Phytochemical and Antioxidant Variability in Some Black Mulberry, Chokeberry, and Elderberry Cultivars in Relation to Cultivar, Plant Part, and Extraction Solvent
Abstract
1. Introduction
2. Materials and Methods
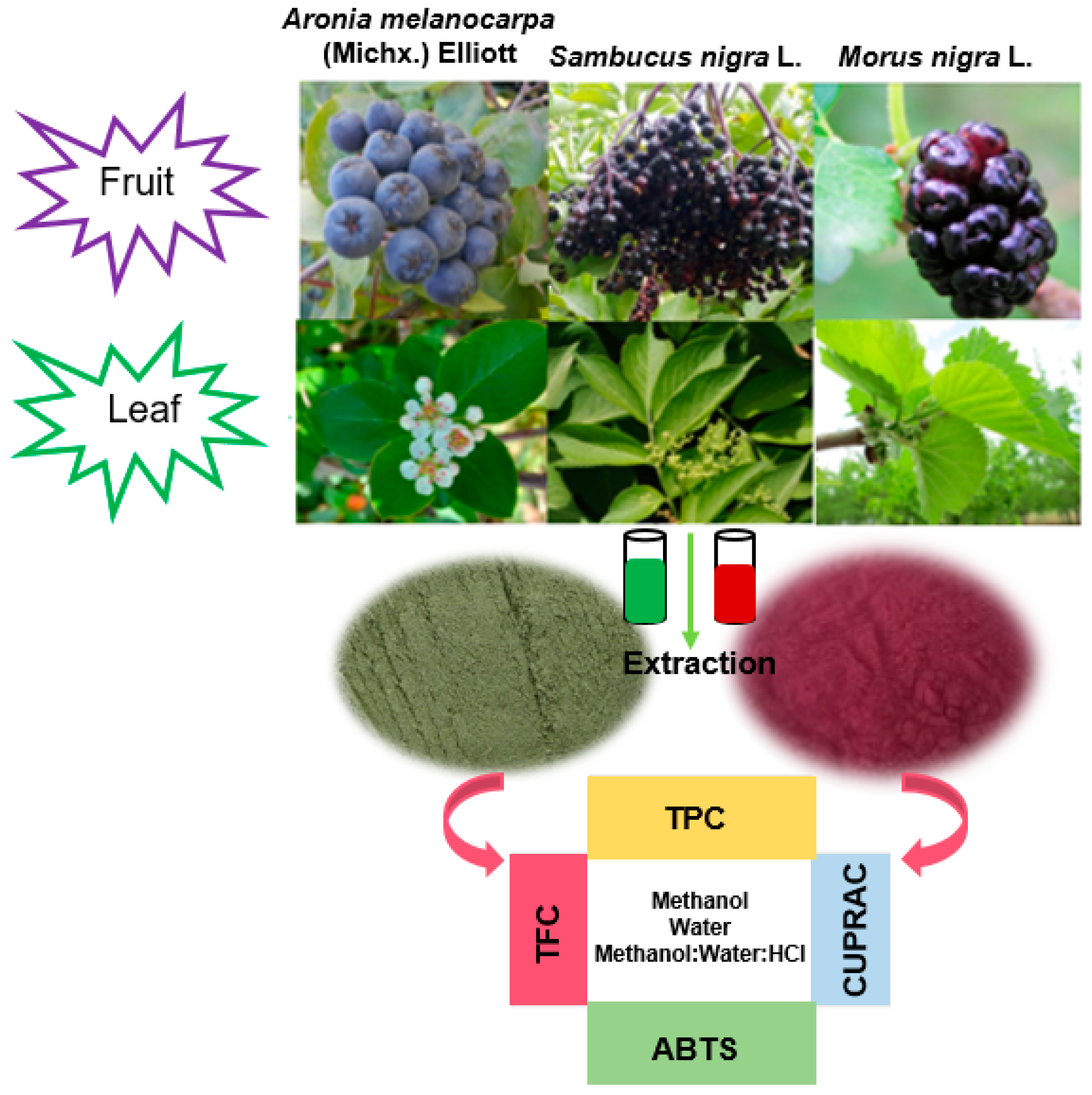
2.1. Plant Material
2.2. Sample Preparation and Extraction
2.3. Total Phenolic Content
2.4. Antioxidant Capacity
2.5. Total Flavonoid Content
2.6. Statistical Analyses
3. Results
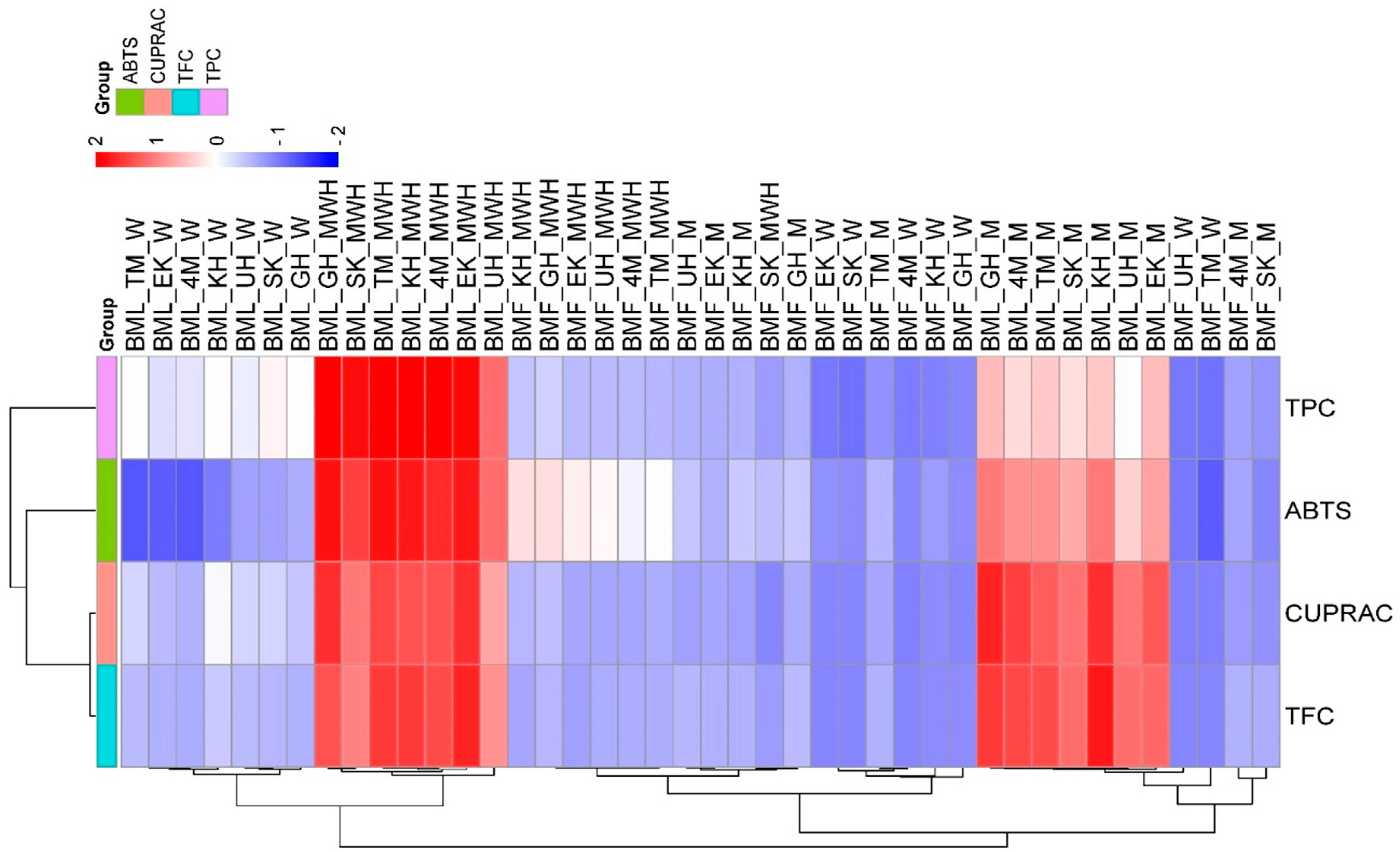
3.1. Effects of Plant Part, Cultivar, and Solvent on TPC, TFC, ABTS, and CUPRAC in Different Extracts of Black Mulberry
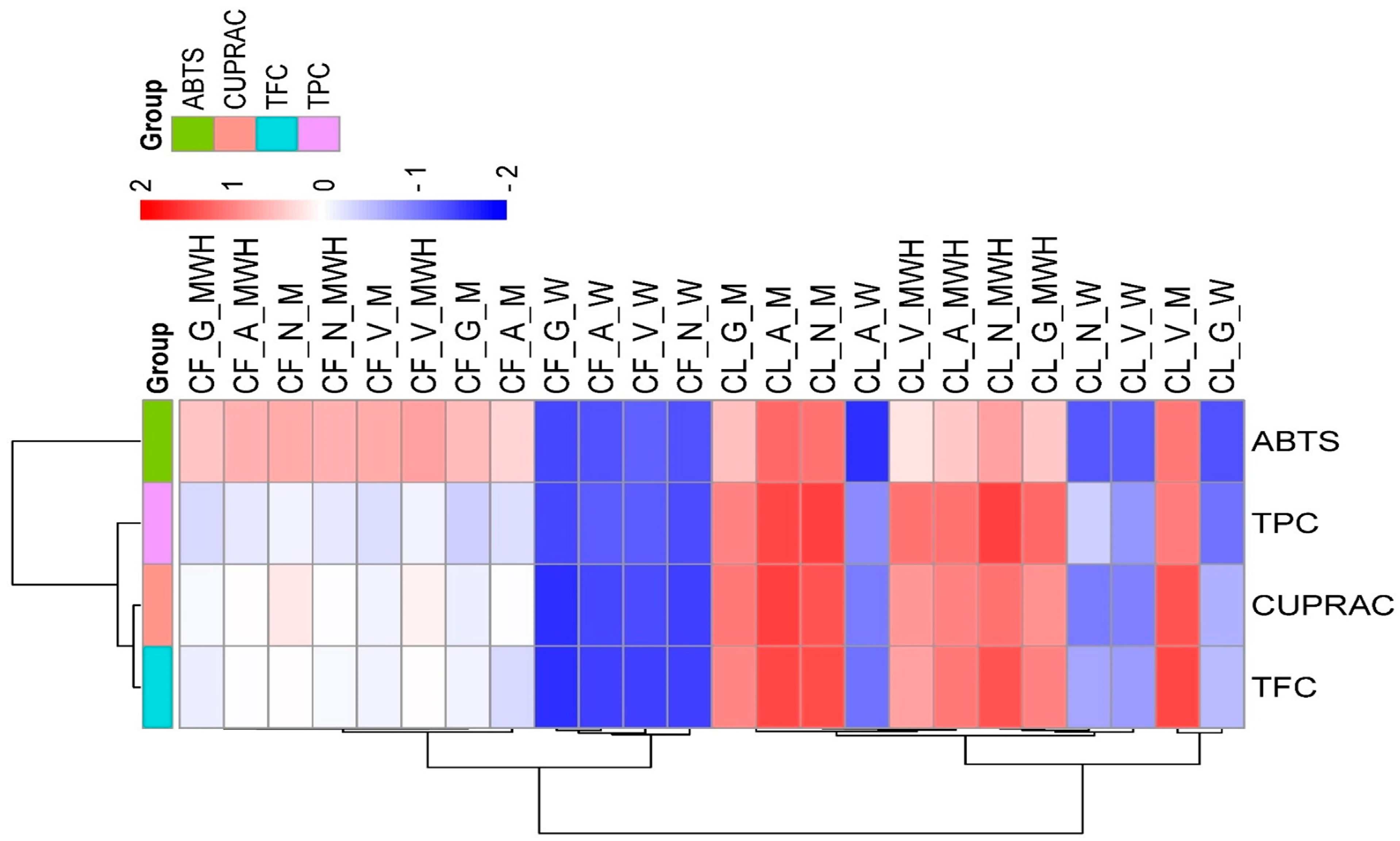
3.2. Effects of Plant Part, Cultivar, and Solvent on Total Phenolic Content (TPC), Total Flavonoid Content (TFC), and Antioxidant Capacity (ABTS and CUPRAC) in Different Extracts of Chokeberry
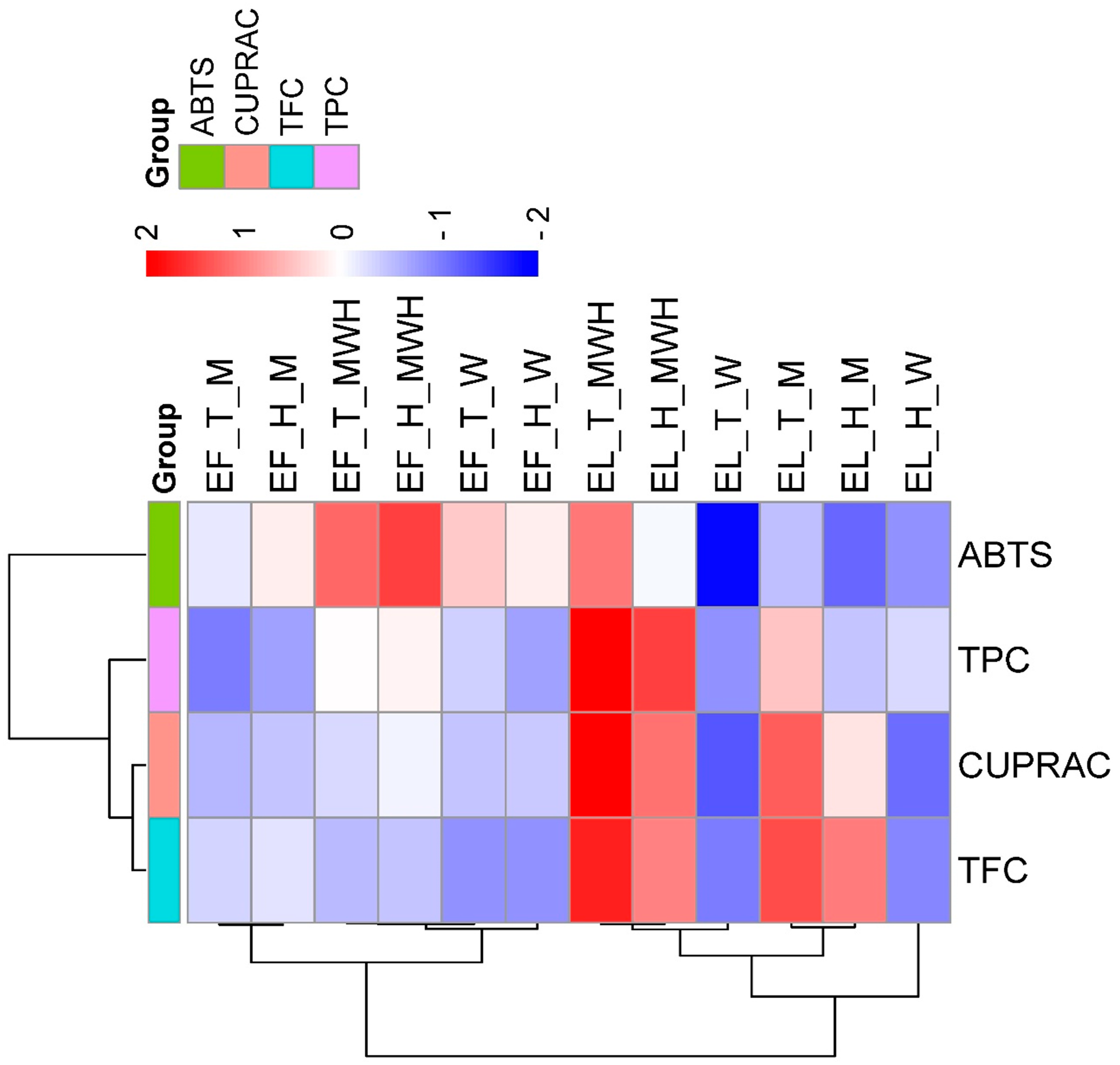
3.3. Effects of Plant Part, Cultivar, and Solvent on Total Phenolic Content (TPC), Total Flavonoid Content (TFC), and Antioxidant Capacity (ABTS and CUPRAC) in Different Extracts of Elderberry
3.4. General Evaluation
4. Discussion
4.1. Effects of Plant Part, Cultivar, and Solvent on TPC, TFC, ABTS, and CUPRAC in Different Extracts of Black Mulberry
4.2. Effects of Plant Part, Cultivar, and Solvent on TPC, TFC, and ABTS in Different Extracts of Chokeberry
4.3. Effects of Plant Part, Cultivar, and Solvent on TPC, TFC, and ABTS in Different Extracts of Elderberry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zengin, R.; Erdoğan, S.; Özhan, O.; Karaca, E.T.; Özçınar, S.; Yılmaztekin, Y.; Uyumlu, A.B. Effects of black mulberry, chokeberry, and elderberry extracts on the healing of burn wounds. Burns 2025, 51, 107391. [Google Scholar] [CrossRef] [PubMed]
- Çalışkan, Z.; Yıldız, E.; Güldaş, M.; Gürbüz, O. Bioactive and anti-carcinogenic properties of kombucha prepared with Aronia melanocarpa juice. Yeni Yüzyıl J. Med. Sci. 2023, 4, 198–206. [Google Scholar]
- Güzel, A.; Uğur, Y.; Öner, E.; Kolaç, T. Comparative analysis of phytochemical content and antioxidants, anti-cholinesterase, anti-atherogenic and molecular docking of Turkish Pelargonium (Pelargonium endlicherianum Fenzl). S. Afr. J. Bot. 2025, 179, 124–133. [Google Scholar] [CrossRef]
- Uğur, Y. Extraction and quantification of melatonin in Cornelian cherry (Cornus mas L.) by ultra-fast liquid chromatography coupled to fluorescence detector (UFLC-FD). Acta Chromatogr. 2023, 35, 219–226. [Google Scholar] [CrossRef]
