Use of In Situ Soil Solution Electric Conductivity to Evaluate Mineral N in Commercial Orchards: Preliminary Results
Abstract
1. Introduction
2. Material and Methods
2.1. Orchard Description
2.2. Probes Installation and Characteristics
2.3. Soil Sampling and Analysis
2.4. Laboratory Determination
2.5. Statistical Analysis
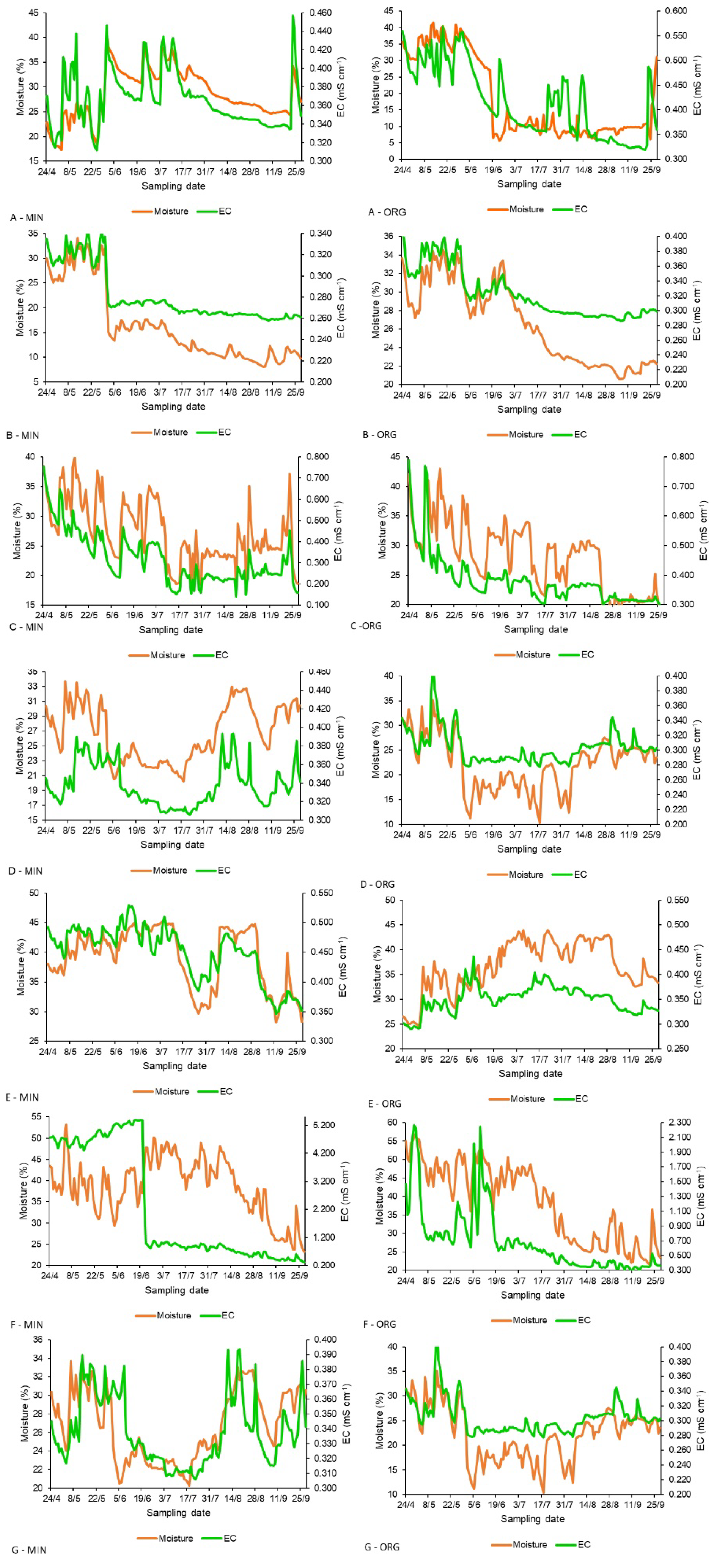
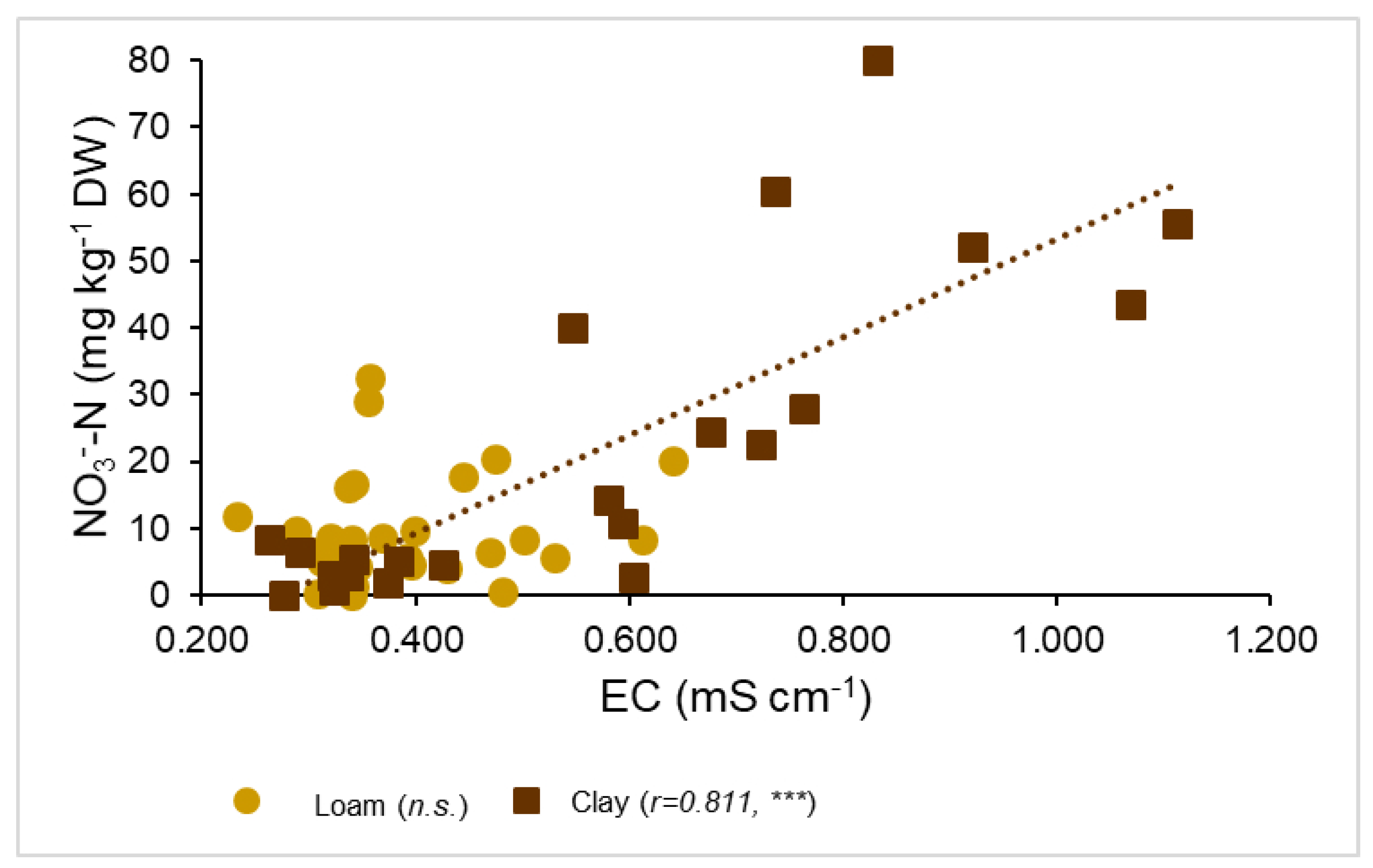
3. Results
3.1. Soil Characteristics
3.2. Trends of Soil Nitrate-N and EC
3.3. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations; The Food and Agriculture Organization (FAO): Rome, Italy, 2020. [Google Scholar]
- Yu, C.; Huang, X.; Chen, H.; Yu, C.Q.; Godfray, H.G.J.; Wright, S.J.; Hall, W.J.; Gong, P.; Ni, S.Q.; Huang, G.R.; et al. Managing nitrogen to restore water quality in China. Nature 2019, 567, 516–520. [Google Scholar] [CrossRef] [PubMed]
- McBratney, A.; Whelan, B.; Ancev, T.; Bouma, J. Future directions of precision agriculture. Precis. Agric. 2005, 6, 7–23. [Google Scholar] [CrossRef]
- Corwin, D.L.; Lesch, S.M. Application of soil electrical conductivity to precision agriculture. Agron. J. 2003, 95, 455–471. [Google Scholar]
- Pascual, M.; Rufat, J.; Arbonés, A.; Escolà, A.; Rosell, J.R.; Martinez-Casasnovas, J.A.; Josep, M.; Villar, M.; Vilarrasa, M. Use of soil EC a measurement for evaluating soil spatial variability for nitrogen management in super-intensive olive oil tree orchards. In Proceedings of the Efficient Use of Different Sources of Nitrogen in Agriculture–From Theory to Practice, Skara, Sweden, 27–29 June 2016; p. 335. [Google Scholar]
- Tagliavini, M.; Scudellari, D.; Marangoni, B.; Toselli, M. Nitrogen fertilization management in orchards to reconcile productivity and environmental aspects. Fertil. Res. 1996, 43, 93–102. [Google Scholar] [CrossRef]
- Stevenson, F.J. Cycles of Soil: The Nitrogen Cycle in Soil—Global and Ecological Aspects; John Wiley & Sons: Hoboken, NJ, USA, 1986; pp. 106–151. [Google Scholar]
- Toselli, M.; Perry, R.L.; Flore, J.A. Evaluation of nitrate-N leaching from lysimeter-grown bearing apple trees. Soil Sci. 2011, 176, 280–287. [Google Scholar] [CrossRef]
- Leggo, P.J.; Ledésert, B. The stimulation of nitrification in an organically enriched soil by zeolitic tuff and its effect on plant growing. Ann. Agrar. Sci. 2009, 7, 1–9. [Google Scholar]
