Reproductive and Pollination Characteristics of Three Understory Impatiens Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methodology
2.2.1. Flowering Phenology and Morphometric Measurements
2.2.2. Pollen Viability and Nectar Sugar Concentration
2.2.3. Pollen–Ovule Ratio and Out-Crossing Index (OCI)
2.2.4. Breeding System Analysis
2.2.5. Observation of Pollination Characteristics and Effects of Floral Components on Seed Setting
2.2.6. Floral Volatile Collection
2.2.7. Data Analysis
3. Results
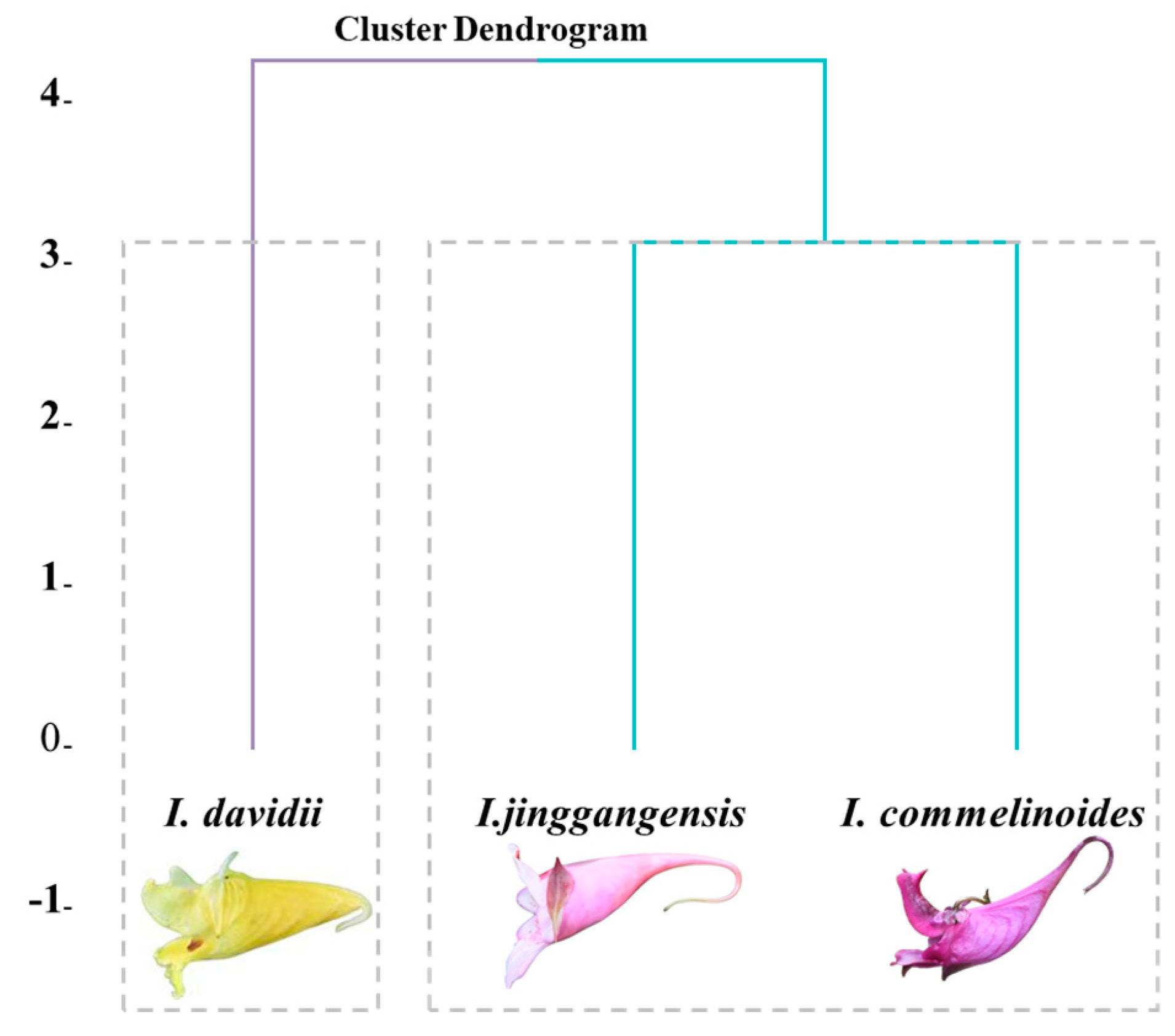
3.1. Analysis of Flowering Phenology, Plant Traits, and Floral Traits in Three Species of Impatiens
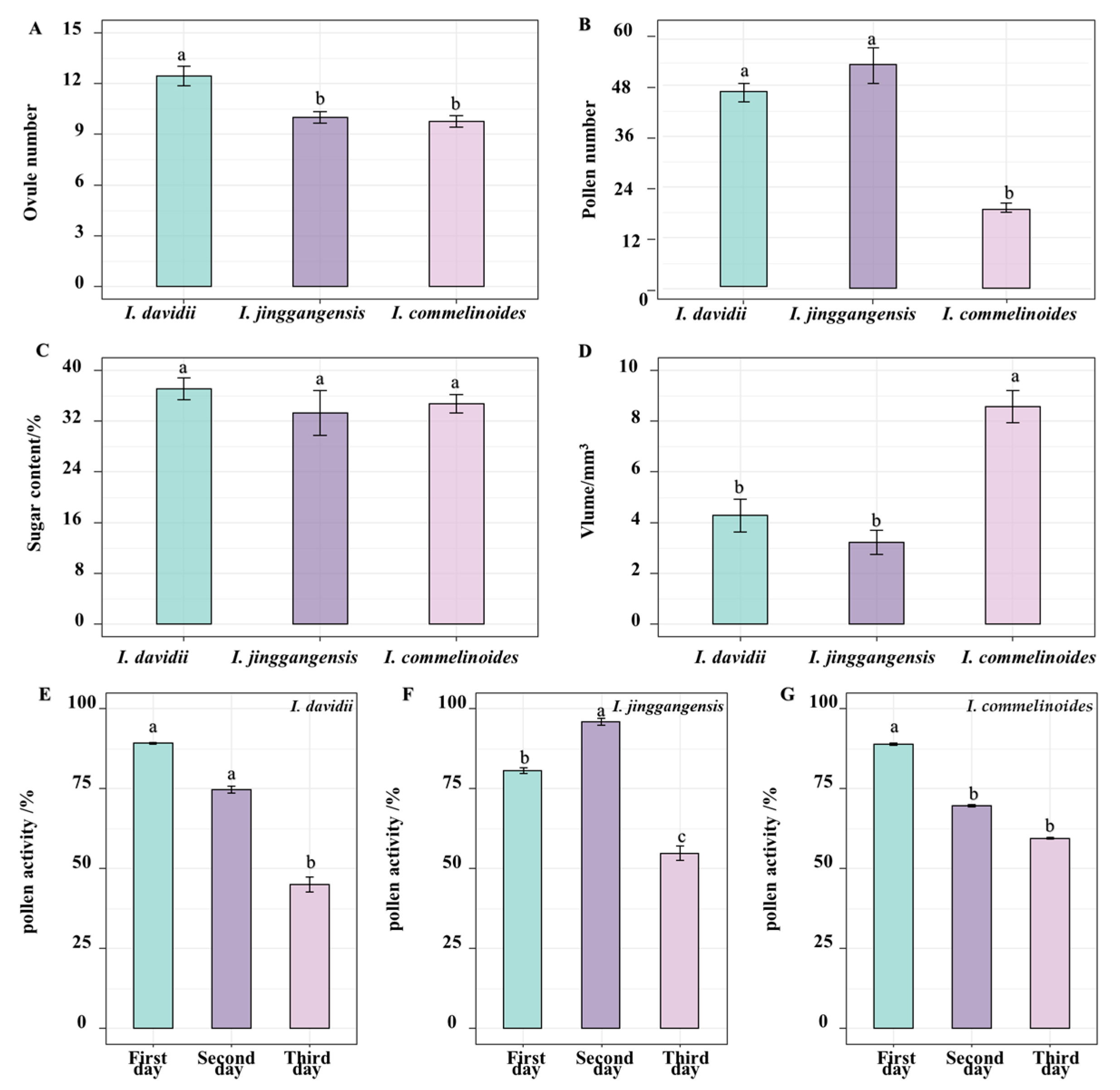
3.2. Pollen Viability, Nectar Volume, and Nectar Sugar Concentration
3.3. Pollen–Ovule Ratio (P/O) and Out-Crossing Index (OCI)
3.4. Breeding System and Floral Trait Effects on Fruit Set
3.5. Pollinator Observations and Foraging Behavior
3.6. Composition of and Variation in Floral Volatile Organic Compounds (VOCs) in Three Impatiens Species
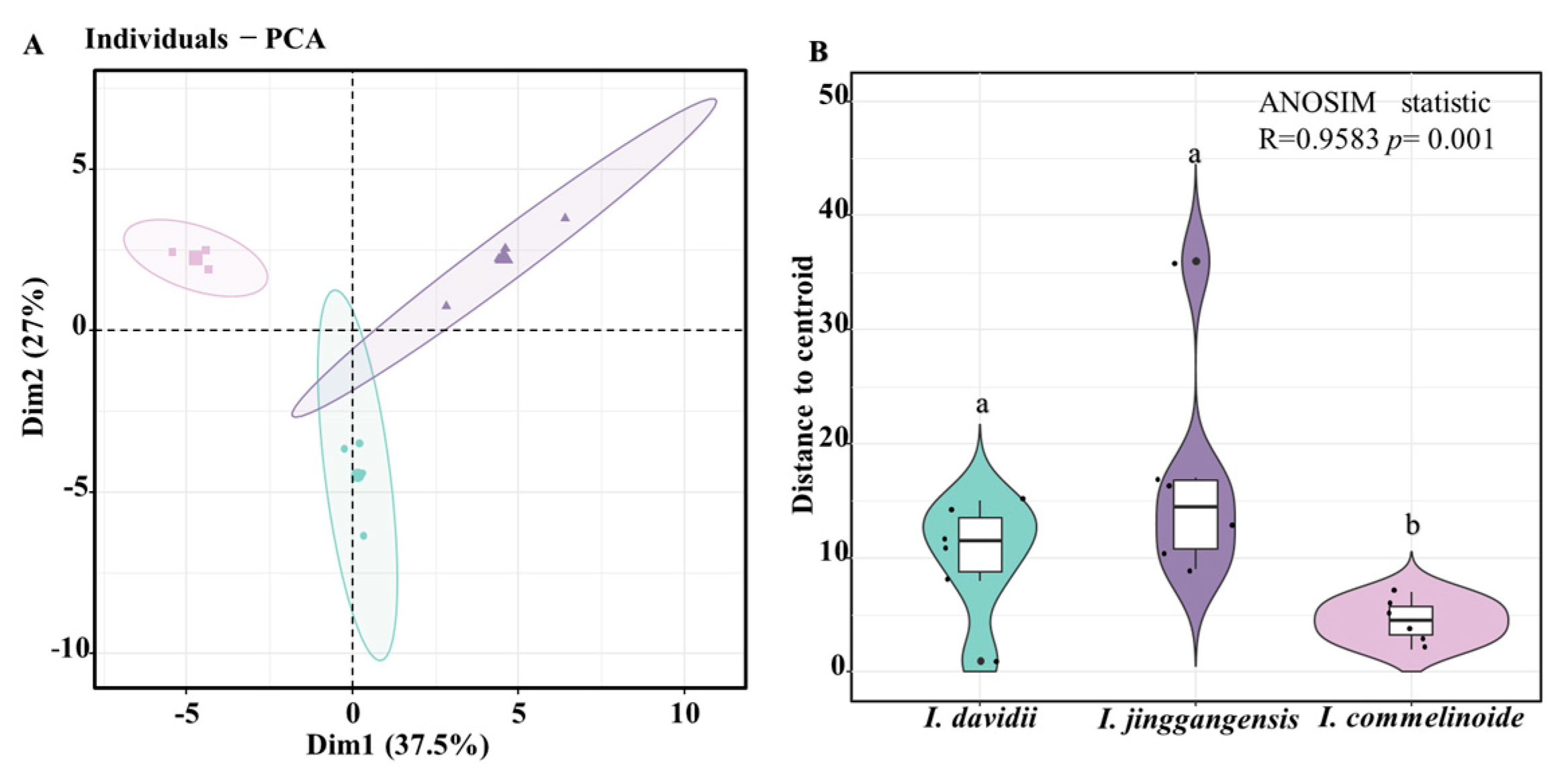
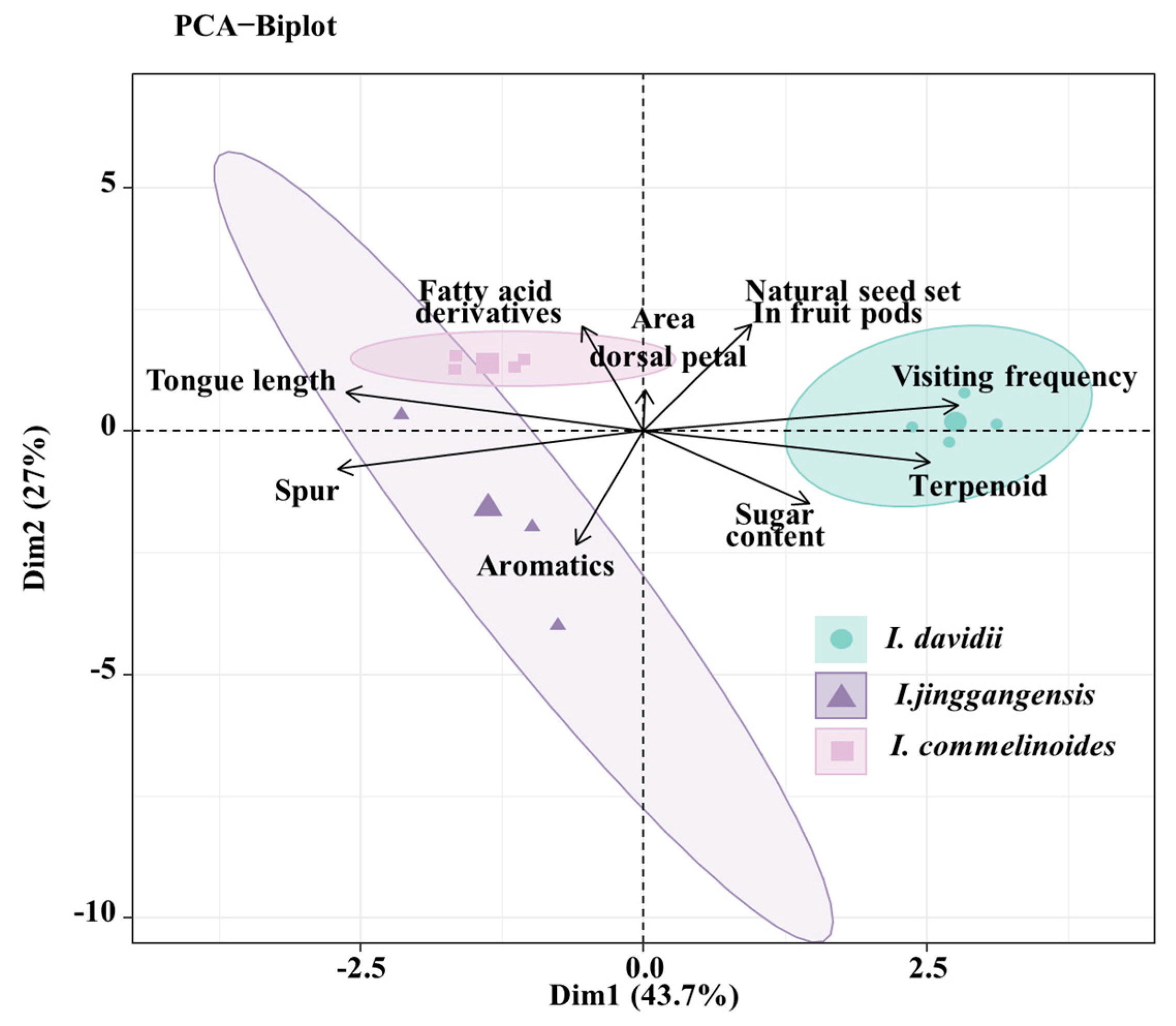
3.7. Floral Trait Differentiation Among Three Impatiens Pollination Strategies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, F.M.; Jiang, C.; Zeng, X.L.; Ren, Y.; Jin, Y.Q.; Feng, D.F.; Chen, Y.Y.; Liu, C.G.; Chen, S.J. Seasonal dynamics of understory vegetation diversity and biomass allocationa cross different tropical land use types. Acta Ecol. Sin. 2024, 44, 9379–9390. [Google Scholar]
- Liang, W.; Wei, X. Relationships between ecosystems above and below ground including forest structure, herb diversity and soil properties in the mountainous area of Northern China. Glob. Ecol. Conserv. 2020, 24, e01228. [Google Scholar] [CrossRef]
- Gilliam, F.S. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- Wayman, R.B.; North, M. Initial response of a mixed-conifer understory plant community to burning and thinning restoration treatments. For. Ecol. Manag. 2007, 239, 32–44. [Google Scholar] [CrossRef]
- Nilsson, M.C.; Wardle, D.A. Understory vegetation as a forest ecosystem driver: Evidence from the northern Swedish boreal forest. Front. Ecol. Environ. 2005, 3, 421–428. [Google Scholar] [CrossRef]
- Gonzalez, M.; Augusto, L.; Gallet-Budynek, A.; Xue, J.; Yauschew-Raguenes, N.; Guyon, D.; Trichet, P.; Delerue, F.; Niollet, S.; Andreasson, F.; et al. Contribution of understory species to total ecosystem aboveground and belowground biomass in temperate Pinus pinaster Ait. forests. For. Ecol. Manag. 2013, 289, 38–47. [Google Scholar] [CrossRef]
