Water-Stress Influences on Three New Promising HLB-Tolerant Citrus Rootstocks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
2.2. Water-Stress Treatments and Experimental Design
2.3. Chlorophyll Index (SPAD) Evaluation
2.4. Plant Growth Evaluation
2.5. Biomass
2.6. Plant Water Status
2.6.1. Leaf Water Potential
2.6.2. Stomatal Conductance
2.6.3. Relative Water Content
2.6.4. Electrolyte Leakage
2.7. Data Analysis
3. Results
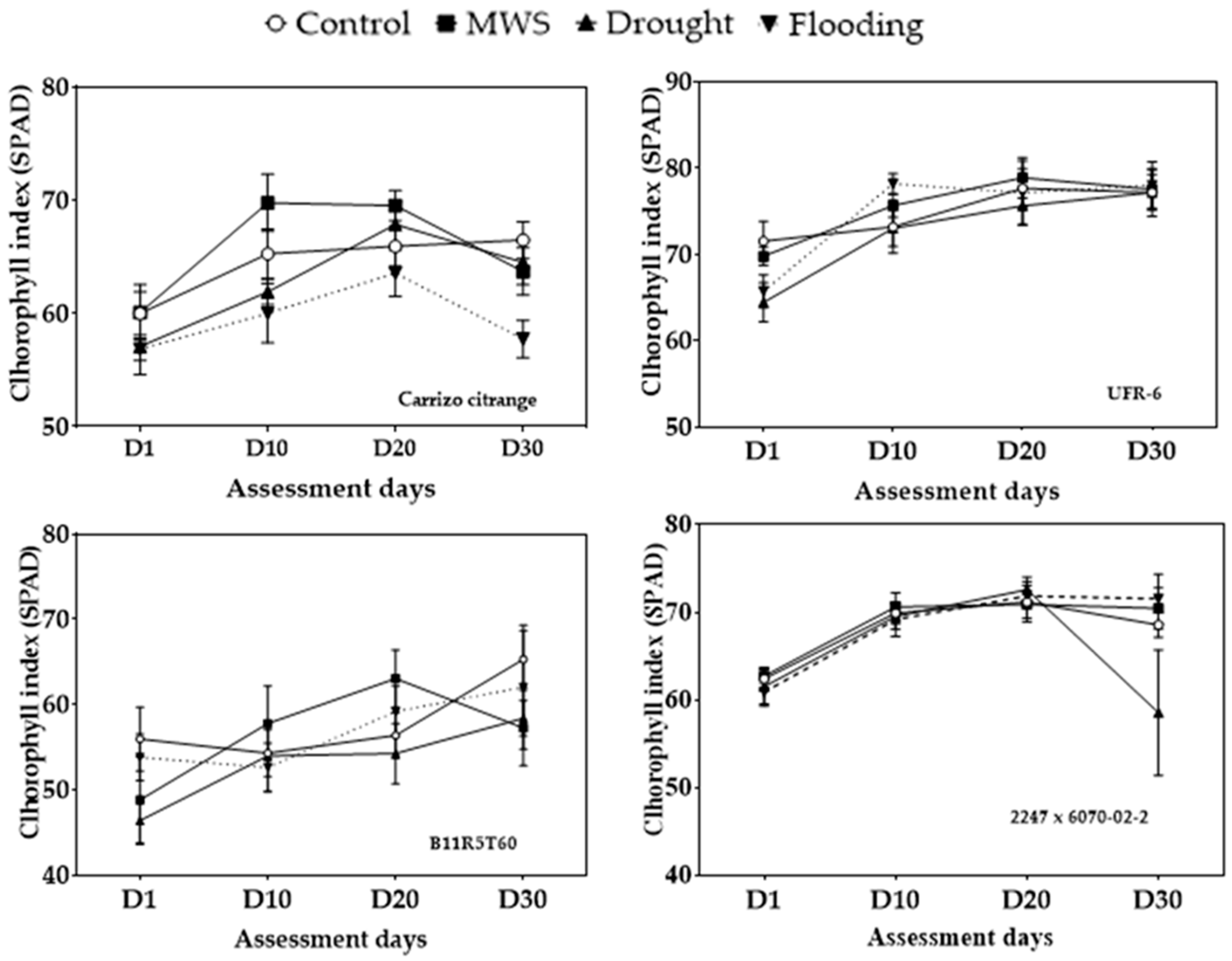
3.1. Chlorophyll Index (SPAD)
3.2. Plant Growth
3.3. Biomass
3.4. Plant Water Status
3.4.1. Leaf Water Potential
3.4.2. Stomatal Conductance
3.4.3. Relative Water Content
3.4.4. Electrolyte Leakage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. 2021. Available online: http://www.fao.org/faostat/es/#data/QC (accessed on 4 April 2021).
