Abstract
The cultivation of table grapes in Brazil is economically significant, with production influenced by edaphoclimatic factors and rootstock selection. The cultivar ‘BRS Núbia’ (Vitis vinifera L. x Vitis labrusca L.) is a promising alternative; however, its phenological behavior, thermal requirements, and compatibility with different rootstocks under subtropical conditions require further evaluation. This study aimed to assess the duration of phenological stages, thermal requirement, and ripening dynamics of ‘BRS Núbia’ grapevines grafted onto the rootstocks ‘IAC 572 Jales’, ‘IAC 766 Campinas’, and ‘Paulsen 1103’. The experiment was conducted in São Manuel, São Paulo, Brazil during the 2021 and 2022 production cycles using a split-plot experimental design (3 × 2). Evaluations included the duration of phenological stages from pruning to budburst, flowering, fruit set, onset of ripening, and harvest, as well as the ripening curve and thermal accumulation from pruning to harvest. Rootstocks did not significantly affect (p > 0.05) the duration of phenological stages; however, differences were observed between production cycles. The 2022 cycle was longer (167.7 days) compared to 2021 (142.6 days), with greater thermal accumulation (1871.7 GDDs vs. 1743.4 GDDs). The analysis of phenological stages revealed that, across both production cycles evaluated, the ‘BRS Núbia’ cultivar required an average accumulation of 1807.5 growing degree days from pruning to harvest. Soluble solids content ranged from 17.43 to 18.50°Brix, and titratable acidity decreased throughout maturation. The maturation index was highest in vines grafted onto ‘Paulsen 1103’, indicating its positive influence on fruit quality. The ‘BRS Núbia’ grapevine exhibited a mean thermal requirement of 1807.5 growing degree days (GDDs) to complete its phenological cycle, which lasted approximately 150 days under subtropical conditions.
1. Introduction
The grapevine (Vitis vinifera L. x Vitis labrusca L.) is a perennial, climbing plant belonging to the Vitaceae family, widely cultivated in various regions of the world, especially in temperate and subtropical climates [1,2]. The grapevine exhibits a well-defined phenological cycle and is sensitive to factors such as temperature, photoperiod, and water availability [3,4,5]. This plant has great adaptability and is classified into different cultivars, intended both for table grape production and for processing into juice, wine, and raisins [6,7].
In addition to its agronomic relevance, the grapevine plays a significant role from both nutritional and socioeconomic perspectives. Grapes are rich in bioactive compounds such as polyphenols, flavonoids, and resveratrol—substances associated with reduced cardiovascular disease risk, antioxidant activity, and anti-inflammatory properties [8,9,10,11]. From a socioeconomic standpoint, viticulture drives important production chains, generating direct and indirect employment, fostering rural tourism, and promoting the development of communities in wine-producing regions [12,13,14]. Thus, grape cultivation represents not only an important source of agricultural income but also an asset for both public health and the local and global economy.
Viticulture is a strategically significant agricultural sector in South America, contributing substantially to the economies of countries such as Argentina, Brazil, Chile, Peru, and Uruguay; likewise, vineyard areas are integrated into and contribute to the landscape, providing social ecosystem services as a socio-cultural outcome [15,16,17]. In 2023, the global vineyard area reached 7.3 million hectares, resulting in a total grape production of 79.4 million tons, of which 43.3% was destined for fresh consumption [18]. In Brazil, viticulture accounts for approximately 1.45 million tons annually, with 52% of production dedicated to table grapes [19]. Beyond its economic relevance, table grape viticulture plays a crucial social role, generating employment in both large agro-industrial enterprises and small family-run farms [20].
Changes in environmental factors may elicit markedly different physiological and developmental responses among plant cultivars and genotypes, depending on their adaptive capacity and genetic background [21,22]. In the context of global climate change, which has been affecting orchard yields, the success of table grape production is directly related to the selection of appropriate rootstocks and cultivars that are well-adapted to local edaphoclimatic conditions and agricultural management practices [23,24,25]. Lack of or excess rainfall, extreme temperatures, hail, and frost affect grapevine crops worldwide [26]. For this reason, continuous assessment of the long-term evolution of climatic factors and their influence on vine production and quality is essential. The use of rootstocks is a well-established practice in viticulture, playing a crucial role in adapting vines to environmental conditions, controlling vegetative vigor, and mitigating biotic and abiotic stresses [27,28]. Furthermore, the interaction between the scion and the rootstock directly affects phenology, thermal requirements, yield, and fruit quality, serving as a key determinant for vineyard longevity and production efficiency.
In addition to their effects on phenology, vegetative vigor, and stress mitigation, rootstocks also significantly influence the physicochemical characteristics and sensory quality of grapes, particularly in warm-climate viticulture. Studies have shown that rootstock–scion interactions can affect berry size, skin thickness, sugar and acid content, phenolic composition, and aromatic profiles, thereby altering the sensory attributes and market value of grapes [28,29,30]. In tropical and subtropical regions, where climatic conditions accelerate ripening and increase disease pressure, selecting rootstocks that promote favorable fruit quality traits is critical for achieving commercial standards and consumer acceptance [30,31]. Therefore, assessing the compatibility of rootstocks with scions such as ‘BRS Núbia’ under these conditions is essential to ensure not only agronomic performance but also high-quality fruit production.
In recent years, Brazilian viticulture has expanded into non-traditional regions, such as the Northeast, driven by technological innovations and market demands [27,32]. In this context, selecting rootstocks compatible with different cultivars and growing environments is fundamental to ensuring productive success and the sustainability of viticultural activities. Among the widely used rootstocks in subtropical regions, ‘IAC 572 Jales’ and ‘IAC 766 Campinas’ stand out for imparting vigor to plants and providing resistance to foliar diseases [28,33,34,35]. Meanwhile, ‘Paulsen 1103’ exhibits high tolerance to water deficit and pest resistance, making it widely employed in regions with greater water restrictions, such as the state of São Paulo [36,37].
‘BRS Núbia’ grape, released by Embrapa in 2013, is a promising alternative for table grape production, characterized by a medium cycle of 120 days, uniform clusters, medium-sized berries, firm texture, and neutral flavor [38]. Although recommended for tropical growing conditions, its adaptation to subtropical regions still requires validation, particularly regarding compatibility with the most commonly used rootstocks in these areas. Analyzing the phenology, thermal requirements, and ripening of ‘BRS Núbia’ grafted onto different rootstocks under subtropical conditions is essential to determining its agronomic viability and establishing technical recommendations for its production.
Table grapes have specific thermal requirements to ensure proper development and high fruit quality, requiring higher temperatures during the production cycle, especially at the phenological stages of budburst, flowering, and ripening [39,40]. Heat accumulation over time is measured in growing degree days (GDDs), which is essential for the efficient completion of the crop cycle. In hot and dry climates, cultivation is favored, as these conditions reduce disease incidence and contribute to uniform ripening and market-quality fruit [39].
Characterizing grapevine phenological development allows for the refinement of management practices, optimizing input use and scheduling agricultural activities. Furthermore, understanding the cultivar’s thermal requirements enables the prediction of its production cycle duration under different climatic conditions, facilitating decision making to maximize productivity and fruit quality [41,42].
Given the above, this study aimed to evaluate the duration of phenological stages, thermal requirements, and ripening of ‘BRS Núbia’ grape grafted onto different rootstocks under subtropical conditions in the state of São Paulo, Brazil.
2. Materials and Methods
2.1. Experimental Site Location
The experiment was conducted over two production cycles (2021 and 2022) at the São Manuel Experimental Farm, located in the municipality of São Manuel, São Paulo, Brazil (22°44′28″ S, 48°34′37″ W) at an altitude of 740 m. The region has a humid subtropical climate (Cfa, according to the Köppen classification), characterized by an average annual precipitation of 1433 mm, an average annual temperature of 22.4 °C, and a relative humidity of 71%. The soil at the experimental site is classified as a Red Nitosol [43].
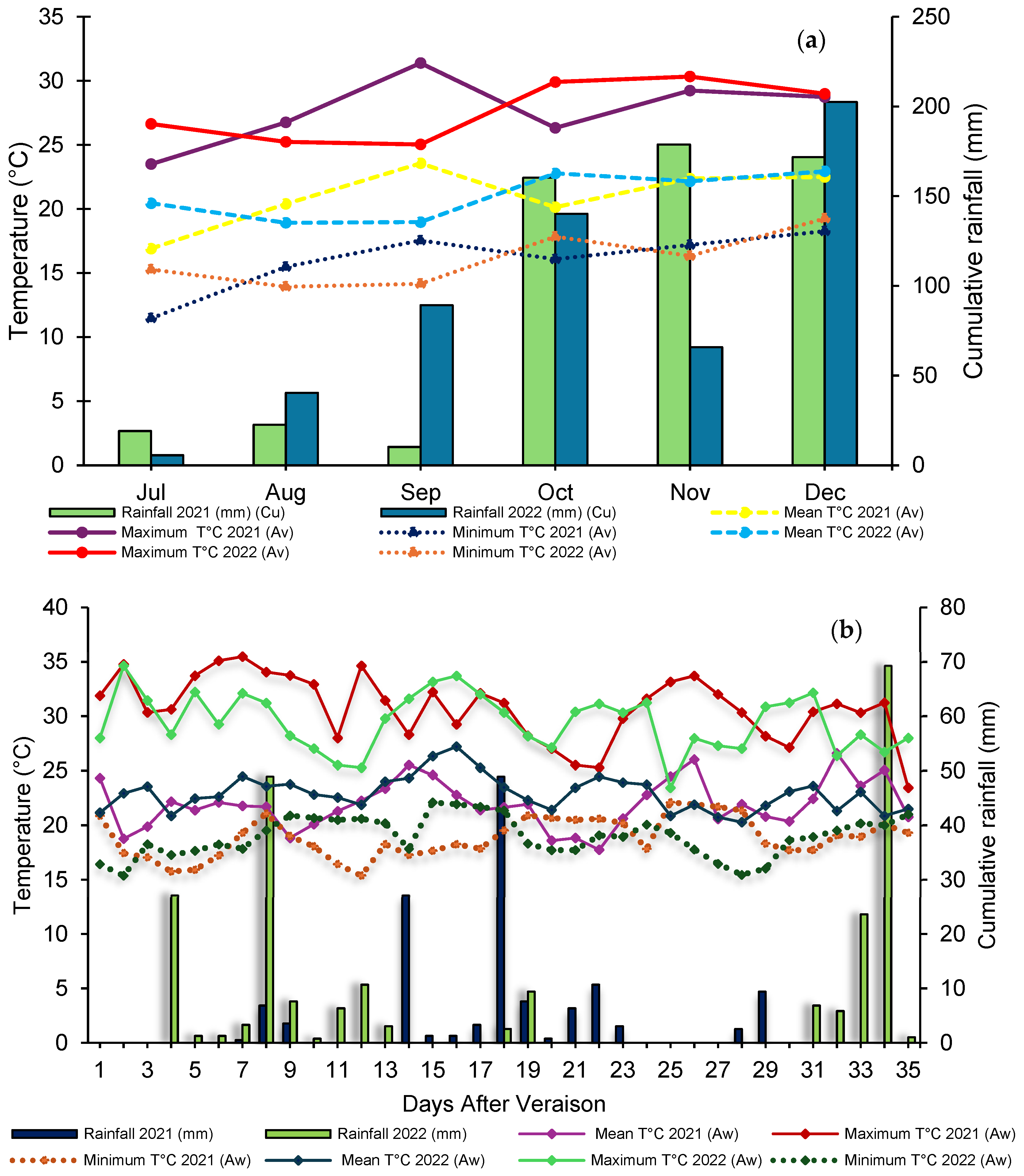
For both production cycles, pruning was carried out in July, while harvest occurred in December. Throughout the study, meteorological variables were monitored, including air temperature (mean, minimum, and maximum) and rainfall, with monthly records during the production cycle. During the berry ripening period, climate measurements were intensified, with daily records for both production cycles (Figure 1a,b).