- Fenster, C.B.; Armbruster, W.S.; Wilson, P.; Dudash, M.R.; Thomson, J.D. Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 375–403. [Google Scholar] [CrossRef]
- Woźniak, N.J.; Sicard, A. Evolvability of flower geometry: Convergence in pollinator-driven morphological evolution of flowers. Semin. Cell Dev. Biol. 2018, 79, 3–15. [Google Scholar] [CrossRef]
- Abdusalam, A.; Liao, W.J.; Zhang, Z.Q.; Li, Q.J. Pollinator shifts along an elevation gradient mediate different response in self-pollination in heterostylous Primula nivalis. J. Syst. Evol. 2022, 60, 186–195. [Google Scholar] [CrossRef]
- Lehmann, L.J.; Maruyama, P.K.; Joaquim Bergamo, P.; Maglianesi, M.A.; Rahbek, C.; Dalsgaard, B. Relative effectiveness of insects versus hummingbirds as pollinators of Rubiaceae plants across elevation in Dominica, Caribbean. Plant Biol. 2019, 21, 738–744. [Google Scholar] [CrossRef]
- Toon, A.; Cook, L.G.; Crisp, M.D. Evolutionary consequences of shifts to bird-pollination in the Australian pea-flowered legumes (Mirbelieae and Bossiaeeae). BMC Evol. Biol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Li, Q.X.; Zhang, M.R.; Gu, C.H.; Li, T.P.; Wan, Q.; Gao, L.; Zhang, T.Y.; Zhao, N.N.; Cai, Y.H. Floral syndrome and breeding system of Stewartia rostrata. J. Zhejiang AF Univ. 2022, 39, 830–837. [Google Scholar]
- Cao, M.H.; Zhao, Q.Y.; Wei, C.M.; Huang, H.Q.; Huang, M.J. Study on the flowering traits and breeding systems of three Impatiens species. Plant Sci. J. 2022, 40, 291–301. [Google Scholar] [CrossRef]
- Fan, X.L.; Barrett, S.C.; Lin, H.; Chen, L.L.; Zhou, X.; Gao, J.Y. Rain pollination provides reproductive assurance in a deceptive orchid. Ann. Bot. 2012, 110, 953–958. [Google Scholar] [CrossRef]
- Spigler, R.B.; Charles, A. Inbreeding reduces floral longevity and flower size in the mixed-mating biennial Sabatia angularis. Int. J. Plant Sci. 2023, 184, 157–163. [Google Scholar] [CrossRef]
- Shi, H.Y.; Wu, J.; Li, J.L.; An, J.D. Foraging preference of the bumblebee Bombus hypocrita (Hymenoptera: Apidae). Acta Entomol. Sin. 2008, 9, 946–952. [Google Scholar]
- Ruchisansakun, S.; Mertens, A.; Janssens, S.B.; Smets, E.F.; Van Der Niet, T. Evolution of pollination syndromes and corolla symmetry in Balsaminaceae reconstructed using phylogenetic comparative analyses. Ann. Bot. 2021, 127, 267–280. [Google Scholar] [CrossRef]
- Huang, Z.H. The Pollination System and Floral Evolution of Genus Rhododendron (Ericaceae). Ph.D. Thesis, Wuhan University, Wuhan, China, 2015. [Google Scholar]