- Ruiz, I.; Almagro, M.; del Mar Solà, M.; Sanz, M.J. Assessment of sustainable land management practices in Mediterranean rural regions. J. Environ. Manag. 2020, 276, 111293. [Google Scholar] [CrossRef]
- Puigdefábregas, J.; Mendizabal, T. Perspectives on desertification: Western Mediterranean. J. Arid Environ. 1998, 39, 209–224. [Google Scholar] [CrossRef]
- Safriel, U.N. Status of Desertification in the Mediterranean Region. In Water Scarcity, Land Degradation and Desertification in the Mediterranean Region; Rubio, J.L., Safriel, U., Daussa, R., Blum, W.E.H., Pedrazzini, F., Eds.; NATO Science for Peace and Security Series C: Environmental Security; Springer: Dordrecht, The Netherlands, 2009; pp. 33–73. [Google Scholar]
- Kijne, J.W. Abiotic stress and water scarcity: Identifying and resolving conflicts from plant level to global level. Field Crop. Res. 2006, 97, 3–18. [Google Scholar] [CrossRef]
- Holzapfel, E.A.; Pannunzio, A.; Lorite, I.; de Oliveira, A.S.S.; Farkas, I. Design and management of irrigation systems. Chil. J. Agric. Res. 2009, 69, 17–25. [Google Scholar] [CrossRef] [Green Version]
- EPA. What Climate Change Means for Florida. 2016. Available online: https://www.epa.gov/sites/default/files/2016-08/documents/climate-change-fl.pdf (accessed on 9 September 2021).
- Vincent, C.; Morillon, R.; Arbona, V.; Gómez-Cadenas, A. Citrus in Changing Environments; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128122174. [Google Scholar]
- Sharif, P.; Seyedsalehi, M.; Paladino, O.; Van Damme, P.; Sillanpää, M.; Sharifi, A.A. Effect of drought and salinity stresses on morphological and physiological characteristics of canola. Int. J. Environ. Sci. Technol. 2018, 15, 1859–1866. [Google Scholar] [CrossRef]
- Colmenero-Flores, J.M.; Arbona, V.; Morillon, R.; Gómez-Cadenas, A. Salinity and Water Deficit. In The Genus Citrus; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128121634. [Google Scholar]
- Arbona, V.; Marco, A.J.; Iglesias, D.J.; López-Climent, M.F.; Talon, M.; Gómez-Cadenas, A. Carbohydrate depletion in roots and leaves of salt-stressed potted Citrus clementina L. Plant Growth Regul. 2005, 46, 153–160. [Google Scholar] [CrossRef]
- De Magalhães Erismann, N.; Machado, E.C.; Tucci, M.L.S.A. Photosynthetic limitation by CO2 diffusion in drought stressed orange leaves on three rootstocks. Photosynth. Res. 2008, 96, 163–172. [Google Scholar] [CrossRef]
- Arbona, V.; Iglesias, D.J.; Jacas, J.; Primo-Millo, E.; Talon, M.; Gómez-Cadenas, A. Plant and Soil (2005) 270: 73-82 Hydrogel substrate amendment alleviates drought effects on young citrus plants. Plant Soil 2005, 270, 73–82. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Responses of woody plants to flooding and salinity. Tree Physiol. 2012, 17, 490. [Google Scholar] [CrossRef]
- Brakke, M.; Allen, L.H. Gas exchange of Citrus seedlings at different temperatures, vapor-pressure deficits, and soil water contents. J. Am. Soc. Hortic. Sci. 1995, 120, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Pedroso, F.K.J.V.; Prudente, D.A.; Carolina, A.; Bueno, R.; Machado, E.C.; Ribeiro, R.V. Drought tolerance in citrus trees is enhanced by rootstock-dependent changes in root growth and carbohydrate availability. Environ. Exp. Bot. 2014, 101, 26–35. [Google Scholar] [CrossRef]
