoamino acid metabolism, and phenylalanine metabolism. T5 significantly enriched butanoate metabolism, the pentose phosphate pathway, taurine and hypotaurine metabolism, styrene degradation, and nicotinate and nicotinamide metabolism. T6 significantly enriched tryptophan metabolism. T7 significantly enriched the bacterial secretion system.
3.7. Correlation Between Rhizosphere Microbial Community Structure and Soil Physical and Chemical Properties and Soil Enzyme Activities
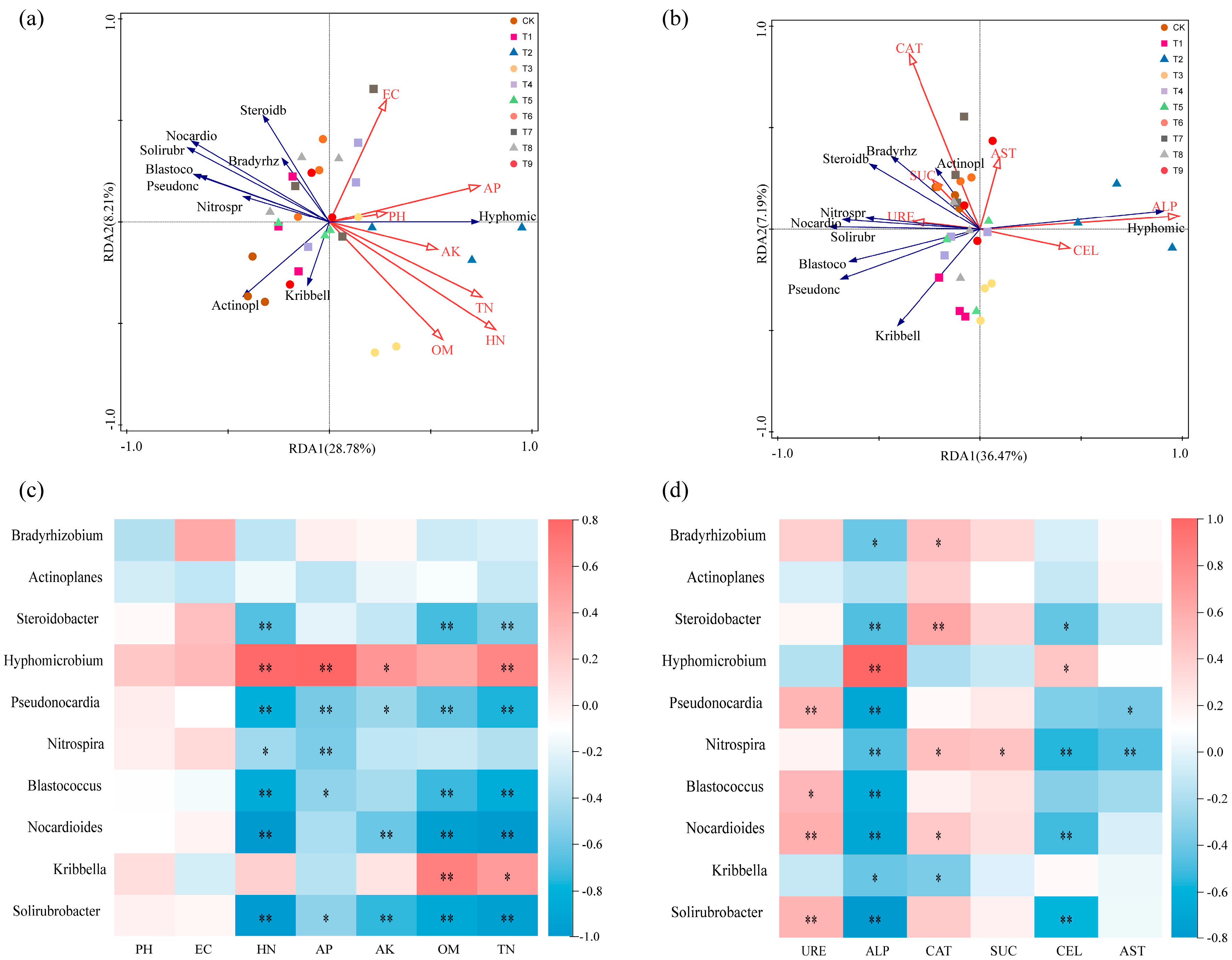
The microbial community composition, soil environmental factors, and soil enzyme activities of different treatments were analyzed by RDA. The effects of seven physical and chemical factors (pH, EC, HN, AP, AK, OM, and TN) and six soil enzyme activities (URE, ALP, CAT, SUC, CEL, and AST) on the tomato rhizosphere bacterial community were investigated. The results showed that RDA1 and RDA2 explained 28.78% and 8.21% of the microbial community structure and soil physical and chemical factors, respectively. pH, HN, and AP were the main environmental factors affecting the microbial community structure in the rhizosphere soil (Figure 7a). RDA1 and RDA2 explained 36.47% and 7.19% of the microbial community structure and soil enzyme activity at the genus level, respectively. ALP and CAT were the main soil enzyme activities that significantly affected the microbial community structure of the rhizosphere soil at the genus level (Figure 7b).
