Genome Wide Analysis of GH Gene Family Reveals Vvgh9 Positively Regulates Sugar Accumulation under Low Sugar Content in Grape
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants and Growth Conditions
2.2. Identification and Phylogenetic Analysis of GH Genes
2.3. Gene Location and Duplication Analysis of GH Genes in the Grape Genome
2.4. RNA-seq Data Analysis
2.5. Total RNA Extraction and Quantitative Real-Time PCR (qRT-PCR) Assays
2.6. Exogenous Sugar Treatment of Grape Berry via Injection
2.7. Treatment of Grape Calli with Different Concentrations of Glucose
2.8. Cloning of VvGH9 Gene
2.9. Transformation of Grapevine Calli and Tomato
2.10. Sugar Determination
3. Results
3.1. Identification and Phylogenetic Analysis of Eleven GH Genes in Grape
3.2. Chromosome Localization and Gene Duplication Analysis of Vvgh Genes
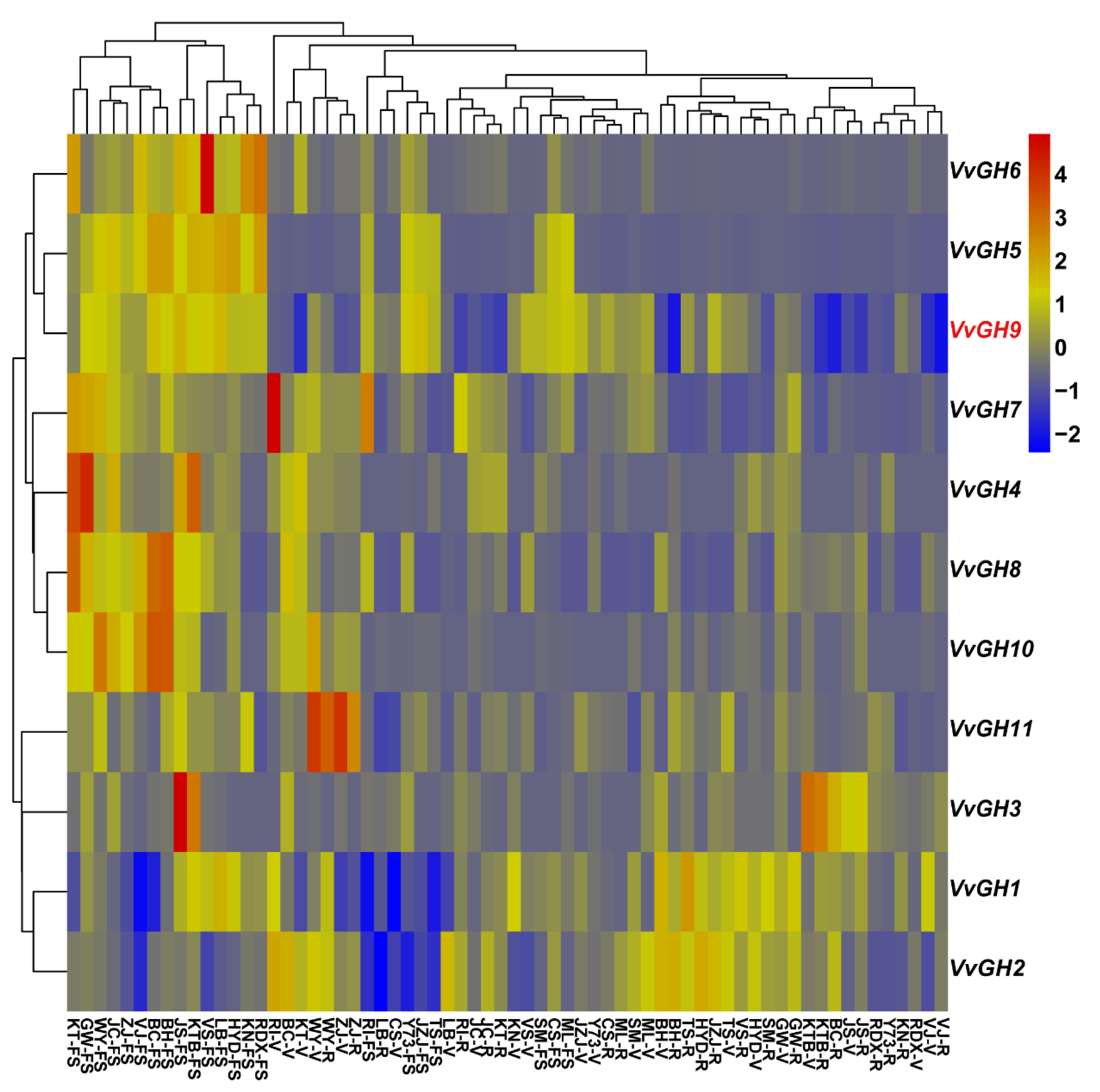
3.3. Expression Profiles of Vvgh Genes at Different Developmental Stages in Grape Berries
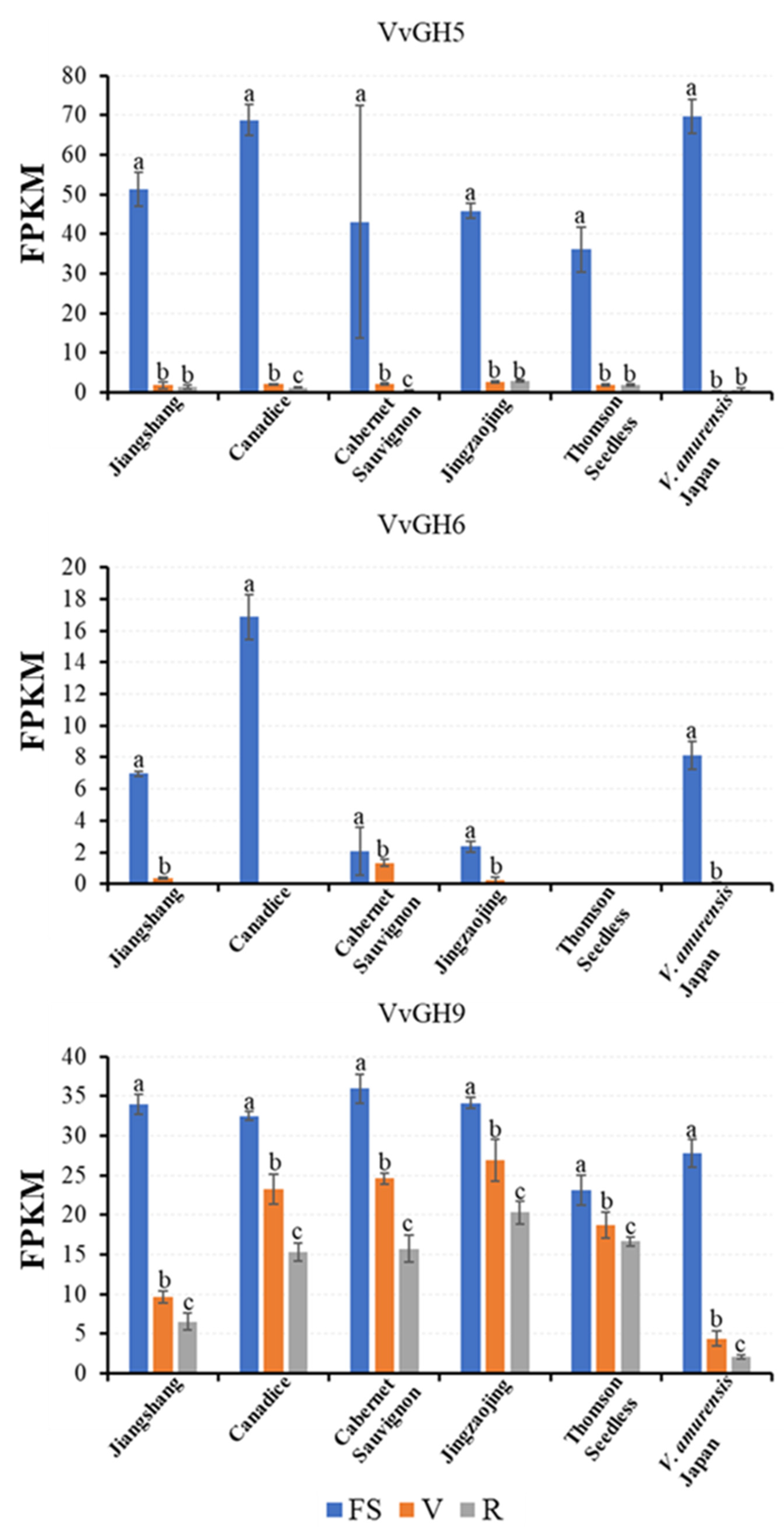
3.4. Exogenous Sugar Treatment by Injection Improves the Expression Level of Vvgh9 in Grape Berries
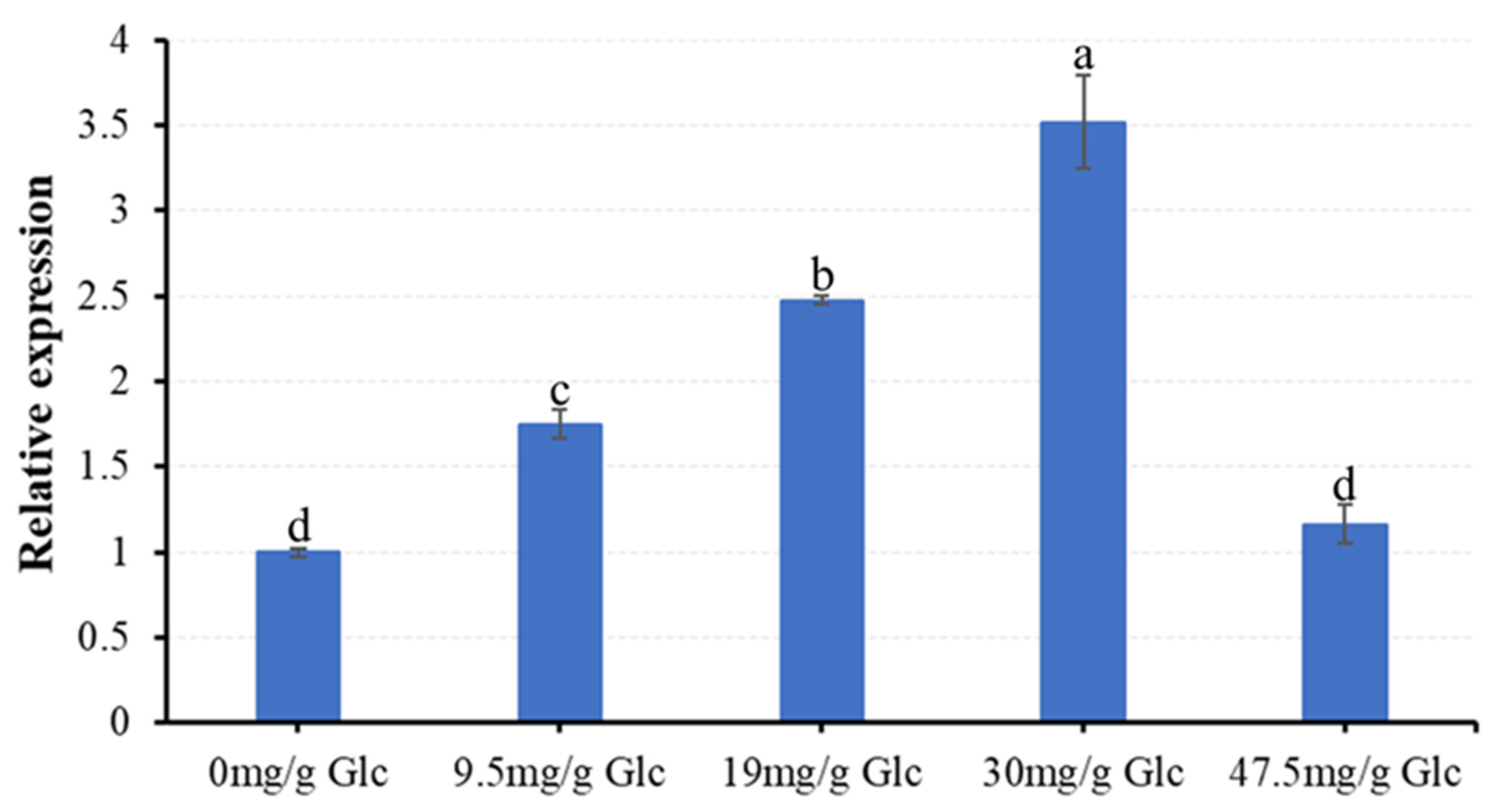
3.5. The Influence of Different Glucose Concentrations on the Expression Pattern of Vvgh9 in ‘41B’ Calli
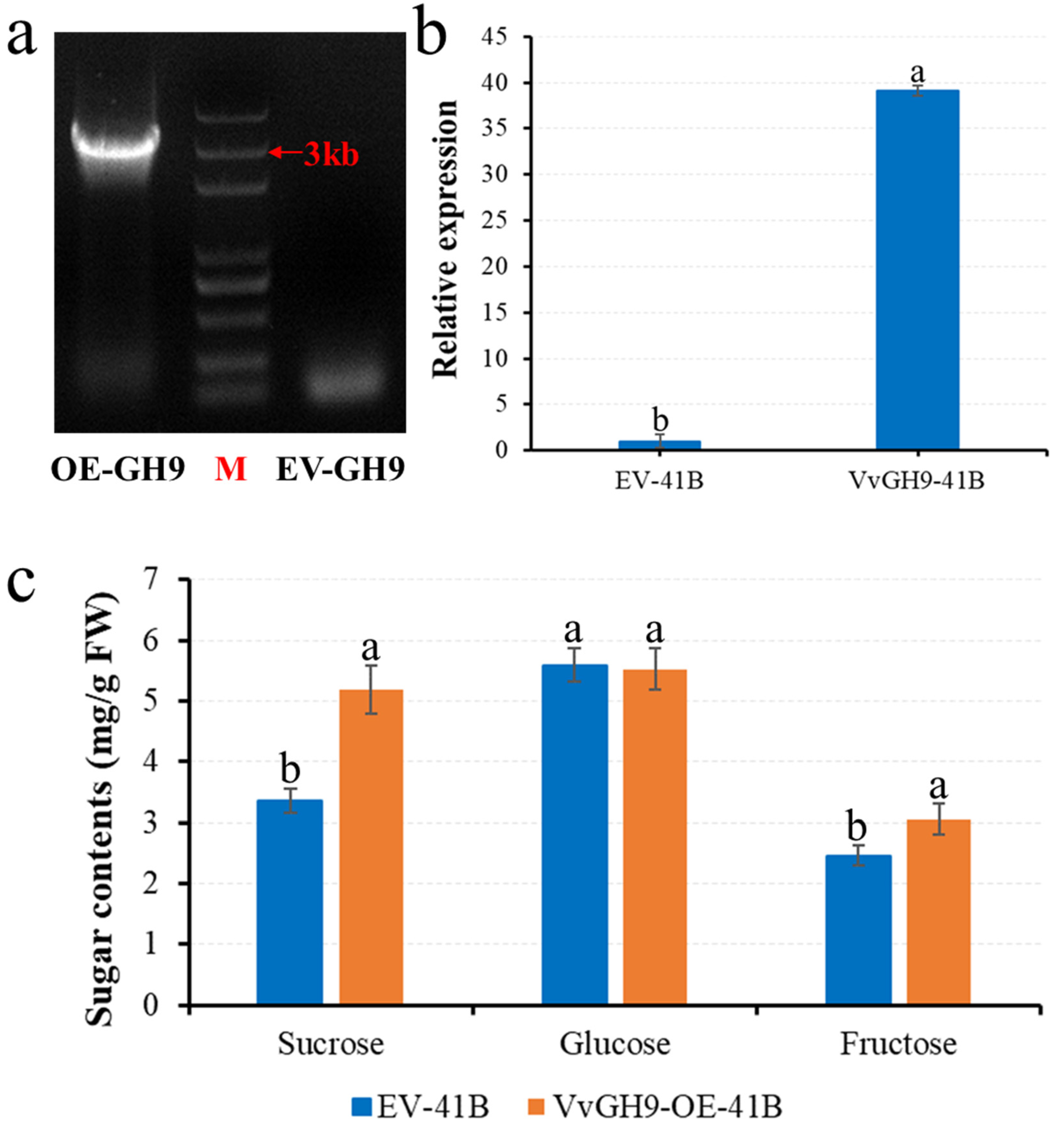
3.6. Overexpression of VvGH9 Gene Improved Sugar Content in 41B Calli
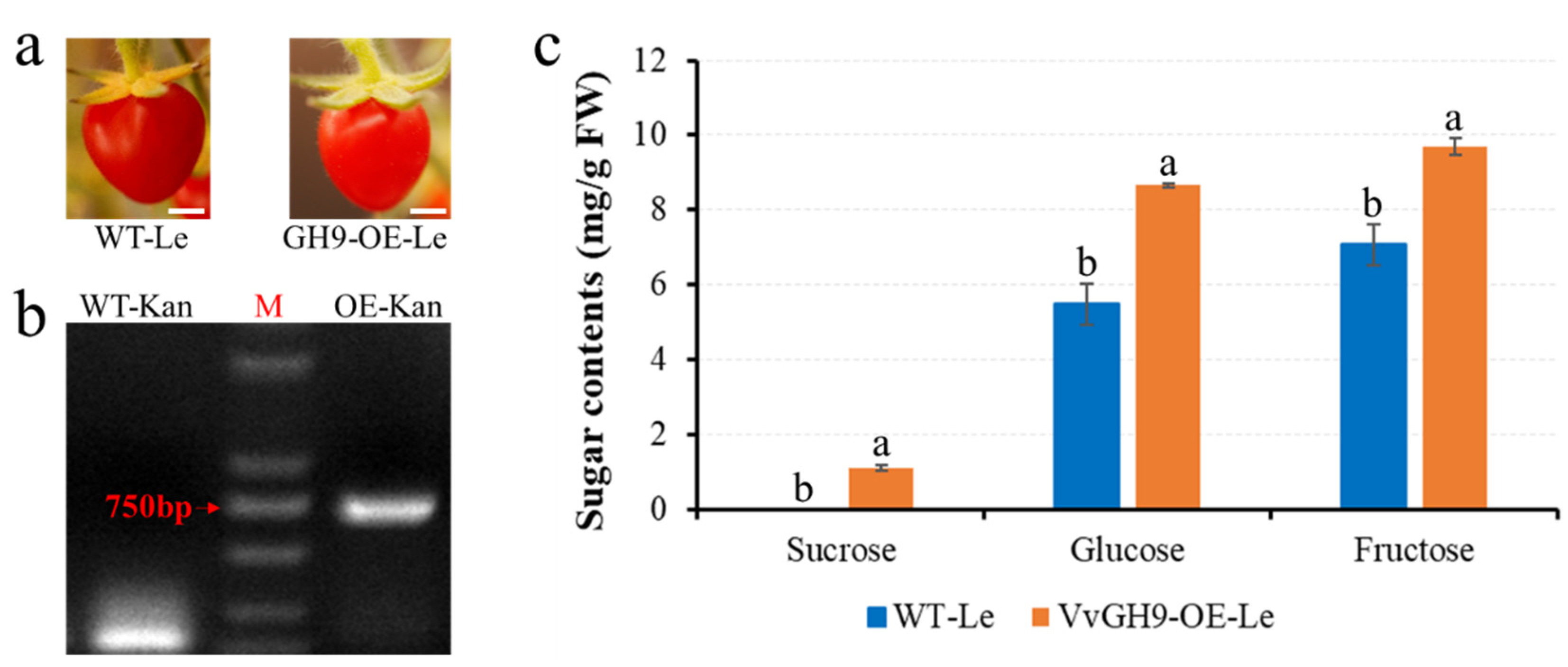