- Ford, H.W. Poorly drained citrus soils. In Proceedings of the 1st International Citrus Symposium, Oakland, CA, USA, 16–26 March 1968; Volume 3, pp. 71–76. [Google Scholar]
- Sullivan, M.; VanToai, T.; Fausey, N.; Beuerlein, J.; Parkinson, R.; Soboyejo, A. Evaluating on-farm flooding impacts on soybean. Crop Sci. 2001, 41, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Alcántara, B.; Jover, S.; Quiñones, A.; Forner-Giner, M.Á.; Rodríguez-Gamir, J.; Legaz, F.; Primo-Millo, E.; Iglesias, D.J. Flooding affects uptake and distribution of carbon and nitrogen in citrus seedlings. J. Plant Physiol. 2012, 169, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Arbona, V.; Gómez-Cadenas, A. Hormonal modulation of citrus responses to flooding. J. Plant Growth Regul. 2008, 27, 241–250. [Google Scholar] [CrossRef]
- Yang, F.; Han, C.; Li, Z.; Guo, Y.; Chan, Z. Dissecting tissue- and species-specific responses of two Plantago species to waterlogging stress at physiological level. Environ. Exp. Bot. 2015, 109, 177–185. [Google Scholar] [CrossRef]
- Pucciarello, C.; Voesenek, L.A.C.J.; Perata, P.; Rashmi, S. Plant responses to flooding. Plant Sci. 2014, 5, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Sánchez, F.; Syvertsen, J.P.; Gimeno, V.; Botía, P.; Perez-Perez, J.G. Responses to flooding and drought stress by two citrus rootstock seedlings with different water-use efficiency. Physiol. Plant. 2007, 130, 532–542. [Google Scholar] [CrossRef]
- Reighard, G.L.; Parker, M.L.; Krewer, G.W.; Beckman, T.G.; Wood, B.W.; Smith, J.E.; Whiddon, J. Impact of hurricanes on peach and pecan orchards in the Southeastern United States. HortScience 2001, 36, 250–252. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Powell, C.A.; Duan, Y.; Shatters, R.G.; Lin, Y.; Zhang, M. Mitigating citrus huanglongbing via effective application of antimicrobial compounds and thermotherapy. Crop Prot. 2016, 84, 150–158. [Google Scholar] [CrossRef]
- Bové, J.M.; Ayres, A.J. Etiology of three recent diseases of citrus in São Paulo State: Sudden death, variegated chlorosis and huanglongbing. IUBMB Life 2007, 59, 346–354. [Google Scholar] [CrossRef]
- Jagoueix, S.; Bove, J.M.; Garnier, M. The phloem-limited bacterium of greening disease of citrus is a member of the α subdivision of the Proteobacteria. Int. J. Syst. Bacteriol. 1994, 44, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Ichinose, K.; Hoa, N.V.; Bang, D.V.; Tuan, D.H.; Dien, L.Q. Limited efficacy of guava interplanting on citrus greening disease: Effectiveness of protection against disease invasion breaks down after one year. Crop Prot. 2012, 34, 119–126. [Google Scholar] [CrossRef]
- Gottwald, T.R. Current epidemiological understanding of Citrus Huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siverio, F.; Marco-Noales, E.; Bertolini, E.; Teresani, G.R.; Peñalver, J.; Mansilla, P.; Aguín, O.; Pérez-Otero, R.; Abelleira, A.; Guerra-García, J.A.; et al. Survey of huanglongbing associated with “Candidatus Liberibacter” species in Spain: Analyses of citrus plants and Trioza erytreae. Phytopathol. Mediterr. 2017, 56, 98–110. [Google Scholar] [CrossRef]
