Abstract
Copper (Cu) is a naturally occurring element in soils, and at adequate concentrations, it is essential for plant survival. However, excessive Cu can lead to contamination, impairing soil quality and affecting the development of living organisms. The present study aimed to evaluate the physiological responses of Stizolobium aterrimum plants grown in soils contaminated with increasing doses of copper. The experiment was conducted in a greenhouse under controlled temperature conditions. Five treatments were applied (0, 30, 60, 240, and 480 mg dm−3). After 51 days of cultivation, the plants were harvested, and their tissues were separated into leaves, roots, and nodules. Nitrogen compounds were extracted, and the contents of total soluble amino acids, ureides, and soluble proteins were quantified. The activity of the nitrogenase enzyme was analyzed in vivo. The results indicate that Stizolobium aterrimum is partially tolerant to copper contamination, exhibiting adequate growth and metabolism in the presence of moderate Cu concentrations. However, increasing Cu levels in the soil reduce fresh biomass production and lead to higher copper accumulation in the root system. High soil Cu concentrations also affect the absorption of other nutrients, in addition to copper itself. Cu doses around 240 mg dm3 can already be considered toxic.
1. Introduction
Copper (Cu) is an essential micronutrient for plants; it is present in plastocyanins, ceruloplasmin, and various enzymes, such as phenolase, laccase, polyphenoloxidase [1,2,3]. In this way, it plays an important role in the biological and physiological processes of plants, including photosynthesis, protein, carbohydrate and cell wall metabolism, iron mobilisation [4,5], and in symbiotic N2 fixation [6].
Cu deficiency exerts a discernible impact on various physiological processes, consequently influencing plant productivity. Conversely, the potential for copper toxicity within plants manifests when soil copper levels fluctuate within the range of 25 to 40 mg/kg [7], and excess of this metal can alter the photosynthetic and respiratory processes, enzyme activity, DNA, membrane integrity, reduced growth and yield [1,5]. Cu pollution may occur due to metal mining [8,9], fertilizers and fungicides [1,10], which may be a potential risk to the health of living beings, either through direct (through contaminated soil, air and water) and/or indirect contact [9].
Symbiotic nitrogen (N2) fixation is an essential process for plant growth and can be carried out by certain plants in partnership with bacteria of the Rhizobium genus. These bacteria live inside nodules in the roots of leguminous plants, where they convert atmospheric nitrogen into ammonia, a form of nitrogen that plants can use. In return, the plants provide the bacteria with carbohydrates and other nutrients [11,12]. The availability of Cu indirectly influences symbiotic nitrogen fixation by supporting copper-dependent processes, such as microaerobic respiration via cytochrome cbb3 oxidase, which are essential for maintaining the energy supply required for nitrogenase activity [13].
Cu deficiency impairs nitrogenase activity, limiting the plant’s ability to fix nitrogen. On the other hand, an excess of this element disrupts the symbiosis between the plant and nitrogen-fixing microorganisms, hindering nodulation and reducing process efficiency. Therefore, adequate Cu availability in the soil enables biological nitrogen fixation (BNF) and supports healthy plant growth [14,15,16].
Stizolobium aterrimum is an herbaceous legume that can be used as green manure in agricultural systems. In addition, this plant has characteristics of high nitrogen fixation capacity and biomass production, rapid growth, abiotic tolerance and was previously shown to be tolerant to lead and boron [17,18]. The assessment of legume tolerance and phytoextraction potential in areas contaminated by heavy metals is essential to understanding their adaptability and resistance. These plants play a key role in phytoextraction, helping to restore degraded soils and promoting environmental sustainability [19,20,21].
Several studies have demonstrated the potential of legumes in Cu phytoextraction in contaminated areas, being used as green manure in various crops. Research indicates that species such as Canavalia ensiformis, Cajanus cajan, and Vachellia campechiana have the ability to accumulate and tolerate high levels of Cu [22,23,24,25]. Studies show that these legumes not only absorb copper from the soil but also exhibit physiological and biochemical strategies that minimize the metal’s toxic effects, such as antioxidant production and the activation of exclusion and compartmentalization mechanisms to sequester Cu in less sensitive tissues [26].
From the hypothesis that Stizolobium aterrimum is a plant with Cu phytoremediation potential, this study seeks to identify the changes that occur in relation to nitrogen metabolism and whether it really has the potential to be a Cu phytoremediation species.
2. Materials and Methods
2.1. Experimental Site and Experimental Design
The experiment was carried out under greenhouse conditions at a controlled temperature of 27 ± 2 °C at the School of Engineering, Ilha Solteira, São Paulo State University (UNESP), SP, Brazil.
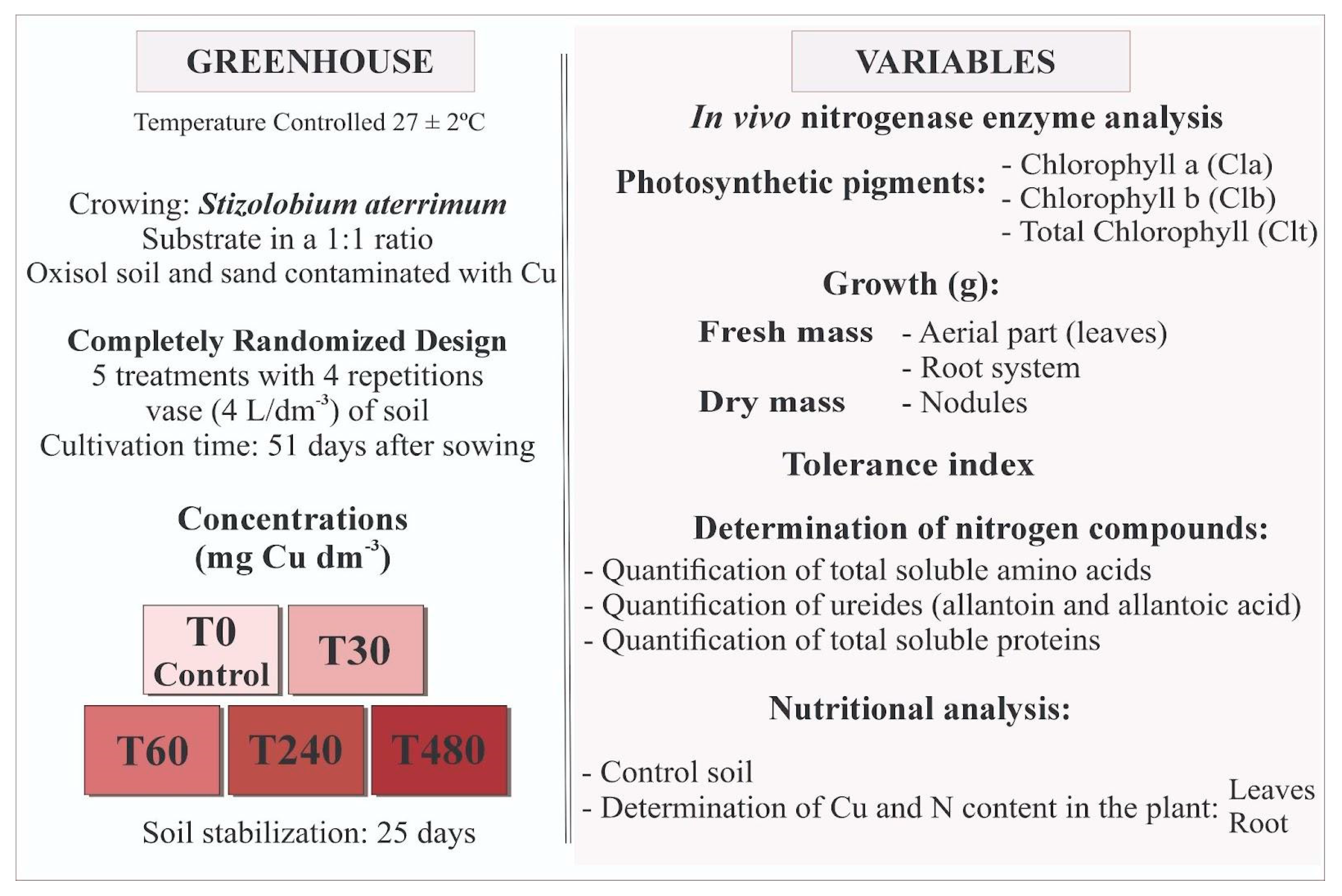
A completely randomized experimental design consisted of up to five treatments and four replications, composed of the following levels of Cu: 0, 30, 60, 240, and 480 mg dm−3 applied to the soil (CuSO4·5H2O, Labsynth® Farmoquímica, Diadema, SP, Brazil). Each pot contained two plants, giving a final population of eight plants per treatment (Figure 1).
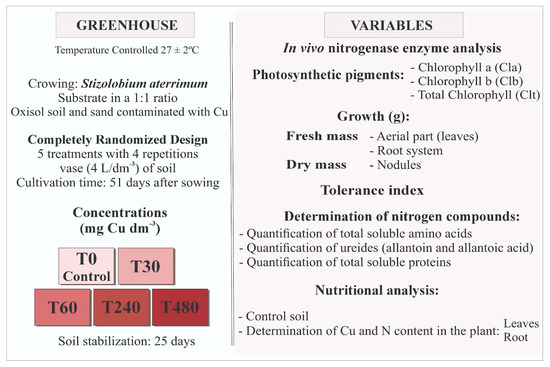
Figure 1.
Diagram of the experimental design and types of evaluations carried out on Stizolobium aterrimum plants grown in different concentrations of Cu in the soil over a period of 51 days.
