Selenium Enrichment of Green and Red Lettuce and the Induction of Radical Scavenging Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Se Concentration and Elemental Composition
2.3. Preparation of Plant Extract
2.4. 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic Acid) (ABTS) Radical Scavenging Assay
2.5. Cytotoxicity Assay against Monkey Kidney (VERO) Cells
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mroczek-Zdyrska, M.; Strubińska, J.; Hanaka, A. Selenium improves physiological parameters and alleviates oxidative stress in shoots of lead-exposed Vicia faba L. minor plants grown under phosphorus-deficient conditions. J. Plant Growth Regul. 2017, 36, 186–199. [Google Scholar] [CrossRef] [Green Version]
- Gratão, P.L.; Alves, L.R.; Lima, L.W. Heavy metal toxicity and plant productivity: Role of metal scavengers. In Plant-Metal Interactions; Srivastava, S., Srivastava, A., Suprasanna, P., Eds.; Springer: Cham, Switzerland, 2019; pp. 49–60. [Google Scholar]
- Jóźwiak, W.; Politycka, B. Effect of selenium on alleviating oxidative stress caused by a water deficit in cucumber roots. Plants 2019, 8, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal 2012, 16, 705–743. [Google Scholar] [CrossRef] [Green Version]
- Bae, M.; Kim, H. The role of Vitamin C, vitamin D, and selenium in immune system against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef]
- Kieliszek, M.; Lipinski, B. Selenium supplementation in the prevention of coronavirus infections (COVID-19). Med. Hypotheses 2020, 143, 109878. [Google Scholar] [CrossRef]
- Haug, A.; Graham, R.D.; Christophersen, O.A.; Lyons, G.H. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb. Ecol. Health Dis. 2007, 19, 209–228. [Google Scholar] [PubMed] [Green Version]