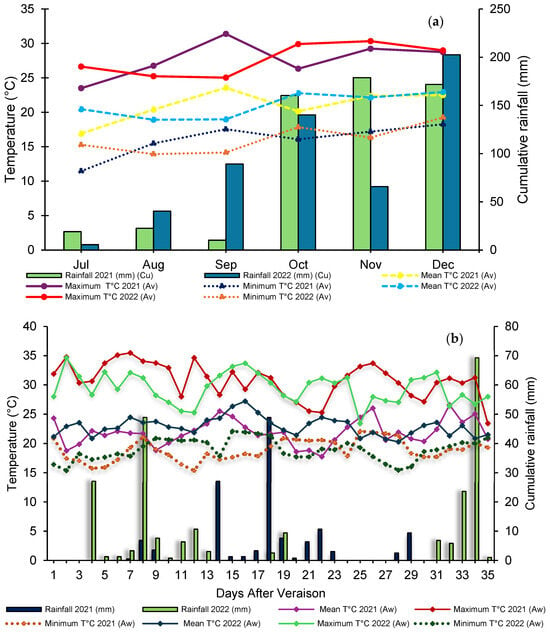
Figure 1.
Annual meteorological data of maximum, minimum, and average temperature and precipitation during the production (a) and maturation (b) cycles of ‘BRS Núbia’ grapes in cycle I and cycle II, São Manuel, São Paulo, Brazil. Source: Department of Soils and Environmental Resources (FCA/UNESP).
2.2. Experimental Site Setup and Management
The planting of grafted ‘BRS Núbia’ grapevines (‘Michele Palieri’ x ‘Arkansas 2095’) was carried out in August 2020. The seedlings were grafted onto the rootstocks ‘IAC 572 Jales’ (‘101-14 Mgt’ (Vitis riparia x Vitis rupestris) x Vitis caribaea), ‘IAC 766 Campinas’ (V. riparia x V. rupestris) x V. cordifolia ‘106-8 Mgt’ x Vitis caribaea), and ‘Paulsen 1103’ (V. berlandieri x V. rupestris). The vines were trained in the Y-trellis system (Open Gable) using a metal structure and spaced at 3.0 m × 2.0 m.
Short pruning (two buds per cane) was adopted in both production cycles, followed by the application of 2.5% hydrogen cyanamide (Dormex®, BASF, Ludwigshafen am Rhein, Germany) to stimulate budburst. After budburst, a single productive shoot per cane was maintained, and the following cultural practices were performed: shoot thinning, tying shoots to the trellis wires, removal of lateral shoots (disbudding), and partial defoliation to enhance aeration and light interception.
Additional management practices included micro-sprinkler irrigation, weed control (mechanical and chemical), and regular applications of fungicides and insecticides for pest and disease control. Nutrient management was based on chemical fertilization, adjusted according to soil chemical analysis and recommendations from Technical Bulletin 100 of the Agronomic Institute [44].
2.3. Experimental Design and Treatments
The experiment was conducted in a randomized complete block design with split plots, where rootstocks (‘IAC 572 Jales’, ‘IAC 766 Campinas’ and ‘Paulsen 1103’) were assigned to the main plots and production cycles I and II (2021 and 2022) to the subplots. The experiment was structured with seven blocks, and each experimental unit comprised three ‘BRS Núbia’ grapevines.
2.4. Evaluated Variables
2.4.1. Phenological Stages
The phenological development of the grapevines was evaluated according to the modified Eichhorn and Lorenz (E–L) scale proposed by [45] and the methodology described by [46]. Visual observations were performed three times per week, always in the morning (between 7:00 and 10:00 a.m.) by the same trained observer to ensure consistency and reduce subjectivity. The recorded phenological stages included:
(i) Budburst, defined as the emergence of green tissue in approximately 50% of the buds;
(ii) Full flowering, when around 50% of the flowers in the cluster were fully open;
(iii) Fruit set, identified by the presence of visible and developing fruitlets after petal fall;
(iv) Onset of ripening (veraison), characterized by the beginning of berry color change and softening, depending on cultivar characteristics;
(v) Harvest, determined when total soluble solids reached 16 to 18°Brix, according to the cultivar.
All phenological stages were expressed as days after pruning (DAP). The monitoring period extended from winter pruning until harvest, covering the full annual cycle of vine development.
2.4.2. Physiological Maturation and Physicochemical Quality of ‘BRS Núbia’ Grape Must
The physiological maturation of ‘BRS Núbia’ grapes was monitored weekly, starting when 50% of the berries reached the initial ripening stage, characterized by the onset of softening and color change. For this purpose, ten grape clusters were marked, from which six berries per cluster were collected, totaling sixty berries per experimental plot, at intervals of 0, 7, 14, 21, 28, and 35 days after the onset of ripening (DAOR). Sampling continued until harvest, when soluble solids stabilized at 17°Brix.
During ripening, the following variables were analyzed in the grape must: soluble solids content (SS), measured with a Reichert® digital refractometer (model r2i300, Depew, NY, USA); pH, determined directly in the must using a (Tecnal® model Tec-10 potentiometer, Piracicaba, Brazil)) [47]; and titratable acidity (TA), obtained by titration with 0.1 N NaOH, expressed as a percentage of tartaric acid per milliliter of must, according to the methodology described by [48]. The maturity index was calculated as the ratio of SS to TA.
2.4.3. Thermal Requirement and Growing Degree Days
The thermal requirement of the grapevines was determined based on the accumulation of growing degree days (GDDs) following the equation proposed by [49,50] using a base temperature of 10 °C [51] according to the phenological stages of the vine:
where:
GDD = ∑ (Tm − 10 °C) × number of days from pruning to harvest
- GDD: Accumulated growing degree days;
- Tm: Daily mean temperature (°C).
2.5. Statistical Analyses
Data were subjected to analysis of variance (ANOVA) to assess the effects of rootstocks and climatic conditions on the production cycles. The means of phenological stages and thermal requirements were compared using Tukey’s test (p < 0.05). To analyze the effect of rootstocks on grape ripening and harvest timing, regression analysis was applied using Sisvar, version 6.0 software [52]. Additionally, to characterize the effects of rootstocks and assess the correlation between variables, principal component analysis (PCA) was performed using Statistical Analysis Software version 4.0 (SAS).
3. Results and Discussion
3.1. Duration of Phenological Stages and Thermal Requirement of ‘BRS Núbia’ Grapevine
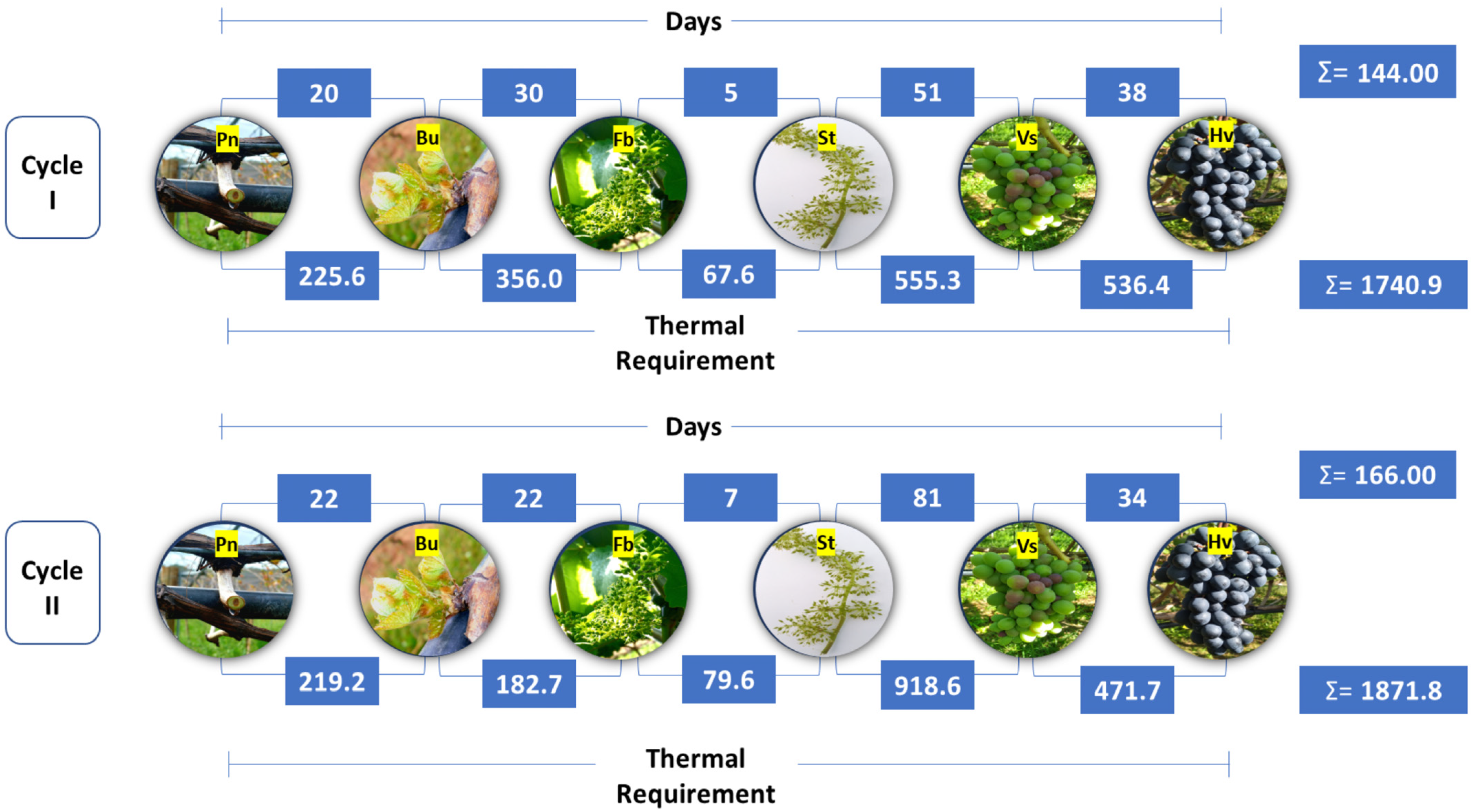
The duration of the phenological stages of the ‘BRS Núbia’ grapevine did not show a significant interaction (p > 0.05) between the rootstocks and production cycles considering the period from pruning to harvest. However, significant differences were observed between production cycles in the duration of phenological phases, including budburst (Bu), full bloom (Fb), fruit set (St), veraison (Vs), and harvest (Hv) (Table 1).

Table 1.
Duration of phenological stages (days after pruning) and thermal requirement of ‘BRS Núbia’ grape under different rootstocks during production cycle I and cycle II, São Manuel, São Paulo, Brazil.
The analysis of phenological stages showed that the shortest duration was observed in the interval between full bloom and fruit set, whereas the longest duration occurred between fruit set and the onset of ripening. The shorter duration between flowering and fruit set can be attributed to the rapid cell growth and differentiation of berry tissues, processes driven by the mobilization of carbohydrate reserves. In contrast, the transition from fruit set to the onset of ripening involves more complex physiological processes, such as the initiation of sugar accumulation and the gradual degradation of organic acids, which may explain the longer duration of this phase.