- Luo, S.F.; Jiang, K.; Huang, W.C. Advances in the convergent evolution of phenotypes and diversification of developmental mechanisms of floral spurs. Biodivers. Sci. 2023, 31, 194–205. [Google Scholar] [CrossRef]
- Lu, G.M.; Lu, N.N.; Ma, Y.; Gao, C.F. Floral traits and their manipulation of foraging behaviors of pollinators of two sympatric Pedicularis species. J. Lanzhou Univ. Nat. Sci. 2021, 57, 338–343. [Google Scholar]
- Pyke, G.H. Floral nectar: Pollinator attraction or manipulation? Trends Ecol. Evol. 2016, 31, 339–341. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Zhu, Y.; Li, Q.Q.; Wang, Y.C.; Tao, L.; Li, L. Pollination biology of two Chinese endemic and sympatric Impatiens (Balsaminaceae) species. Guihaia 2024, 44, 741–755. [Google Scholar] [CrossRef]
- Van der Niet, T.; Peakall, R.; Johnson, S.D. Pollinator-driven ecological speciation in plants: New evidence and future perspectives. Ann. Bot. 2014, 113, 199–212. [Google Scholar] [CrossRef]
- Van der Niet, T.; Johnson, S.D. Phylogenetic evidence for pollinator-driven diversification of angiosperms. Trends Ecol. Evol. 2012, 27, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Dupont, Y.L.; Hansen, D.M.; Rasmussen, J.T.; Olesen, J.M. Evolutionary changes in nectar sugar composition associated with switches between bird and insect pollination: The Canarian bird-flower element revisited. Funct. Ecol. 2004, 18, 670–676. [Google Scholar] [CrossRef]
- Tong, Z.Y.; Huang, S.Q. Potential selection driving evolution of long corolla tubes and case studies. Plant Sci. J. 2023, 41, 719–728. [Google Scholar]
- Yu, S.X.; Janssens, S.B.; Zhu, X.Y.; Lidén, M.; Gao, T.G.; Wang, W. Phylogeny of Impatiens (Balsaminaceae): Integrating molecular and morphological evidence into a new classification. Cladistics 2016, 32, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.H.; Li, M.J.; Ren, L.Y.; Huang, R.X.; Chen, Y.; Bai, X. A dataset on the diversity and geographical distributions of wild Impatiens in China. Biodivers. Sci. 2022, 30, 118–122. [Google Scholar] [CrossRef]
- Wang, J.S.; Lu, Y.F.; Xu, Y.L.; Jin, S.H.; Jin, X.F. Impatiens wuyiensis (Balsaminaceae), a new species from Fujian of Southeast China, based on morphological and molecular evidences. Bot. Stud. 2020, 61, 29. [Google Scholar] [CrossRef]
- Tiwari, U.L. Impatiens tajoensis (Balsaminaceae): A new species from Arunachal Pradesh, India. Taiwania 2023, 68, 39–43. [Google Scholar]
- Tian, J.; Yuan, T.H.; Peng, S.; Yang, Z.Z.; Hu, G.W.; Wang, Q.F. A new species of Impatiens (Balsaminaceae) from Southern Xizang, China with supplementary knowledge on I. leptocarpa Hook. f. Phytotaxa 2024, 661, 47–62. [Google Scholar] [CrossRef]