2.2. Cultivation Conditions
A mixed Oxisol soil [24,25] with washed sand, in a 1:1 ratio, was used. The soil sample was collected from the experimental area of the Teaching, Research, and Extension Farm (FEPE), plant production sector, in Selvíria—MS (20°20′24.9″ S, 51°24′19.7″ W), at a depth of 0–0.2 m, forming a composite sample. It was crumbled, air-dried, and sieved (4.0 mm), and shown to have the following chemical attributes: pH (CaCl2) 5.6, organic matter 21 g dm−3, P (resin) 6 mg dm−3, K 0.9 mmolc dm−3, Ca 24 mmolc dm−3, Mg 18 mmolc dm−3, potential acidity (H + Al) 21 mmolc dm−3, Al 0 mmolc dm−3, sum of bases 42.9 mmolc dm−3, cation exchange capacity 63.9 mmolc dm−3, and base saturation 67%. The determination of the parameters followed the soil laboratory protocol.
The pots used were polypropylene with a capacity of 4 dm3. The soil mixture was contaminated with CuSO4.5H2O in the respective proportions of each treatment. Then the pots were sealed with plastic bags and remained at rest for 25 days.
2.3. Experimental Process
The seeds of Stizolobium aterrimum were sterilized for 15 min in a 10% NaClO solution (commercial solution) and then soaked in distilled water for one hour before sowing. Six seeds per pot were sown at a depth of 3 cm. After 10 days, the seedlings were thinned to three representative seedlings per pot, selecting those with greater vigor and uniform size.
During the experiment, replenishment of evapotranspiration water for the plots was achieved using suspended micro-sprinklers, which were activated twice a day (morning and afternoon). At the end of the experiment, that is, 51 days after sowing, a series of traits were measured.
2.4. Growth and Partitioned Biomass Production
One plant per experimental unit was used to determine the fresh biomass of the shoot, root, and nodules (g plant−1). The number of nodules per plant was recorded. Subsequently, the same plants were separated into leaves, stems, shoot (leaves + stem) and roots and oven-dried at 65 °C for 72 h to determine the dry mass [26]. Based on the plant’s dry weight, the tolerance index (TI) was calculated using the equation below:
The remaining plants from each pot were used to determine the content of chlorophylls and total soluble compounds (amino acids, ureides, allantoin, allantoic acid, and proteins) in the tissues of the leaves, roots, and nodules, and the activity of nitrogenase in vivo in the roots. To do this, the samples were stored in a freezer after being wrapped in aluminum foil until they were analyzed.
2.5. Chemical Analysis of Plant Tissue
The dry leaves, stalks, and roots were ground in a Wiley mill and the total concentrations of Cu were determined [27].
To determine the concentration of Cu, the samples were digested in nitric-perchloric acid (65% HNO3 and 70% HClO4). Atomic absorption spectrometry was used to determine the concentrations of Cu.
2.6. Analysis of Chlorophylls
For the extraction and quantification of chlorophylls, dimethyl sulfoxide (DMSO) was used as the extractor, due to its greater stability, which allows the plant extract to be stored for up to 5 days without significant loss of pigment content [28]. The chlorophyll contents were obtained after incubation at 65 °C for 30 min. Following extraction, the absorbance (ABS) of the solvent was measured at wavelengths specific to the different pigments by spectrophotometry. Chlorophyll a (Chl a), chlorophyll b (Chl b), and total chlorophyll (Clt) contents were expressed as μg mL−1 and calculated according to Equations (2)–(4) [28]:
2.7. Analysis of Nitrogen Metabolism and Amino Acids
The nitrogenase enzyme activity was determined in vivo in the roots using the N-Fixation Package system (Qubit Systems—Canada) through the quantification of H2 released, which is a byproduct of the nitrogenase enzyme found in legume nodules [29].
For the quantification of nitrogen compounds, extraction was performed in MCW (methanol, chloroform, and water for amino acids and ureides), followed by extraction in 0.1 N NaOH (for proteins) [30]. The aqueous phase was used for the quantification of amino acids and ureides (allantoin and allantoic acid [31,32]. The concentrations of amino acids and ureides were obtained after readings at 570 and 545 nm, respectively, compared with standard curves of leucine (for amino acids) and allantoin (for ureides). Protein quantification was also performed [33].
2.8. Data Analysis
Data were subjected to analysis of variance (ANOVA) using the F test (p ≤ 0.05). When significant, the traits were subjected to the Tukey test (p < 0.05). Principal component analysis (PCA) and Pearson’s correlation (p < 0.05) with multiple comparisons at 5% probability were calculated using the corrplot, FactoMineR, and factoextra package (Posit Team 2023). All statistical analyses of the data was performed using protocols developed in the R version 4.2.1 software (R Core Team 2023, Vienna, Austria).
3. Results
3.1. Nitrogen Metabolism and Amino Acids
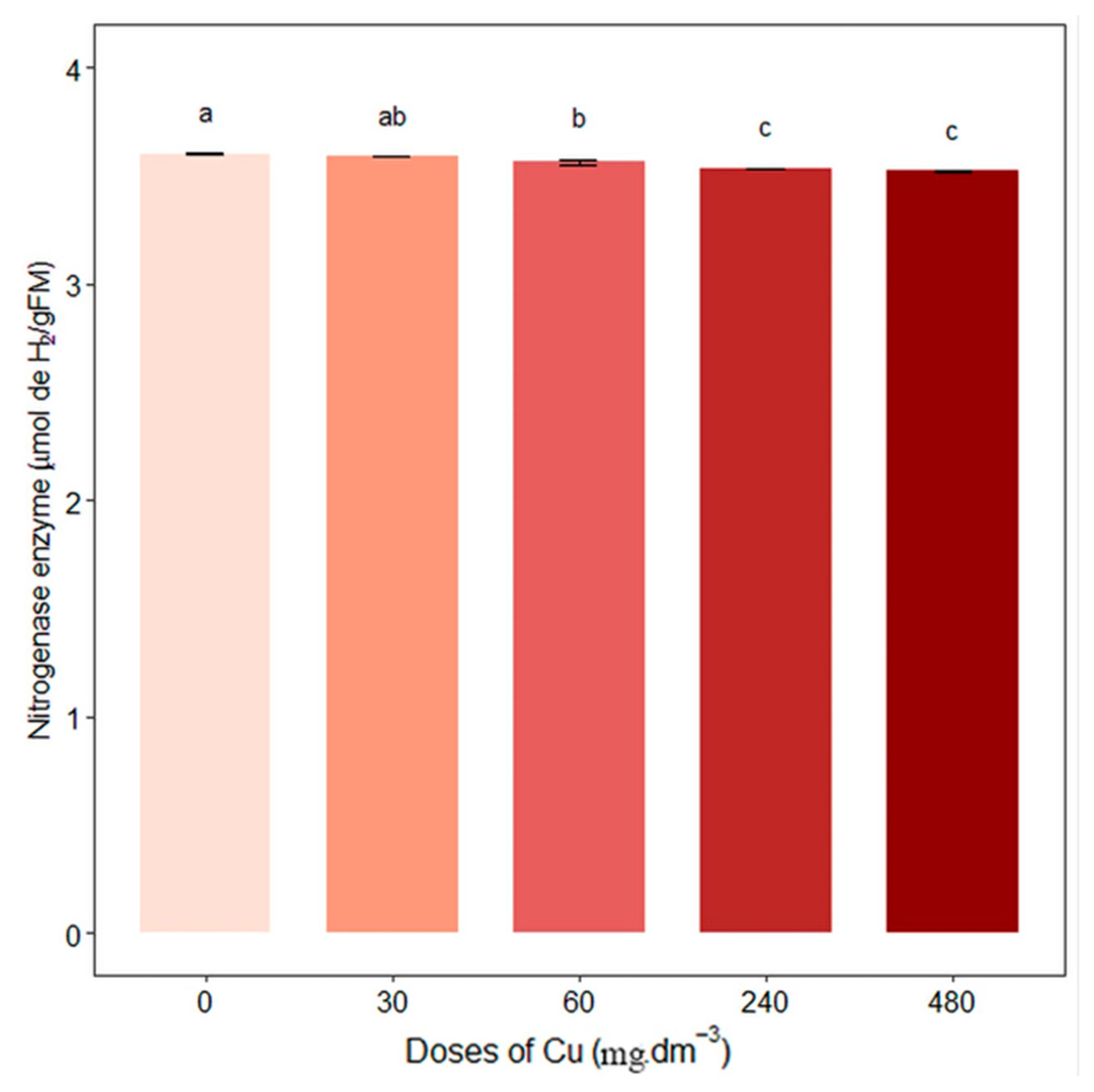
The activity of the nitrogenase enzyme decreased with increased copper concentration, and 60, 240, and 480 mg Cu dm−3 treatments differed significantly from the control (0 mg Cu·dm−3), which presented an activity of 3.59 ± 0.006 (Figure 2).
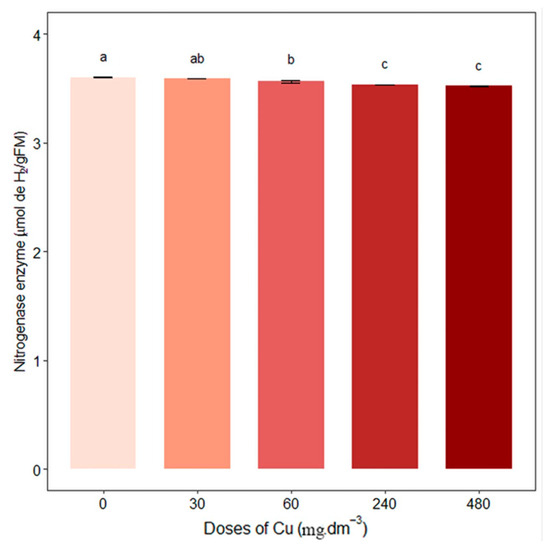
Figure 2.