- Arenas-Arenas, F.J.; Duran-Vila, N.; Quinto, J.; Hervalejo, Á. Is the presence of Trioza erytreae, vector of huanglongbing disease, endangering the Mediterranean citrus industry? Survey of its population density and geographical spread over the last years. J. Plant Pathol. 2018, 100, 567–574. [Google Scholar] [CrossRef]
- Arenas-Arenas, F.J.; Duran-Vila, N.; Quinto, J.; Hervalejo, Á. Geographic spread and inter-annual evolution of populations of Trioza erytreae in the Iberian Peninsula. J. Plant Pathol. 2019, 101, 1151–1157. [Google Scholar] [CrossRef]
- Tallón Vila, C.I. Biotechnology Applied to the Genetic Improvement of Citrus Rootstocks. Development of a Protocol for Micropropagation and Adventitious Regeneration for Use in Generating Salt Toleran Mutant Lines. Ph.D. Thesis, Universidad de Murcia, Murcia, Spain, 2015; p. 105. [Google Scholar]
- Yelenosky, G.; Vu, J.C.V.; Wutscher, H.K. Influence of paclobutrazol in the soil on growth, nutrient elements in the leaves, and flood/freeze tolerance of citrus rootstock seedlings. J. Plant Growth Regul. 1995, 14, 129–134. [Google Scholar] [CrossRef]
- Castle, W.S.; Bowman, K.D.; Grosser, J.W.; Futch, S.H.; Graham, J.H. Florida citrus rootstock selection guide. Univ. Fla. Coop. Ext. Publ. 2016, SP248. Available online: https://edis.ifas.ufl.edu/pdf%5CHS%5CHS126000.pdf (accessed on 9 September 2021).
- Hussain, S.; Khalid, M.F.; Saqib, M.; Ahmad, S.; Zafar, W.; Rao, M.J.; Morillon, R.; Anjum, M.A. Drought tolerance in citrus rootstocks is associated with better antioxidant defense mechanism. Acta Physiol. Plant. 2018, 40, 1–10. [Google Scholar] [CrossRef]
- Florida Citrus Rootstock Selection Guide, 4th ed.; University of Florida: Gainesville, FL, USA, 2021; Available online: https://crec.ifas.ufl.edu/extension/citrus_rootstock/tables.html (accessed on 30 August 2021).
- Poorter, H. Interspecific variation in relative growth rate: On ecological causes and physiological consequences. In Causes and Consequences of Variation in Growth Rate and Productivity of Higher Plants; SPB Academic Publishing: Hague, The Netherlands, 1989; pp. 45–68. [Google Scholar]
- Vincent, J.M. Distortion of fungal hyphæ in the presence of certain inhibitors. Nature 1947, 159, 850. [Google Scholar] [CrossRef]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap Pressure in Vascular Plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Rewald, B.; Raveh, E.; Gendler, T.; Ephrath, J.E.; Rachmilevitch, S. Phenotypic plasticity and water flux rates of Citrus root orders under salinity. J. Exp. Bot. 2012, 63, 2717–2727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, J.M. Osmoregulation and water stress in higher plants. Annu. Rev. plant Physiol. 1984, 35, 299–319. [Google Scholar] [CrossRef]
- Bowman, K.D.; Joubert, J. Citrus rootstocks. In The Genus Citrus; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128121634. [Google Scholar]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef]