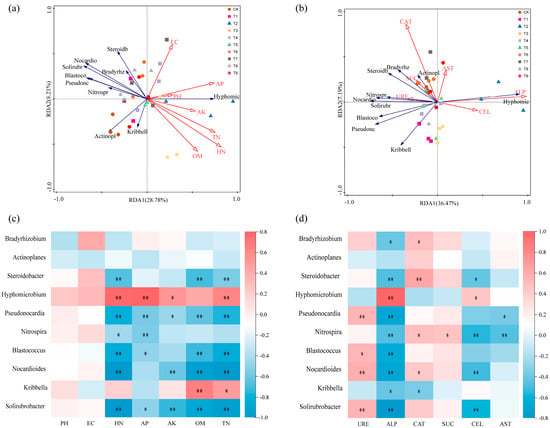
Figure 7.
Correlation analysis of soil dominant bacteria with environmental factors and soil enzyme activities: (a) redundancy analysis of the correlation between microbial community and environmental factors at the genus level; (b) redundancy analysis of the correlation between microbial community and soil enzyme activity; (c) heat map of the correlation between microbial community and environmental factors at the genus level; (d) heat map of the correlation between microbial community at genus level and soil enzyme activity. The positive correlation is expressed in red, and the negative correlation is expressed in blue. The significant correlation was expressed as an asterisk (* p < 0.05; ** p < 0.01).
In order to further analyze the relationship between various environmental factors and microbial communities, a heat map of the correlation between environmental factors and microbial communities was constructed. It can be seen from the figure that soil HN, AP, AK, TN, ALP, and CEL were significantly positively correlated with Hyphomicrobium and significantly negatively correlated with Pseudonocardia, Nocardioides, and Solirubrobacter. Soil OM and TN were significantly positively correlated with Kribbella and significantly negatively correlated with Steroidobacter, Pseudonocardia, Blastococcus, Nocardioides, and Solirubrobacter. Pseudonocardia, Blastococcus, Nocardioides, and Solirubrobacter were significantly positively correlated with URE. CAT was positively correlated with Bradyrhizobium, Steroidobacter, Nitrospira, and Nocardioides and negatively correlated with Kribbella. Nitrospira was significantly positively correlated with SUE (Figure 7c,d).