3.7. Heterologous Overexpression of Vvgh9 Increased the Sugar Content of Tomato Berries
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, M.; Li, Y.F.; Liu, T.X.; Yang, G.S. Research progress in sugar accumulation in grape berries. Hunan Agric. Sci. 2020, 11, 91–95. [Google Scholar]
- Zhang, Q.; Chen, Q.S.; Liu, Y.T.; Yin, P.; Meng, Z.F. Research progress on saccharide composition and quality characters of grape fruit. Hubei Agric. Sci. 2012, 51, 4978–4981. [Google Scholar]
- Roitsch, T. Source-sink regulation by sugar and stress. Curr. Opin. Plant Biol. 1999, 2, 198–206. [Google Scholar] [CrossRef]
- Henrissat, B.; Callebaut, I.; Fabrega, S.; Lehn, P.; Mornon, J.P.; Davies, G. Conserved catalytic machinery and the prediction of a common fold for several families of glycosyl hydrolases. Proc. Natl. Acad. Sci. USA 1995, 92, 7090–7094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarter, J.D.; Withers, S.G. Mechanisms of enzymatic glycoside hydrolysis. Curr. Opin. Struct. Biol. 1994, 4, 885–892. [Google Scholar] [CrossRef]
- Semenza, G.; Auricchio, S. Small-intestinal disaccharidases. In The Metabolic Basis of Inherited Diseases; Scrives, C.R., Beaudet, A.L., Eds.; McGraw-Hill: New York, NY, USA, 1989; pp. 2975–2977. [Google Scholar]
- Neufeld, E.F. Lysosomal storage diseases. Annu. Rev. Biochem. 1991, 60, 257–280. [Google Scholar] [CrossRef]
- Bauer, M.W.; Driskill, L.E.; Kelly, R.M. Glycosyl hydrolases from hyperthermophilic microorganisms. Curr. Opin. Biotechnol. 1998, 9, 141–145. [Google Scholar] [CrossRef]