- Schiavon, M.; Pilon-Smits, E.A.H. Selenium biofortification and phytoremediation phytotechnologies: A review. J. Environ. Qual. 2017, 46, 10–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.; Waterland, N.; Moon, Y.; Tou, J.C. Selenium biofortification of agricultural crops and effects on plant nutrients and bioactive compounds important for human health and disease prevention—A review. Plant Food Hum. Nutr. 2019, 74, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium biofortification and interaction with other elements in plants: A review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef] [PubMed]
- Tinggi, U. Selenium: Its role as antioxidant in human health. Environ. Health Prev. Med. 2008, 13, 102–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feinberg, A.; Stenke, A.; Peter, T.; Hinckley, E.-L.S.; Driscoll, C.T.; Winkel, L.H.E. Reductions in the deposition of sulfur and selenium to agricultural soils pose a risk of future nutrient deficiencies. Commun. Earth Environ. 2021, 2, 101. [Google Scholar] [CrossRef]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H.E. Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puccinelli, M.; Malorgio, F.; Pezzarossa, B. Selenium enrichment of horticultural crops. Molecules 2017, 22, 933. [Google Scholar] [CrossRef] [PubMed]
- Wiesner-Reinhold, M.; Schreiner, M.; Baldermann, S.; Schwarz, D.; Hanschen, F.S.; Kipp, A.P.; Rowan, D.D.; Bentley-Hewitt, K.L.; McKenzie, M.J. Mechanisms of selenium enrichment and measurement in Brassicaceous vegetables, and their application to human health. Front. Plant Sci. 2017, 8, 1365. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, M.A.; Meschede, C.A.C.; Mühling, K.H. Selenium foliar application alters patterns of glucosinolate hydrolysis products of pak choi Brassica rapa L. var. chinensis. Sci. Hortic. 2020, 273, 109614. [Google Scholar] [CrossRef]