4. Discussion
In this study, by comparing the effects of different amendments on tomato continuous cropping soil, the potential role and mechanism of amendments in alleviating continuous cropping obstacles were discussed, mainly focusing on the changes in soil physical and chemical properties, enzyme activity, microbial community structure, and function. The results showed that the application of amendments significantly improved the physical and chemical properties and biological characteristics of the soil, especially the treatment of farm manure + Bacillus subtilis + Trichoderma harzianum (T2) and plant straw decomposed soil + Bacillus subtilis + Trichoderma harzianum (T3). The T2 treatment significantly increased tomato yield, soil pH, the EC value, the organic matter content, and key nutrient levels such as hydrolyzed nitrogen, available phosphorus, and available potassium. The contents of soil alkali-hydrolyzable nitrogen, available potassium, organic matter, and total nitrogen in the T3 treatment also increased significantly. The significant increase in these indicators indicates that amendments play an important role in alleviating soil acidification and nutrient imbalance. This result is consistent with previous studies that show that the application of organic fertilizers and functional microorganisms can significantly improve soil fertility and the acid–base balance and promote the healthy growth of crops [16]. In terms of soil enzyme activity, the T2 and T3 treatments significantly increased phosphatase activity. The increase in phosphatase indicates that the transformation ability of phosphorus in soil is enhanced, and the formation of phosphorus ions in soil needs the participation of phosphatase. The secretion of phosphatase can greatly improve the utilization of phosphorus in soil by plants [17]. The enhancement of phosphatase activity optimizes the effective utilization of phosphorus and supports the phosphorus metabolic capacity of Proteobacteria and Bacteroidetes. The farmyard manure/plant-derived straw decomposed soil + Bacillus subtilis + Trichoderma harzianum improver increased soil nutrient content, improved soil enzyme activity, and could effectively increase crop yield. The reason for this may be related to the large amount of organic matter and unmineralized nutrient elements in the decomposed soil of farm manure or plant straw, which effectively improves the organic matter and nutrient content of the soil. The increase in soil organic matter can enhance the activity of soil microorganisms, improve soil enzyme activity, promote the decomposition of soil organic matter and nutrients, and then increase soil available nutrients [18]. In addition, bio-amendments contain beneficial microorganisms, including Bacillus subtilis, which can decompose organic matter and convert macromolecular organic matter in the soil into nutrients that can be easily absorbed by crops [19], providing favorable conditions for crop growth. Trichoderma harzianum is a widely used soil fungus capable of enhancing plant stress resistance and nutrient absorption through symbiosis with plant roots. The increase in catalase activity indicates that the microbial community has made an adaptive response to oxidative stress, which may enhance the degradation ability of harmful substances and improve the soil environment.
The metagenomic sequencing analysis showed that the application of amendments significantly affected the composition and diversity of soil microbial communities. At the phylum level, the T2 and T3 treatments significantly increased the relative abundance of Proteobacteria and Bacteroidetes. Proteobacteria are beneficial bacteria in soil, participating in various organic carbon and nitrogen cycles [20] and playing a major role in organic matter decomposition and the soil phosphorus cycle [21], and there are many nitrogen-fixing bacteria in Proteobacteria. Some members of Bacteroidetes are considered to play a key role in the decomposition of refractory organic matter and the inhibition of soil pathogenic organisms [22,23]. This suggests that the application of T2 and T3 may promote the formation of more diverse microbial communities in the soil nutrient cycle and the decomposition of organic matter. This study also found that amendments can increase the abundance of Chloroflexi in the soil, which may be due to the higher available potassium content in the soil. Studies have shown that there is a significant positive correlation between soil available potassium content and the relative abundance of Chloroflexi [24], which is consistent with the conclusions in this study. At the genus level, the T2 treatment significantly increased the relative abundance of beneficial bacteria such as Hyphomicrobium, Rhodomicrobium, and Rhodoplanes. These bacteria are closely related to the nitrogen cycle and organic matter decomposition. Studies have shown that Hyphomicrobium are crucial components of methanol-utilizing denitrification [25]. Rhodomicrobium is a nitrogen-fixing microorganism enriched in the rice rhizosphere [26].
In addition, by analyzing the functional metabolic pathways of different treatments, the significant differences in the function of microbial communities were revealed, and the change trend of key metabolic pathways was discussed. It is very important to compare the correlation and distribution of microbial community functional pathways between groups [27]. Compared with the control group (CK), the functional pathways of multiple treatment groups were significantly reconstructed, especially in metabolic-related pathways (such as amino acid metabolism and carbohydrate metabolism) and environmental information processing (such as membrane transport and signal transduction). The redistribution of this function may be a strategy for microbial communities to adapt to changes in the external environment [28]. We used heat maps to show the relative abundance differences of each group on functional pathways and revealed the similarity between treatment groups through a cluster analysis. The results showed that different treatments may induce changes in microbial community function in a specific direction [29]. At the same time, specific treatments may promote the synthesis of stress-resistant or antibacterial-related secondary metabolites by microorganisms [30]. Cell motility and quorum sensing: flagellar assembly and quorum sensing functions were significantly enhanced in the T2 and T4 groups, which may be related to the enhanced environmental adaptability of microorganisms. The enrichment of these amino acids and vitamin metabolism, such as tryptophan, vitamin B6, and nicotinic acid metabolism, may reflect the enhanced regulation of microbial communities in energy metabolism and nutrient synthesis. Different treatments significantly affected the functional structure of the microbial community. These functional changes may be closely related to the response mechanism of microorganisms to environmental stress. Especially in the case of enhanced secondary metabolite synthesis and cell motility, microorganisms may adapt to new niches by increasing competitiveness or collaboration capabilities. In addition, the enhanced function of antibiotic metabolism and drug resistance also indicates that external interventions may drive the evolutionary selection pressure of microbial communities to a certain extent.