- Lim, S.H.; Choi, C.I. Pharmacological properties of Morus nigra L. (Black mulberry) as a promising nutraceutical resource. Nutrients 2019, 11, 437. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Liu, X.; Chen, X.; Ding, C.; Dong, L.; Xiao, F. Chokeberry (Aronia melanocarpa) as a new functional food relationship with health: An overview. J. Future Foods 2021, 1, 168–178. [Google Scholar] [CrossRef]
- Mota, A.H.; Andrade, J.M.; Rodrigues, M.J.; Custódio, L.; Bronze, M.R.; Duarte, N.; Reis, C.P. Synchronous insight of in vitro and in vivo biological activities of Sambucus nigra L. extracts for industrial uses. Ind. Crops Prod. 2020, 154, 112709. [Google Scholar] [CrossRef]
- Uğur, Y.; Zengin, R.; Ernim, C.; Günhan, Z.İ.; Şalva, E.; Erdoğan, S. Changes in the phenolic, melatonin, sugar contents and antioxidant capacity, depending on ripening stage in different Cornelian cherry (Cornus mas L.) fruits. ChemistrySelect 2024, 9, e202304682. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Bouyahya, A.; Omari, N.E.; El Hachlafi, N.; Jemly, M.E.; Hakkour, M.; Balahbib, A.; Zengin, G. Chemical compounds of berry-derived polyphenols and their effects on gut microbiota, inflammation, and cancer. Molecules 2022, 27, 3286. [Google Scholar] [CrossRef]
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Xue, S.J.; Forney, C.F.; Lim, L.T. Influence of extraction conditions on ultrasound-assisted recovery of bioactive phenolics from blueberry pomace and their antioxidant activity. Molecules 2018, 23, 1685. [Google Scholar] [CrossRef] [PubMed]
- Pascariu, O.E.; Dias, L.G.; Israel-Roming, F. Optimization of extraction method of bioactive compounds from elderberries (Sambucus nigra L.) and testing extract stability. Horticulturae 2024, 10, 743. [Google Scholar] [CrossRef]
- Negreanu-Pirjol, B.S.; Oprea, O.C.; Negreanu-Pirjol, T.; Roncea, F.N.; Prelipcean, A.M.; Craciunescu, O.; Popoviciu, D.R. Health benefits of antioxidant bioactive compounds in the fruits and leaves of Lonicera caerulea L. and Aronia melanocarpa (Michx.) Elliot. Antioxidants 2023, 12, 951. [Google Scholar] [CrossRef]
- Zengin, R.; Maraş, Z.; Uğur, Y.; Özhan, O.; Karaat, F.E.; Erdoğan, S. Determination of phytochemical composition in fruits and leaves from different origins: Black mulberry, chokeberry and elderberry genotypes. Anal. Lett. 2024, 1–23. [Google Scholar] [CrossRef]
- Liu, D.; He, X.Q.; Wu, D.T.; Li, H.B.; Feng, Y.B.; Zou, L.; Gan, R.Y. Elderberry (Sambucus nigra L.): Bioactive compounds, health functions, and applications. J. Agric. Food Chem. 2022, 70, 4202–4220. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, Z.; Zeng, Y.; Luo, M.; Yang, J. Molecular mechanism and health role of functional ingredients in blueberry for chronic disease in human beings. Int. J. Mol. Sci. 2018, 19, 2785. [Google Scholar] [CrossRef]