- Hořák, D.; Janeček, Š. A geographical perspective on the relationship between Impatiens spur lengths and bill lengths of sunbirds in Afrotropical mountains. Ecol. Evol. 2021, 11, 3120–3129. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.L. The Pollination Biology of Four Species of Impatiens L. Master’s Thesis, Hunan Normal University, Changsha, China, 2009. [Google Scholar]
- Zhong, Y.F.; Zhang, Z.; Song, X.Q.; Zhou, Z.D. Pollination biology of Impatiens hainanensis (Balsaminaceae) populations at different altitudes. Biodivers. Sci. 2014, 22, 467–475. [Google Scholar]
- Tian, J.; Liu, K.; Hu, G. Pollination ecology and pollination system of Impatiens reptans (Balsaminaceae) endemic to China. Ann. Bot. 2004, 93, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.X.; Ming, K. Floral Traits Pollination System of Impatiens chinensis (Balsaminaceae). Bull. Bot. Res. 2009, 29, 164–168. [Google Scholar]
- Mao, Z.B.; Boehler, C.; Ge, X.J. Pollination ecology and breeding system of Impatiens lateristachys (Balsaminaceae) endemic to China. Guihaia 2011, 31, 160–166. [Google Scholar]
- Tang, Y.F.; Fang, Y.; Liu, C.Q.; Lu, Q.B.; Hu, X.H. The long spur of Impatiens macrovexilla may reflect adaptation to diurnal hawkmoth pollinators despite diversity of floral visitors. Flora 2020, 266, 151599. [Google Scholar] [CrossRef]
- Li, D.F.; Yan, X.C.; Lin, Y.; Wang, L.; Wang, Q. Do flowers removed of either nectar or pollen attract fewer bumblebee pollinators? An experimental test in Impatiens oxyanthera. AoB Plants 2021, 13, plab029. [Google Scholar] [CrossRef]
- Fischer, E.; Abrahamczyk, S.; Holstein, N.; Janssens, S.B. Evolution of Impatiens (Balsaminaceae) in the Albertine Rift-The endemic Impatiens purpureoviolacea complex consists of ten species. Taxon 2021, 70, 1273–1299. [Google Scholar] [CrossRef]
- Abrahamczyk, S.; Humphreys, A.M.; Trabert, F.; Droppelmann, F.; Gleichmann, M.; Krieger, V.; Linnartz, M.; Lozada-Gobilard, S.; Rahelivololona, M.E.; Schubert, M.; et al. Evolution of brood-site mimicry in Madagascan Impatiens (Balsaminaceae). Perspect. Plant Ecol. Evol. Syst. 2021, 49, 125590. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Yi, Y. Features of floral odor and nectar in the distylous Luculia pinceana (Rubiaceae) promote compatible pollination by hawkmoths. Ecol. Evol. 2023, 13, e9920. [Google Scholar] [CrossRef]
- Mundi, O.; Awa Ii, T.; Chmel, K.; Ewome, F.L.; Uceda-Gómez, G.; Janečková, P.; Janeček, Š. The ornithophily of Impatiens sakeriana does not guarantee a preference by sunbirds. Biol. J. Linn. Soc. 2022, 137, 240–249. [Google Scholar] [CrossRef]