Soil organic matter, nutrient content, and pH significantly affect the composition and function of microbial communities. Microbial community structure can also affect the physical and chemical properties of soil. Soil enzyme activity is an indicator of microbial metabolic processes and reflects the activity and function of microbial communities. There is a close relationship between soil enzyme activity and microbial communities, and different functional microbial communities produce specific enzymes to decompose organic matter in the soil. Organic fertilizers, microbial amendments, etc., can increase the organic matter in the soil, provide more nutrients for microorganisms, promote the growth of certain microbial communities, change the enzyme activity in the soil, and improve soil fertility. The correlation analysis showed that hydrolytic nitrogen, available phosphorus, pH, phosphatase, and catalase were the most important factors affecting the changes in the microbial community. Nitrogen and phosphorus are the main factors affecting soil microorganisms, and when the organic matter content is relatively high, the soil microbial community structure is more abundant. The increase in the soil hydrolyzed nitrogen and available phosphorus content directly promotes the proliferation of beneficial microorganisms [31]. The increase in the pH value reduced the degree of soil acidification and provided a suitable living environment for more functional bacteria. This further verified the core mechanism of the amendment in alleviating continuous cropping obstacles, that is, by improving soil physical and chemical properties and promoting the growth of beneficial microorganisms to achieve the improvement in soil health and the increase in crop yield. In this study, the treatment effect of farm fertilizer + Bacillus subtilis + Trichoderma hartzii was the most significant, which was analyzed because farm fertilizer could provide nutrients for organic matter and microorganisms, Bacillus subtilis had the function of decomposing organic matter, promoting plant growth and inhibiting disease, and Trichoderma hartzii played a positive role in improving rhizosphere microbial community and promoting plant nutrient absorption. The effects of different amendments on soil microbial community composition and metabolic pathways were clarified, which provided a scientific basis for the further optimization of the formulation and application of amendments. However, there are still some limitations in this study, such as the uniformity of environmental conditions and the lack of testing and long-term effects. Therefore, future studies can carry out long-term monitoring under multi-environment and multi-crop conditions to further reveal the profound impact of amendments on soil ecosystems.
5. Conclusions
The continuous cropping obstacle is an important challenge in tomato facility production, which seriously affects the yield and quality of tomato and restricts the sustainable development of tomato. In this study, the treatment of farm fertilizer/plant-derived straw decomposed soil + Bacillus subtilis + Trichoderma harziana significantly improved tomato yield, soluble solid matter, soil hydrolyzed nitrogen, available phosphorus, available potassium, organic matter and total nitrogen content, and soil phosphatase activity; changed the structure and diversity of the soil microbial community; and induced the transformation of soil microbial community function. This combined approach not only improves soil fertility but also improves plant resistance and reduces soil disease, thereby promoting sustainable agricultural production. Compared with single microbial or amendment treatment, this composite treatment method can significantly improve soil health and crop yield, improve soil structure, and enhance soil water retention and permeability, which is particularly important for intensive planting and has strong adaptability and long-term effects. This composite microbial treatment is used in soil management in horticultural systems with regular application of farm manure in combination with Bacillus subtilis and Trichoderma harziana. Studying the response of different crops to improve the application dose can ensure the maximum benefit in intensive management. Therefore, the treatment of farm manure/plant-derived straw decomposed soil + Bacillus subtilis + Trichoderma harziensis is helpful in improving the ecological environment of soil and improving the long-term productivity of soil. Compared with traditional fertilizers and pesticides, this method not only reduces the negative impact on the environment but also promotes the restoration and regeneration of soil health, providing a new solution for sustainable agricultural development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11050446/s1, Table S1. Map of planting greenhouses.
Author Contributions
Conceptualization, Resources, Funding Acquisition, Project Administration, J.W.; Methodology, Software, Writing—Original Draft Preparation, Data Curation, Y.W.; Validation, W.T.; Investigation, D.Z.; Formal Analysis, C.X.; Writing—Review and Editing, Y.L.; Supervision, Z.W.; Visualization, S.L. and J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Agriculture Research System (grant number CARS-23-G01) and the Key Project of Innovative Engineering of the Heilongjiang Academy of Agricultural Sciences (grant number CX23GG05).