- Razali, N.; Mat-Junit, S.; Abdul-Muthalib, A.F.; Subramaniam, S.; Abdul-Aziz, A. Effects of various solvents on the extraction of antioxidant phenolics from the leaves, seeds, veins and skins of Tamarindus indica L. Food Chem. 2012, 131, 441–448. [Google Scholar] [CrossRef]
- Kallithraka, S.; Garcia-Viguera, C.; Bridle, P.; Bakker, J. Survey of solvents for the extraction of grape seed phenolics. Phytochem. Anal. 1995, 6, 265–267. [Google Scholar] [CrossRef]
- Özel, M.Z.; Göğüş, F. Subcritical water as a green solvent for plant extraction. In Alternative Solvents for Natural Products Extraction; Chemat, F., Vian, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 105–123. [Google Scholar]
- Yaman, R. Bazı Üzümsü Meyve Türlerinin Karakteristik Fitokimyasal Bileşenlerinin Tayini, In Vitro Biyoerişebilirliğinin ve In Vivo Yanık Yarası Iyileşmesi Üzerine Subakut Etkilerinin Araştırılması. Master’s Thesis, İnönü University, Malatya, Türkiye, 2022. [Google Scholar]
- Bae, S.H.; Suh, H.J. Antioxidant activities of five different mulberry cultivars in Korea. LWT-Food Sci. Technol. 2007, 40, 955–962. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Özgen, M.; Serçe, S.; Kaya, C. Phytochemical and antioxidant properties of anthocyanin-rich Morus nigra and Morus rubra fruits. Sci. Hortic. 2009, 119, 275–279. [Google Scholar] [CrossRef]
- Ghasemi, K.; Ghasemi, Y.; Ebrahimzadeh, M.A. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak. J. Pharm. Sci. 2009, 22, 277–281. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Wang, H.; Cao, G.; Prior, R.L. Total antioxidant capacity of fruits. J. Agric. Food Chem. 1996, 44, 701–705. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Rani, P.; Khullar, N. Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multi-drug-resistant Salmonella typhi. Phytother. Res. 2004, 18, 670–673. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Musharraf, S.G.; Choudhary, M.I.; Rahman, A.U. Application of analytical methods in authentication and adulteration of honey. Food Chem. 2022, 372, 131339. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- Capanoglu, M.V.A.R.E. The evaluation of bioactive compounds and antioxidant capacity of chokeberry (Aronia melanocarpa) products. J. Food Nutr. Res. 2013, 52, 219–229. [Google Scholar]
- Demir, Y. Zeytinyağı ve çam reçinesi karışımının ratlarda deneysel yara iyileşmesi üzerine etkisi. Ph.D. Thesis, Gazi University, Ankara, Türkiye, 2017. [Google Scholar]
- Jakobek, L.; Drenjančević, M.; Jukić, V.; Šeruga, M. Phenolic acids, flavonols, anthocyanins and antiradical activity of “Nero”, “Viking”, “Galicianka” and wild chokeberries. Sci. Hortic. 2012, 147, 56–63. [Google Scholar] [CrossRef]