- Miyamoto, T.; Kameyama, K.; Iwata, Y. Monitoring electrical conductivity and nitrate concentrations in an Andisol field using time domain reflectometry. Jpn. Agric. Res. Q. 2015, 49, 261–267. [Google Scholar] [CrossRef][Green Version]
- De Neve, S.; Van de Steene, J.; Hartmann, R.; Hofman, G. Using time domain reflectometry for monitoring mineralization of nitrogen from soil organic matter. Eur. J. Soil Sci. 2000, 51, 295–304. [Google Scholar] [CrossRef]
- Nissen, H.H.; Moldrup, P.; Henriksen, K. Time domain reflectometry measurements of nitrate transport in manure-amended soil. Soil Sci. Soc. Am. J. 1998, 62, 99–109. [Google Scholar] [CrossRef]
- Patriquin, D.G.; Blaikie, H.; Patriquin, M.J.; Yang, C. On-farm measurements of pH, electrical conductivity and nitrate in soil extracts for monitoring coupling and decoupling of nutrient cycles. Biol. Agric. Hortic. 1993, 9, 231–272. [Google Scholar] [CrossRef]
- Available online: www.RegioneEmilia-Romagna.it (accessed on 1 May 2020).
- Maynard, D.G.; Kalra, Y.P. Nitrate and exchangeable ammonium nitrogen. In Soil Sampling and Methods of Analysis; Carter, M.R., Ed.; Canadian Society of Soil Science, Lewis Publishers: Boca Raton, FL, USA, 1993; pp. 25–37. [Google Scholar]
- Mengel, K. Available nitrogen in soils and its determination by the ‘Nmin-method’ and by electro ultrafiltration (EUF). Fertil. Res. 1991, 28, 251–262. [Google Scholar] [CrossRef]
- Baldi, E.; Marcolini, G.; Quartieri, M.; Sorrenti, G.; Muzzi, E.; Toselli, M. Organic fertilization in nectarine (Prunus persica var. nucipersica) orchard combines nutrient management and pollution impact. Nutr. Cycl. Agroecosyst. 2016, 105, 39–50. [Google Scholar] [CrossRef]
- Tagliavini, M.; Millard, P.; Quartieri, M.; Marangoni, B. Foliar nitrogen uptake and withdrawal from peach leaves during senescence. In Proceedings of the III International Symposium on Mineral Nutrition of Deciduous Fruit Trees 448, Zaragoza, Spain, 27 May 1996; pp. 459–466. [Google Scholar]
- Toselli, M.; Baldi, E.; Cavani, L.; Sorrenti, G. Nutrient management in fruit crops: An organic way. In Fruit Crops; Srivastava, A.K., Chengxiao, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 379–392. [Google Scholar]