During the berry ripening stage, the average temperature was 21.9 °C in cycle I and 22.9 °C in cycle II, with an average minimum temperature of 18.9 °C and 19.0 °C and an average maximum temperature of 30.8 °C and 29.5 °C, respectively (Figure 1b). Maximum temperature during the ripening phase directly affects the respiration rate and metabolism of phenolic compounds in grapes, which can influence fruit coloration and chemical composition [53].
The accumulated rainfall was higher in cycle II (229.1 mm) compared to cycle I (133.4 mm), indicating a greater influence of climatic conditions on vine development (Figure 1a).
Although rootstocks did not significantly affect the duration of phenological stages (Table 1), the production cycles exhibited substantial differences in the thermal requirements of the grapevines (Figure 2).
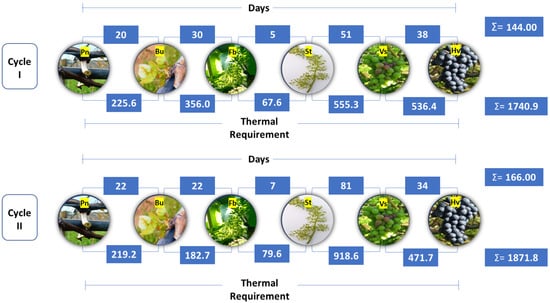
Figure 2.
Duration of the phenological stages and thermal requirement of the ‘BRS Núbia’ grapevine on different rootstocks during production cycles Iand cycle II, São Manuel, São Paulo, Brazil. Note: Pn: Pruning; Bu: Budburst; Fb: Full bloom; St: Fruit set; Vs: Veraison; and Hv: Harvest.
The period between pruning and the onset of budburst was longer in cycle II (20.3 days) than in cycle I (19.7 days), which may be related to temperature variations during this period (Figure 2). Lower temperatures can delay bud dormancy release and reduce vine metabolic activity, postponing budburst. This phenomenon is associated with the plant’s hormonal regulation, particularly the balance between abscisic acid and cytokinins, which control bud induction [54].
Higher temperatures during the first cycle favored vine development, as increased temperatures after pruning can advance budburst and promote greater phenological uniformity [55]. Additionally, the effect of hydrogen cyanamide on budburst advancement may have been more efficient in the first cycle due to higher temperatures, promoting a greater hormonal stimulus for dormancy release [56].
Although temperature was the predominant environmental factor associated with the year-to-year differences observed in phenology and thermal accumulation, other physiological and environmental mechanisms may also have contributed to these variations [57]. Specifically, the depth of bud dormancy and the chilling requirement of the ‘BRS Núbia’ cultivar could have influenced the timing of budburst and subsequent developmental stages, particularly in response to variable winter conditions between cycles [58,59,60,61]. Moreover, sensitivity to photoperiod may also modulate hormonal regulation and sink-source dynamics, thereby affecting the pace of berry development and ripening [62]. Considering these additional factors provides a more comprehensive and mechanistic interpretation of the phenological variability observed across growing seasons.
The first cycle had a longer duration between pruning and full bloom (46.5 days), which was 4.2 days longer than the second cycle (42.3 days). Similar differences were observed between pruning and fruit set, with durations of 51.4 days in cycle I and 50.2 days in cycle II (Table 1). Despite higher average temperatures in cycle I, the duration of these phases was not solely influenced by temperature, suggesting that other factors, such as solar radiation, also played a role in development and sugar accumulation in the fruit. Solar radiation directly influences photosynthetic rate and plant metabolism, regulating carbohydrate biosynthesis and accelerating energy reserve accumulation. Thus, greater light availability can result in more efficient plant metabolism, promoting vegetative and reproductive development [63]. The production cycle duration also varied between years. In cycle II, berry ripening began 132.7 days after pruning, while in cycle I, this stage was reached in 106.6 days, representing a difference of 32.1 days (Table 1). Harvest in cycle II occurred 167.7 days after pruning, whereas in cycle I, it was performed at 142.6 days, resulting in a 25.1-day longer production cycle in the second year.
This variation may be attributed to climatic conditions, particularly temperature. In cycle II, a cooler environment likely slowed plant development, increasing the time required for berries to reach maturity and delaying harvest. Temperature directly influences plant growth rate and phenological stage development. In general, higher temperatures accelerate plant metabolism, shortening the cycle, while lower temperatures slow this process, extending the cycle. These factors explain how thermal variations can affect the cycle length of ‘BRS Núbia’ grapevines.
The variation in ripening time between production cycles has important practical implications for vineyard management and commercial planning. Early ripening may allow harvest to occur under more favorable climatic conditions, potentially improving fruit integrity and reducing disease pressure [64,65]. It also facilitates better labor scheduling and allocation of resources during peak harvest periods. In contrast, delayed ripening, as observed in the second cycle, may increase exposure to late-season rainfall, elevate disease risk, and shorten the marketing window, particularly for table grapes with limited shelf life [66,67]. Therefore, understanding the factors that control ripening dynamics is essential not only for improving fruit quality but also for optimizing harvest logistics and aligning production with market demands.
The duration of the ‘BRS Núbia’ grapevine cycle in the two evaluated years (167.7 days in cycle II and 142.6 days in cycle I) was longer than reported in other regions. In northern Paraná, cycles of 130 to 135 days were recorded, while in northwestern São Paulo, duration ranged from 125 to 130 days [68]. Under Cerrado conditions, the production cycle lasted approximately 140 days, highlighting the influence of climate on grapevine phenological development [69].
The increase in thermal accumulation is associated with a reduction in the grapevine production cycle, advancing ripening and harvest, which may impact fruit quality due to the shorter time available for reserve compound development and the assimilation of photoassimilates and sugars [70]. During both production cycles, cumulative precipitation was 1047.7 mm and 1083.3 mm, respectively (Figure 1a).
In the critical period between October and December, the accumulated rainfall index was 510.9 mm in cycle I and 408.4 mm in cycle II. During the vegetative stage, the higher precipitation in cycle I likely favored vine growth, providing more water for root, leaf, and shoot development, which in turn boosted the photosynthetic rate and resulted in more vigorous vegetative growth. Conversely, in cycle II, with lower precipitation, the plants exhibited more balanced growth, avoiding risks associated with excessive moisture. This condition provided a more controlled environment for fruit ripening, favoring more uniform berry maturation, which resulted in a shorter and more efficient production cycle.
Thermal requirements varied significantly between production cycles, being 7.36% higher in cycle II (1871.7 GDDs) compared to cycle I (1743.4 GDDs) (Table 1). This variation was primarily attributed to temperature differences throughout the cycle, with higher thermal requirements between fruit set and the onset of ripening as well as from ripening to harvest. Budburst, ripening, and harvest were the stages with the highest thermal requirement, regardless of the production cycle, highlighting the importance of thermal accumulation in these periods. Additionally, accumulated thermal sum during ripening can impact the phenolic compound content in the grape skin, influencing color stability and fruit flavor intensity [71].
The influence of climate on degree-day accumulation has been observed in other grapevine cultivars. For ‘Niagara Rosada’ cultivated under subtropical conditions, thermal requirements of 1465.2 to 1615.1 GD were recorded over two production cycles [72]. Moreover, there is evidence that rootstock can influence grapevine thermal requirements [73,74]. The fact that ‘BRS Núbia’ exhibited similar thermal requirements among the tested rootstocks suggests that scion genetic factors may have a greater influence on phenological regulation than specific rootstock characteristics.
In subtropical regions, the optimal average temperature for grapevine cultivation is estimated at 27 °C [75], considering its impact on phenology and thermal accumulation. An increase in minimum temperatures can reduce the vegetative phase and advance fruit ripening and harvest [55,76], which was evident in the analyzed production cycles.
The ‘BRS Núbia’ grapevine demonstrated adaptation to local climatic conditions and was classified as having an intermediate cycle. The evaluation of phenological stages indicated an average thermal requirement of 1807.5 GD between pruning and harvest, considering the two analyzed production cycles (Table 1). The phenological stability of ‘BRS Núbia’ and its ability to complete the cycle within consistent patterns reinforce its potential for cultivation in subtropical regions. However, specific management practices, such as advancing pruning in years with expected higher temperatures or adjusting irrigation during critical periods, may be effective strategies to optimize yield and fruit quality.
3.2. Evolution of ‘BRS Núbia’ Grape Ripening
The ripening of ‘BRS Núbia’ grapes began at 106.6 and 132.7 days after pruning (DAP) in production cycle I and cycle II, respectively. No significant interaction was observed between rootstocks and berry sampling periods for the ripening curve, allowing the adjustment of regression models to analyze variations in soluble solids content, titratable acidity, pH, and the maturity index as a function of days after pruning.
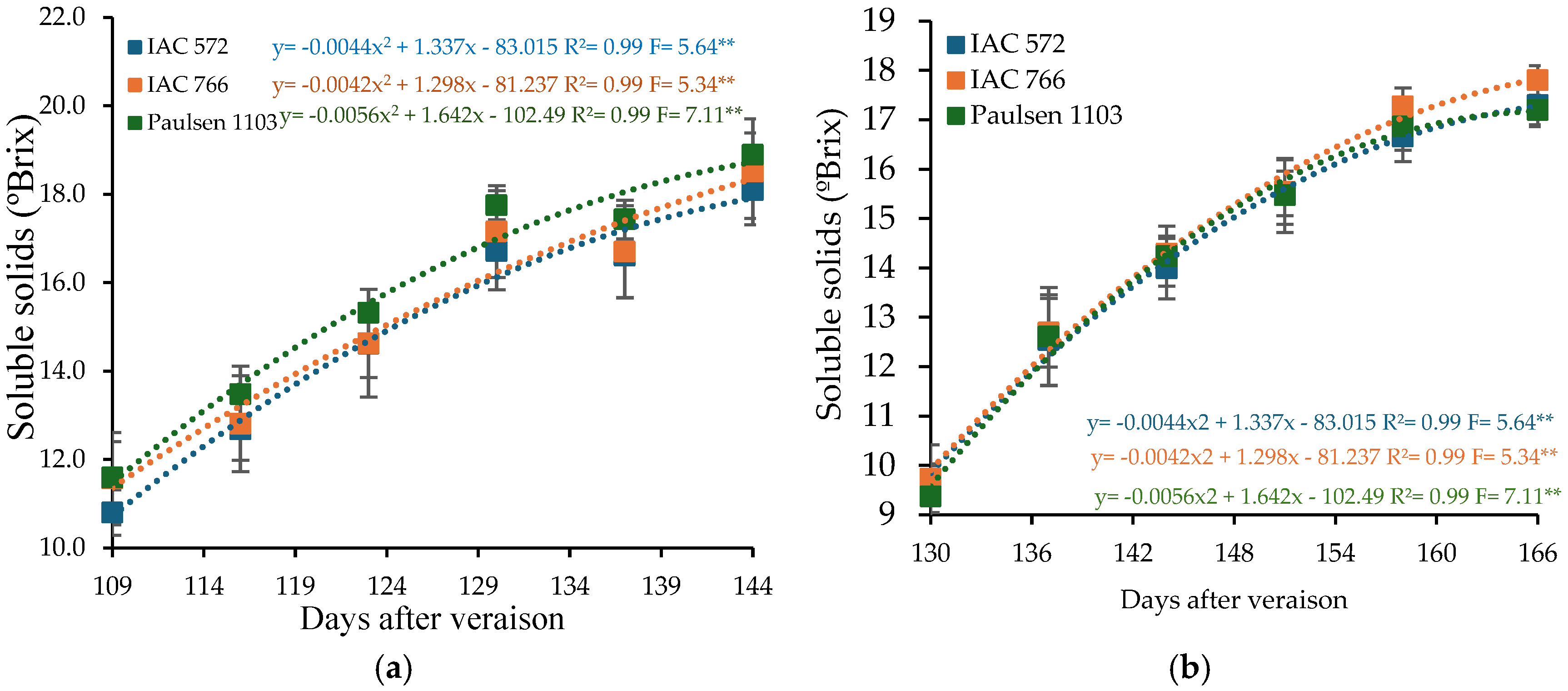
The soluble solids content in the must of ‘BRS Núbia’ grapes at harvest was 18.50°Brix in cycle I and 17.43°Brix in cycle II, both exceeding the minimum requirement for table grape cultivars (16°Brix) according to Brazilian regulations [68,77,78]. Under tropical conditions, ‘BRS Núbia’ grapes exhibit soluble solids content ranging from 17 to 19°Brix [35]. Temperature and rainfall frequency directly impact ripening, reducing sugar concentration in the fruit [79,80] (Figure 3).