- Ahmad, W.; Zeenat, F.; Hasan, A.; Abdullah, A.; Nargis, A.; Tarannum, T. Mazu (Quercus infectoria Oliv)—An overview. Indian J. Unani Med. 2011, 4, 17–22. [Google Scholar]
- Sharma, N.; Verma, S. Medicinal plants and wound healing. Int. J. Appl. Res. 2017, 3, 385–389. [Google Scholar]
- Yan, J.; Ruan, J.; Huang, P.; Sun, L.; Li, X.; Zhong, S.; Xie, B. The structure–activity relationship review of the main bioactive constituents of Morus genus plants. J. Nat. Med. 2020, 74, 331–340. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food 2010, 13, 255–269. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Barchielli, L.; Balsamo, V.; Bini, L.; Menicagli, R.; Zinnai, A.; Venturi, F. Chokeberry (Aronia melanocarpa L.) and bilberry (Vaccinium myrtillus L.) functional ingredients: Health effects, analytical determination and applications in the food industry. Foods 2022, 11, 1124. [Google Scholar]
- Rugină, D.; Sconta, Z.; Leopold, N.; Pintea, A.; Bunea, A.; Socaciu, C.; Diehl, H.A. Antioxidant activities of Aronia melanocarpa L. fruit juice in human subjects of different age. J. Med. Food 2012, 15, 952–958. [Google Scholar]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef] [PubMed]
- Petrova, I.; Petkova, N.; Ivanov, I.; Denev, P. Antioxidant activity and polyphenolic content of elderberry juice. Bulgarian Chem. Commun. 2016, 48, 53–57. [Google Scholar]
- Hossen, M.M.; Ali, M.Y.; Jahurul, M.H.A.; Abdel-Daim, M.M.; Gan, S.H.; Khalil, M.I.; Alam, M.T. Beneficial roles of Morus spp. in cancer prevention and treatment. Oxid. Med. Cell. Longev. 2021, 2021, 9932876. [Google Scholar]
| Cultivar | Solvent | TPC | TFC | ABTS | CUPRAC | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fruit | Leaf | Fruit | Leaf | Fruit | Leaf | Fruit | Leaf | ||
| Tohma Medik | Methanol:Water:HCl | 14.40 ± 0.55 o–q | 44.29 ± 0.50 b | 6.84 ± 0.15 op | 39.94 ± 0.38 c | 26.39 ± 0.54 kl | 46.92 ± 0.58 a | 27.38 ± 0.38 n | 104.22 ± 2.36 c |
| Methanol | 11.36 ± 0.40 t | 25.99 ± 0.17 f | 7.63 ± 0.33 mn | 38.36 ± 0.44 d | 20.04 ± 0.49 n–p | 36.34 ± 0.86 e | 26.28 ± 0.14 o–q | 98.58 ± 0.41 e | |
| Water | 8.85 ± 0.41 y | 21.33 ± 0.95 i | 2.79 ± 0.09 st | 8.94 ± 0.14 j | 12.35 ± 0.23 x | 11.62 ± 0.11 x | 16.80 ± 0.16 v | 39.78 ± 0.28 j | |
| Karacaköy Horum | Methanol:Water:HCl | 15.83 ± 0.55 m | 44.73 ± 0.76 b | 6.33 ± 0.20 pq | 39.95 ± 1.18 c | 29.49 ± 1.01 hi | 46.39 ± 0.65 a | 30.40 ± 0.48 m | 101.49 ± 1.66 d |
| Methanol | 14.06 ± 0.32 q | 25.74 ± 0.20 f | 7.62 ± 0.48 mn | 43.93 ± 0.49 a | 21.70 ± 0.33 m | 38.85 ± 1.74 d | 25.06 ± 0.63 pq | 109.23 ± 1.72 b | |