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wu, Z.; Jiang, J.; Dong, W.; Cui, S. The Spatiotemporal Characteristics and Driving Factors of Soil Degradation in the Black Soil Region of Northeast China. Agronomy 2024, 14, 2870. [Google Scholar] [CrossRef]
- Han, S.; Ji, X.; Huang, L.; Liu, G.; Ye, J.; Wang, A. Effects of aftercrop tomato and maize on the soil microenvironment and microbial diversity in a long-term cotton continuous cropping field. Front. Microbiol. 2024, 15, 1410219. [Google Scholar] [CrossRef]
- Zhao, X.; Xie, P.; Zhang, X.; Ou, Z.; Ma, H.; Suo, C.; Ma, J.; Wan, P. Characteristics of different aged plantations of Ormosia hosiei with regards to soil microbial biomass and enzymatic activities. J. For. Res. 2024, 35, 119. [Google Scholar] [CrossRef]
- Kumar, A.; Das, A.; Singh, D.; Das, M.K.; Srivastava, G.P.; Singh, J.P.; Tilgam, J.; Thapa, S.; Das, S.; Chakdar, H. Soil health restoration in degraded lands: A microbiological perspective. Land Degrad. Dev. 2023, 34, 5155–5170. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, G.; Cheng, Y.; Shi, P.; Yang, C.; Yang, H.; Xu, Z. Soil acidification in continuously cropped tobacco alters bacterial community structure and diversity via the accumulation of phenolic acids. Sci. Rep. 2019, 9, 12499. [Google Scholar] [CrossRef]
- Song, X.; Huang, L.; Li, Y.; Zhao, C.; Tao, B.; Zhang, W. Characteristics of soil fungal communities in soybean rotations. Front. Plant Sci. 2022, 13, 926731. [Google Scholar] [CrossRef] [PubMed]
- Stefan, L.; Hartmann, M.; Engbersen, N.; Six, J.; Schöb, C. Positive effects of crop diversity on productivity driven by changes in soil microbial composition. Front. Microbiol. 2021, 12, 660749. [Google Scholar] [CrossRef]
- Sanguineti, D.; Zampieri, G.; Treu, L.; Campanaro, S. Metapresence: A tool for accurate species detection in metagenomics based on the genome-wide distribution of mapping reads. mSystems 2024, 9, e00213-24. [Google Scholar] [CrossRef]
- Prosser, J.I. Dispersing misconceptions and identifying opportunities for the use of ‘omics’ in soil microbial ecology. Nat. Rev. Microbiol. 2015, 13, 439–446. [Google Scholar] [CrossRef]
- Liao, H.; Ji, Y.; Sun, Y. High-resolution strain-level microbiome composition analysis from short reads. Microbiome 2023, 11, 183. [Google Scholar] [CrossRef]
- Sun, S.; Badgley, B.D. Changes in microbial functional genes within the soil metagenome during forest ecosystem restoration. Soil Biol. Biochem. 2019, 135, 163–172. [Google Scholar] [CrossRef]
- Heijden, M.G.A.V.D.; Bardgett, R.D.; Van Straalen, N.M.V. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zu, C.; Riaz, M.; Li, C.; Zhu, Q.; Xia, H.; Dong, Q.; Shen, J. Influences of tobacco straw return with lime on microbial community structure of tobacco-planting soil and tobacco leaf quality. Environ. Sci. Pollut. Res. 2024, 21, 30959–30971. [Google Scholar] [CrossRef]
- Hera, O.; Sturzeanu, M.; Vîjan, L.E.; Tudor, V.; Teodorescu, R. Biochemical Evaluation of Some Fruit Characteristics of Blueberry Progenies Obtained from ‘Simultan × Duke’. ACS Omega 2023, 8, 18603–18616. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, J.; Liu, L.; Chen, L.; Yin, X.; Tan, W.; Yan, L.; Shen, T. Synergistic changes in bacterial community composition, function, and soil characteristics of tomato rhizosphere soil under long-term monoculture conditions. Rhizosphere 2024, 31, 100950. [Google Scholar] [CrossRef]
- Wei, X.; Xie, B.; Wan, C.; Song, R.; Zhong, W.; Xin, S.; Song, K. Enhancing soil health and plant growth through microbial fertilizers: Mechanisms, benefits, and sustainable agricultural practices. Agronomy 2024, 14, 609. [Google Scholar] [CrossRef]