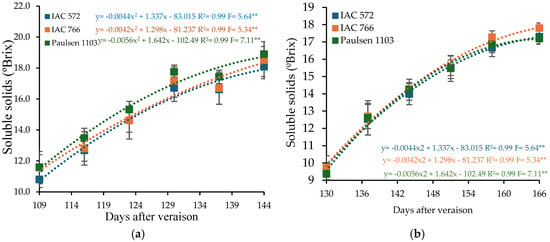
Figure 3.
Soluble solids content (SS) in the must of ‘BRS Núbia’ grapes cultivated during production cycle I (a) and cycle II (b), São Manuel-SP, 2023. Significant F-value at p < 0.05 (n = 6). The rootstocks ‘IAC 572’, ‘IAC 766’, and ‘Paulsen 1103’ are represented by blue, orange, and green colors, respectively. ** p > 0.05.
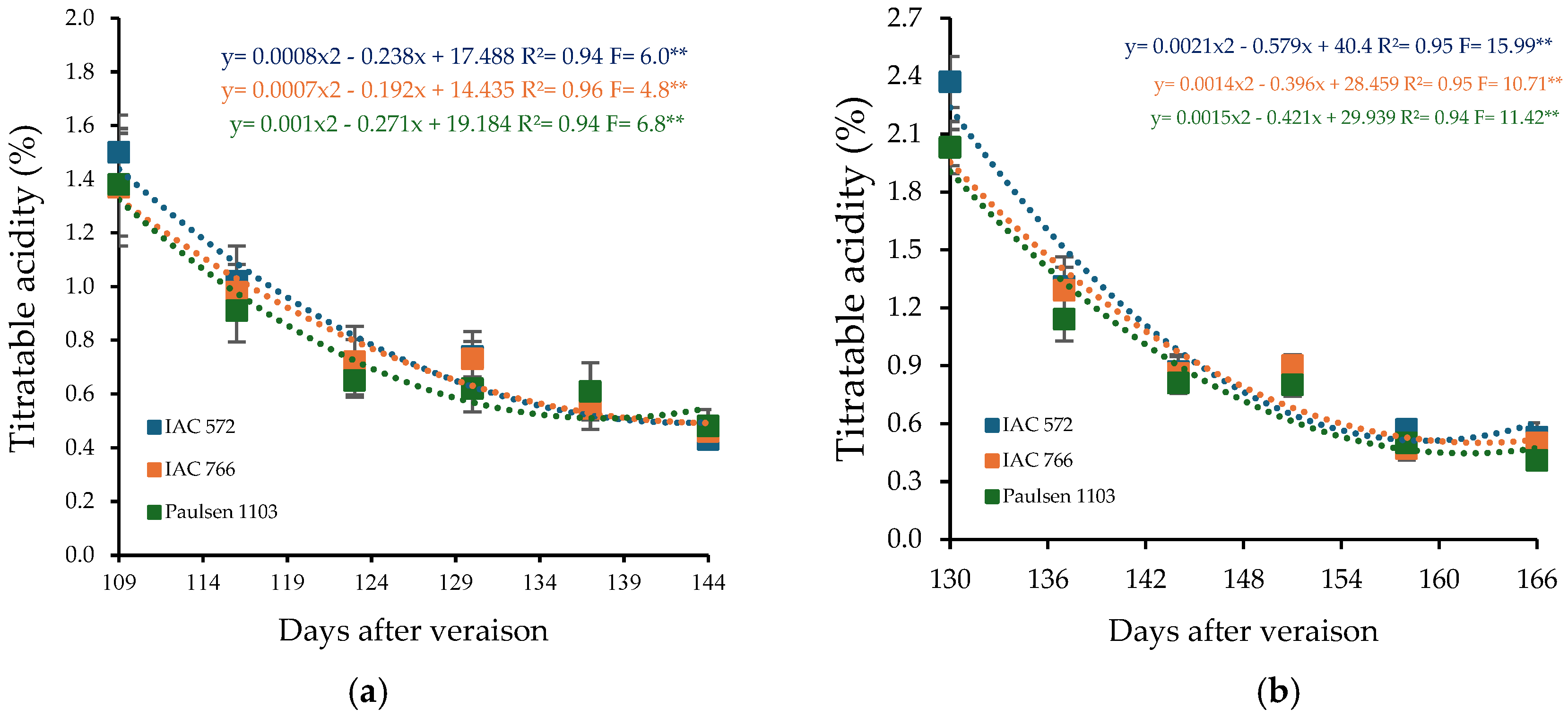
Titratable acidity decreased throughout the cycle, with quadratic regression models indicating an inverse correlation with soluble solids content (Figure 4). In the first cycle, the minimum titratable acidity points occurred at 144, 137, and 136 DAP for the rootstocks ‘IAC 572 Jales’, ‘IAC 766 Campinas’, and ‘Paulsen 1103’, respectively. In the second cycle, minimum values were recorded at 138, 141, and 140 DAP (Figure 4b).
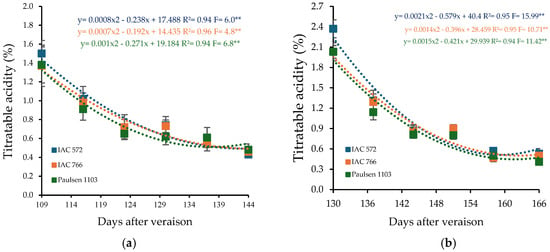
Figure 4.
Titratable acidity (TA) in the must of ‘BRS Núbia’ grapes cultivated during production cycle I (a) and cycle II (b), São Manuel-SP, 2023. Significant F-value at p < 0.05 (n = 6). The rootstocks ‘IAC 572’, ‘IAC 766’, and ‘Paulsen 1103’ are represented by blue, orange, and green colors, respectively. ** p > 0.05.
Initial titratable acidity was 1.42% in cycle I, decreasing to 0.46% at the end of ripening. In cycle II, initial acidity was higher (2.14%) but also decreased, reaching 0.48% at harvest (Figure 4b). The reduction in acidity throughout the cycle is an expected process during grape maturation, as organic acids, especially malic acid, are metabolized and utilized in cellular respiration [81]. Cycle II, with slightly lower temperatures, may have favored the maintenance of higher acidity levels, as lower temperatures slow acid degradation and extend their retention in the fruit.
Variations in titratable acidity between cycles were influenced by climatic conditions, including average temperatures of 28.7 °C in cycle I and 27.8 °C in cycle II as well as accumulated precipitation (Figure 1b). The rootstock ‘IAC 572 Jales’ resulted in the highest titratable acidity values at the end of ripening (Figure 4b), directly impacting the sensory profile of ‘BRS Núbia’ grapes, as acidity influences fruit flavor perception for fresh consumption. The rootstock can affect grape chemical composition by regulating mineral absorption and translocation, particularly potassium, which directly influences must acidity. The acid-base composition of must is primarily determined by tartaric and malic acid levels as well as potassium concentration in the soil and plant, which affect grape stability and quality [82].
The lower temperatures observed during cycle II, particularly between July and September (Figure 1b), coincided with the final stage of berry filling and the onset of ripening, delaying organic acid degradation and resulting in higher acidity at harvest. This demonstrates that temperature not only affects the rate of sugar accumulation but also influences the dynamics of organic acid degradation, reinforcing the need for climate monitoring to predict the final characteristics of the harvest.
Similar results have been reported for ‘BRS Núbia’ grapes in the Brazilian Cerrado, where average titratable acidity levels were 0.52% and 0.51% tartaric acid per 100 mL of must [69,83]. These values are consistent with those obtained for other table grape cultivars grown in subtropical regions, such as ‘BRS Ísis’ [31,39], ‘BRS Melodia’ [84], ‘Niagara Rosada’ [72], and ‘BRS Vitória’ [37,85].
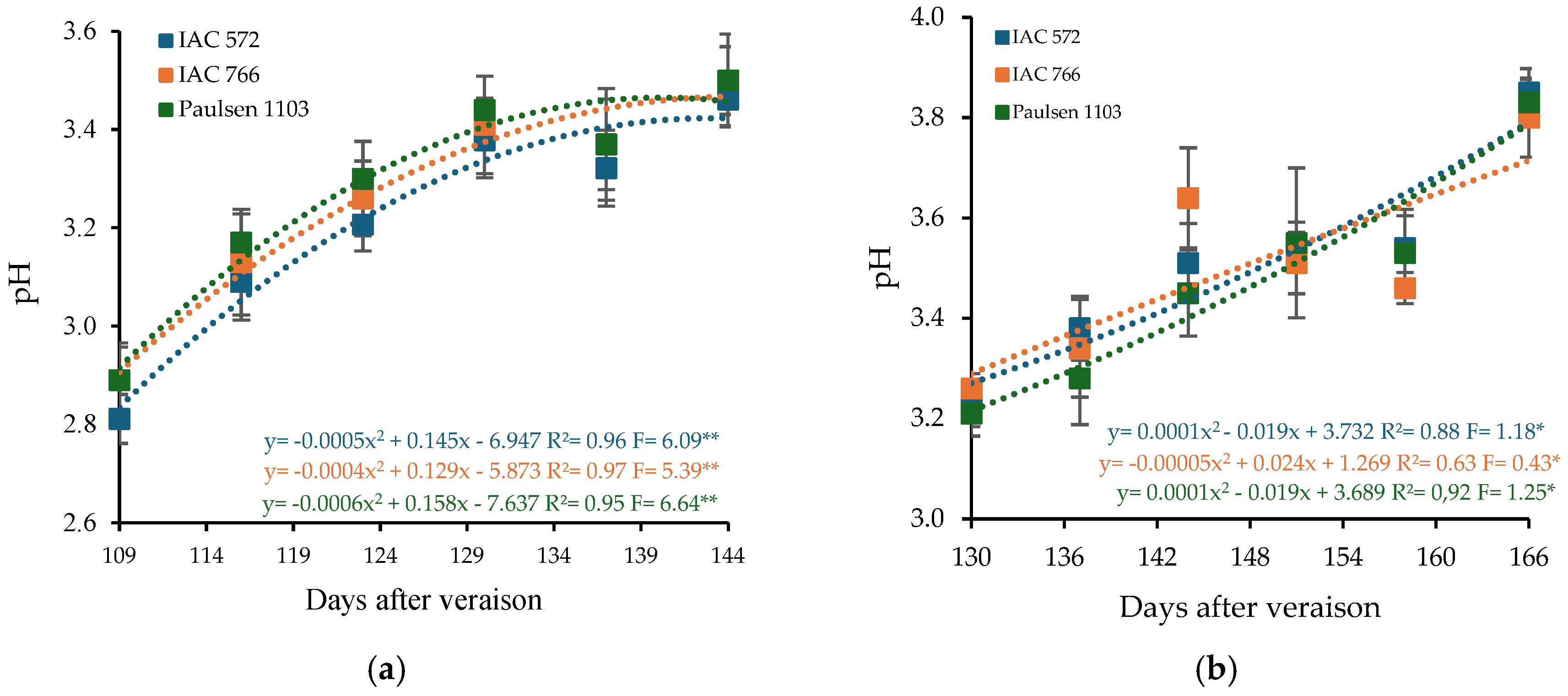
The pH of ‘BRS Núbia’ grape must varied according to the rootstock and production cycle. In the first cycle, the must pH values were 3.21, 3.26, and 3.28 for the rootstocks ‘IAC 572 Jales’, ‘IAC 766 Campinas’, and ‘Paulsen 1103’, respectively. In the second cycle, these values increased to 3.51, 3.50, and 3.48 (Figure 5), aligning with results obtained for grapevines cultivated in the Cerrado [69]. Quadratic regression models revealed that maximum pH values were reached at 144, 144, and 132 DAP in cycle I and at 158, 166, and 143 DAP in cycle II, depending on the rootstock used (Figure 5).