| Water | 10.12 ± 0.11 uv | 21.35 ± 0.17 i | 2.98 ± 0.07 st | 10.53 ± 0.09 i | 17.59 ± 0.40 st | 15.07 ± 0.24 vw | 19.84 ± 0.11 st | 50.13 ± 0.10 i | |
| Gümüşhacıköy Horum | Methanol:Water:HCl | 16.82 ± 0.24 l | 45.78 ± 0.24 a | 8.00 ± 0.14 k–m | 37.66 ± 0.87 e | 29.62 ± 0.73 h | 47.14 ± 0.73 a | 33.26 ± 0.79 l | 109.46 ± 0.83 b |
| Methanol | 14.35 ± 0.06 pq | 27.12 ± 0.37 e | 8.70 ± 0.54 j | 40.31 ± 0.17 c | 21.59 ± 0.34 m | 38.71 ± 0.15 d | 27.43 ± 0.47 n | 112.63 ± 1.09 a | |
| Water | 10.37 ± 0.66 u | 21.02 ± 0.19 i | 3.14 ± 0.04 s | 7.77 ± 0.06 l–n | 16.48 ± 0.22 tu | 18.95 ± 0.13 p–r | 17.62 ± 0.17 uv | 35.76 ± 0.31 k | |
| Ürgüp Horum | Methanol:Water:HCl | 15.05 ± 0.22 no | 34.19 ± 0.14 d | 6.93 ± 0.13 op | 30.64 ± 0.50 h | 27.40 ± 0.57 jk | 39.50 ± 0.95 d | 26.52 ± 0.65 op | 77.77 ± 0.73 h |
| Methanol | 14.27 ± 0.47 pq | 20.90 ± 0.10 i | 8.36 ± 0.17 j–l | 34.95 ± 0.80 f | 21.13 ± 0.42 mn | 30.81 ± 0.71 g | 24.85 ± 1.13 q | 90.37 ± 1.09 g | |
| Water | 8.97 ± 0.29 y | 19.68 ± 0.17 j | 2.80 ± 0.03 st | 8.54 ± 0.15 jk | 14.31 ± 0.08 w | 18.21 ± 0.32 rs | 16.82 ± 0.10 v | 38.99 ± 0.55 j | |
| 44 MRK 01 | Methanol:Water:HCl | 14.87 ± 0.36 n–p | 44.37 ± 0.62 b | 6.80 ± 0.16 op | 38.49 ± 0.42 d | 25.43 ± 1.03 l | 45.03 ± 1.30 b | 26.34 ± 0.66 op | 101.16 ± 1.09 d |
| Methanol | 12.83 ± 0.28 r | 24.62 ± 0.49 g | 7.73 ± 0.27 l–n | 38.75 ± 0.32 d | 18.48 ± 1.46 q–s | 36.49 ± 0.38 e | 23.46 ± 0.35 r | 104.80 ± 1.07 c | |
| Water | 9.68 ± 0.33 vw | 18.69 ± 0.20 k | 2.52 ± 0.09 st | 6.89 ± 0.07 op | 15.68 ± 0.19 uv | 11.62 ± 0.12 x | 16.85 ± 0.16 v | 29.50 ± 1.10 m | |
| Erzincan Karadut | Methanol:Water:HCl | 15.15 ± 0.35 n | 42.11 ± 0.34 c | 5.85 ± 0.52 q | 42.91 ± 0.13 b | 28.35 ± 0.82 ij | 46.37 ± 0.43 a | 26.92 ± 0.35 n | 109.28 ± 0.61 b |
| Methanol | 13.88 ± 0.27 q | 27.26 ± 0.32 e | 7.27 ± 0.26 no | 35.41 ± 0.33 f | 19.63 ± 0.44 o–q | 34.90 ± 0.55 f | 27.10 ± 0.14 n | 99.71 ± 1.40 e | |
| Water | 9.39 ± 0.19 wx | 18.22 ± 0.48 k | 2.75 ± 0.06 st | 7.26 ± 0.13 no | 16.62 ± 0.02 tu | 12.23 ± 0.11 x | 18.00 ± 0.50 uv | 32.13 ± 0.40 l | |
| Şelale Karadut | Methanol:Water:HCl | 12.16 ± 0.28 s | 41.80 ± 0.49 c | 5.20 ± 0.06 r | 31.94 ± 0.41 g | 20.77 ± 0.75 m–o | 43.26 ± 1.82 c | 18.57 ± 0.20 tu | 90.95 ± 1.53 g |
| Methanol | 11.69 ± 0.28 st | 24.03 ± 0.32 g | 6.89 ± 0.19 op | 34.90 ± 0.40 f | 16.01 ± 0.44 uv | 34.31 ± 0.59 f | 20.08 ± 0.40 s | 92.69 ± 0.93 f | |
| Water | 8.81 ± 0.28 y | 22.11 ± 0.12 h | 2.41 ± 0.02 t | 7.94 ± 0.18 k–n | 16.44 ± 0.30 tu | 18.28 ± 0.15 rs | 17.33 ± 0.03 uv | 40.34 ± 0.41 j | |
| Average of Cultivar | Tohma Medik | 11.54 ± 2.44 | 30.53 ± 1.53 | 5.75 ± 2.26 | 29.08 ± 2.12 | 19.59 ± 1.10 | 31.63 ± 1.69 | 23.49 ± 1.04 | 80.86 ± 1.93 |
