Light Quality Environment and Photomorphological Responses of Young Olive Trees
Abstract
:1. Introduction
2. Materials and Methods
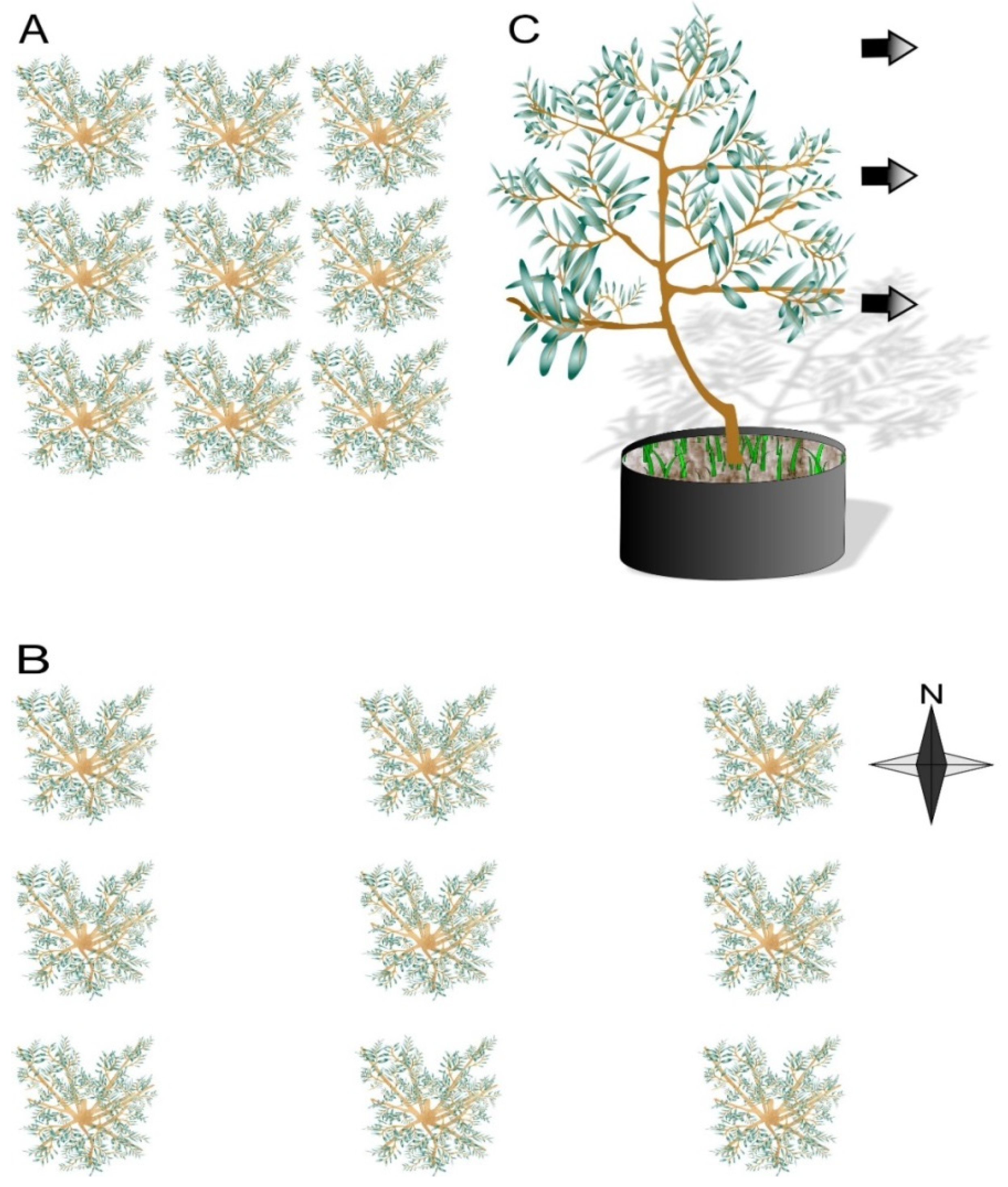
2.1. Light Quality Response to Leaf Area Index of Neighboring Trees
2.2. Morphological Responses to Light Quality
2.2.1. Plant Material
2.2.2. Experimental Design and Light Quality Treatments
2.2.3. Light, Temperature, and Relative Humidity Measurements
2.2.4. Morphological and Biomass Measurements
2.3. Statistical Analyses
3. Results
3.1. Light Quality Characterization in Response to Leaf Area Index
3.2. Morphological Responses to Light Quality
3.2.1. Light Quality Characterization of Far-Red Mirrors and Green Fences
3.2.2. Morphological Responses to Far-Red Mirrors
3.2.3. Morphological Responses to Green Fences
3.2.4. Cluster and Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Connor, D.; Gomez-Del-Campo, M.; Rousseaux, M.C.; Searles, P.S. Structure, management and productivity of hedgerow olive orchards: A review. Sci. Hortic. 2014, 169, 71–93. [Google Scholar] [CrossRef]
- Farinelli, D.; Tombesi, S. Performance and oil quality of ‘Arbequina’ and four Italian olive cultivars under super high density hedgerow planting system cultivated in central Italy. Sci. Hortic. 2015, 192, 97–107. [Google Scholar] [CrossRef]
- Mariscal, M.J.; Orgaz, F.; Villalobos, F.J. Modelling and measurement of radiation interception by olive canopies. Agric. For. Meteorol. 2000, 100, 183–197. [Google Scholar] [CrossRef]
- Gómez-Del-Campo, M.; Connor, D.J.; Trentacoste, E.R. Long-term Effect of Intra-Row Spacing on Growth and Productivity of Super-High Density Hedgerow Olive Orchards (cv. Arbequina). Front. Plant Sci. 2017, 8, 1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-del-Campo, M.; Trentacoste, E.R.; Connor, D.J. Long-term effects of row spacing on radiation interception, fruit characteristics and production of hedgerow olive orchard (cv. Arbequina). Sci. Hortic. 2020, 272, 109583. [Google Scholar] [CrossRef]
- Cherbiy-Hoffmann, S.U.; Hall, A.J.; Rousseaux, M.C. Fruit, yield, and vegetative growth responses to photosynthetically active radiation during oil synthesis in olive trees. Sci. Hortic. 2013, 150, 110–116. [Google Scholar] [CrossRef]
- Cherbiy-Hoffmann, S.U.; Hall, A.J.; Searles, P.S.; Rousseaux, M.C. Responses of olive tree yield determinants and components to shading during potentially critical phenological phases. Sci. Hortic. 2015, 184, 70–77. [Google Scholar] [CrossRef]
- Gregoriou, K.; Pontikis, K.; Vemmos, S. Effects of reduced irradiance on leaf morphology, photosynthetic capacity, and fruit yield in olive (Olea europaea L.). Photosynthetica 2007, 45, 172–181. [Google Scholar] [CrossRef]
- Larbi, A.; Vázquez, S.; El-Jendoubi, H.; Msallem, M.; Abadia, J.; Abadia, A.; Morales, F. Canopy light heterogeneity drives leaf anatomical, eco-physiological, and photosynthetic changes in olive trees grown in a high-density plantation. Photosynth. Res. 2015, 123, 141–155. [Google Scholar] [CrossRef] [Green Version]