- Crisosto, C.H.; Mitchell, F.G.; Johnson, S. Factors in fresh market stone fruit quality. Postharvest News Inf. 1995, 6, 17–21. [Google Scholar]
- Daane, K.; Johnson, R.; Michailides, T.; Crisosto, C.; Dlott, J.; Ramirez, H.; Jokota, G.; Morgan, D. Excess nitrogen raises nectarine susceptibility to disease and insects. Calif. Agric. 1995, 49, 13–18. [Google Scholar] [CrossRef][Green Version]
- Crisosto, C.H.; Johnson, S.; DeJong, T.; Day, K.R. Orchard factors affecting postharvest stone fruit quality. HortScience 1997, 32, 820–823. [Google Scholar] [CrossRef]
- Das, B.S.; Wraith, J.M.; Inskeep, W.P. Nitrate concentrations in the root zone estimated using time domain reflectometry. Soil Sci. Soc. Am. J. 1999, 63, 1561–1570. [Google Scholar] [CrossRef]
- Koumanov, K.; Stoilov, G.; Dochev, D. The “Nitrate nitrogen–electrical conductivity” relationship in non-saline soils under fertigation. In Proceedings of the ICID 19th European Regional Conference “Sustainable Use of Land and Water”, Brno, Czech Republic, 4–8 June 2001; Volume 136, pp. 4–8. [Google Scholar]
- Baldi, E.; Toselli, M. Mineralization dynamics of different commercial organic fertilizers from agro-industry organic waste recycling: An incubation Fexperiment. Plant Soil Environ. 2014, 60, 93–99. [Google Scholar] [CrossRef]




| Species | Variety | Rootstock | Planting Year | Planting Distance (m) |
|---|---|---|---|---|
| Prunus domestica L. | September Yummy | GF677 (P. persica × P. dulcis) | 2015 | 4 × 1.5 |
| Prunus armeniaca L. | Faralia® | Mirobalan 29C (P. cerasifera) | 2014 | 4.8 × 2.5 |
| Prunus persica Batsch var. nucipersica | Romagna Red | Ishtara® (P. persica × P. cerasifera) | 2012 | 3.5 × 1.2 |
| Malus domestica Borkh | Rosy Glow | M9 | 2012 | 3.5 × 1 |
| Pyrus comunis L. | Abbé Fetél | Self-rooted | 2001 | 3.9 × 2.2 |
| Actinidia chinensis chinensis | Dorì | Hayward | 2017 | 4.5 × 2 |
| Actinidia chinensis deliciosa | Hayward | Self-rooted | 2016 | 5 × 2 |
| Variety | Sand (%) | Loam (%) | Clay (%) | Total N (‰ DWz) | OM (% DW) | CEC (Meq 100 g−1) | Texture | WRB |
|---|---|---|---|---|---|---|---|---|
| Apricot | 32 | 48 | 20 | 1.67 | 2.37 | 23.1 | loam | Fluvic Cambisols |
| Plum | 32 | 48 | 20 | 1.67 | 2.37 | 23.1 | loam | Fluvic Cambisols |