| Karacaköy Horum | 13.34 ± 2.55 | 30.61 ± 3.77 | 5.64 ± 2.09 | 31.47 ± 3.81 | 22.92 ± 1.26 | 33.44 ± 4.19 | 25.10 ± 4.59 | 86.95 ± 2.84 | |
| Gümüşhacıköy Horum | 13.85 ± 2.84 | 31.31 ± 2.17 | 6.61 ± 2.64 | 28.58 ± 1.66 | 22.56 ± 1.75 | 34.94 ± 2.54 | 26.10 ± 3.86 | 85.95 ± 3.67 | |
| Ürgüp Horum | 12.77 ± 2.88 | 24.92 ± 1.97 | 6.03 ± 2.50 | 24.71 ± 3.28 | 20.95 ± 5.68 | 29.51 ± 3.29 | 22.73 ± 4.54 | 69.04 ± 2.20 | |
| 44 MRK 01 | 12.46 ± 2.28 | 29.23 ± 1.65 | 5.68 ± 2.41 | 28.04 ± 3.87 | 19.86 ± 1.44 | 31.04 ± 1.05 | 22.22 ± 4.23 | 78.48 ± 3.79 | |
| Erzincan Karadut | 12.81 ± 2.63 | 29.20 ± 1.45 | 5.29 ± 2.02 | 28.53 ± 1.28 | 21.53 ± 1.30 | 31.17 ± 3.05 | 24.01 ± 4.52 | 80.38 ± 2.43 | |
| Şelale Karadut | 10.89 ± 1.59 | 29.31 ± 3.40 | 4.83 ± 1.96 | 24.93 ± 2.81 | 17.74 ± 2.33 | 31.95 ± 2.00 | 18.66 ± 1.21 | 74.66 ± 2.76 | |
| Average of Solvent | Methanol:Water:HCl | 14.90 ± 1.40 | 42.47 ± 3.74 | 6.56 ± 0.87 | 37.36 ± 4.28 | 26.78 ± 2.98 | 44.94 ± 2.75 | 27.05 ± 4.31 | 99.19 ± 1.80 |
| Methanol | 13.21 ± 1.22 | 25.10 ± 2.10 | 7.74 ± 0.65 | 38.09 ± 3.19 | 19.80 ± 2.01 | 35.77 ± 2.73 | 24.89 ± 2.45 | 101.14 ± 2.87 | |
| Water | 9.46 ± 0.67 | 20.34 ± 1.46 | 2.77 ± 0.24 | 8.27 ± 1.16 | 15.64 ± 1.69 | 15.14 ± 3.18 | 17.61 ± 1.05 | 38.09 ± 4.36 | |
| Overall Mean | 12.52 ± 2.55 | 29.30 ± 2.93 | 5.69 ± 2.23 | 27.91 ± 1.34 | 20.74 ± 1.15 | 31.95 ± 1.88 | 23.19 ± 4.99 | 79.48 ± 3.68 | |
| Cultivar | Solvent | TPC | TFC | ABTS | CUPRAC | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fruit | Leaf | Fruit | Leaf | Fruit | Leaf | Fruit | Leaf | ||
| Nero | Methanol:Water:HCl | 39.63 ± 0.59 f | 69.22 ± 0.95 a | 39.79 ± 2.21 ef | 65.96 ± 1.54 a | 100.80 ± 0.48 de | 105.15 ± 0.41 c | 128.03 ± 3.12 hi | 193.13 ± 3.34 c |
| Methanol | 41.58 ± 0.11 e | 68.93 ± 0.74 a | 40.95 ± 1.71 e | 66.43 ± 2.32 a | 102.52 ± 1.92 cd | 116.12 ± 3.17 ab | 140.00 ± 2.73 f | 206.62 ± 2.24 b | |
| Water | 18.58 ± 0.82 n | 36.31 ± 1.47 i | 13.39 ± 0.24 k | 27.41 ± 0.51 i | 38.21 ± 0.77 kl | 40.30 ± 0.59 jk | 45.74 ± 0.65 n | 72.06 ± 1.18 l | |
| Viking | Methanol:Water:HCl | 41.67 ± 0.61 e | 62.89 ± 0.13 c | 40.77 ± 2.86 e | 54.97 ± 1.59 d | 104.78 ± 0.87 c | 87.60 ± 1.20 i | 134.32 ± 4.32 g | 176.36 ± 1.73 e |
| Methanol | 38.41 ± 0.53 gh | 61.24 ± 0.60 d | 39.08 ± 0.20 ef | 67.19 ± 2.63 a | 102.72 ± 1.78 cd | 115.27 ± 0.75 b | 123.52 ± 3.99 ij | 206.09 ± 3.96 b | |
| Water | 20.88 ± 0.21 m | 28.85 ± 0.75 j | 13.32 ± 0.44 k | 26.28 ± 0.23 i | 41.99 ± 0.21 j | 41.21 ± 0.56 jk | 51.28 ± 0.21 m | 72.36 ± 0.58 l | |
| Galicjanka | Methanol:Water:HCl | 37.52 ± 0.06 h | 64.27 ± 0.28 b | 37.78 ± 0.43 f | 59.01 ± 1.70 bc | 95.40 ± 0.56 gh | 94.49 ± 2.99 h | 126.23 ± 3.56 h–j | 179.04 ± 4.02 e |