- Deng, X.L.; Liu, Y.C.; Wu, Y. Interconnection among dominant plant populations of Castanopsis community in Jinggang mountain nature reserve. Acta Phytoecol. Sin. 2003, 27, 531–536. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Community Ecology Package. R Package Version 2013, 2, 321–326. [Google Scholar]
- Ginestet, C. ggplot2: Elegant graphics for data analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2011, 174, 245–246. [Google Scholar] [CrossRef]
- Rossi, G.S.; Alaa, E.; Jerrica, J.; Kenneth, C.; Welch, J. Heat exposure limits pentose phosphate pathway activity in bumblebees. Conserv. Physiol. 2024, 12, coae031. [Google Scholar] [CrossRef]
- Huang, X.B.; Li, H.; Dai, X.Y.; Wu, G.A.; Zhou, H.; Chen, H.; Zheng, L.; Zhai, Y.F. Research Progress of Bumblebee Behavior and Pollination Application. Shandong Agric. Sci. 2021, 53, 130–137. [Google Scholar]
- Herrera, C.M. Components of pollinator “quality”: Comparative analysis of a diverse insect assemblage. Oikos 1987, 50, 79–90. [Google Scholar] [CrossRef]
- Kahnt, B.; Hattingh, W.N.; Theodorou, P.; Wieseke, N.; Kuhlmann, M.; Glennon, K.L.; van der Niet, T.; Paxton, R.; Cron, G.V. Should I stay or should I go? Pollinator shifts rather than cospeciation dominate the evolutionary history of South African Rediviva bees and their Diascia host plants. Mol. Ecol. 2019, 28, 4118–4133. [Google Scholar] [CrossRef]
- Kang, X.S.; Pan, B.; Duan, S.; Suo, F.Y.; Zhang, Y.Z.; Shi, W.; Gulnur, S. Comparison of Flowering Phenology and Reproductive Traits of Four Species of Calligonum L. (Polygonaceae) under Ex-situ Conservation at the Turpan Eremophytes Botanical Garden in Xinjiang. J. Desert Res. 2012, 32, 1315–1327. [Google Scholar]
- Hu, J.; Jiang, J.L.; Cheng, W.N.; Wei, L.N.; Wang, Y.; Hu, F.C.; Deng, X.X.; Li, Y. Pollination biology and breeding system of endangred plant Petrocosmea qinlingensis. Acta Ecol. Sin. 2024, 44, 8595–8604. [Google Scholar]
- Perazza, G.; Decarli, M. Monitoring of Cypripedium calceolus (Orchidaceae) in the Adamello-Brenta Natural Park (Italy). Nat. Conserv. Res. 2020, 5, 178–184. [Google Scholar] [CrossRef]
- Zhou, Z.L.; Li, Z.J.; Gong, W.S.; Gao, S. Study on the Flowering Phenology in Populus euphratica and Populus pruinosa. J. Wuhan Bot. Res. 2005, 23, 163–168. [Google Scholar]
- Charlesworth, D.; Brian, C. Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Syst. 1987, 18, 237–268. [Google Scholar] [CrossRef]
- Moore, D. Insects of palm flowers and fruits. In Insects on Palms; CABI: Wallingford, UK, 2001; pp. 233–266. [Google Scholar]