- Rousseaux, M.C.; Cherbiy-Hoffmann, S.U.; Hall, A.J.; Searles, P.S. Fatty acid composition of olive oil in response to fruit canopy position and artificial shading. Sci. Hortic. 2020, 271, 109477. [Google Scholar] [CrossRef]
- Wang, Q.W.; Robson, T.M.; Pieristè, M.; Oguro, M.; Oguchi, R.; Murai, Y.; Kurokawa, H. Testing trait plasticity over the range of spectral composition of sunlight in forb species differing in shade tolerance. J. Ecol. 2020, 108, 1923–1940. [Google Scholar] [CrossRef]
- Casal, J.J. Shade Avoidance. Arab. Book 2012, 10, e0157. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, T.; Folta, K.M. Green light augments far-red-light-induced shade response. Plant Growth Regul. 2015, 77, 147–155. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Sanchez, R.A.; Scopel, A.L.; Casal, J.J.; Ghersa, C.M. Early detection of neighbour plants by phytochrome perception of spectral changes in reflected sunlight. Plant Cell Environ. 1987, 10, 551–557. [Google Scholar] [CrossRef]
- Keuskamp, D.H.; Keller, M.M.; Ballaré, C.L.; Pierik, R. Blue light regulated shade avoidance. Plant Signal. Behav. 2012, 7, 514–517. [Google Scholar] [CrossRef] [Green Version]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R.; et al. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Pierik, R. The shade-avoidance syndrome: Multiple signals and ecological consequences. Plant Cell Environ. 2017, 40, 2530–2543. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, M.C.; Jordan, E.T.; Vierstra, R.D.; Ballaré, C.L. Directed overexpression of PHYA locally suppresses stem elongation and leaf senescence responses to far-red radiation. Plant Cell Environ. 1997, 20, 1551–1558. [Google Scholar] [CrossRef]
- Sasidharan, R.; Chinnappa, C.C.; Staal, M.; Elzenga, J.T.M.; Yokoyama, R.; Nishitani, K.; Voesenek, L.A.C.J.; Pierik, R. Light Quality-Mediated Petiole Elongation in Arabidopsis during Shade Avoidance Involves Cell Wall Modification by Xyloglucan Endotransglucosylase/Hydrolases. Plant Physiol. 2010, 154, 978–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Van Westreenen, A.; He, L.; Evers, J.B.; Anten, N.P.R.; Marcelis, L.F.M. Light from below matters: Quantifying the consequences of responses to far-red light reflected upwards for plant performance in heterogeneous canopies. Plant Cell Environ. 2021, 44, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.; Aas, O.T.; Jetmundsen, M.R.; Lee, Y.; Torre, S.; Fløistad, I.S.; Olsen, J.E. Day Extension with Far-Red Light Enhances Growth of Subalpine Fir (Abies lasiocarpa (Hooker) Nuttall) Seedlings. Forests 2018, 9, 175. [Google Scholar] [CrossRef] [Green Version]
- Schettini, E.; De Salvador, F.R.; Scarascia-Mugnozza, G.; Vox, G. Radiometric properties of photoselective and photoluminescent greenhouse plastic films and their effects on peach and cherry tree growth. J. Hortic. Sci. Biotechnol. 2011, 86, 79–83. [Google Scholar] [CrossRef]