| Methanol | 36.33 ± 0.22 i | 60.54 ± 1.43 d | 38.80 ± 0.66 ef | 58.10 ± 0.48 c | 98.91 ± 3.10 ef | 97.61 ± 2.42 fg | 122.07 ± 3.37 j | 188.83 ± 4.34 cd | |
| Water | 17.72 ± 0.94 n | 23.82 ± 0.22 l | 11.20 ± 0.53 k | 30.39 ± 0.30 h | 35.33 ± 1.07 l | 38.22 ± 1.40 kl | 39.53 ± 0.40 o | 92.96 ± 0.60 k | |
| Aron | Methanol:Water:HCl | 39.56 ± 0.19 f | 62.84 ± 0.49 c | 40.08 ± 0.88 ef | 60.73 ± 0.64 b | 101.32 ± 1.20 de | 94.53 ± 0.92 h | 130.79 ± 1.01 gh | 185.43 ± 2.96 d |
| Methanol | 38.75 ± 0.46 fg | 68.63 ± 0.37 a | 35.04 ± 2.71 g | 67.25 ± 2.32 a | 92.41 ± 2.44 h | 118.61 ± 3.03 a | 128.99 ± 1.66 h | 212.47 ± 4.64 a | |
| Water | 20.61 ± 0.51 m | 27.09 ± 0.14 k | 13.09 ± 0.32 k | 20.11 ± 0.37 j | 38.97 ± 0.41 k | 30.23 ± 0.93 m | 47.50 ± 1.16 mn | 71.57 ± 0.72 l | |
| Average of Cultivar | Nero | 33.26 ± 1.06 | 58.15 ± 1.41 | 31.38 ± 1.57 | 53.27 ± 1.45 | 80.51 ± 1.75 | 87.19 ± 3.52 | 104.59 ± 4.49 | 157.27 ± 6.21 |
| Viking | 33.66 ± 3.69 | 50.99 ± 1.63 | 31.06 ± 1.40 | 49.48 ± 1.25 | 83.16 ± 3.91 | 81.36 ± 3.42 | 103.04 ± 3.21 | 151.60 ± 6.85 | |
| Galicjanka | 30.52 ± 2.63 | 49.55 ± 1.37 | 29.26 ± 1.56 | 49.16 ± 1.11 | 76.55 ± 3.99 | 76.78 ± 2.02 | 95.95 ± 2.42 | 153.61 ± 4.78 | |
| Aron | 32.97 ± 1.29 | 52.85 ± 1.49 | 29.40 ± 1.51 | 49.36 ± 2.16 | 77.57 ± 2.24 | 81.13 ± 3.60 | 102.43 ± 1.22 | 156.49 ± 4.81 | |
| Average of Solvent | Methanol:Water:HCl | 39.60 ± 1.58 | 64.81 ± 2.77 | 39.60 ± 1.97 | 60.17 ± 2.30 | 100.58 ± 3.58 | 95.44 ± 2.71 | 129.85 ± 4.22 | 183.49 ± 2.26 |
| Methanol | 38.77 ± 1.98 | 64.83 ± 2.20 | 38.47 ± 2.64 | 64.74 ± 1.41 | 99.14 ± 4.80 | 111.90 ± 1.98 | 128.65 ± 2.81 | 203.50 ± 4.81 | |
| Water | 19.45 ± 1.51 | 29.02 ± 1.84 | 12.75 ± 1.00 | 26.05 ± 1.92 | 38.63 ± 2.54 | 37.49 ± 1.59 | 46.01 ± 3.47 | 77.24 ± 2.51 | |
| Overall Mean | 32.60 ± 2.59 | 52.89 ± 1.56 | 30.27 ± 1.72 | 50.32 ± 1.98 | 79.45 ± 2.51 | 81.61 ± 3.07 | 101.50 ± 4.18 | 154.74 ± 5.86 | |
| Cultivar | Solvent | TPC | TFC | ABTS | CUPRAC | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fruit | Leaf | Fruit | Leaf | Fruit | Leaf | Fruit | Leaf | ||
| Tokat (T1) | Methanol:Water:HCl | 37.06 ± 0.39 d | 55.34 ± 0.71 a | 16.68 ± 0.83 g | 47.11 ± 0.58 a | 60.20 ± 1.08 b | 58.98 ± 0.40 b | 79.88 ± 0.32 f | 166.63 ± 1.54 a |
| Methanol | 28.64 ± 0.44 g | 40.68 ± 0.14 c | 19.54 ± 0.17 e | 42.69 ± 0.84 b | 46.84 ± 1.31 e | 43.41 ± 0.22 f | 68.66 ± 0.76 i | 139.45 ± 0.42 b | |
| Water | 33.92 ± 0.11 e | 29.75 ± 1.14 fg | 12.23 ± 0.19 h | 10.24 ± 0.44 i | 52.68 ± 0.63 c | 30.16 ± 1.16 i | 74.49 ± 0.65 gh | 42.56 ± 0.34 k | |