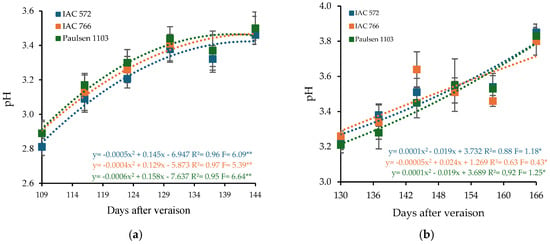
Figure 5.
Hydrogen potential (pH) in the must of ‘BRS Núbia’ grapes cultivated during production cycle I (a) and cycle II (b), São Manuel-SP, 2023. Significant F-value at p < 0.05 (n = 6). The rootstocks ‘IAC 572’, ‘IAC 766’, and ‘Paulsen 1103’ are represented by blue, orange, and green colors, respectively. * p > 0.01, ** p > 0.05.
The increase in pH during ripening is directly related to the degradation of malic acid and the accumulation of cations, such as potassium, in berry tissues, which reduces acidity perception and alters the acid-base balance of the must [86].
Variations in pH may be associated with climatic factors and the composition of organic acids. The increase in pH during the second cycle suggests a possible reduction in acidity due to lower temperatures and solar radiation (Figure 1b), affecting the sensory profile of the grapes. Temperature plays a crucial role in plant metabolism, influencing must pH, as higher temperatures can reduce acidity and accelerate ripening [87,88].
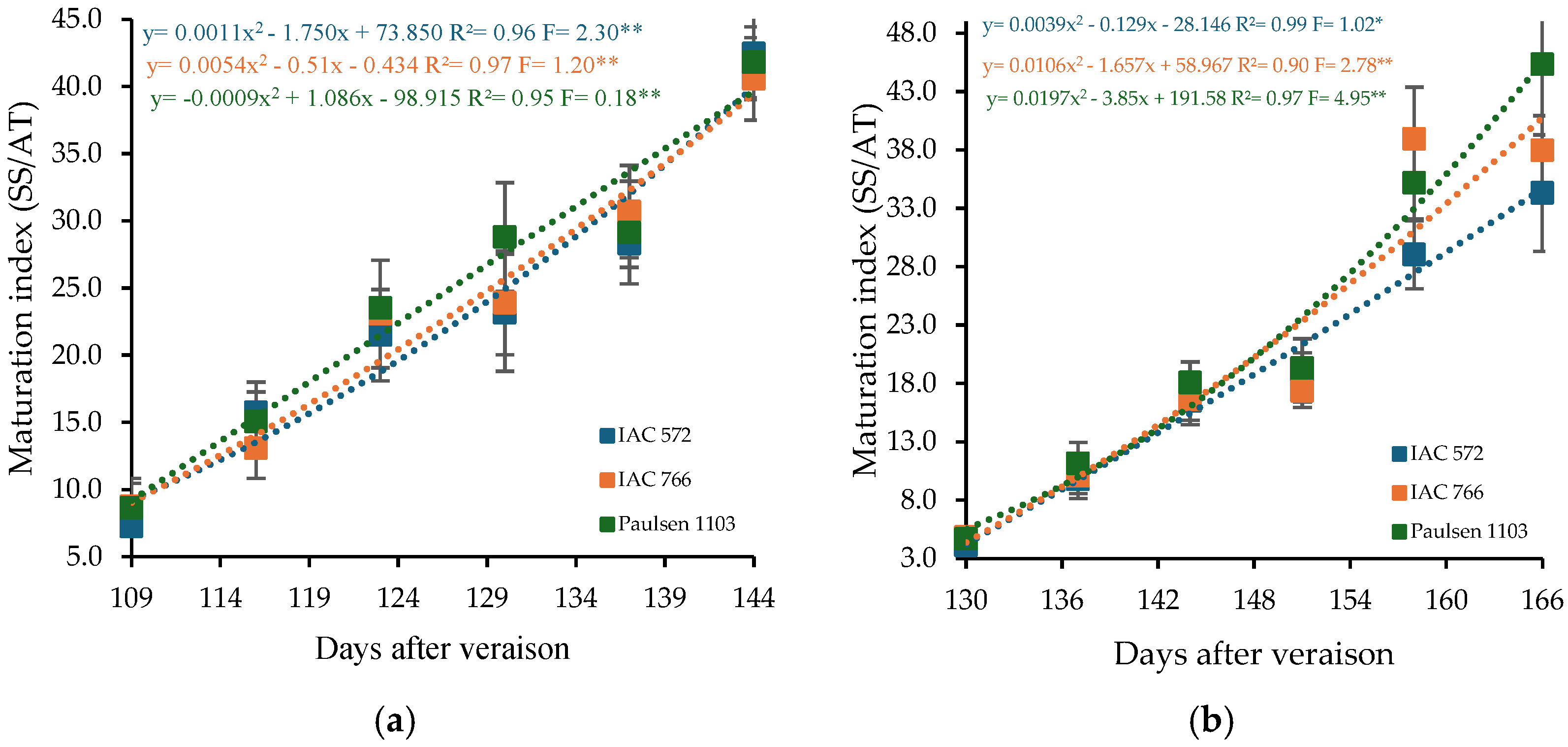
The maturity index of ‘BRS Núbia’ grapes ranged from 8.21 to 41.61 in cycle I and from 4.54 to 39.20 in cycle II (Figure 6). Quadratic regression models indicated that the maximum maturity index points occurred at 144 DAP in cycle I and at 166 DAP in cycle II. The ratio between soluble solids and total acidity is a crucial parameter for determining the optimal harvest time, as it reflects the balance between sweetness and acidity in the fruit.
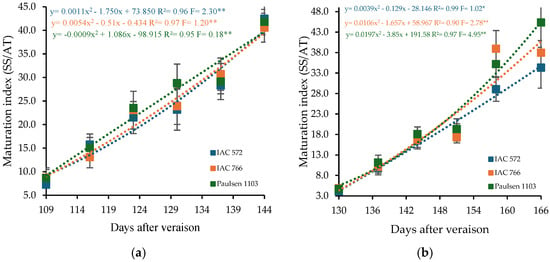
Figure 6.
Maturity index (MI) in the must of ‘BRS Núbia’ grapes cultivated during production cycle I (a) and cycle II (b), São Manuel-SP, 2023. Significant F-value at p < 0.05 (n = 6). The rootstocks ‘IAC 572’, ‘IAC 766’, and ‘Paulsen 1103’ are represented by blue, orange, and green colors, respectively. * p > 0.01 ** p > 0.05.
The rootstock ‘Paulsen 1103’ resulted in the highest maturity indices, with values of 41.80 in cycle I and 45.32 in cycle II (Figure 6a,b), whereas ‘IAC 766 Campinas’ advanced full ripening to 28 DAIM in the second cycle. This superior maturation index associated with ‘Paulsen 1103’ may be linked to physiological traits of this rootstock, including its efficient water uptake capacity and vigorous root system, which enhance nutrients and assimilate translocation to the scion [89]. Furthermore, ‘Paulsen 1103’ is known to influence abscisic acid (ABA) signaling in the berries, a hormone that plays a key role in sugar accumulation and acid degradation during ripening [90,91,92]. The elevated maturity index may thus reflect an accelerated or more synchronized ripening process triggered by hormonal crosstalk and improved hydraulic conductance, contributing to higher °Brix and lower acidity at harvest. The maturity index is an important indicator of grape quality since soluble solids increase with ripening while acidity decreases (Figure 3 and Figure 4). High temperatures can advance phenological stages, impacting must composition and potentially compromising final grape quality [93].
The appropriate selection of rootstocks and the monitoring of climatic conditions are essential to ensuring fruit quality. Management strategies should be adopted to mitigate the effects of climatic fluctuations on grape ripening and chemical balance [94,95]. These factors directly influence sugar content and acidity, determining the acceptance of ‘BRS Núbia’ grapes in the table grape market.
3.3. Principal Component Analysis (PCA)
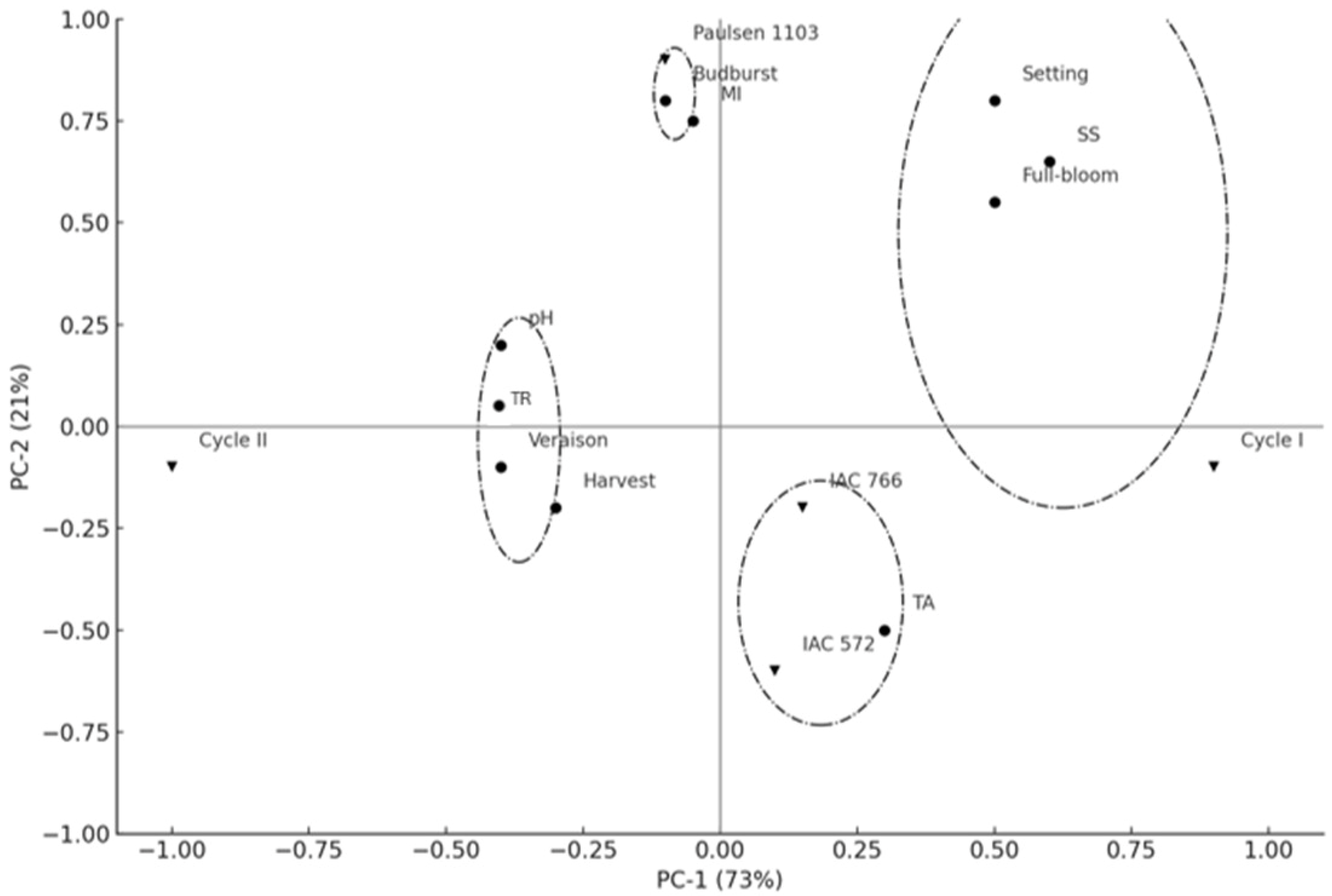
Principal Component Analysis (PCA) enabled the visualization of variability among rootstocks (‘IAC 572 Jales’, ‘IAC 766 Campinas’, and ‘Paulsen 1103’) and production cycles (cycle I and cycle II) of the ‘BRS Núbia’ cultivar. The first two principal components together explained 94% of the total data variability (PC-1 = 73%; PC-2 = 21%), indicating a robust representation of the interrelationships among the analyzed factors (Figure 7).