- González, C.V.; Jofré, M.F.; Vila, H.F.; Stoffel, M.; Bottini, R.; Giordano, C.V. Morphology and Hydraulic Architecture of Vitis vinifera L. cv. Syrah and Torrontés Riojano Plants Are Unaffected by Variations in Red to Far-Red Ratio. PLoS ONE 2016, 11, e0167767. [Google Scholar] [CrossRef]
- Aphalo, P.J.; Rikala, R. Spacing of silver birch seedlings grown in containers of equal size affects their morphology and its variability. Tree Physiol. 2006, 26, 1227–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamage, H.K.; Ashton, M.S.; Singhakumara, B.M.P. Leaf structure of Syzygium spp. (Myrtaceae) in relation to site affinity within a tropical rain forest. Bot. J. Linn. Soc. 2003, 141, 365–377. [Google Scholar] [CrossRef]
- Dudley, S.A.; Schmitt, J. Genetic Differentiation in Morphological Responses to Simulated Foliage Shade between Populations of Impatiens capensis from Open and Woodland Sites. Funct. Ecol. 1995, 9, 655. [Google Scholar] [CrossRef]
- Kasulin, L.; Agrofoglio, Y.; Botto, J.F. The receptor-like kinase ERECTA contributes to the shade-avoidance syndrome in a background-dependent manner. Ann. Bot. 2013, 111, 811–819. [Google Scholar] [CrossRef] [Green Version]
- Botto, J.F. Plasticity to simulated shade is associated with altitude in structured populations of Arabidopsis thaliana. Plant Cell Environ. 2015, 38, 1321–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hitz, T.; Hartung, J.; Graeff-Hönninger, S.; Munz, S. Morphological Response of Soybean (Glycine max (L.) Merr.) Cultivars to Light Intensity and Red to Far-Red Ratio. Agronomy 2019, 9, 428. [Google Scholar] [CrossRef] [Green Version]
- Searles, P.S.; Agüero Alcaras, L.M.; Rousseaux, M.C. El consumo del agua por el cultivo de olivo (Olea europaea L.) en el noroeste de Argentina: Una comparación con la cuenca mediterránea. Ecol. Austral. 2011, 21, 15–28. [Google Scholar]
- Miserere, A.; Searles, P.S.; Manchó, G.; Maseda, P.H.; Rousseaux, M.C. Sap Flow Responses to Warming and Fruit Load in Young Olive Trees. Front. Plant Sci. 2019, 10, 1199. [Google Scholar] [CrossRef] [PubMed]
- Proietti, P.; Nasini, L.; Reale, L.; Caruso, T.; Ferranti, F. Productive and vegetative behavior of olive cultivars in super high-density olive grove. Sci. Agric. 2015, 72, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Barranco, D.; Cimato, A.; Fiorino, P.; Rallo, L.; Touzani, A.; Castañeda, C.; Serafn, F.; Trujillo, I. Word Catalogue of Olive Varieties, 1st ed.; International Olive Oil Council: Madrid, Spain, 2000. [Google Scholar]
- Rousseaux, M.C.; Hall, A.J.; Sanchez, R.A. Far-red enrichment and photosynthetically active radiation level influence leaf senescence in field-grown sunflower. Physiol. Plant 1996, 96, 217–224. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2020. Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. Available online: http://www.infostat.com.ar (accessed on 1 September 2020).