- Naumoff, D.G. Conserved sequence motifs in levansucrases and bifunctional β-xylosidases and α-L-arabinases. FEBS Lett. 1999, 448, 177–179. [Google Scholar] [CrossRef] [Green Version]
- Ermakova, S.P.; Ivanova, E.P.; Bakunina, I.Y.; Mikhailov, V.V.; Zvyagintseva, T.N. Effect of Brown Algae metabolites on the synthesis of O-glycosyl hydrolases by bacteria degrading the thallus of Fucus evanescens. Microbiology 2012, 81, 396–402. [Google Scholar] [CrossRef]
- Sathya, T.A.; Khan, M. Diversity of glycosyl hydrolase enzymes from metagenome and their application in food industry. J. Food Sci. 2014, 79, 11. [Google Scholar] [CrossRef]
- Chen, H.C.; Jin, X.; Zhu, L.J.; Lu, Y.L.; Ma, Z.; Liu, S.J.; Chen, X.L. Glycosyl hydrolase catalyzed glycosylation in unconventional media. Appl. Microbiol. Biotechnol. 2020, 104, 9523–9534. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, D.L.; Liu, Y.S.; Xi, Y.M.; Wang, X.L.; Meng, S. Analysis of Populus glycosyl hydrolase family I members and their potential role in the ABA treatment and drought stress response. Plant Physiol. Biochem. 2021, 163, 178–188. [Google Scholar] [CrossRef]
- Yang, J.F.; Ma, L.; Jiang, W.B.; Yao, Y.; Tang, Y.H.; Pang, Y.Z. Comprehensive identification and characterization of abiotic stress and hormone responsive glycosyl hydrolase family 1 genes in Medicago truncatula. Plant Physiol. Biochem. 