Average nitrogenase enzyme content (µmol de H2/gFM). Different letters indicate statistical differences between treatments (p < 0.05).
3.2. Chlorophyll, Biomass and Nodules in Plants
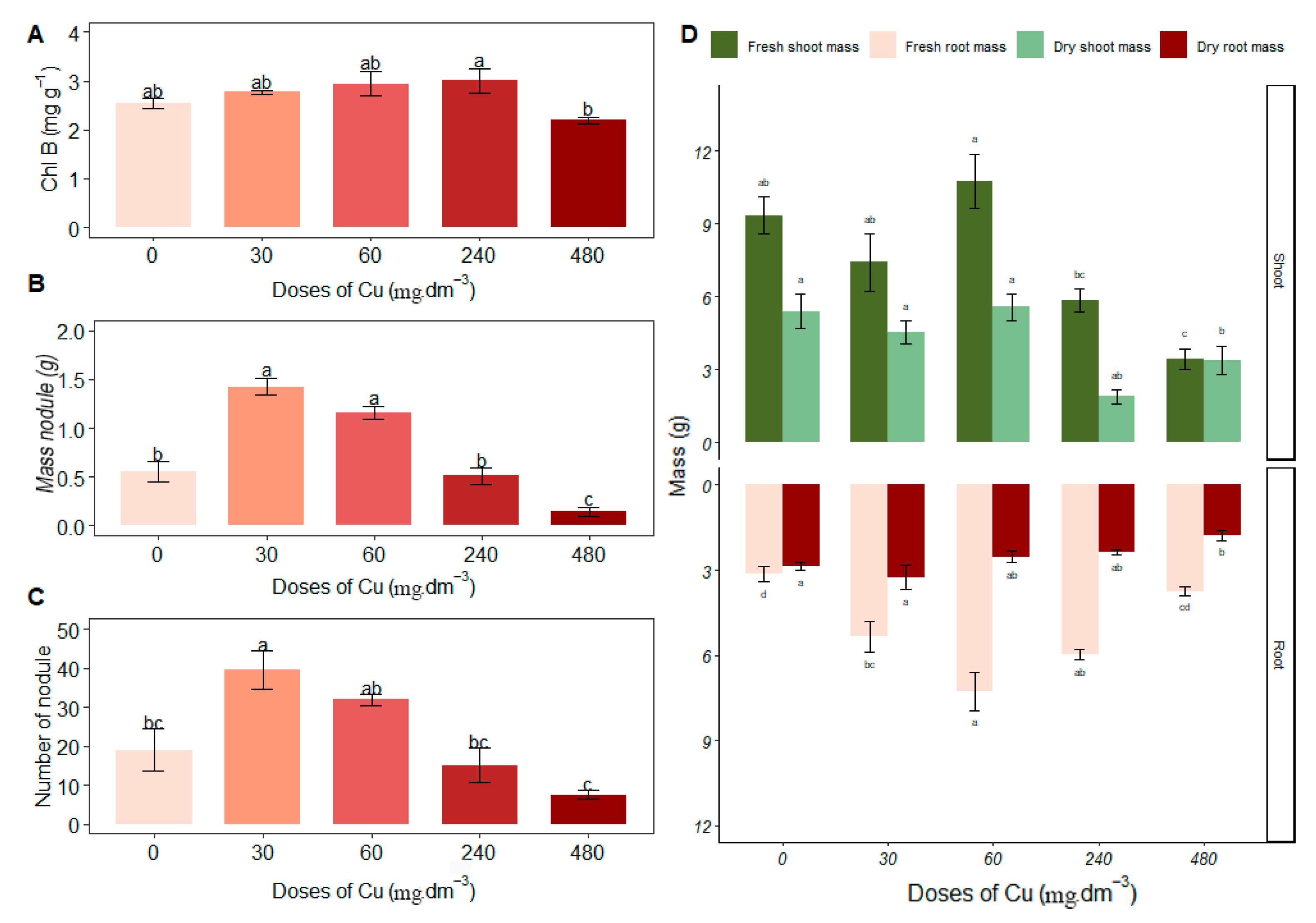
Excess copper (Cu) caused an increase in Chl b in Stizolobium aterrimum at 240 mg Cu dm−3 (3.01 ± 0.50), with no statistical difference compared to control treatments of 0, 30 and 60 mg Cu dm−3 (Figure 3A). There were no significant differences in Chl a and Clt levels in response to excess Cu.
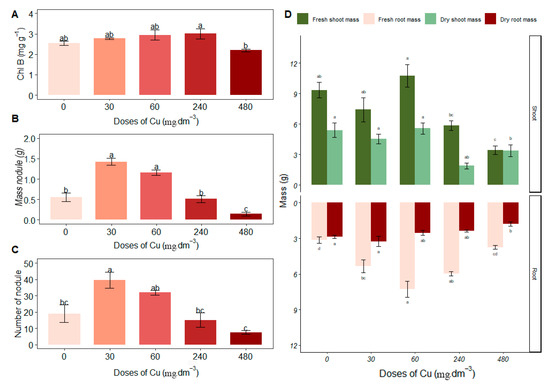
Figure 3.
Average values of (A) chlorophyll B—Chl B (mg·g−1); (B) nodule mass (g); (C) number of nodules; (D) fresh and dry mass of shoots and roots (g) under different concentrations of copper. Different letters indicate statistical differences between treatments (p < 0.05).
In the biometric evaluations (Figure 3D), Stizolobium aterrimum presented greater shoot fresh mass in the 60 mg Cu dm−3 treatment (10.75 ± 2.22); despite being statistically equal to the control, this treatment also increased root fresh mass compared to the control (7.28 ± 1.33). In the 60 mg Cu dm−3 treatment, Cu stimulated the growth of plant biomass with increases in shoot fresh mass, root fresh mass, and shoot dry mass. Although shoot dry mass did not have the highest average, it did not differ significantly from the control (0 mg Cu dm−3; 5.57 ± 1.11).
The fresh root mass increased in the 60 mg Cu dm−3 treatment (7.28 ± 1.33), differing from the 240 and 480 mg Cu dm−3 treatments. This suggests a larger volume occupied by the root system in the soil and a greater contact surface, favoring interaction with rhizobial strains. The root dry mass was higher at 30 mg Cu dm−3 (3.25 ± 0.86) compared to the 480 mg Cu dm−3 treatment (Figure 3D).
There was an increase in root fresh mass and root dry mass at the 30 mg Cu dm−3 treatment (5.35 ± 1.10 and 3.25 ± 0.86, respectively). Nodule mass at 30 and 60 mg Cu dm−3 showed no significant difference (1.42 ± 0.17 and 1.16 ± 0.13, respectively; Figure 3B), indicating higher nodule biomass under these treatments. Additionally, nodules were more numerous in the 30 mg Cu dm−3 treatment (Figure 3C).
3.3. Nitrogen Compounds: Amino Acids and Proteins
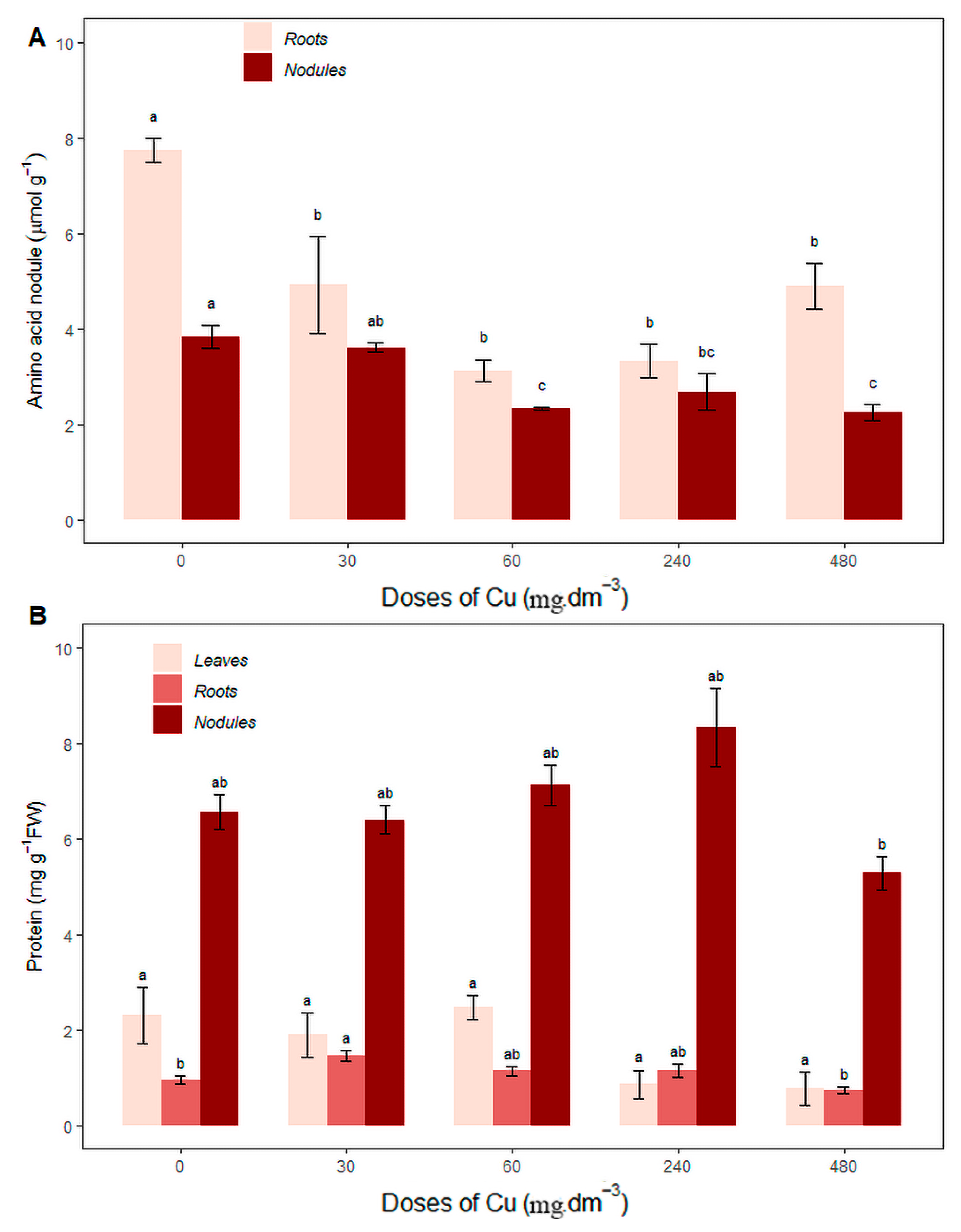
There was an increase in the amino acid concentration in the roots of plants grown in soil without the addition of Cu (0 mg Cu dm−3) compared to the other treatments, with a value of 7.73 ± 0.50, while the amino acid concentration in control nodules (3.82 ± 0.46) did not differ significantly from the 30 mg Cu dm−3 treatment (3.60 ± 0.18), both showing higher average values (Figure 4A), suggesting that the metabolism of amino acids was activated in order to increase the synthesis of amino acids to meet the nitrogen needs of the plant, given that nitrogen synthesis from biological nitrogen fixation is not sufficient for plant growth.