- Piao, J.; Ju, H.G.; Piao, Z.Y. Floral syndrome and breeding system of Atractylodes japonica. Guihaia 2015, 35, 166–172. [Google Scholar]
- Issaly, E.A.; Sersic, A.N.; Pauw, A.; Cocucci, A.A.; Traveset, A.; Benitez-Vieyra, S.M.; Paiaro, V. Reproductive ecology of the bird-pollinated Nicotiana glauca across native and introduced ranges with contrasting pollination environments. Biol. Invasions 2020, 22, 485–498. [Google Scholar] [CrossRef]
- Zhang, J.D.; Wang, L.; Sui, J.; Tian, X.H.; Ren, Y. Self-pollination Mechanism of Actaea asiatica (Ranunculaceae). CBB 2018, 53, 212–218. [Google Scholar]
- Del Carmen Salas-Arcos, L.; Lara, C.; Castillo-Guevara, C.; Cuautle, M.; Ornelas, J.F. “Pro-bird” floral traits discourage bumblebee visits to Penstemon gentianoides (Plantaginaceae), a mixed-pollinated herb. Sci. Nat. 2019, 106, 1. [Google Scholar] [CrossRef]
- Liang, H.; Zhao, Y.H.; Rafferty, N.E.; Ren, Z.X.; Zhong, L.; Li, H.D.; Li, D.Z.; Wang, H. Evolutionary and ecological factors structure a plant–bumblebee network in a biodiversity hotspot, the Himalaya–Hengduan Mountains. Funct. Ecol. 2021, 35, 2523–2535. [Google Scholar] [CrossRef]
- Micheneau, C.; Fournel, J.; Humeau, L.; Pailler, T. Orchid–bird interactions: A case study from Angraecum (Vandeae, Angraecinae) and Zosterops (white-eyes, Zosteropidae) on Reunion Island. Botany 2008, 86, 1143–1151. [Google Scholar] [CrossRef]
- Micheneau, C.; Fournel, J.; Warren, B.H.; Hugel, S.; Gauvin-Bialecki, A.; Pailler, T.; Strasberg, D.; Chase, M.W. Orthoptera, a new order of pollinator. Ann. Bot. 2010, 105, 355–364. [Google Scholar] [CrossRef]
- Minet, J.; Basquin, P.; Haxaire, J.; Lees, D.C.; Rougerie, R. A new taxonomic status for Darwin’s “predicted” pollinator: Xanthopan praedicta stat. nov. Antenor 2021, 8, 69–86. [Google Scholar]
- Huber, F.K.; Kaiser, R.; Sauter, W.; Schiestl, F.P. Floral scent emission and pollinator attraction in two species of Gymnadenia (Orchidaceae). Oecologia 2005, 142, 564–575. [Google Scholar] [CrossRef]
- Jürgens, A. The hidden language of flowering plants: Floral odours as a key for understanding angiosperm evolution? New Phytol. 2009, 183, 240–243. [Google Scholar] [CrossRef]
- Ramírez, S.R.; Eltz, T.; Fujiwara, M.K.; Gerlach, G.; Goldman-Huertas, B.; Tsutsui, N.D.; Pierce, N.E. Asynchronous diversification in a specialized plant-pollinator mutualism. Science 2011, 333, 1742–1746. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Viljoen, A.M. Linalool-a review of a biologically active compound of commercial importance. Nat. Prod. Commun. 2008, 3, 1183–1192. [Google Scholar] [CrossRef]