2021, 158, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Vasu, P.; Persson, S.; Mort, A.J.; Somerville, C.R. Development and application of a suite of polysaccharide-degrading enzymes for analyzing plant cell walls. Proc. Natl. Acad. Sci. USA 2006, 103, 11417–11422. [Google Scholar] [CrossRef] [Green Version]
- Bourne, Y.; Henrissat, B. Glycoside hydrolases and glycosyltransferases: Families and functional modules. Curr. Opin. Struct. Biol. 2001, 11, 593–600. [Google Scholar] [CrossRef]
- Tricone, A. Uncommon glycosidases for the enzymatic preparation of glycosides. Biomolecules 2015, 5, 2160–2183. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.G.; Domoto, P.A.; Jane, J.L. Structures and functional properties of apple (Malus. domestica Borkh) fruit starch. Carbohydr. Polym. 2006, 63, 432–441. [Google Scholar] [CrossRef]
- Bertin, N.; Causse, M.; Brunel, B.; Tricon, D.; Génard, M. Identification of growth processes involved in QTLs for tomato fruit size and composition. J. Exp. Bot. 2009, 60, 237–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Kuang, S.; Zhang, A.D.; Zhang, W.S.; Chen, M.J.; Yin, X.R.; Chen, K.S. Characterization of starch degradation related genes in postharvest Kiwifruit. Int. J. Mol. Sci. 2016, 17, 2112. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S.K.; Datta, S. β-Glucosidase from the hyperthermophilic archaeon Thermococcus sp. Is a salt-tolerant enzyme that is stabilized by its reaction product glucose. Appl. Microbiol. Biotechnol. 