- Casal, J.J.; Sanchez, R.A.; Deregibus, V.A. The effect of plant density on tillering: The involvement of R/FR ratio and the proportion of radiation intercepted per plant. Environ. Exp. Bot. 1986, 26, 365–371. [Google Scholar] [CrossRef]
- Libenson, S.; Rodriguez, V.; López Pereira, M.; Sánchez, R.A.; Casal, J.J. Low Red to Far-Red Ratios Reaching the Stem Reduce Grain Yield in Sunflower. Crop Sci. 2002, 42, 1180–1185. [Google Scholar] [CrossRef]
- Bastías, R.M.; Corelli-Grappadelli, L. Light quality management in fruit orchards: Physiological and technological aspects. Chil. J. Agric. Res. 2012, 72, 574–581. [Google Scholar] [CrossRef]
- Escribano-Rocafort, A.G.; Ventre-Lespiaucq, A.B.; Granado-Yela, C.; de Casas, R.R.; Delgado, J.A.; Escudero, A.; Balaguer, L. Intraindividual variation in light-related functional traits: Magnitude and structure of leaf trait variability across global scales in Olea europaea trees. Trees 2017, 31, 1505–1517. [Google Scholar] [CrossRef]
- Connor, D.J. Adaptation of olive (Olea europaea L.) to water-limited environments. Aust. J. Agric. Res. 2005, 56, 1181–1189. [Google Scholar] [CrossRef]
- Baldini, E.; Facini, O.; Nerozzi, F.; Rossi, F.; Rotondi, A. Leaf characteristics and optical properties of different woody species. Trees 1997, 12, 73–81. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Scopel, A.L.; Sánchez, R.A. Far-Red Radiation Reflected from Adjacent Leaves: An Early Signal of Competition in Plant Canopies. Science 1990, 247, 329–332. [Google Scholar] [CrossRef]
- Franklin, K.A.; Whitelam, G.C. Phytochromes and Shade-avoidance Responses in Plants. Ann. Bot. 2005, 96, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Rapoport, H.F.; Fabbri, A.; Sebastiani, L. Olive Biology. In The Olive Tree Genome, Compendium of Plant Genomes; Rugini, E., Baldoni, L., Muleo, R., Sebastiani, L., Eds.; Springer: Cham, Switzerland, 2016; pp. 13–25. [Google Scholar]
- Besnard, G.; Terral, J.-F.; Cornille, A. On the origins and domestication of the olive: A review and perspectives. Ann. Bot. 2018, 121, 385–403. [Google Scholar] [CrossRef] [Green Version]
- Albarracín, V.; Hall, A.J.; Searles, P.S.; Rousseaux, M.C. Responses of vegetative growth and fruit yield to winter and summer mechanical pruning in olive trees. Sci. Hortic. 2017, 225, 185–194. [Google Scholar] [CrossRef]
- Ladux, F.J.; Trentacoste, E.R.; González, C.; Searles, P.S.; Rousseaux, M.C. Photomorphological Responses of Young Olive Trees to Red/Far-Red Ratio and Photosynthetically Active Radiation. Ph.D. Thesis, 2021. in preparation. [Google Scholar]
- Aphalo, P.J.; Lehto, T. Effect of lateral far-red light supplementation on the growth and morphology of birch seedlings and its interaction with mineral nutrition. Trees 2001, 15, 297–303. [Google Scholar] [CrossRef]