- Raguso, R.A.; Pichersky, E.R. New Perspectives in Pollination Biology: Floral Fragrances. A day in the life of a linalool molecule: Chemical communication in a plant-pollinator system. Part 1: Linalool biosynthesis in flowering plants. Plant Spec. Biol. 1999, 14, 95–120. [Google Scholar] [CrossRef]
- Gong, W.C.; Xu, S.J.; Liu, Y.H.; Wang, C.M.; Martin, K.; Meng, L.Z. Chemical composition of floral scents from three Plumeria rubra L. (Apocynaceae) forms linked to petal color proprieties. Biochem. Syst. Ecol. 2019, 85, 54–59. [Google Scholar] [CrossRef]
- He, S.T. Gene Expression and Pollinator Response of Ordor Volatiles from Azadirachta indica Flowers. Ph.D. Thesis, Institute of Chemical Industry of Forest Products, Beijing, China, 2022. [Google Scholar]
- Galen, C. The effects of nectar thieving ants on seedset in floral scent morphs of Polemonium viscosum. Oikos 1983, 41, 245–249. [Google Scholar] [CrossRef]


| Spcise | Companion Plant | Longitude and Latitude | Altitude/m |
|---|---|---|---|
| I. davidii | Rubus sp.1, Mallotus barbatus, Boehmeria platanifolia, Iris tectorum | 26.551850° N, 114.126558° E | 920 |
| I. jinggangensis | I. davidii, Equisetum hyemale, Lonicera japonica, Cnidium monnieri | 26.647942° N, 114.174698° E | 838 |
| I. commelinoides | Hypolepis punctata, Gonostegia hirta, Oenanthe javanica | 26.557789° N, 114.131217° E | 915 |
| Blooming Period | I. davidii | I. jinggangensis | I. commelinoides |
|---|---|---|---|
| Flowering period of simple flower | 5.44 ± 0.25 d | 4.80 ± 0.4 d | 5.36 + 0.69 d |
| The stamen stage | 3.06 ± 0.14 d | 3.80 ± 0.89 d | 3.43 ± 0.24 d |
| Pistil stage | 2.39 ± 0.27 d | 1.00 ± 0.79 d | 1.93 ± 0.39 d |
| Full bloom stage | September–October | July–August | July–August |
| Community flowering period | August–October | June–October | June–October |
| Fruit stage | September–November | August–November | July–November |
| Floral Traits | I. davidii | I. jinggangensis | I. commelinoides |
|---|---|---|---|
| Symmetrical | yes | yes | yes |
| Group | Section Crena | Section Racemus | Section Laxiflora |
| Flower color | yellow | red | purple |
| Length of dorsal petal | 13.23 ± 1.84 b | 11.58 ± 1.65 b | 17.34 ± 1.93 a |
| Width of dorsal petal | 11.85 ± 1.40 b | 9.38 ± 1.13 c | 14.00 ± 1.86 a |
| Height of channel | 6.22 ± 0.56 b | 7.65 ± 0.82 c | 6.67 ± 1.02 a |
| Depth of channel | 29.04 ± 3.47 b | 30.27 ± 4.24 b | 34.57 ± 4.17 a |
| Width of channel | 7.84 ± 0.96 a | 8.13 ± 0.76 a | 8.14 ± 0.77 a |
| Length of lateral sepal | 11.64 ± 1.18 a | 11.71 ± 1.00 a | 8.81 ± 1.02 b |
| Width of lateral sepal | 9.48 ± 1.35 a | 4.37± 0.46 c | 7.18 ± 0.81 b |