2016, 100, 8399–8409. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.D.; Long, L.F.; Liang, M.F.; Li, H.B.; Chen, Y.H.; Zheng, M.J.; Ni, H.; Li, Q.B.; Zhu, Y.B. Characterization of a glucose-stimulated β-glucosidases from Microbulbifer sp. ALW1. Microbiol. Res. 2021, 251, 126840. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Wang, Y.; Tong, Q.; Xu, G.Z.; Xu, M.L.; Li, S.H.; Fan, P.G.; Li, P.G.; Liang, Z.C. Transcriptomic analysis of grapevine Dof transcription factor gene family in response to cold stress and functional analyses of the VaDof17d gene. Planta 2021, 253, 55. [Google Scholar] [CrossRef] [PubMed]
- Di, F.F.; Jian, H.J.; Wang, T.Y.; Chen, X.P.; Ding, Y.R.; Du, H.; Lu, K.; Li, J.N.; Liu, L.Z. Genome-wide analysis of the PYL gene family and identification of PYL genes that respond to abiotic stress in Brassica napus. Genes 2018, 9, 156. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.L.; Tong, Q.; Wang, Y.; Wang, Z.M.; Xu, G.Z.; Elias, G.K.; Li, S.H.; Liang, Z.C. Transcriptomic Analysis of the Grapevine LEA Gene Family in Response to Osmotic and Cold Stress Reveals a Key Role for VamDHN3. Plant Cell Physiol. 2020, 61, 775–786. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, R.; Liang, Z.C.; Li, S.H. Grape-RNA: A database for the collection, evaluation, treatment, and data sharing of grape RNA-seq datasets. Genes 2020, 11, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaillon, O.; Aury, J.M.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C.; et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 2007, 449, 463–467. [Google Scholar]