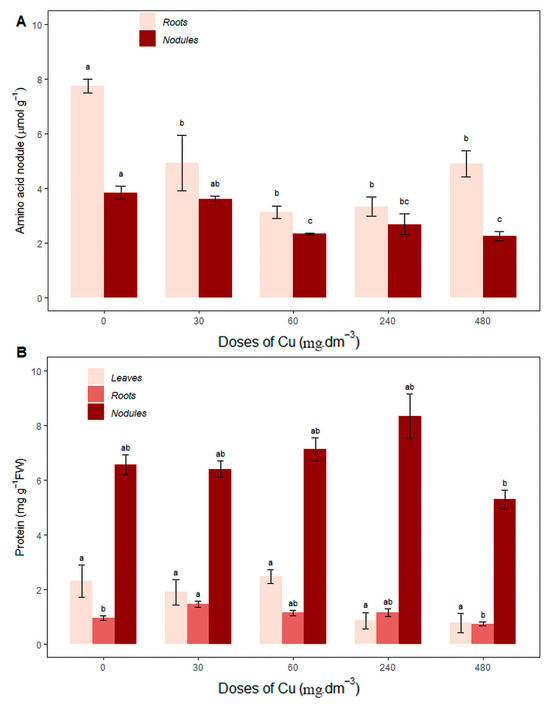
Figure 4.
Average content of (A) root and nodule amino acids (mg·g−1FW); (B) protein from leaves, roots, and nodules (mg·g−1FW) under different copper concentrations. Different letters indicate statistical differences between treatments (p < 0.05).
Total protein concentration (Figure 4B) in the leaves was the same for all treatments, and root proteins increased in the 30 mg Cu dm−3 treatment (1.45 ± 0.21) compared to the control (0 mg Cu dm−3) and the 480 mg Cu dm−3 treatment. Nodule proteins increased in the 240 mg Cu dm−3 treatment (8.32 ± 1.62), not differing statistically from the control (0 mg Cu dm−3) or the 30 and 60 mg Cu dm−3 treatments.
The total protein concentration did not vary in the leaves across all treatments, but there was an increase in the roots and nodules (Figure 4B). Root protein levels increased in the 30 mg Cu dm−3 treatment (1.45 ± 0.21) compared to the control (0 mg Cu dm−3) and the 480 mg Cu dm−3 treatment, while nodule protein levels increased in the 240 mg Cu dm−3 treatment (8.32 ± 1.62), not differing statistically from the control (0 mg Cu dm−3) or the 30 and 60 mg Cu dm−3 treatments.
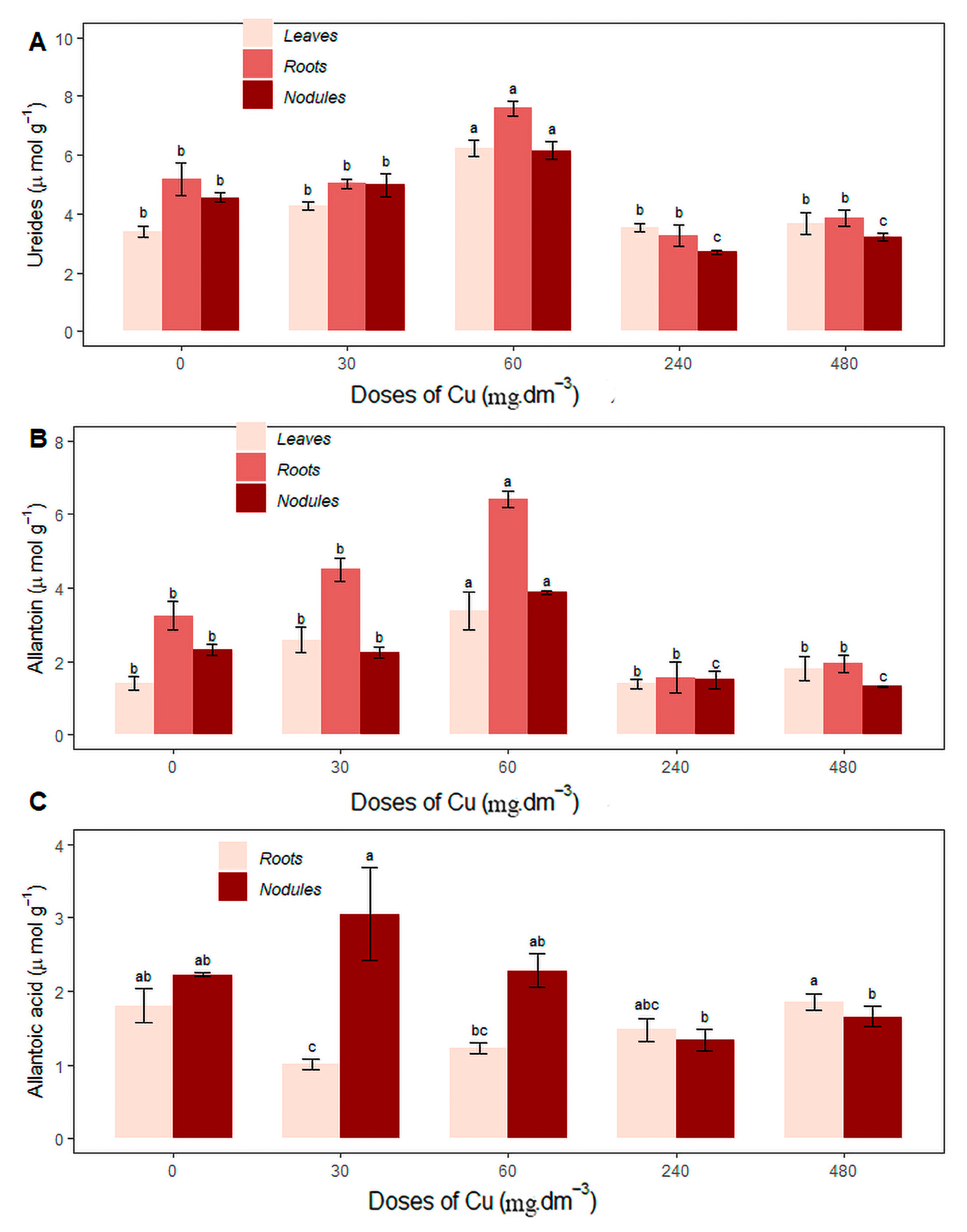
3.4. Nitrogenous Transport Compounds: Ureides, Allantoin and Allantoic Acid
The 60 mg Cu dm−3 treatment stood out with regard to increasing the ureide concentration in leaves, roots, and nodules, with values of 6.24 ± 0.51, 7.59 ± 0.48, and 6.16 ± 0.57, respectively, compared to the control (0 mg Cu dm−3) and the other treatments (Figure 5A). The same trend occurred for allantoin concentrations, with values of 3.38 ± 1.02, 6.43 ± 0.44, and 3.87 ± 0.11, respectively, in the same organs (Figure 5B).