| Length of spur | 6.13 ± 1.05 c | 25.03 ± 6.00 a | 22.47 ± 3.51 b |
| Length of basal petal | 10.28 ± 1.45 b | 9.23 ± 1.92 b | 15.70 ± 2.77 a |
| Width of basal petal | 19.70 ± 2.80 b | 21.27 ± 5.64 a | 21.40 ± 3.06 a |
| Length of upper petal | 9.35 ± 1.72 a | 8.71 ± 2.85 a | 7.25 ± 0.97 a |
| Width of upper petal | 11.02 ± 3.63 b | 9.81 ± 2.31 b | 13.22 ± 1.51 a |
| Ring diameter length of spur | 1.53 ± 0.25 b | 1.58 ± 0.33 b | 1.72 ± 0.27 a |
| Corolla length | 18.29 ± 3.57 b | 27.25 ± 3.26 a | 26.95 ± 2.77 a |
| Protrusion of tongue | 12.34 ± 0.33 c | 27.47 ± 0.79 a | 19.33 ± 0.84 b |
| Body length | 18.16 ± 0.41 b | 18.28 ± 0.64 b | 16.42 ± 0.36 a |
| Breast height | 5.00 ± 0.13 a | 5.45 ± 0.29 a | 4.94 ± 0.09 a |
| Species | Height\cm | Leaf Length\mm | Leaf Width\mm | Aspect Ratio | Basal Diameter\mm |
|---|---|---|---|---|---|
| I. davidii | 71.57 ± 24.05 b | 91.81 ± 18.29 b | 49.06 ± 12.31 a | 1.91 ± 0.26 b | 11.52 ± 3.95 b |
| I. jinggangensis | 107.15 ± 16.77 a | 126.47 ± 16.65 a | 37.02 ± 5.14 b | 3.43 ± 0.21 a | 15.5 ± 5.38 a |
| I. commelinoides | 52.7 ± 16.22 c | 43.37 ± 9.54 c | 22.91 ± 4.49 c | 1.9 ± 0.26 b | 2.65 ± 0.59 c |
| Species | Pollen Number | Ovule Number | Ovule Ratio |
|---|---|---|---|
| I. davidii | 460,930.30 ± 81,977.65 a | 12.45 ± 3.34 a | 37,504.26 ± 6791.32 b |
| I. jinggangensis | 528,233.33 ± 126,452.9 a | 10.00 ± 0.8 9b | 52,918.13 ± 11,869.74 a |
| I. commelinoides | 186,485.00 ± 42,639.78 b | 9.75 ± 2.18 b | 17,141.79 ± 4152.89 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Su, Q.; Zhang, Y.; Zhang, S.; Li, H.; Liao, L.; Zeng, J.; Huang, W.; Xiao, Y. Reproductive and Pollination Characteristics of Three Understory Impatiens Species. Horticulturae 2025, 11, 453. https://doi.org/10.3390/horticulturae11050453
Xue Y, Su Q, Zhang Y, Zhang S, Li H, Liao L, Zeng J, Huang W, Xiao Y. Reproductive and Pollination Characteristics of Three Understory Impatiens Species. Horticulturae. 2025; 11(5):453. https://doi.org/10.3390/horticulturae11050453
Chicago/Turabian StyleXue, Yuxi, Qitao Su, Yuxin Zhang, Shujian Zhang, Heng Li, Leiqin Liao, Jia Zeng, Weiyuan Huang, and Yian Xiao. 2025. "Reproductive and Pollination Characteristics of Three Understory Impatiens Species" Horticulturae 11, no. 5: 453. https://doi.org/10.3390/horticulturae11050453
APA StyleXue, Y., Su, Q., Zhang, Y., Zhang, S., Li, H., Liao, L., Zeng, J., Huang, W., & Xiao, Y. (2025). Reproductive and Pollination Characteristics of Three Understory Impatiens Species. Horticulturae, 11(5), 453. https://doi.org/10.3390/horticulturae11050453