- Zaher-Ara, T.; Boroomand, N.; Sadat-Hosseini, M. Physiological and morphological response to drought stressin seedlings of ten citrus. Trees 2016, 30, 985–993. [Google Scholar] [CrossRef]
- Rodríguez-Gamir, J.; Ancillo, G.; González-Mas, M.C.; Primo-Millo, E.; Iglesias, D.J.; Forner-Giner, M.A. Root signalling and modulation of stomatal closure in flooded citrus seedlings. Plant Physiol. Biochem. 2011, 49, 636–645. [Google Scholar] [CrossRef]
- Arbona, V.; López-Climent, M.F.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Maintenance of a high photosynthetic performance is linked to flooding tolerance in citrus. Environ. Exp. Bot. 2009, 66, 135–142. [Google Scholar] [CrossRef]
- Partiya, R.; Ghazvini, R.F.; Fifaei, R.; Ghasemnezhad, M. Response of different citrus genotypes to continuous flooding conditions. Int. J. Hortic. Sci. Technol. 2018, 5, 253–263. [Google Scholar] [CrossRef]
- Ponnamperuma, F.N. The Chemistry of Submerged Soils. Adv. Agron. 1972, 24, 29–96. [Google Scholar] [CrossRef]
- Romero, P.; Navarro, J.M.; Pérez-Pérez, J.; García-Sánchez, F.; Gómez-Gómez, A.; Porras, I.; Martinez, V.; Botía, P. Deficit irrigation and rootstock: Their effects on water relations, vegetative development, yield, fruit quality and mineral nutrition of Clemenules mandarin. Tree Physiol. 2006, 26, 1537–1548. [Google Scholar] [CrossRef]
- Santana-Vieira, D.D.S.; Freschi, L.; da Hora Almeida, L.A.; de Moraes, D.H.S.; Neves, D.M.; Dos Santos, L.M.; Bertolde, F.Z.; dos Santos Soares Filho, W.; Coelho Filho, M.A.; da Silva Gesteira, A. Survival strategies of citrus rootstocks subjected to drought. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Zollinger, N.; Kjelgren, R.; Cerny-Koenig, T.; Kopp, K.; Koenig, R. Drought responses of six ornamental herbaceous perennials. Sci. Hortic. 2006, 109, 267–274. [Google Scholar] [CrossRef]
- Lei, Y.; Yin, C.; Li, C. Differences in some morphological, physiological, and biochemical responses to drought stress in two contrasting populations of Populus przewalskii. Physiol. Plant. 2006, 127, 182–191. [Google Scholar] [CrossRef]
- Rodríguez-Gamir, J.; Primo-Millo, E.; Forner, J.B.; Forner-Giner, M.A. Citrus rootstock responses to water stress. Sci. Hortic. 2010, 126, 95–102. [Google Scholar] [CrossRef]
- García-Sánchez, F.; Syvertsen, J.P. Salinity tolerance of Cleopatra mandarin and Carrizo citrange citrus rootstock seedlings is affected by CO2 enrichment during growth. J. Am. Soc. Hortic. Sci. 2006, 131, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, K.; Ito, Y.; Yamaguchi-Shinozaki, K. Update on abiotic stresses in arabidopsis and grasses transcriptional regulatory networks in response to abiotic stresses in arabidopsis and grasses 1. Plant Physiol. 2009, 149, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, W.J.; Zhang, J. Root signals and the regulation of growth and development of plants in drying soil. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 55–76. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dat, J.F.; Capelli, N.; Folzer, H.; Bourgeade, P.; Badot, P.-M. Sensing and signalling during plant flooding. Plant Physiol. Biochem. 2004, 42, 273–282. [Google Scholar] [CrossRef]