| Haschberg | Methanol:Water:HCl | 37.79 ± 0.64 d | 48.97 ± 1.59 b | 18.09 ± 0.55 f | 37.49 ± 0.14 c | 62.98 ± 1.20 a | 47.85 ± 1.07 e | 87.22 ± 0.15 e | 133.64 ± 1.67 c |
| Methanol | 30.74 ± 0.45 f | 33.07 ± 2.40 e | 21.40 ± 0.67 d | 37.79 ± 0.60 c | 49.74 ± 0.56 d | 37.04 ± 0.54 h | 73.21 ± 0.27 h | 99.78 ± 0.65 d | |
| Water | 31.04 ± 0.99 f | 34.36 ± 0.28 e | 12.30 ± 0.12 h | 11.41 ± 0.13 h | 49.83 ± 0.51 d | 40.14 ± 0.73 g | 75.14 ± 0.40 g | 47.74 ± 1.09 j | |
| Average of Cultivar | Tokat (T1) | 33.21 ± 3.69 | 41.92 ± 11.14 | 16.15 ± 3.22 | 33.35 ± 17.44 | 53.24 ± 5.87 | 44.19 ± 2.51 | 74.34 ± 4.89 | 116.22 ± 56.48 |
| Haschberg | 33.19 ± 3.51 | 38.80 ± 7.78 | 17.26 ± 4.01 | 28.90 ± 13.12 | 54.18 ± 6.64 | 41.67 ± 4.87 | 78.52 ± 6.58 | 93.72 ± 37.49 | |
| Average of Solvent | Methanol:Water:HCl | 37.43 ± 0.62 | 52.15 ± 3.66 | 17.38 ± 1.00 | 42.30 ± 5.28 | 61.59 ± 1.83 | 53.42 ± 6.14 | 83.55 ± 4.03 | 150.14 ± 18.12 |
| Methanol | 29.69 ± 1.22 | 36.88 ± 4.44 | 20.47 ± 1.11 | 40.24 ± 2.76 | 48.29 ± 1.83 | 40.23 ± 3.51 | 70.93 ± 2.54 | 119.62 ± 21.74 | |
| Water | 32.48 ± 1.70 | 32.05 ± 2.63 | 12.27 ± 0.14 | 10.83 ± 0.70 | 51.26 ± 1.64 | 35.15 ± 5.53 | 74.82 ± 0.60 | 45.15 ± 2.93 | |
| Overall Mean | 33.20 ± 3.50 | 40.36 ± 9.46 | 16.71 ± 3.57 | 31.12 ± 15.15 | 53.71 ± 6.10 | 42.93 ± 9.30 | 76.43 ± 6.02 | 104.97 ± 47.92 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zengin, R.; Uğur, Y.; Erdoğan, S.; Yavuz, Ç.; Hatterman-Valenti, H.; Kaya, O. Phytochemical and Antioxidant Variability in Some Black Mulberry, Chokeberry, and Elderberry Cultivars in Relation to Cultivar, Plant Part, and Extraction Solvent. Horticulturae 2025, 11, 455. https://doi.org/10.3390/horticulturae11050455
Zengin R, Uğur Y, Erdoğan S, Yavuz Ç, Hatterman-Valenti H, Kaya O. Phytochemical and Antioxidant Variability in Some Black Mulberry, Chokeberry, and Elderberry Cultivars in Relation to Cultivar, Plant Part, and Extraction Solvent. Horticulturae. 2025; 11(5):455. https://doi.org/10.3390/horticulturae11050455
Chicago/Turabian StyleZengin, Rukiye, Yılmaz Uğur, Selim Erdoğan, Çiğdem Yavuz, Harlene Hatterman-Valenti, and Ozkan Kaya. 2025. "Phytochemical and Antioxidant Variability in Some Black Mulberry, Chokeberry, and Elderberry Cultivars in Relation to Cultivar, Plant Part, and Extraction Solvent" Horticulturae 11, no. 5: 455. https://doi.org/10.3390/horticulturae11050455
APA StyleZengin, R., Uğur, Y., Erdoğan, S., Yavuz, Ç., Hatterman-Valenti, H., & Kaya, O. (2025). Phytochemical and Antioxidant Variability in Some Black Mulberry, Chokeberry, and Elderberry Cultivars in Relation to Cultivar, Plant Part, and Extraction Solvent. Horticulturae, 11(5), 455. https://doi.org/10.3390/horticulturae11050455