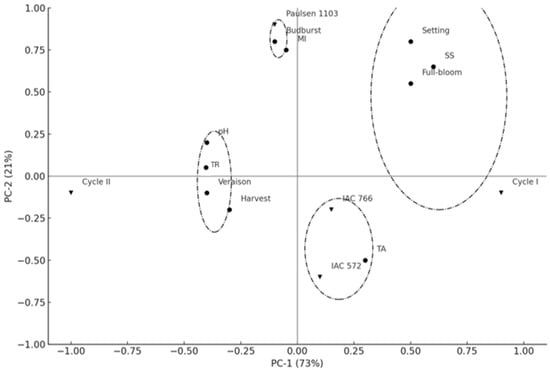
Figure 7.
Principal component analysis of ‘BRS Núbia’ vines on different rootstocks. Note: SS: Soluble solids; TR: Thermal requirement; TA: Titratable acidity; MI: Maturation index. The symbols (triangles, circles, and squares) represent different combinations of cultivars and rootstocks, and are used solely for visual distinction.
The separation between production cycles indicates a strong influence of environmental conditions on phenology and fruit ripening. Cycle I (2021) showed a greater association with phenological events such as full bloom, fruit set, and soluble solids accumulation (SS), suggesting a more favorable plant development and higher sugar accumulation, possibly due to higher temperatures and lower precipitation. Conversely, cycle II did not show a strong correlation with these variables, indicating a potential delay in fruit ripening or a lower sugar accumulation rate, potentially due to differences in climatic conditions. These results highlight the importance of environmental variables in grapevine physiology, demonstrating that thermal and water fluctuations can directly impact photosynthetic efficiency and carbohydrate metabolism in fruits [96].
Rootstocks also significantly influenced grapevine phenology. ‘Paulsen 1103’ was associated with the budburst event, indicating that this scion/rootstock combination may advance the start of the phenological cycle. Meanwhile, the rootstocks ‘IAC 572 Jales’ and ‘IAC 766 Campinas’ were correlated with titratable acidity (TA), suggesting greater retention of organic acids during ripening, an important factor in maintaining the sensory balance of the fruit. Additionally, ‘IAC 766 Campinas’ was closely related to pH, suggesting an influence on the regulation of the grape’s acid profile.
The distribution of rootstocks in different quadrants reinforces their distinct influences on the metabolism of ‘BRS Núbia’ grapevines. The separate clustering of ‘Paulsen 1103’ from the other rootstocks indicates a differentiated phenological behavior, favoring early budburst and potentially influencing the duration of the production cycle. On the other hand, the rootstocks ‘IAC 572 Jales’ and ‘IAC 766 Campinas’ exhibited similar effects on acidity retention, which may directly impact the final quality of the fruit.
These findings reinforce the hypothesis that ‘Paulsen 1103’ may enhance early developmental stages due to its high hydraulic conductivity and potential stimulation of scion vigor. This rootstock is known for promoting earlier budburst and increasing canopy activity, which can lead to greater light interception and enhanced carbohydrate biosynthesis during the initial phenological phases. These physiological traits may contribute to the higher maturity index values and earlier ripening observed for ‘Paulsen 1103’ in this study.
In contrast, the association of ‘IAC 572 Jales’ and ‘IAC 766 Campinas’ with higher titratable acidity may reflect their moderate vigor and potentially lower rates of abscisic acid (ABA) signaling involved in ripening onset. These rootstocks may promote a more gradual accumulation of sugars and slower degradation of organic acids, particularly under subtropical climate conditions, thereby preserving acidity and contributing to better sensory balance in the berries.
4. Conclusions
The ‘BRS Núbia’ grapevine exhibited a mean thermal requirement of 1807.5 growing degree days (GDDs) to complete its phenological cycle, which lasted approximately 150 days under subtropical conditions. Although the rootstocks ‘IAC 572 Jales’, ‘IAC 766 Campinas’, and ‘Paulsen 1103’ did not significantly alter the duration of phenological stages, they had notable effects on fruit quality parameters, particularly soluble solids content, titratable acidity, and maturation index. Differences observed in phenological progression and thermal accumulation between the two production cycles were primarily influenced by climatic variability, especially temperature and precipitation levels during key developmental stages. These findings reinforce the physiological plasticity and adaptability of the ‘BRS Núbia’ cultivar to subtropical environments, supporting its suitability for regions with similar agroclimatic conditions. Moreover, the results highlight the importance of integrating rootstock selection with climate monitoring to optimize fruit quality and harvest timing in subtropical viticulture systems.
Author Contributions
H.S.A.M. and M.A.T. planned and designed the experiment. H.S.A.M., S.d.N.S.B., J.C.A., D.E.F.F., P.H.H.C. and C.R.M. performed the plant physiological analyses and chemical, biochemical, and enzyme analyses. H.S.A.M., C.A.P.C.S., F.J.D.N. and M.A.T. performed data analyses. H.S.A.M., F.J.D.N. and C.A.P.C.S. created the tables and figures. H.S.A.M., F.J.D.N., C.A.P.C.S. and M.d.S.S. wrote and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Coordination for the Improvement of Higher Education Personnel), CAPES process no. 88887.669920/2022-00 through granting the scholarship for the first author, several programs that supported the study in setting up the experimental area Fundação de Amparo à Pesquisa do Estado de São Paulo (Research Support Foundation of the State of São Paulo), (FAPESP process no. 2020/12152-3), and the CNPq for the Research Productivity Scholarship (process no. 307377/2021-0).
Data Availability Statement
The original contributions presented in the study are included in the article material; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Candar, S.; Uysal, T.; Ayaz, A.; Akdemir, U.; Korkutal, İ.; Bahar, E. Viticulture tradition in Turkey. Vitic. Stud. (VIS) 2021, 1, 39–54. [Google Scholar] [CrossRef]
- Monteiro, A.I.; Malheiro, A.C.; Bacelar, E.A. Morphology, physiology and analysis techniques of grapevine bud fruitfulness: A review. Agriculture 2021, 11, 127. [Google Scholar] [CrossRef]
- Palliotti, A.; Tombesi, S.; Silvestroni, O.; Lanari, V.; Gatti, M.; Poni, S. Changes in vineyard establishment and canopy management urged by earlier climate-related grape ripening: A review. Sci. Hortic. 2014, 178, 43–54. [Google Scholar] [CrossRef]
- Wilson, T.G.; Kustas, W.P.; Alfieri, J.G.; Anderson, M.C.; Gao, F.; Prueger, J.H.; Alstad, K.P. Relationships between soil water content, evapotranspiration, and irrigation measurements in a California drip-irrigated Pinot noir vineyard. Agric. Water Manag. 2020, 237, 106186. [Google Scholar] [CrossRef]
- Evangelista, T.Y.L.; Pereira, G.A. Climatic potential, phenology, and thermal needs of vines grown at different times in the semi-arid region. Bragantia 2024, 84, e20240189. [Google Scholar]
- Zombardo, A.; Mica, E.; Puccioni, S.; Perria, R.; Valentini, P.; Mattii, G.B.; Storchi, P. Berry quality of grapevine under water stress as affected by rootstock–scion interactions through gene expression regulation. Agronomy 2020, 10, 680. [Google Scholar] [CrossRef]
- Ranade, Y.; Pathak, P.; Chandrashekar, M.; Saha, S. Diversity analysis of culturable epiphytic microbial consortia of table grape berry surface. Food Biotechnol. 2023, 37, 54–73. [Google Scholar] [CrossRef]
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape bioactive molecules, and the potential health benefits in reducing the risk of heart diseases. Food Chem. X 2021, 12, 100149. [Google Scholar] [CrossRef]
- Abi Rached, R.; Perra, M.; Manca, M.L.; Rajha, H.N.; Louka, N.; Maroun, R.G.; Manconi, M. Exploring the efficacy and industrial potential of polyphenol products from grapes and their by-products. Sustain. Chem. Pharm. 2024, 42, 101805. [Google Scholar] [CrossRef]
- Liu, X.; Hu, K.K.; Haritos, V.S. Enzymatic production of cello-oligosaccharides with potential human prebiotic activity and release of polyphenols from grape marc. Food Chem. 2024, 435, 137562. [Google Scholar] [CrossRef]
- Nirmal, N.; Mahale, K.R.; Rathod, N.B.; Siddiqui, S.A.; Dhar, B.K. Winery waste: A sustainable approach for bioactive compound extraction and various industrial applications. Process Saf. Environ. Prot. 2025, 193, 760–771. [Google Scholar] [CrossRef]
- Alston, J.M.; Sambucci, O. Grapes in the world economy. In The Grape Genome; Springer: Cham, Switzerland, 2019; pp. 1–24. [Google Scholar]
- Vukovic, D.B.; Maiti, M.; Vujko, A.; Shams, R. Residents’ perceptions of wine tourism on the rural destinations development. Br. Food J. 2020, 122, 2739–2753. [Google Scholar] [CrossRef]
- García-Navarro, F.J.; Jiménez-Ballesta, R.; Perales, J.A.L.; Perez, C.; Amorós, J.A.; Bravo, S. Sustainable Viticulture in the Valdepeñas Protected Designation of Origin: From Soil Quality to Management in Vitis vinifera. Sustainability 2023, 15, 9339. [Google Scholar] [CrossRef]
- Straffelini, E.; Carrillo, N.; Schilardi, C.; Aguilera, R.; Orrego, M.J.E.; Tarolli, P. Viticulture in Argentina under extreme weather scenarios: Actual challenges, future perspectives. Geogr. Sustain. 2023, 4, 161–169. [Google Scholar] [CrossRef]
- Myga-Piątek, U.; Rahmonov, O. Winery regions as the oldest cultural landscapes: Remnants, signs, and metamorphoses. Misc. Geogr. Reg. Stud. Dev. 2018, 22, 69–80. [Google Scholar] [CrossRef]
- Gutiérrez Gamboa, G.; Pszczólkowski, P.; Fourment, M. Opening Remarks and General Overview of the Current Scientific Scenario of Latin American Vitiviniculture: A Critical View. In Latin American Viticulture Adaptation to Climate Change: Perspectives and Challenges of Viticulture Facing up Global Warming; Springer: Cham, Switzerland, 2024; pp. 1–18. [Google Scholar]
- OIV. The International Organisation of Vine and Wine. Statistics. 2023. Available online: https://www.oiv.int/index.php/what-we-do/statistics (accessed on 15 January 2025).