- Balemi, T.; Negisho, K. Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: A review. J. Soil Sci. Plant Nutr. 2012, 12, 547–562. [Google Scholar] [CrossRef]
- Toan, N.S.; Nguyen, T.D.P.; Thu, T.T.N.; Lim, D.T.; Dong, P.D.; Gia, N.T.; Khoo, K.; Chew, K.; Show, P.L. Soil mineralization as effects of plant growth promoting bacteria isolated from microalgae in wastewater and rice straw application in a long-term paddy rice in Central Viet Nam. Environ. Technol. Innov. 2021, 24, 101982. [Google Scholar] [CrossRef]
- Mokemiabeka, N.S.; Niebi, A.L.N.J.; Kayath, C.A.; Boundzou, K.G.E.; Nguibi, E. Involvement of Bacillus species in the understanding of the softening process of safou pulp (Dacryodes edulis HJ Lam). Int. J. Sci. Res. 2021, 10, 355–359. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Liu, T.; Wang, H.; Zhu, S. Analysis of bacterial and fungal communities in continuous-cropping ramie (Boehmeria nivea L. gaud) fields in different areas in china. Sci. Rep. 2020, 10, 3264. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, C.P.; Schadt, W.C.; Chang, X.S.; et al. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Change Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef]
- Roley, S.S. Diazotrophic nitrogen fixation in the rhizosphere and endosphere. In Rhizosphere Biology: Interactions Between Microbes and Plants; Springer: Singapore, 2021; pp. 93–108. [Google Scholar] [CrossRef]
- Hou, Y.; Jia, R.; Zhou, L.; Zhang, L.; Li, B.; Zhu, J. Different Fish Farming Patterns in Paddy Fields Substantially Impact the Bacterial Community Composition, Stability, and Assembly Processes in Paddy Water. Agriculture 2024, 14, 2306. [Google Scholar] [CrossRef]
- Tian, T.; Chen, Z.; Tian, Y.; Gao, L. Microbial diversity in solar greenhouse soils in Round-Bohai Bay-Region, China: The influence of cultivation year and environmental condition. Environ. Sci. Pollut. Res. 2017, 24, 23236–23249. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, A.J.; Ojala, A.; Fred, T.; Toivonen, J.; Tiirola, M. Methylophilaceae and Hyphomicrobium as target taxonomic groups in monitoring the function of methanol-fed denitrification biofilters in municipal wastewater treatment plants. J. Ind. Microbiol. Biotechnol. 2017, 44, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, W.; Beebout, S.S.; Zhang, H.; Liu, L.; Yang, J.; Zhang, J. Grain yield, water and nitrogen use efficiencies of rice as influenced by irrigation regimes and their interaction with nitrogen rates. Field Crops Res. 2016, 193, 54–69. [Google Scholar] [CrossRef]
- Jansson, J.K.; Baker, E.S. A multi-omic future for microbiome studies. Nat. Microbiol. 2016, 1, 16049. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Ge, F.; Li, J.; Tu, S.; Yang, H.; Ren, Y.; Gong, X.; Yao, C. Unveiling the regulatory mechanism of poly-γ-glutamic acid on soil characteristics under drought stress through integrated metagenomics and metabolomics analysis. Front. Microbiol. 2024, 15, 1387223. [Google Scholar] [CrossRef]
- Logue, J.B.; Findlay, S.E.G.; Comte, J. Editorial: Microbial responses to environmental changes. Front. Microbiol. 2015, 6, 204–221. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, S.; Wang, L.; Chen, S.; Li, S.; Lei, X.; Sun, X.; Qin, L. Salt stress-induced changes in microbial community structures and metabolic processes result in increased soil cadmium availability. Sci. Total Environ. 2021, 782, 147125. [Google Scholar] [CrossRef]
- Li, C.; Yu, J.; Li, Z.; Yang, J.; Chen, S.; Yang, Y.; Yang, C.; Hu, Y. Exogenous application of sodium hydrosulfide suppresses bacterial wilt and regulates the soil microecology. Preprints 2021. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).