- Diowksz, A.; Kordialik-Bogacka, E.; Ambroziak, W. Se-enriched sprouted seeds as functional additives in sourdough fermentation. LWT-Food Sci. Technol. 2014, 56, 524–528. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets-iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Broadley, M.R.; Alcock, J.; Alford, J.; Cartwright, P.; Foot, I.; Fairweather-Tait, S.J.; Hart, D.J.; Hurst, R.; Knott, P.; Mcgrath, S.P.; et al. Selenium biofortification of high-yielding winter wheat (Triticum aestivum L.) by liquid or granular Se fertilization. Plant Soil 2010, 332, 5–18. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Li, F.; Wenzel-Storjohann, A.; Sulieman, S.; Tasdemir, D.; Mühling, K.H. Comparative metabolite profile, biological activity and overall quality of three lettuce (Lactuca sativa L., Asteraceae) cultivars in response to sulfur nutrition. Pharmaceutics 2021, 13, 713. [Google Scholar] [CrossRef]
- Shalaby, T.; Bayoumi, Y.; Alshaal, T.; Elhawat, N.; Sztrik, A.; El-Ramady, H. Selenium fortification induces growth, antioxidant activity, yield and nutritional quality of lettuce in salt-affected soil using foliar and soil applications. Plant Soil 2017, 421, 245–258. [Google Scholar] [CrossRef]
- Schiavon, M.; Lima, L.W.; Jiang, Y.; Hawkesford, M.J. Effects of Selenium on Plant Metabolism and Implications for Crops and Consumers. In Selenium in Plants; Pilon-Smits, E., Winkel, L., Lin, Z.Q., Eds.; Plant Ecophysiology; Springer: Cham, Switzerland, 2017; Volume 11. [Google Scholar]
- Eloff, J.N. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 1998, 60, 1–8. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- McGaw, L.J.; Steenkamp, V.; Eloff, J.N. Evaluation of Athrixia bush tea for cytotoxicity, antioxidant activity, caffeine content and presence of pyrrolizidine alkaloids. J. Ethnopharm. 