- IBGE. Instituto Brasileiro de Geografia e Estatística. In Levantamento Sistemático da Produção Agrícola (LSPA); IBGE: Rio de Janeiro, Brazil, 2023. Available online: https://sidra.ibge.gov.br/pesquisa/lspa/tabelas (accessed on 18 April 2024).
- Dinis, L.T.; Bernardo, S.; Yang, C.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. Mediterranean viticulture in the context of climate change. Ciênc. Téc. Vitivinoícol. 2022, 37, 139–158. [Google Scholar] [CrossRef]
- Aydin, D.; Coskun, O.F. Comparison of edta-enhanced phytoextraction strategies with Nasturtium officinale (Watercress) on an artificially arsenic contaminated water. Pak. J. Bot. 2013, 45, 1423–1429. [Google Scholar]
- Coşkun, Ö.F. Association Mapping for Drought Tolerance in Watermelons (Citrullus lanatus L.). Horticulturae 2025, 11, 193. [Google Scholar] [CrossRef]
- Dry, I.B.; Davies, C.; Dunlevy, J.D.; Smith, H.M.; Thomas, M.R.; Walker, A.R.; Walker, R.R.; Clingeleffer, P.R. Development of new wine-, dried-and tablegrape scions and rootstocks for Australian viticulture: Past, present and future. Aust. J. Grape Wine Res. 2022, 28, 177–195. [Google Scholar] [CrossRef]
- Massano, L.; Fosser, G.; Gaetani, M.; Bois, B. Assessment of climate impact on grape productivity: A new application for bioclimatic indices in Italy. Sci. Total Environ. 2023, 905, 167134. [Google Scholar] [CrossRef]
- Kabato, W.; Getnet, G.T.; Sinore, T.; Nemeth, A.; Molnár, Z. Towards climate-smart agriculture: Strategies for sustainable agricultural production, food security, and greenhouse gas reduction. Agronomy 2025, 15, 565. [Google Scholar] [CrossRef]
- Leonel, S.; Roberto, S.R.; da Silva, S.R. Orchard Management Under Climate Change. Horticulturae 2025, 11, 98. [Google Scholar] [CrossRef]
- Leão, P.C.S.; Nascimento, J.H.B.; Moraes, D.S.; Souza, E.R. Yield components of the new seedless table grape ‘BRS Ísis’ as affected by the rootstock under semi-arid tropical conditions. Sci. Hortic. 2020, 263, 109114. [Google Scholar] [CrossRef]
- Domingues Neto, F.J.; Tecchio, M.A.; Borges, C.V.; Rodrigues, J.D.; Ono, E.O.; Lima, G.P.P.; Leonel, M. Yield Performance and Quality Assessment of Brazilian Hybrid Grapes Influenced by Rootstocks and Training Systems. Horticulturae 2024, 10, 909. [Google Scholar] [CrossRef]
- Callili, D.; Tecchio, M.A.; Sánchez, C.A.P.C.; Neto, F.J.D.; Bonfim, F.P.G.; de Souza Silva, M.; Campos, O.P. Table grape ‘BRS Vitória’yield performance, vigor, and quality as influenced by rootstocks in a subtropical region. Aust. J. Crop Sci. 2025, 119, 284–291. [Google Scholar]
- Gonçalves, B.; Aires, A.; Oliveira, I.; Afonso, S.; Morais, M.C.; Correia, S.; Silva, A.P. Sweet cherry. In Temperate Fruits; Apple Academic Press: Cambridge, MA, USA, 2021; pp. 333–415. [Google Scholar]
- Sánchez, C.A.P.C.; Tecchio, M.A.; Callili, D.; da Silva, M.J.R.; Basílio, L.S.P.; Leonel, S.; Alonso, J.C.; Lima, G.P.P. Productivity and physicochemical properties of the BRS Isis grape on various rootstocks under subtropical climatic conditions. Agriculture 2023, 13, 2113. [Google Scholar] [CrossRef]
- Leão, P.C.S.; Carvalho, J.N. Tropical Viticulture in Brazil: São Francisco Valley as an Important Supplier of Table Grapes to the World Market. In Latin American Viticulture Adaptation to Climate Change: Perspectives and Challenges of Viticulture Facing up Global Warming; Springer International Publishing: Cham, Switzerland, 2024; pp. 47–59. [Google Scholar]
- Campos, L.F.C.; Alves, J., Jr.; Campos, C.M.d.A.; Casaroli, D.; Evangelista, A.W.P.; Seleguini, A. Sistema radicular do porta-enxerto IAC 572 ‘JALES’ sob Niágara rosada nas condições do Cerrado Goiano. Irriga 2017, 22, 723–734. [Google Scholar] [CrossRef]
- Tecchio, M.A.; Hernandes, J.L.; Paioli Pires, E.J.; Terra, M.M.; Moura, M.F. Cultivo da videira para mesa, vinho e suco. In Cultivo de Fruteiras Clima Temperado em Regiões Subtropicais e Tropicais, 2nd ed.; Pio, R., Ed.; UFLA: Lavras, Brazil, 2018; pp. 512–584. [Google Scholar]
- Viana, A.P.; Rodrigues, D.L.; Santos, E.A. Porta-enxerto, cultivares de mesa e de vinho. In Uva: Do Plantio à Colheita; Motoike, S., Borém, A., Eds.; Editora UFV: Viçosa, Brazil, 2018; pp. 49–60. [Google Scholar]
- Tecchio, M.A.; Moura, M.F.; Teixeira, L.A.J.; Pires, E.J.; Leonel, S. Influence of rootstocks and pruning times on yield and nutrients content and extraction in ‘Niagara Rosada’ grapevine. Pesqui. Agropecu. Bras. 2014, 49, 340–348. [Google Scholar] [CrossRef]
- Callili, D.; Sanchez, C.A.P.C.; Campos, O.P.; Carneiro, D.C.d.S.; ScudelettI, A.C.B.; Tecchio, M.A. Phenology, thermal demand, and maturation development of the ‘BRS Vitória’ grape cultivated on different rootstocks in subtropical conditions. Rev. Bras. Frutic. 2023, 45, e-999. [Google Scholar] [CrossRef]
- Leão, P.C.d.S.; Lima, M.A.C.; de Cultivar, B.R.S. Núbia: Produtividade e Qualidade da Uva no Submédio do Vale do São Francisco. Circular Técnica n 172; Embrapa Semiárido: Petrolina, Brazil, 2017; pp. 1–4. [Google Scholar]
- Ahmed, S.; Roberto, S.R.; Shahab, M.; Colombo, R.C.; Silvestre, J.P.; Koyama, R.; Souza, R.T. Proposal of double-cropping system for ‘BRS Isis’ seedless grape grown in subtropical area. Sci. Hortic. 2019, 251, 118–126. [Google Scholar] [CrossRef]
- Salama, A.M.; Ezzat, A.; El-Ramady, H.; Alam-Eldein, S.M.; Okba, S.K.; Elmenofy, H.M.; Holb, I.J. Temperate fruit trees under climate change: Challenges for dormancy and chilling requirements in warm winter regions. Horticulturae 2021, 7, 86. [Google Scholar] [CrossRef]
- Yin, Y.; Deng, H.; Wu, S. Spatial-temporal variations in the thermal growing degree-days and season under climate warming in China during 1960–2011. Int. J. Biometeorol. 2019, 63, 649–658. [Google Scholar] [CrossRef]
- Charalampopoulos, I.; Polychroni, I.; Psomiadis, E.; Nastos, P. Spatiotemporal estimation of the olive and vine cultivations’ Growing Degree days in the Balkans region. Atmosphere 2021, 12, 148. [Google Scholar] [CrossRef]
- Embrapa; Empresa Brasileira de Pesquisa Agropecuária. Sistema Brasileiro de Classificação de Solos; Centro Nacional de Pesquisa de Solos: Rio de Janeiro, Brazil, 2018. [Google Scholar]
- Tecchio, M.A.; Teixeira, L.A.J.; Terra, M.M.; Paioli-Pires, E.J.; Hernandes, J.L. Uvas comuns para mesa e vinho (Vits lambusca). In Boletim 100: Recomendações de Adubação e Calagem Para o Estado de São Paulo, 2nd ed.; Cantarella, H., Quaggio, J.A., Mattos, D., Jr., Boaretto, R.M., Van Raij, B., Eds.; Instituto Agronômico: Campinas, Brazil, 2022; pp. 303–308. [Google Scholar]
- Eichhorn, K.W.; Lorenz, D.H. Phaenologische Entwicklungsstadien der Rebe. Eur. Mediterr. Plant Prot. Organ. 1984, 14, 295–298. [Google Scholar]
- Coombe, B.G. Growth Stages of the Grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- Instituto Adolfo Lutz. Métodos Físico-Químicos Para Análise de Alimentos; Intituto Adolfo Lutz: São Paulo, Brazil, 2008; 1020p.