- Sun, H.J.; Uchii, S.; Watanabe, S.; Ezura, H. A highly efficient transformation protocol for Micro-Tom, a model cultivar for tomato functional genomics. Plant Cell Physiol. 2006, 47, 426–431. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, L.M.; Ren, C.; Ren, F.R.; Wang, Y.; Fan, P.G.; Li, S.H.; Liang, Z.C. VvSWEET10 mediates sugar accumulation in grapes. Genes 2019, 10, 255. [Google Scholar] [CrossRef] [Green Version]
- Coombe, B.G. Growth Stages of the Grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Blake, C.C.F.; Koening, D.F.; Mair, G.A.; North, A.C.T.; Phillips, D.C.; Sarma, V.R. Structure of hen egg white lysozyme. A three-dimensional Fourier synthesis at 2 Å resolution. Nature 1965, 206, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Henrissat, B.; Bairoch, A. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 1993, 293, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.J.; Wilson, K.S.; Henrissat, B. Nomenclature for sugar-binding subsites in glycosyl hydrolases. Biochem. J. 1997, 321, 557–559. [Google Scholar] [CrossRef]
- White, A.; Rose, D.R. Mechanism of catalysis by retaining β-glycosyl hydrolases. Curr. Opin. Struct. Biol. 1997, 7, 645–651. [Google Scholar] [CrossRef]