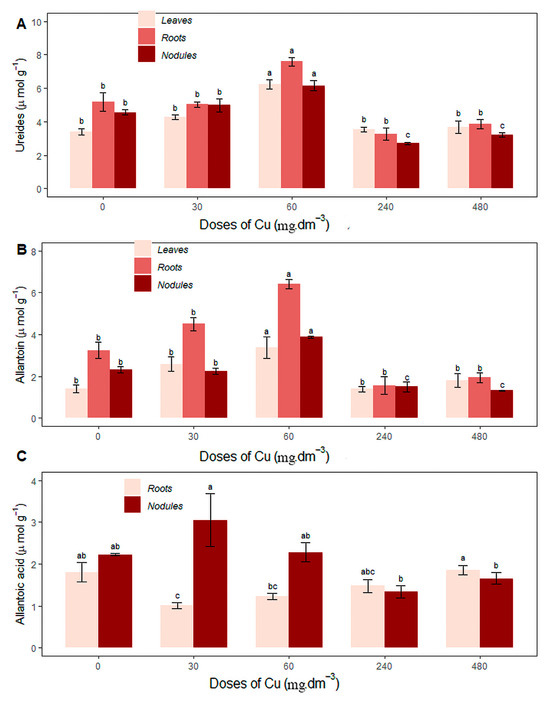
Figure 5.
Average content of (A) ureides in leaves, roots, and nodules (µmol·g−1); (B) allantoin in leaves, roots, and nodules (µmol·g−1); (C) allantoic acid (µmol·g−1) under different copper concentrations. Different letters indicate statistical differences between treatments (p < 0.05).
The highest allantoic acid concentration in the roots occurred in the 480 mg Cu dm−3 treatment, not differing statistically from the control (0 mg Cu dm−3), at 1.86 ± 0.22, respectively. In the nodules, the highest average was recorded in the 30 mg Cu dm−3 treatment (3.05 ± 1.25), and did not differ statistically from the control (0 mg Cu dm−3) and the 60 mg Cu dm−3 treatment (Figure 5C). There were no significant differences in the allantoic acid concentration in the leaves in response to excess Cu.
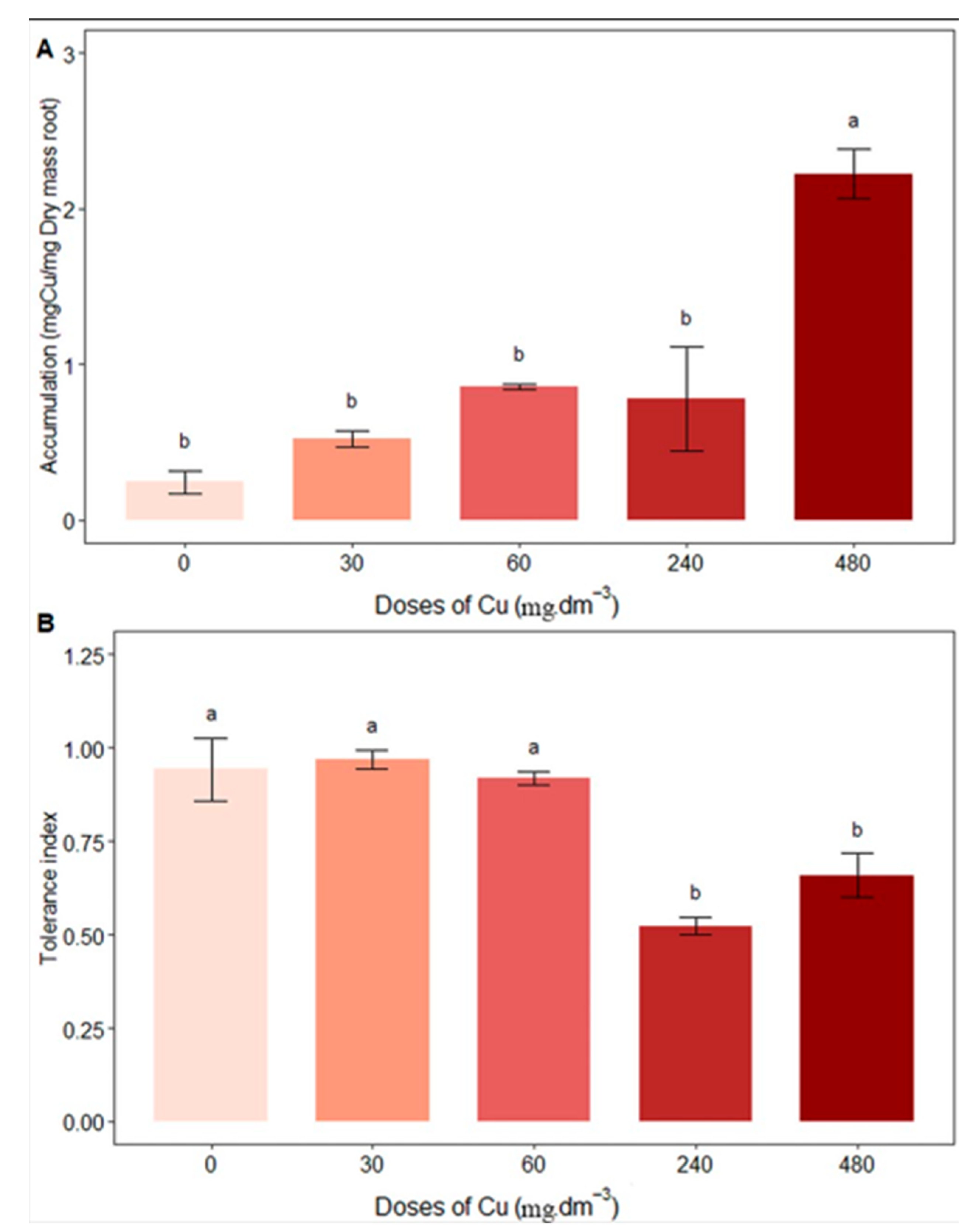
3.5. Copper Accumulation and Tolerance Index of Plants
The root was the organ that accumulated the most Cu in the plant tissue, and the 480 mg Cu dm−3 treatment presented the greatest accumulation (2.22 ± 0.31) (Figure 6A). This value corresponds to an 11% increase in Cu content in plant biomass compared to the control (0 mg Cu dm−3). There were no significant differences in shoot Cu accumulation between treatments. The values obtained were 0.94 ± 0.16, 0.96 ± 0.04, 0.91 ± 0.03, 0.52 ± 0.04, and 0.65 ± 0.11 for the control and 30, 60, 240, and 480 mg Cu dm−3 treatments, respectively. The control and 30 and 60 mg Cu dm−3 treatments differed significantly from the others (Figure 6B).
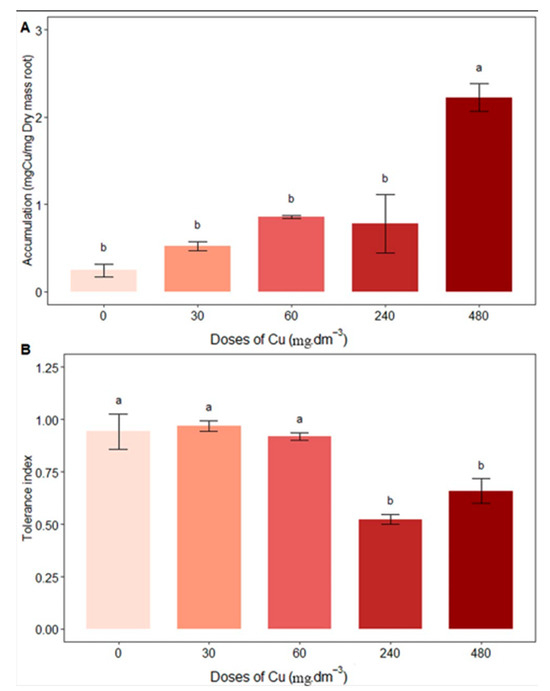
Figure 6.
Average content of (A) accumulation in roots (mg Cu/ mg dry mass); (B) tolerance index under different copper concentrations. Different letters indicate statistical differences between treatments (p < 0.05).