- de La Rosa, T.M.; Aphalo, P.J.; Lehto, T. Effects of far-red light on the growth, mycorrhizas and mineral nutrition of Scots pine seedlings. Plant Soil 1998, 201, 17–25. [Google Scholar] [CrossRef]
- Day, M.E.; Zazzaro, S.; Perkins, L.B. Seedling ontogeny and environmental plasticity in two co-occurring shade-tolerant conifers and implications for environment-population interactions. Am. J. Bot. 2014, 101, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Walters, M.B.; Reich, P.B. Seed size, nitrogen supply, and growth rate affect tree seedling survival in deep shade. Ecology 2000, 81, 1887–1901. [Google Scholar] [CrossRef]
- Courbier, S.; Pierik, R. Canopy Light Quality Modulates Stress Responses in Plants. iScience 2019, 22, 441–452. [Google Scholar] [CrossRef] [Green Version]
- González, C.V.; Jerez, D.N.; Jofré, M.F.; Guevara, A.; Prieto, J.A.; Mazza, C.; Williams, L.E.; Giordano, C.V. Blue light attenuation mediates morphological and architectural acclimation of Vitis vinifera cv. Malbec to shade and increases light capture. Environ. Exp. Bot. 2019, 157, 112–120. [Google Scholar] [CrossRef]







| Variables | Cultivar | |||||
|---|---|---|---|---|---|---|
| Arbequina | Coratina | Arauco | ||||
| FR | Control | FR | Control | FR | Control | |
| Main stem elongation (cm) | 22.9 ± 3.2 bc | 29.5 ± 3.4 c | 18.7 ± 3.2 ab | 22.8 ± 3.2 bc | 18.4 ± 3.8 ab | 12.3 ± 3.6 a |
| Internode length (cm) | 3.5 ± 0.4 abc | 3.7 ± 0.5 bc | 3.1 ± 0.4 ab | 4.4 ± 0.4 c | 3.2 ± 0.5 abc | 2.2 ± 0.5 a |
| Increase in main stem nodes (#) | 6.6 ± 0.5 b | 8.0 ± 0.5 c | 5.9 ± 0.5 ab | 5.0 ± 0.5 a | 6.0 ± 0.5 ab | 5.8 ± 0.5 ab |
| Axillary shoots per plant (#) | 3.2 ± 0.5 bc | 4.3 ± 0.5 c | 0.5 ± 0.5 a | 0.4 ± 0.5 a | 1.9 ± 0.6 ab | 1.6 ± 0.6 a |
| Axillary shoot angle (°) | 61.0 ± 3.3 b | 56.4 ± 3.6 ab | 52.2 ± 3.3 ab | 49.8 ± 3.3 a | 53.1 ± 4.1 ab | 53.9 ± 3.8 ab |
| Variables | Cultivar | |||||
|---|---|---|---|---|---|---|
| Arbequina | Coratina | Arauco | ||||
| FR | Control | FR | Control | FR | Control | |
| Leaves (g) | 4.7 ± 0.6 b | 6.6 ± 0.6 c | 2.9 ± 0.6 a | 5.4 ± 0.6 bc | 4.3 ± 0.7 ab | 4.5 ± 0.7 ab |
| Stems (g) | 3.4 ± 0.3 ab | 4.2 ± 0.3 b | 2.9 ± 0.3 a | 3.6 ± 0.3 ab | 2.7 ± 0.4 a | 2.7 ± 0.3 a |
| Roots (g) | 5.8 ± 0.7 a | 5.3 ± 0.7 a | 5.6 ± 0.7 a | 6.1 ± 0.8 a | 5.3 ± 0.8 a | 4.7 ± 0.8 a |
| Aboveground (g) | 8.1 ± 0.8 ab | 10.8 ± 0.9 c | 5.8 ± 0.8 a | 9.1 ± 0.8 bc | 7.1 ± 1.0 ab | 7.3 ± 0.9 ab |
| Aboveground/belowground | 1.5 ± 0.2 ab | 2.1 ± 0.2 c | 1.1 ± 0.2 a | 1.6 ± 0.2 bc | 1.4 ± 0.2 ab | 1.6 ± 0.2 bc |
| Total plant (g) | 13.9 ± 1.3 abc | 16.0 ± 1.4 c | 11.5 ± 1.3 a | 15.1 ± 1.3 bc | 12.3 ± 1.6 ab | 12.0 ± 1.5 ab |