- Bielsa, B.; Hewitt, S.; Reyes-Chin-Wo, S.; Dhingra, A.; Rubio-Cabetas, M.J. Identification of water use efficiency related genes in “Garnem” almond-peach rootstock using time-course transcriptome analysis. PLoS ONE 2018, 13, 1–24. [Google Scholar] [CrossRef]
- Bajji, M.; Kinet, J.M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef] [PubMed]






| Water Treatments | Rootstock | FW | DW | ||||
|---|---|---|---|---|---|---|---|
| R ± SE | S ± SE | L ± SE | R ± SE | S ± SE | L ± SE | ||
| MWS | Carrizo citrange | 33.35 ± 8.86 a | 32.92 ± 6.44 a | 33.17 ± 8.77 a | 14.03 ± 5.21 ab | 30.12 ± 7.02 a | 24.24 ± 8.80 b |
| UFR-6 | 0.00 ± 0.00 b | 9.07 ± 3.57 b | 3.68 ± 2.21 b | 4.00 ± 2.89 b | 12.34 ± 5.56 bc | 53.54 ± 3.74 a | |
| B11R5T60 | 20.83 ± 5.83 a | 18.91 ± 5.52 b | 31.40 ± 4.79 a | 19.21 ± 4.60 a | 23.73 ± 5.08 ab | 36.21 ± 5.79 ab | |
| 2247 x 6070-02-2 | 28.01 ± 4.44 a | 9.10 ± 2.54 b | 4.40 ± 2.99 b | 5.83 ± 2.65 b | 2.78 ± 1.82 c | 6.21 ± 4.67 c | |
| Drought | Carrizo citrange | 54.15 ± 7.62 b | 63.98 ± 3.61 a | 65.35 ± 3.72 ns | 9.69 ± 6.28 b | 35.04 ± 6.04 a | 33.33 ± 6.06 ab |
| UFR-6 | 58.47 ± 7.45 ab | 59.80 ± 6.10 ab | 63.24 ± 6.09 ns | 6.38 ± 2.84 b | 23.86 ± 6.21 ab | 65.15 ± 2.53 a | |
| B11R5T60 | 45.39 ±7.65 b | 50.64 ± 4.43 b | 59.88 ± 5.83 ns | 9.15 ± 5.27 b | 30.51 ± 6.99 ab | 42.41 ± 4.41 b | |
| 2247 x 6070-02-2 | 77.42 ± 2.60 a | 61.97 ± 3.72 ab | 72.71 ± 2.25 ns | 35.00 ± 4.67 a | 16.67 ± 5.94 b | 27.88 ± 4.53 c | |
| Flooding | Carrizo citrange | 52.68 ± 6.41 bc | 40.99 ± 5.18 a | 44.55 ± 6.17 ab | 55.10 ± 5.12 ab | 30.12 ± 5.55 a | 34.85 ± 6.85 b |
| UFR-6 | 39.11 ± 5.99 c | 19.12 ± 7.58 b | 31.25 ± 4.19 b | 53.15 ± 8.79 ab | 11.53 ± 6.95 b | 67.17 ± 1.78 a | |
| B11R5T60 | 61.70 ± 5.85 ab | 46.15 ± 3.75 a | 54.94 ± 8.37 a | 51.22 ± 7.37 b | 20.34 ± 4.00 ab | 35.78 ± 7.63 b | |
| 2247 x 6070-02-2 | 70.97 ± 3.11 a | 41.31 ± 5.36 a | 30.77 ± 4.07 b | 70.00 ± 3.09 a | 29.17 ± 7.25 b | 19.85 ± 5.78 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aparicio-Durán, L.; Gmitter Jr., F.G.; Arjona-López, J.M.; Calero-Velázquez, R.; Hervalejo, Á.; Arenas-Arenas, F.J. Water-Stress Influences on Three New Promising HLB-Tolerant Citrus Rootstocks. Horticulturae 2021, 7, 336. https://doi.org/10.3390/horticulturae7100336
Aparicio-Durán L, Gmitter Jr. FG, Arjona-López JM, Calero-Velázquez R, Hervalejo Á, Arenas-Arenas FJ. Water-Stress Influences on Three New Promising HLB-Tolerant Citrus Rootstocks. Horticulturae. 2021; 7(10):336. https://doi.org/10.3390/horticulturae7100336
Chicago/Turabian StyleAparicio-Durán, Lidia, Frederick G. Gmitter Jr., Juan M. Arjona-López, Rocío Calero-Velázquez, Áurea Hervalejo, and Francisco J. Arenas-Arenas. 2021. "Water-Stress Influences on Three New Promising HLB-Tolerant Citrus Rootstocks" Horticulturae 7, no. 10: 336. https://doi.org/10.3390/horticulturae7100336
APA StyleAparicio-Durán, L., Gmitter Jr., F. G., Arjona-López, J. M., Calero-Velázquez, R., Hervalejo, Á., & Arenas-Arenas, F. J. (2021). Water-Stress Influences on Three New Promising HLB-Tolerant Citrus Rootstocks. Horticulturae, 7(10), 336. https://doi.org/10.3390/horticulturae7100336