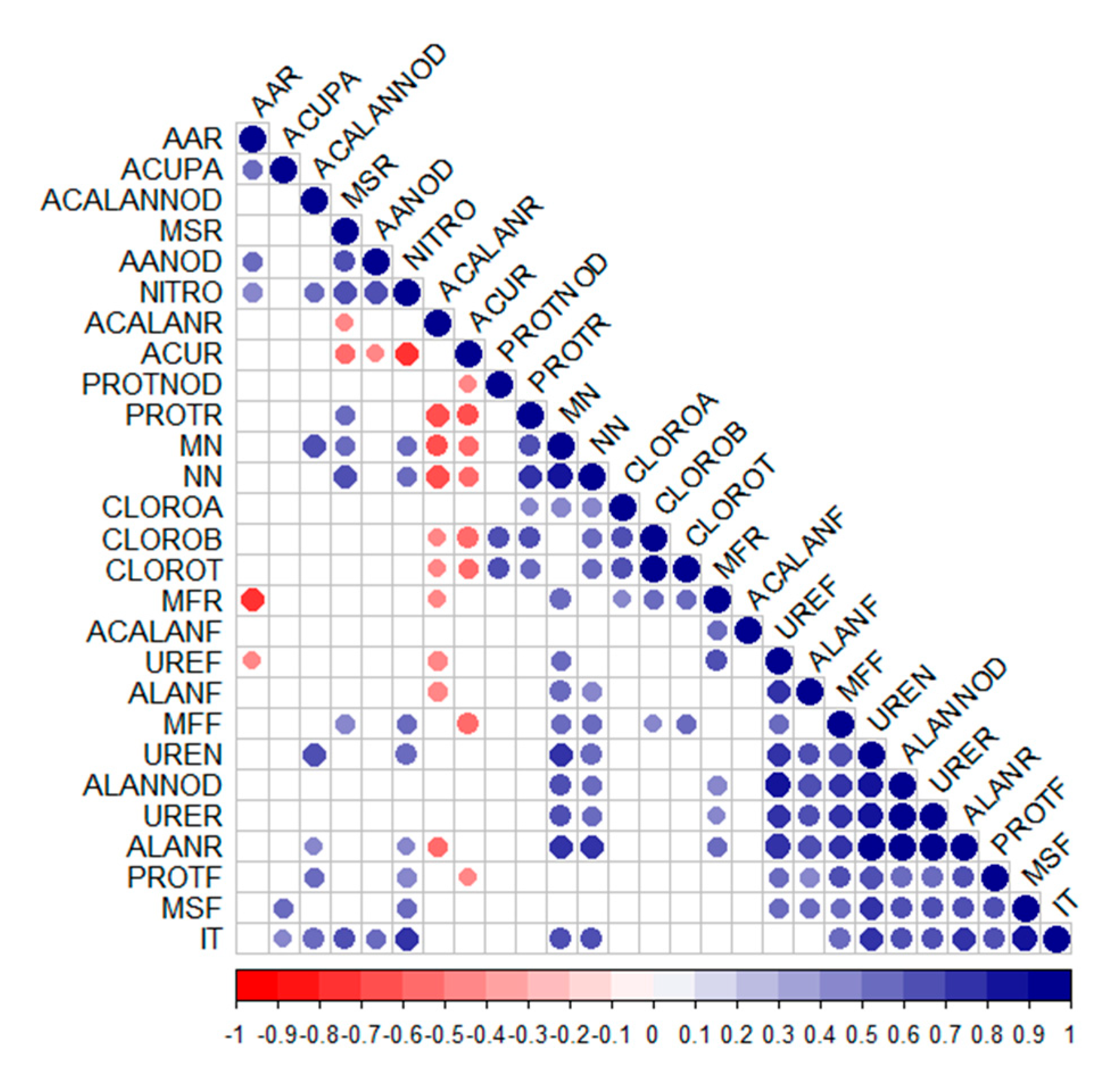
The tolerance index, shoot dry mass, and leaf proteins showed positive correlations with each other (Figure 7) and with several other variables, such as the quantification of allantoin in roots and nodules, ureides in roots and nodules, shoot fresh mass, and nitrogenase activity. Additionally, strong positive correlations were observed between the variable mass of nodules and number of nodules, chlorophyll a, root fresh mass and shoot fresh mass, ureides in leaves, nodules, and roots, allantoin in nodules, roots, and leaves, and tolerance index.
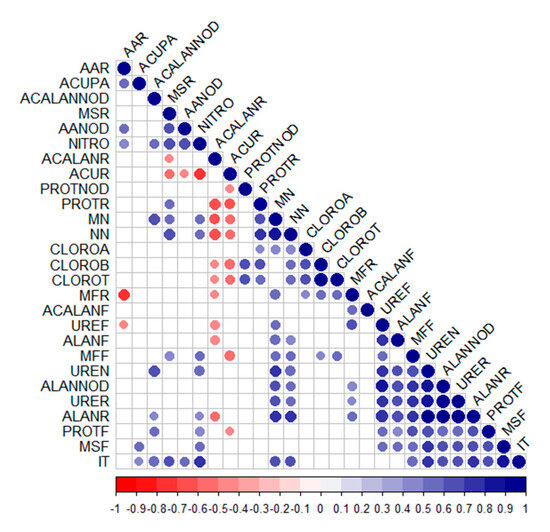
Figure 7.
Correlation matrix. Blank spaces indicate non-significant correlations according to Pearson’s correlation test (p < 0.05). AAR—amino acids in roots. ACUPA—shoot copper accumulation. ACALANNOD—allantoic acid in nodules. MSR—root dry mass. AANOD—amino acids in nodules. NITRO—nitrogenase enzyme. ACALANR—allantoic acid in roots. ACUR—root copper accumulation. PROTNOD—proteins in nodules. PROTR—proteins in roots. MN—mass of nodules. NN—number of nodules. CLOROA—chlorophyll a. CLOROB—chlorophyll b. CLOROT—total chlorophyll. MFR—root fresh mass. ACALANF—allantoic acid in leaves. UREF—ureides in leaves. ALANF—allantoin in leaves. MFF—leaf fresh mass. UREN—ureides in nodules. ALLANOD—allantoin in nodules. URER—ureides in roots. ALANR—allantoin in roots. PROTF—proteins in leaves. MSF—leaf dry mass. IT—tolerance index.
The two variables that showed the strongest negative correlations were allantoic acid in roots and copper accumulation in roots; both were negatively related to root proteins, mass of nodules, number of nodules, chlorophyll b, total chlorophyll content, and root dry mass.
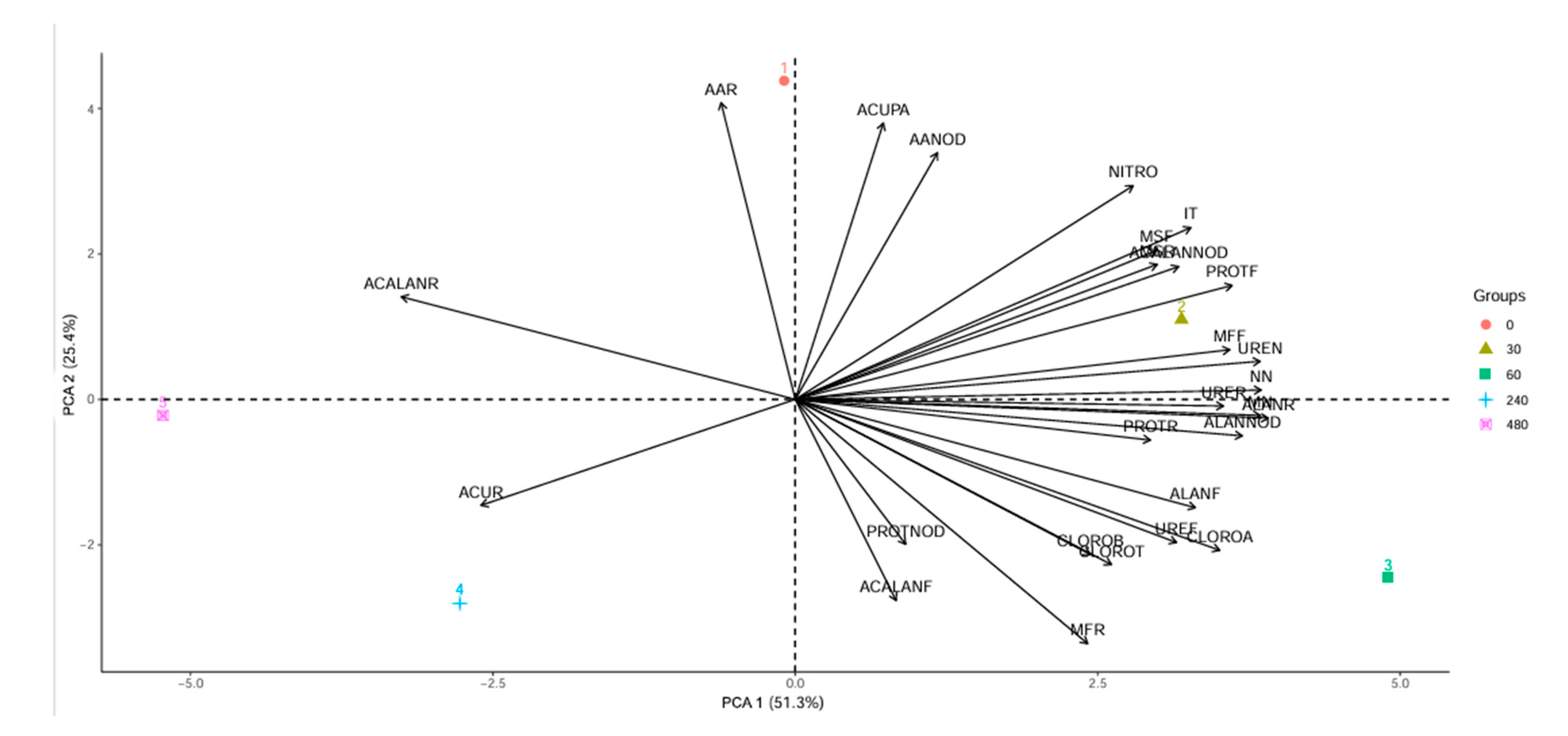
The first two principal components explained 76.7% of the data variation (Figure 8). The control treatment contributed to the variation in amino acid levels in roots and nodules, as well as to copper accumulation in shoots. In contrast, accumulation in roots showed a concentration-dependent response to copper, with the highest accumulation observed at 240 mg Cu dm−3. Leaf protein levels were significantly affected by the 30 mg Cu dm−3 concentration. Chlorophyll a, ureides, and allantoin levels in leaves were primarily associated with the 60 mg Cu dm−3 treatment.
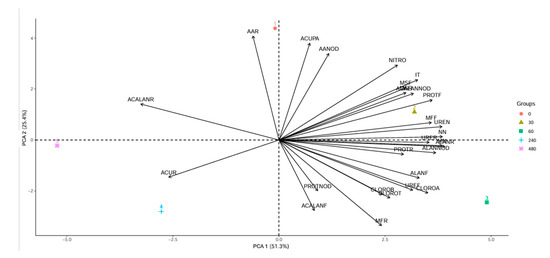
Figure 8.