| Specific leaf mass (mg cm−2) | 22.7 ± 0.8 a | 24.9 ± 0.8 a | 23.50 ± 0.8 a | 24.7 ± 0.8 a | 22.8 ± 0.9 a | 22.6 ± 0.9 a |
| Variables | Cultivar | |||||
|---|---|---|---|---|---|---|
| Arbequina | Coratina | Arauco | ||||
| GF | Control | GF | Control | GF | Control | |
| Main stem elongation (cm) | 27.3 ± 3.6 b | 29.2± 3.4 b | 22.4 ± 3.4 ab | 25.5 ± 3.4 b | 12.5 ± 3.8 a | 14.4 ± 3.8 a |
| Internode length (cm) | 3.8 ± 0.7 ab | 3.7 ± 0.7 ab | 3.7 ± 0.7 ab | 5.1 ± 0.7 b | 2.8 ± 0.8 a | 3.1 ± 0.8 ab |
| Increase in main stem nodes (#) | 7.7 ± 0.7 b | 7.9 ± 0.6 b | 5.7 ± 0.6 a | 5.3 ± 0.6 a | 6.3 ± 0.8 ab | 5.5 ± 0.7 a |
| Axillary shoots per plant (#) | 4.3 ± 0.6 c | 3.9 ± 0.6 bc | 1.1 ± 0.5 a | 1.0 ± 0.5 a | 2.3 ± 0.8 ab | 2.8 ± 0.7 abc |
| Axillary shoot angle (°) | 58.1 ± 4.0 a | 59.2 ± 3.8 a | 49.9 ± 3.8 a | 55.1 ± 3.8 a | 53.0 ± 4.2 a | 59.8 ± 4.2 a |
| Variables | Cultivar | |||||
|---|---|---|---|---|---|---|
| Arbequina | Coratina | Arauco | ||||
| GF | Control | GF | Control | GF | Control | |
| Leaves (g) | 5.6 ± 0.7 bcd | 6.9 ± 0. 7 d | 4.6 ± 0.6 abc | 6.2 ± 0.7 cd | 3.1 ± 0.7 a | 3.9 ± 0.7 ab |
| Stems (g) | 4.1 ± 0.4 b | 4.0 ± 0.4 b | 4.1 ± 0.4 b | 4.6 ± 0.4 b | 2.1 ± 0.4 a | 2.4 ± 0.4 a |
| Roots (g) | 5.9 ± 0.8 ab | 5.5 ± 0.8 a | 6.5 ± 0.8 ab | 8.1 ± 0.8 b | 4.3 ± 0.9 a | 4.3 ± 0.9 a |
| Aboveground (g) | 9.7 ± 1.1 c | 10.9 ± 1.0 c | 8.7 ± 1.0 bc | 10.8 ± 1.0 c | 5.2 ± 1.1 a | 6.3 ± 1.1 ab |
| Aboveground/belowground | 1.7 ± 0.2 ab | 2.2 ± 0.2 b | 1.4 ± 0.2 a | 1.5 ± 0.2 a | 1.4 ± 0.3 a | 1.5 ± 0.3 a |
| Total plant (g) | 15.6 ± 1.7 bc | 16.4 ± 1.6 c | 15.2 ± 1.6 bc | 18.9 ± 1.6 c | 9.5 ± 1.8 a | 10.6 ± 1.8 ab |
| Specific leaf mass (mg cm−2) | 24.1 ± 0.8 a | 23.9 ± 0.8 a | 23.5 ± 0.8 a | 23.5 ± 0.8 a | 23.8 ± 0.8 a | 21.9 ± 0.8 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladux, F.J.; Trentacoste, E.R.; Searles, P.S.; Rousseaux, M.C. Light Quality Environment and Photomorphological Responses of Young Olive Trees. Horticulturae 2021, 7, 369. https://doi.org/10.3390/horticulturae7100369
Ladux FJ, Trentacoste ER, Searles PS, Rousseaux MC. Light Quality Environment and Photomorphological Responses of Young Olive Trees. Horticulturae. 2021; 7(10):369. https://doi.org/10.3390/horticulturae7100369
Chicago/Turabian StyleLadux, Federico J., Eduardo R. Trentacoste, Peter S. Searles, and M. Cecilia Rousseaux. 2021. "Light Quality Environment and Photomorphological Responses of Young Olive Trees" Horticulturae 7, no. 10: 369. https://doi.org/10.3390/horticulturae7100369
APA StyleLadux, F. J., Trentacoste, E. R., Searles, P. S., & Rousseaux, M. C. (2021). Light Quality Environment and Photomorphological Responses of Young Olive Trees. Horticulturae, 7(10), 369. https://doi.org/10.3390/horticulturae7100369