| Pear | 35 | 53 | 12 | 1.37 | 2.03 | 21.2 | silty-loam | Fluvic Cambisols |
| Peach | 16 | 57 | 27 | 1.12 | 1.43 | 14.9 | silty-clay-loam | Fluvic Cambisols |
| Apple | 42 | 43 | 15 | 1.27 | 1.82 | 14.5 | loam | Fluvic Cambisols |
| Dorì kiwifruit | 18 | 46 | 36 | 1.34 | 1.83 | 23.5 | silty-clay-loam | Hypovertic Cambisols |
| Hayward kiwifruit | 18 | 46 | 36 | 1.34 | 1.83 | 23.5 | silty-clay-loam | Hypovertic Cambisols |
| Sampling Date | Fertilization | NO3−-N (mg kg−1 ss) | EC (mS cm−1) | Moisture (%) |
|---|---|---|---|---|
| April 26th | Organic | 18.1 | 0.618 | 36.3 |
| Mineral | 17.4 | 0.442 | 30.3 | |
| Significance | nsz | ns | ns | |
| May 21st | Organic | 7.77 | 0.466 | 36.3 |
| Mineral | 6.16 | 0.389 | 31.9 | |
| Significance | ns | ns | ns | |
| June 26th | Organic | 27.8 | 0.474 | 32.4 |
| Mineral | 15.9 | 0.496 | 33.0 | |
| Significance | ns | ns | ns | |
| August 24th | Organic | 10.6 | 0.375 | 27.6 |
| Mineral | 9.88 | 0.400 | 31.3 | |
| Significance | ns | ns | ns | |
| September 27th | Organic | 34.9 | 0.347 | 26.5 |
| Mineral | 27.0 | 0.319 | 24.8 | |
| Significance | ns | ns | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldi, E.; Quartieri, M.; Muzzi, E.; Noferini, M.; Toselli, M. Use of In Situ Soil Solution Electric Conductivity to Evaluate Mineral N in Commercial Orchards: Preliminary Results. Horticulturae 2020, 6, 39. https://doi.org/10.3390/horticulturae6030039
Baldi E, Quartieri M, Muzzi E, Noferini M, Toselli M. Use of In Situ Soil Solution Electric Conductivity to Evaluate Mineral N in Commercial Orchards: Preliminary Results. Horticulturae. 2020; 6(3):39. https://doi.org/10.3390/horticulturae6030039
Chicago/Turabian StyleBaldi, Elena, Maurizio Quartieri, Enrico Muzzi, Massimo Noferini, and Moreno Toselli. 2020. "Use of In Situ Soil Solution Electric Conductivity to Evaluate Mineral N in Commercial Orchards: Preliminary Results" Horticulturae 6, no. 3: 39. https://doi.org/10.3390/horticulturae6030039
APA StyleBaldi, E., Quartieri, M., Muzzi, E., Noferini, M., & Toselli, M. (2020). Use of In Situ Soil Solution Electric Conductivity to Evaluate Mineral N in Commercial Orchards: Preliminary Results. Horticulturae, 6(3), 39. https://doi.org/10.3390/horticulturae6030039