Principal component analysis. Groups 0 to 480 indicate copper concentrations (mg Cu dm−3). AAR—amino acids in roots. ACUPA—shoot copper accumulation. ACALANNOD—allantoic acid in nodules. MSR—root dry mass. AANOD—amino acids in nodules. NITRO—nitrogenase enzyme. ACALANR—allantoic acid in roots. ACUR—root copper accumulation. PROTNOD—proteins in nodules. PROTR—proteins in roots. MN—mass of nodules. NN—number of nodules. CLOROA—chlorophyll a. CLOROB—chlorophyll b. CLOROT—total chlorophyll. MFR—root fresh mass. ACALANF—allantoic acid in leaves. UREF—ureides in leaves. ALANF—allantoin in leaves. MFF—leaf fresh mass. UREN—ureides in nodules. ALLANOD—allantoin in nodules. URER—ureides in roots. ALANR—allantoin in roots. PROTF—proteins in leaves. MSF—leaf dry mass. IT—tolerance index.
4. Discussion
Cu concentration available in the soil influenced the activity of the nitrogenase enzyme, demonstrating a negative impact on the nitrogen fixation capacity. Phytotoxic concentrations of metal in the soil contribute negatively to nitrogenase activity in species such as Acacia mangium, Enterolobium contortisiliquum and Sesbana virgata, resulting in a decrease in the number of nodules and vegetative growth [34]. The rhizobium–plant interaction also represents an important factor for nitrogenase activity, given that it is proven that some groups of rhizobia are less tolerant to the metal than others. Cu is the most toxic metal for species of symbiotic bacteria that fix nitrogen, justifying the decrease in nitrogenase activity at high concentrations [35,36,37].
Nitrogen fixation and assimilation promote increased plant biomass, especially at the dose of 60 mg Cu·dm−3, which stimulated shoot and root biomass accumulation. However, higher copper doses impaired root growth, reducing nodule formation and, consequently, biological nitrogen fixation, highlighting plant sensitivity to excess Cu.
Inhibition of root growth is one of the fastest responses to Cu toxicity. The reduction in biomass production occurs because, although Cu is an essential micronutrient for plant growth, having important functions in several physiological processes such as respiration, photosynthesis, carbohydrate allocation, nitrogen fixation and protein metabolism [38], when in high concentrations in the soil it can be absorbed in greater quantities by the plant, causing damage to the electron transport chain and a reduction in the number of chloroplasts, which affects negatively photosynthetic activity of the plant [39].
Regarding nitrogen metabolism compounds, allantoin and allantoic acid are two forms of ureides used for nitrogen transport which are used as mechanisms to assess the efficiency of biological fixation. The advantage of transport in the form of allantoin and allantoic acid lies in the efficiency of nitrogen transport in relation to carbon atoms in the molecule [40,41]. The allantoic acid concentrations in root tissue was higher in treatments with high doses of Cu, reaching a higher concentration in these treatments when compared to control. When analyzing the allantoin concentration, it is clear that intermediate doses of copper in the range of 60 mg Cu dm−3 cause greater accumulation of this compound, both in root tissue and in nodules.
It is possible to observe that in the 60 mg Cu dm−3 treatment the element enhances nitrogen fixation via biological fixation, given the increase in soluble compounds. In this treatment, the ureide concentration in leaves, roots and nodules increased, demonstrating that the plant is transporting the fixed nitrogen from the roots (where biological nitrogen fixation occurs) to its other organs.
Amino acid synthesis may have increased to meet the nitrogen needs of the plant, given that nitrogen synthesis from biological nitrogen fixation would not be sufficient for plant growth. Total protein concentration in roots increased at copper concentrations of 30 mg Cu dm−3, demonstrating that nitrogen is present in a usable form for other metabolic processes, which may be structural-, defense-, regulation-, and/or transport-related [22,23].
Copper acts as a cofactor in metalloproteins involved in electron transport in chloroplasts and mitochondria, as well as in defense against oxidative stress through enzymes such as Cu/Zn-superoxide dismutase [42]. The maintenance of protein levels in leaves suggests preservation of photosynthesis and may be related to the stability of photosystem II, since no significant changes were observed in chlorophyll content under Cu stress [43,44]. In contrast, increased protein levels in roots and nodules indicate acclimation through antioxidant and homeostatic mechanisms [45]. The distribution of proteins across tissues may reflect a Cu tolerance strategy aimed at sustaining vital functions under stress.
Cu accumulated in leaves and stems did not show significant variation, and an increase in Cu accumulation was observed only in root tissue, mainly in the 480 mg Cu dm−3 treatment. Higher Cu concentration in root tissues may be related to low translocation of the metal to the aerial part. To be considered phytostabilizers, plants must absorb large amounts of the metal and retain it in the roots, with low translocation to the aerial part [22,46,47].
The tolerance index showed a variation, with an increase in the 60 mg Cu dm−3 treatment, followed by a decrease in the 240 mg Cu dm−3 treatment. This variation indicates that concentrations close to 60 mg Cu dm−3 of Cu in the soil are ideal for plant growth. Although it is not possible to state that at higher doses the plant cannot develop—given that the tolerance index indicates a percentage of 52%, a study with a longer time span and intermediate doses of Cu is required to evaluate the growth capacity during the life cycle.
5. Conclusions
Stizolobium aterrimum has shown tolerance to low concentrations of Cu in the soil with regard to its growth and nitrogen fixation capacity. At high doses, there is a drastic reduction in plant growth. Greater Cu concentration in the soil reduces the production of fresh mass and increases the internal Cu concentration in the root system.
Doses in the range of 240 mg dm3 can already be considered toxic to Stizolobium aterrimum. The species has the potential to be a phytostabilizer, since it demonstrates the capacity to absorb and accumulate copper in the root tissues. However, as a future direction, it is necessary to verify the transfer of copper from the soil to the plant and the translocation of the nutrient throughout the legume body to confirm its use in the decontamination of environments.
Author Contributions
Conceptualization, L.S.d.C.; methodology, B.G.P.C. and J.V.A.; formal analysis, B.G.P.C. and J.V.A.; writing—original draft preparation, B.G.P.C., J.V.A. and B.S.d.S.; writing—review and editing, L.S.d.C., M.L.G.O., R.P.d.S., T.C.F., A.d.M.L. and L.A.d.S.; supervision, L.S.d.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo, (FAPESP; grant number 2020/12421-4 to L.S.C.), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; finance code 001), and the National Council for Scientific and Technological Development (CNPq; grant number 302499/2021-0 to L.S.C.).
Data Availability Statement
Data are contained within the article.
Acknowledgments
This work was carried out with the support of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Goswami, S.; Das, S. Copper Phytoremediation Potential of Calandula officinalis L. and the Role of Antioxidant Enzymes in Metal Tolerance. Ecotoxicol. Environ. Saf. 2016, 126, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Forli, F.; Otto, R.; Vitti, G.C.; do Vale, D.W.; Miyake, R.T.M. Micronutrients Application on Cultivation of Sugarcane Billets. Afr. J. Agric. Res. 2017, 12, 790–794. [Google Scholar] [CrossRef]
- Chia, J.-C.; Yan, J.; Rahmati Ishka, M.; Faulkner, M.M.; Simons, E.; Huang, R.; Smieska, L.; Woll, A.; Tappero, R.; Kiss, A.; et al. Loss of OPT3 Function Decreases Phloem Copper Levels and Impairs Crosstalk between Copper and Iron Homeostasis and Shoot-to-Root Signaling in Arabidopsis Thaliana. Plant Cell 2023, 35, 2157–2185. [Google Scholar] [CrossRef]
- Rehman, M.; Maqbool, Z.; Peng, D.; Liu, L. Morpho-Physiological Traits, Antioxidant Capacity and Phytoextraction of Copper by Ramie (Boehmeria nivea L.) Grown as Fodder in Copper-Contaminated Soil. Environ. Sci. Pollut. Res. Int. 2019, 26, 5851–5861. [Google Scholar] [CrossRef] [PubMed]
- Napoli, M.; Cecchi, S.; Grassi, C.; Baldi, A.; Zanchi, C.A.; Orlandini, S. Phytoextraction of Copper from a Contaminated Soil Using Arable and Vegetable Crops. Chemosphere 2019, 219, 122–129. [Google Scholar] [CrossRef]
- Seliga, H. Nitrogen Fixation in Several Grain Legume Species with Contrasting Sensitivities to Copper Nutrition. Acta Physiol. Plant. 1998, 20, 263–267. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in Abiotic and Biotic Environments; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-0-429-16151-3. [Google Scholar]