- Winkler, A.J. Viticultura; Companhia Editorial Continental: México City, México, 1965. [Google Scholar]
- Villa Nova, N.A.; Pedro Júnior, M.J.; Pereira, A.R.; Ometto, J.C. Estimativa de graus-dia acumulados acima de qualquer temperatura base em função das temperaturas máxima e mínima. Ciênc. Terra 1972, 30, 1–8. [Google Scholar]
- Pedro Júnior, M.J.; Sentelhas, P.C.; Pommer, C.V. Determinação da temperatura-base, graus-dia e índice biometeorológico para a videira ‘Niagara Rosada’. Rev. Bras. Agrometeorol. 1994, 2, 51–56. [Google Scholar]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciênc. Agrotecnol. 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Cataldo, E.; Eichmeier, A.; Mattii, G.B. Effects of Global Warming on Grapevine Berries Phenolic Compounds—A Review. Agronomy 2023, 13, 2192. [Google Scholar] [CrossRef]
- Camargo-Alvarez, H.; Salazar-Gutiérrez, M.; Keller, M.; Hoogenboom, G. Modeling the effect of temperature on bud dormancy of grapevines. Agric. For. Meteorol. 2020, 280, 15. [Google Scholar] [CrossRef]
- Malinovski, L.I.; Pandolfo, C.; Campos, C.G.C.; de Lima, M.; da Silva, A.L.; Vieira, H.J. Clima: Viticultura de elevada altitude do estado de Santa Catarina. In A Cultura da Videira: Viticultura de Altitude; Rufato, L., Filho, J.L.M., Brighenti, A.F., Bogo, A., Kretzsschmar, A.A., Eds.; UDESC: Florianópolis, Brazil, 2021; pp. 27–47. [Google Scholar]
- Mas, I.; de la Fuente, J.L.; Ruiz-Bermejo, M. Temperature effect on aqueous NH4CN polymerization: Relationship between kinetic behaviour and structural properties. Eur. Polym. J. 2020, 132, 109719. [Google Scholar] [CrossRef]
- Rességuier, L.; Mary, S.; Le Roux, R.; Petitjean, T.; Quénol, H.; Van Leeuwen, C. Temperature variability at local scale in the Bordeaux area. Relations with environmental factors and impact on vine phenology. Front. Plant Sci. 2020, 11, 515. [Google Scholar] [CrossRef] [PubMed]
- Schrader, J.A.; Cochran, D.R.; Domoto, P.A.; Nonnecke, G.R. Phenology and winter hardiness of cold-climate grape cultivars and advanced selections in Iowa climate. HortTechnology 2019, 29, 906–922. [Google Scholar] [CrossRef]
- Anzanello, R.; Fogaça, C.M.; Sartori, G.B.D. Induction and overcoming of dormancy of grapevine buds in response to thermal variations in the winter period. Cienc. Rural. 2021, 51, e20200887. [Google Scholar] [CrossRef]
- Velappan, Y.; Chabikwa, T.G.; Considine, J.A.; Agudelo-Romero, P.; Foyer, C.H.; Signorelli, S.; Considine, M.J. The bud dormancy disconnect: Latent buds of grapevine are dormant during summer despite a high metabolic rate. J. Exp. Bot. 2022, 73, 2061–2076. [Google Scholar] [CrossRef]
- Velappan, Y.; Considine, J.A.; Signorelli, S.; Considine, M.J. Contrasting seasonal dynamics of dormancy, respiratory metabolism and cell cycle state in grapevine buds of a subtropical and Mediterranean climate. Food Energy Secur. 2023, 12, e431. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Y.; Fan, P.; Kong, J.; Wang, Y.; Xu, X.; Dai, Z. Grapevine plantlets respond to different monochromatic lights by tuning photosynthesis and carbon allocation. Hortic. Res. 2023, 10, uhad160. [Google Scholar] [CrossRef]
- Domingues Neto, F.J.; Pimentel Junior, A.; Modesto, L.R.; Moura, M.F.; Putti, F.F.; Boaro, C.S.F.; Ono, E.O.; Rodrigues, J.D.; Tecchio, M.A. Photosynthesis, Biochemical and Yield Performance of Grapevine Hybrids in Two Rootstock and Trellis Height. Horticulturae 2023, 9, 596. [Google Scholar] [CrossRef]
- Mirás-Avalos, J.M.; Araujo, E.S. Optimization of vineyard water management: Challenges, strategies, and perspectives. Water 2021, 13, 746. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Greer, D.H.; Liu, Y.; Baby, T.; Xiao, Z. Impact of climate change on grape berry ripening: An assessment of adaptation strategies for the Australian vineyard. Front. Plant Sci. 2022, 13, 1094633. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V.; Vertuccio, L.; Vittoria, V.; Pace, B.; Cefola, M.; Clarizia, G. Active packaging for table grapes: Evaluation of antimicrobial performances of packaging for shelf life of the grapes under thermal stress. Food Packag. Shelf Life 2020, 25, 100545. [Google Scholar] [CrossRef]
- Hazarika, T.K.; Marak, T. Salicylic acid and oxalic acid in enhancing the quality and extending the shelf life of grape cv. Thompson seedless. Food Sci. Technol. Int. 2022, 28, 463–475. [Google Scholar] [CrossRef]
- Maia, J.D.G.; Ritschel, P.; Camargo, U.A.; Souza, R.T.; Farjado, T.V.M.; Girardi, C.L. BRS Núbia Nova Cultivar de Uva de Mesa com Sementes e Coloração Preta Uniforme. Circular Técnica n 139., 1st ed.; Embrapa Uva e Vinho: Bento Gonçalves, Brazil, 2013; p. 12. [Google Scholar]
- Campos, L.; Vendruscolo, E.; Campos, C.; Teramoto, A.; Seleguini, A. Preliminary results on agronomic behavior of table grapes on different rootstocks in brazilian Cerrado conditions. Agric. Conspec. Sci. 2022, 87, 265–276. [Google Scholar]
- Chavarria, G.; Santos, H.P.; Mandelli, F.; Marodin, G.A.B.; Bergamaschi, H.; Cardoso, L.S. Caracterização fenológica e requerimento térmico da cultivar moscato giallo sob cobertura plástica. Rev. Bras. Frutic. 2009, 31, 119–126. [Google Scholar] [CrossRef]
- Blancquaert, E.H.; Oberholster, A.; Ricardo-da-Silva, J.M.; Deloire, A.J. Effects of abiotic factors on phenolic compounds in the grape berry—A review. S. Afr. J. Enol. Vitic. 2019, 40, 1. [Google Scholar] [CrossRef]
- Callili, D.; da Silva, M.J.R.; Sanchez, C.A.P.C.; Watanabe, C.Y.; Macedo, B.M.d.P.; Domingues Neto, F.J. Rootstock and potassium fertilization, in terms of phenology, thermal demand and chemical evolution, of berries on Niagara Rosada grapevine under subtropical conditions. Bragantia 2022, 81, e2022. [Google Scholar] [CrossRef]
- Bruna, E.D.; Back, Á.J. Comportamento da cultivar Niágara Rosada enxertada sobre diferentes porta-enxertos no sul de Santa Catarina, Brasil. Rev. Bras. Frutic. 2015, 37, 924–933. [Google Scholar] [CrossRef]
- Tecchio, M.A.; da Silva, M.J.R.; Paiva, A.P.M.; Moura, M.F.; Terra, M.M.; Pires, E.J.P.; Leonel, S. Phenological, physicochemical, and productive characteristics of “Vênus” grapevine onto rootstocks. Pesqui. Agropecu. Bras. 2019, 54, e00335. [Google Scholar] [CrossRef]
- Grillakis, M.G.; Doupis, G.; Kapetanakis, E.; Goumenaki, E. Future shifts in the phenology of table grapes on Crete under a warming climate. Agric. For. Meteorol. 2022, 318, 108915. [Google Scholar] [CrossRef]
- Wurz, D.A.; Filho, J.L.M.; Rufato, L. Implantação do vinhedo. In A Cultura da Videira: Viticultura de Altitude; Rufato, L., Filho, J.L.M., Brighenti, A.F., Bogo, A., Kretzsschmar, A.A., Eds.; UDESC: Florianópolis, Brazil, 2021; pp. 159–180. [Google Scholar]
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento. In Instrução Normativa Nº 1, de 1º de Fevereiro de 2002; Ministério da Agricultura, Pecuária e Abastecimento: Brasília, Brazil, 2002; p. 5.
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento. In Instrução Normativa Nº 69, de 6 de Novembro de 2018; Ministério da Agricultura, Pecuária e Abastecimento: Brasília, Brazil, 2018; p. 25.
- Irimia, L.M.; Patriche, C.V.; Bucur, G.M.; Quénol, H.; Cotea, V.V. Spatial Distribution of Grapes Sugar Content and its Correlations with Climate Characteristics and Climate Suitability in the Huși (Romania) Wine Growing Region. Not. Bot. Horti Agrobot. 2015, 43, 250–258. [Google Scholar] [CrossRef]
- Drappier, J.; Thibon, C.; Rabot, A.; Geny-Denis, L. Relationship between wine composition and temperature: Impact on Bordeaux wine typicity in the context of global warming-Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in organic acid profiles during fruit development and ripening: Correlation or causation? Front. Plant Sci. 2018, 9, 1689. [Google Scholar] [CrossRef]
- Mota, R.V.; Silva, C.P.C.; Favero, A.C.; Purgatto, E. Composição físico-química de uvas para vinho fino em ciclos de verão e inverno. Rev. Bras. Frutic. 2010, 32, 1127–1137. [Google Scholar] [CrossRef]
- Cardoso, K.S.; Neto, P.L.A.N. Caracterização fenológica de cultivares de videira de mesa sobre o porta-enxerto IAC 572 em Goiânia-GO. Ipê Agron. J. 2022, 6, 7011. [Google Scholar]
- Koyama, R.; Borges, W.F.S.; Colombo, R.C.; Hussain, I.; Souza, R.T.; Roberto, S.R. Phenology and yield of the hybrid seedless grape ‘BRS Melodia’ grown in an annual double cropping system in a subtropical area. Horticulturae 2020, 6, 3. [Google Scholar] [CrossRef]
- Santana, S.; Dos Santos, V.; Tecchio, M.; Pereira, L.; Nunes, A.; Almeida, H.; Pereira, G. Caracterização físico-química de uvas ‘BRS Vitória’ cultivadas em clima subtropical. Rev. Iberoam. Tecnol. Postcosecha 2023, 24, 150–163. [Google Scholar]
- Plantevin, M.; Merpault, Y.; Lecourt, J.; Destrac-Irvine, A.; Dijkstra, L.; van Leeuwen, C. Characterization of varietal effects on the acidity and pH of grape berries for selection of varieties better adapted to climate change. Front. Plant Sci. 2024, 15, 1439114. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.J.; Cook, J.A. General Viticulture, 4th ed.; University of California Press: Berkley, CA, USA, 1974. [Google Scholar]
- Silva, L.; Caldas, L.A.; Ribeiro, M.G.; Almeida, R.V. Obstáculos Epistemológicos no Ensino-Aprendizagem de Química Geral e Inorgânica no Ensino Superior: Resgate da definição ácido-base de Arrhenius e crítica ao ensino das “funções inorgânicas”. Quím. Nova Esc. 2014, 36, 261–268. [Google Scholar]
- Gambetta, G.A.; Manuck, C.M.; Drucker, S.T.; Shaghasi, T.; Fort, K.; Matthews, M.A.; Walker, M.A.; McElrone, A.J. The relationship between root hydraulics and scion vigour across Vitis rootstocks: What role do root aquaporins play? J. Exp. Bot. 2012, 63, 6445–6455. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Zapata, P.J.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Serrano, M.; Guillén, F. Preharvest salicylate treatments enhance antioxidant compounds, color and crop yield in low pigmented-table grape cultivars and preserve quality traits during storage. Antioxidants 2020, 9, 832. [Google Scholar] [CrossRef]
- Navarro-Calderon, A.; Falagan, N.; Terry, L.A.; Alamar, M.C. Biomarkers of postharvest resilience: Unveiling the role of abscisic acid in table grapes during cold storage. Front. Plant Sci. 2023, 14, 1266807. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fei, Y.; Howell, K.; Chen, D.; Clingeleffer, P.; Zhang, P. Rootstocks for grapevines now and into the future: Selection of rootstocks based on drought tolerance, soil nutrient availability, and soil pH. Aust. J. Grape Wine Res. 2024, 2024, 6704238. [Google Scholar] [CrossRef]
- Duchêne, E.; Huard, F.; Dumas, V.; Schneider, C.; Merdinoglu, D. The challenge of adapting grapevine varieties to climate change. Clim. Res. 2010, 41, 193–204. [Google Scholar] [CrossRef]
- Fraga, H.; Santos, J.A.; Moutinho-Pereira, J.; Carlos, C.; Silvestre, J.; Eiras-Dias, J.; Malheiro, A.C. Statistical modelling of grapevine phenology in Portuguese wine regions: Observed trends and climate change projections. J. Agric. Sci. 2016, 154, 795–811. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Ollat, N. An update on the impact of climate change in viticulture and potential adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Rafique, R.; Ahmad, T.; Ahmed, M.; Khan, M.A. Exploring key physiological attributes of grapevine cultivars under the influence of seasonal environmental variability. OENO One 2023, 57, 2. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).