- Nibbering, P.; Petersen, B.; Motawia, M.S.; Jorgensen, B.; Ulvskov, P.; Niittyla, T. Golgi-localized exo-β1,3-galactosidases involved in cell expansion and root growth in Arabidopsis. J. Biol. Chem. 2020, 295, 10581–10592. [Google Scholar] [CrossRef] [PubMed]
- Carqueijeiro, I.; Koudounas, K.; Bernonvile, T.D.; Sepulveda, L.J.; Mosquera, A.; Bomzan, D.P.; Oudin, A.; Lanoue, A.; Besseau, S.; Cruz, P.L.; et al. Alternative splicing creates a pseudo-strictosidine β-D-glucosidase modulating alkaloid synthesis in Catharanthus roseus. Plant Physiol. 2021, 185, 836–856. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Wang, Y.; Ren, C.; Fan, P.; Kuang, Y.; Wang, Y.; Liang, Z. Genome Wide Analysis of GH Gene Family Reveals Vvgh9 Positively Regulates Sugar Accumulation under Low Sugar Content in Grape. Horticulturae 2021, 7, 453. https://doi.org/10.3390/horticulturae7110453
Xu G, Wang Y, Ren C, Fan P, Kuang Y, Wang Y, Liang Z. Genome Wide Analysis of GH Gene Family Reveals Vvgh9 Positively Regulates Sugar Accumulation under Low Sugar Content in Grape. Horticulturae. 2021; 7(11):453. https://doi.org/10.3390/horticulturae7110453
Chicago/Turabian StyleXu, Guangzhao, Yi Wang, Chong Ren, Peige Fan, Yangfu Kuang, Yue Wang, and Zhenchang Liang. 2021. "Genome Wide Analysis of GH Gene Family Reveals Vvgh9 Positively Regulates Sugar Accumulation under Low Sugar Content in Grape" Horticulturae 7, no. 11: 453. https://doi.org/10.3390/horticulturae7110453