- Lam, E.J.; Cánovas, M.; Gálvez, M.E.; Montofré, Í.L.; Keith, B.F.; Faz, Á. Evaluation of the Phytoremediation Potential of Native Plants Growing on a Copper Mine Tailing in Northern Chile. J. Geochem. Explor. 2017, 182, 210–217. [Google Scholar] [CrossRef]
- Ghazaryan, K.; Movsesyan, H.; Ghazaryan, N.; Watts, B.A. Copper Phytoremediation Potential of Wild Plant Species Growing in the Mine Polluted Areas of Armenia. Environ. Pollut. 2019, 249, 491–501. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Ahmar, S.; Ullah Khan, M.H.; Rehman, M.; Maqbool, Z.; Liu, L. Morpho-Physiological Traits, Gaseous Exchange Attributes, and Phytoremediation Potential of Jute (Corchorus capsularis L.) Grown in Different Concentrations of Copper-Contaminated Soil. Ecotoxicol. Environ. Saf. 2020, 189, 109915. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Starker, C.G.; Parra-Colmenares, A.L.; Smith, L.; Mitra, R.M.; Long, S.R. Nitrogen Fixation Mutants of Medicago Truncatula Fail to Support Plant and Bacterial Symbiotic Gene Expression. Plant Physiol. 2006, 140, 671–680. [Google Scholar] [CrossRef]
- Yanlin, M.; Chengbin, X.; Jianquan, L.; Guangpeng, R. Nutrient-dependent regulation of symbiotic nitrogen fixation in legumes. Hortic. Res. 2024, 12, uhae321. [Google Scholar] [CrossRef]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, Toxicity and Tolerance in Plants and Management of Cu-Contaminated Soil. BioMetals 2021, 34, 737–759. [Google Scholar] [CrossRef]
- Mergaert, P.; Kereszt, A.; Kondorosi, E. Gene Expression in Nitrogen-Fixing Symbiotic Nodule Cells in Medicago truncatula and Other Nodulating Plants. Plant Cell 2020, 32, 42–68. [Google Scholar] [CrossRef] [PubMed]
- Yruela, I. Cobre em plantas. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef]
- de Souza, L.A.; de Andrade, S.A.L.; de Souza, S.C.R.; Schiavinato, M.A. Tolerância e potencial fitorremediador de Stizolobium aterrimum associada ao fungo micorrízico arbuscular Glomus etunicatum em solo contaminado por chumbo. Rev. Bras. Ciênc. Solo 2011, 35, 1441–1451. [Google Scholar] [CrossRef]
- Costa, B.G.P.; Justino, G.C.; Aguiar, L.F.; Souza, L.A.; Camargos, L.S. Boron Phytoremediation: Stizolobium aterrimum Is Tolerant and Can Be Used for Phytomanagement of Boron Excess in Soils. Int. J. Environ. Stud. 2019, 76, 329–337. [Google Scholar] [CrossRef]
- da Silva, M.B.; Bomfim, N.C.P.; da Silva, V.N.; de Lima Frachia, C.; de Souza, L.A.; Justino, G.C.; de Camargos, L.S. Response of Cajanus Cajan to Excess Copper in the Soil: Tolerance and Biomass Production. Physiol. Mol. Biol. Plants 2022, 28, 1335–1345. [Google Scholar] [CrossRef]
- Bomfim, N.C.P.; Aguilar, J.V.; Ferreira, T.C.; dos Santos, B.S.; de Paiva, W.d.S.; de Souza, L.A.; Camargos, L.S. Root Development in Leucaena leucocephala (Lam.) de Wit Enhances Copper Accumulation. Environ. Sci. Pollut. Res. 2023, 30, 80245–80260. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, B.S.; Mendonça, G.W.; Ferreira, T.C.; Bomfim, N.C.P.; de Carvalho, I.F.; Aguilar, J.V.; Camargos, L.S. Exploring the Potential of Crotalaria juncea L. for Phytoremediation: Insights from Gas Exchange, Pigment Quantification, and Growth Measurements under Copper Stress. Horticulturae 2024, 10, 746. [Google Scholar] [CrossRef]
- Sousa, R.R.; Ferraz, T.M.; de Oliveira, J.D.; Nascimento, I.d.O.; Reis, F.d.O.; Costa, N.B. Phytoremediation potential of Canavalia ensiformis in copper- and zinc-contaminated soil. Rev. Em Agronegócio E Meio Ambiente 2024, 17, e11022. [Google Scholar]
- Cambrollé, J.; García, J.L.; Figueroa, M.E.; Cantos, M. Evaluating wild grapevine tolerance to copper toxicity. Chemosphere 2015, 120, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Santoyo-Martínez, M.; Mussali-Galante, P.; Hernández-Plata, I.; Valencia-Cuevas, L.; Flores-Morales, A.; Ortiz-Hernández, L.; Flores-Trujillo, K.; Ramos-Quintana, F.; Tovar-Sánchez, E. Heavy Metal Bioaccumulation and Morphological Changes in Vachellia Campechiana (Fabaceae) Reveal Its Potential for Phytoextraction of Cr, Cu, and Pb in Mine Tailings. Environ. Sci. Pollut. Res. 2020, 27, 11260–11276. [Google Scholar] [CrossRef]
- Santoyo-Martínez, M.; Mussali-Galante, P.; Hernández-Plata, I.; Valencia-Cuevas, L.; Rodríguez, A.; Castrejón-Godínez, M.L.; Tovar-Sánchez, E. Phytoremediation Potential of Crotalaria Pumila (Fabaceae) in Soils Polluted with Heavy Metals: Evidence from Field and Controlled Experiments. Plants 2024, 13, 1947. [Google Scholar] [CrossRef]
- Karczewska, A.; Mocek, A.; Goliński, P.; Mleczek, M. Phytoremediation of Copper-Contaminated Soil. In Phytoremediation: Management of Environmental Contaminants, Volume 2; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 143–170. ISBN 978-3-319-10969-5. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Olive Tree, S.A. Assessment of the Nutritional Status of Plants: Principles and Applications, 2nd ed.; Brazilian Association for Potashion and Phosphate Research: Piracicaba, Brazil, 1997. [Google Scholar]
- Hiscox, J.D.; Israelstam, G.F. A Method for the Extraction of Chlorophyll from Leaf Tissue without Maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Bieleski, R.L.; Turner, N.A. Separation and Estimation of Amino Acids in Crude Plant Extracts by Thin-Layer Electrophoresis and Chromatography. Anal. Biochem. 1966, 17, 278–293. [Google Scholar] [CrossRef]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R.E. The Determination of Amino-Acids with Ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- Vogels, G.D.; Van Der Drift, C. Differential Analyses of Glyoxylate Derivatives. Anal. Biochem. 1970, 33, 143–157. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Trannin, I.C.B.; Moreira, F.M.S.; Siqueira, J.O. Crescimento e nodulação de Acacia mangium, Enterolobium contortisiliquum e Sesbania virgata em solo contaminado com metais pesados. Rev. Bras. Ciênc. Solo 2001, 25, 743–753. [Google Scholar] [CrossRef]
- Arguello, J.M.; Raimunda, D.; Padilla-Benavides, T. Mechanisms of Copper Homeostasis in Bacteria. Front. Cell. Infect. Microbiol. 2013, 3, 73. [Google Scholar] [CrossRef]
- Elizalde-Díaz, J.P.; Hernández-Lucas, I.; Medina-Aparicio, L.; Dávalos, A.; Leija, A.; Alvarado-Affantranger, X.; García-García, J.D.; Hernández, G.; Garcia-de los Santos, A. Rhizobium Tropici CIAT 899 copA Gene Plays a Fundamental Role in Copper Tolerance in Both Free Life and Symbiosis with Phaseolus Vulgaris. Microbiology 2019, 165, 651–661. [Google Scholar] [CrossRef]
- Matsuda, A.; Moreira, F.M.d.S.; Siqueira, J.O. Tolerância de rizóbios de diferentes procedências ao zinco, cobre e cádmio. Pesqui. Agropecuária Bras. 2002, 37, 343–355. [Google Scholar] [CrossRef]
- Marques, D.M.; Veroneze Júnior, V.; da Silva, A.B.; Mantovani, J.R.; Magalhães, P.C.; de Souza, T.C. Copper Toxicity on Photosynthetic Responses and Root Morphology of Hymenaea courbaril L. (Caesalpinioideae). Water Air Soil Pollut. 2018, 229, 138. [Google Scholar] [CrossRef]
- Girotto, E.; Ceretta, C.A.; Rossato, L.V.; Farias, J.G.; Brunetto, G.; Miotto, A.; Tiecher, T.L.; de Conti, L.; Lourenzi, C.R.; Schmatz, R.; et al. Biochemical Changes in Black Oat (Avena strigosa Schreb) Cultivated in Vineyard Soils Contaminated with Copper. Plant Physiol. Biochem. 2016, 103, 199–207. [Google Scholar] [CrossRef]
- Lu, M.-Z.; Carter, A.M.; Tegeder, M. Altering Ureide Transport in Nodulated Soybean Results in Whole-Plant Adjustments of Metabolism, Assimilate Partitioning, and Sink Strength. J. Plant Physiol. 2022, 269, 153613. [Google Scholar] [CrossRef]
- Thu, S.W.; Lu, M.-Z.; Carter, A.M.; Collier, R.; Gandin, A.; Sitton, C.C.; Tegeder, M. Role of Ureides in Source-to-Sink Transport of Photoassimilates in Non-Fixing Soybean. J. Exp. Bot. 2020, 71, 4495–4511. [Google Scholar] [CrossRef]
- Gratão, P.L.; Polle, A.; Lea, P.J.; Azevedo, R.A. Making the Life of Heavy Metal-Stressed Plants a Little Easier. Funct. Plant Biol. 2005, 32, 481–494. [Google Scholar] [CrossRef]
- Bertrand, M.; Poirier, I. Photosynthetic Organisms and Excess of Metals. Photosynthetica 2005, 43, 345–353. [Google Scholar] [CrossRef]
- Yruela, I. Copper in Plants: Acquisition, Transport and Interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef]
- Raven, J.; Evans, M.C.W.; Korb, R.E. The Role of Trace Metals in Photosynthetic Electron Transport in O2-Evolving Organisms. Photosynth. Res. 1999, 60, 111–150. [Google Scholar] [CrossRef]
- Nedjimi, B. Phytoremediation: A Sustainable Environmental Technology for Heavy Metals Decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation Techniques for Removal of Heavy Metals from the Soil Contaminated through Different Sources: A Review. Environ. Sci. Pollut. Res. 2020, 27, 1319–1333. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).