2007, 110, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H. German Nutrition Society (DGE) revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Zou, C.; Du, Y.; Rashid, A.; Ram, H.; Savasli, E.; Pieterse, P.J.; Ortiz-Monasterio, I.; Yazici, A.; Kaur, C.; Mahmood, K.; et al. Simultaneous biofortification of wheat with Zinc, Iodine, Selenium, and Iron through foliar treatment of a micronutrient cocktail in six countries. J. Agric. Food Chem. 2019, 67, 8096–8106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ram, H.; Rashid, A.; Zhang, W.; Duarte, A.P.; Phattarakul, N.; Simunji, S.; Kalayci, M.; Freitas, R.; Rerkasem, B.; Bal, R.S.; et al. Biofortification of wheat, rice and common bean by applying foliar zinc fertilizer along with pesticides in seven countries. Plant Soil 2016, 403, 389–401. [Google Scholar] [CrossRef]
- White, P.J.; Bowen, H.C.; Parmaguru, P.; Fritz, M.; Spracklen, W.P.; Spiby, R.E.; Meacham, M.C.; Mead, A.; Harriman, M.; Trueman, L.J.; et al. Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J. Exp. Bot. 2004, 55, 1927–1937. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, M.A.; Sulieman, S.; Mühling, K.H. Regulation of selenium/sulfur interactions to enhance chemopreventive effects: Lessons to learn from Brassicaceae. Molecules 2020, 25, 5846. [Google Scholar] [CrossRef]
- Gigolashvili, T.; Kopriva, S. Transporters in plant sulfur metabolism. Front Plant Sci. 2014, 5, 442. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, M.A.; Mühling, K.H. Plant-derived sulfur containing natural products produced as a response to biotic and abiotic stresses: A review of their structural diversity and medicinal importance. J. Appl. Bot. Food Qual. 2019, 92, 204–215. [Google Scholar]
- Meschede, C.A.C.; Abdalla, M.A.; Mühling, K.H. Sulfur but not nitrogen supply increases the ITC/Nitrile ratio in Pak Choi (Brassica rapa subsp. Chinensis (L.) Hanelt). J. Appl. Bot. Food Qual. 2020, 93, 95–104. [Google Scholar]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, R.; Proietti, P.; Onofri, A.; Regni, L.; Esposto, S.; Servili, M.; Businelli, D.; Selvaggini, R. Biofortification (Se): Does it increase the content of phenolic compounds in virgin olive oil (VOO)? PLoS ONE 2017, 12, e0176580. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; dall’Acqua, S.; Mietto, A.; Pilon-Smits, E.A.H.; Sambo, P.; Masi, A.; Malagoli, M. Selenium fertilization alters the chemical composition and antioxidant constituents of tomato (Solanum lycopersicon L.). J. Agric. Food Chem. 2013, 61, 10542–10554. [Google Scholar] [CrossRef]
- Pezzarossa, B.; Rosellini, I.; Malorgio, F.; Borghesi, E.; Tonutti, P. Effects of selenium enrichment of tomato plants on ripe fruit metabolism and composition. In Proceedings of the 7th International Postharvest Symposium, Kuala Lumpur, Malaysia, 25–29 June 2013; pp. 247–251. [Google Scholar]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C.; et al. Current knowledge on selenium biofortification to improve the nutraceutical profile of food: A comprehensive review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef]
- Galić, L.; Špoljarević, M.; Jakovac, E.; Ravnjak, B.; Teklić, T.; Lisjak, M.; Perić, K.; Nemet, F.; Lončarić, Z. Selenium biofortification of soybean seeds influences physiological responses of seedlings to osmotic stress. Plants 2021, 10, 1498. [Google Scholar] [CrossRef]
- Benincasa, P.; D’Amato, R.; Falcinelli, B.; Troni, E.; Fontanella, M.C.; Frusciante, S.; Guiducci, M.; Beone, G.M.; Businelli, D.; Diretto, G. Grain endogenous selenium and moderate salt stress work as synergic elicitors in the enrichment of bioactive compounds in maize sprouts. Agronomy 2020, 10, 735. [Google Scholar] [CrossRef]
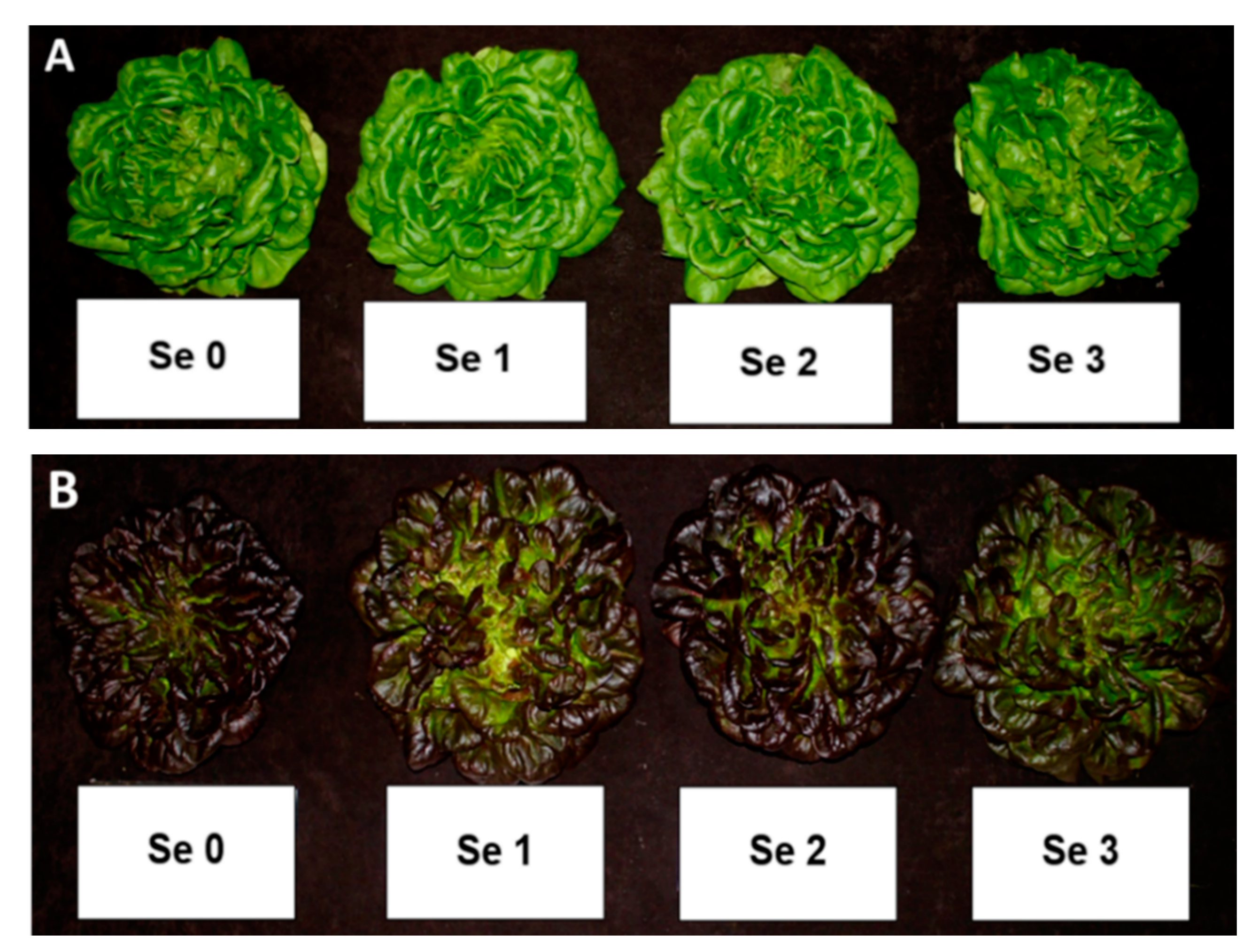
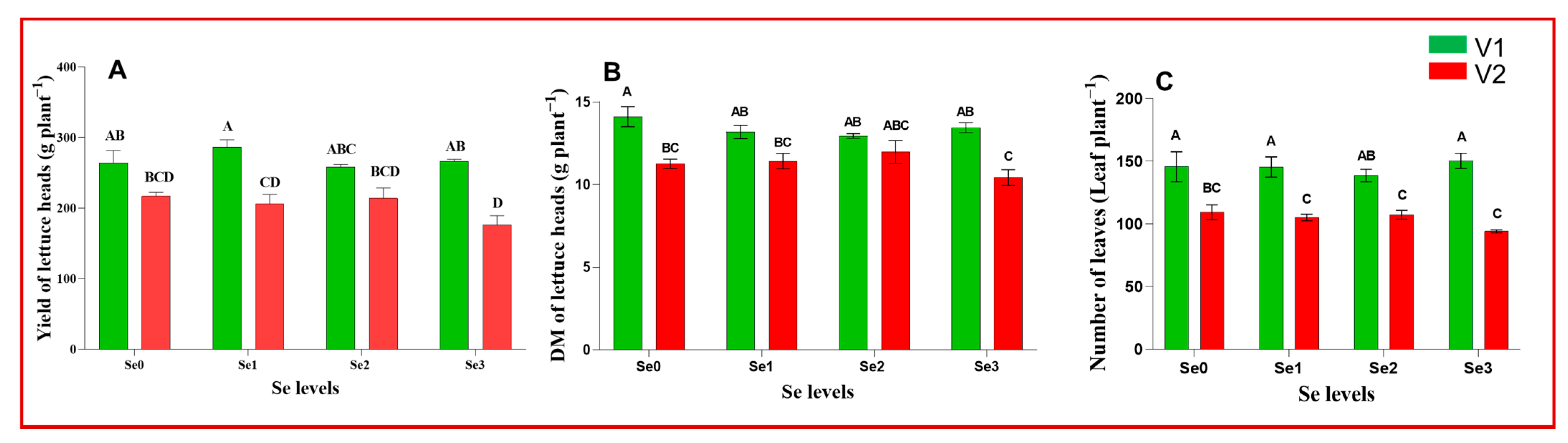
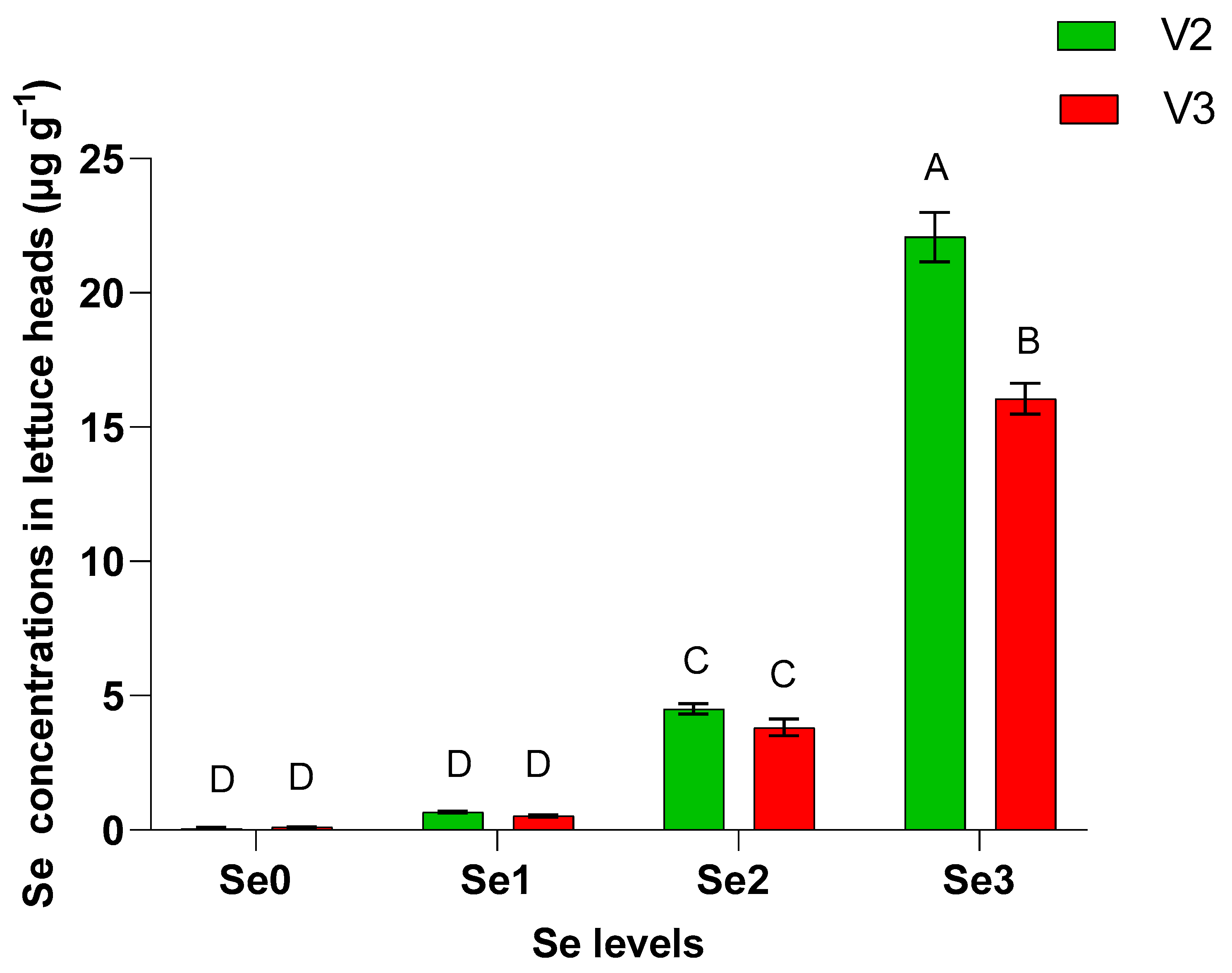

| Multi-Leaf Green Lettuce (Hawking) V1 | Multi-Leaf Red Lettuce (Barlach) V2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Different Selenium Levels | ||||||||
| Se0 | Se1 | Se2 | Se3 | Se0 | Se1 | Se2 | Se3 | |
| Macro in mg/g | ||||||||
| Mg | 3.87 ± 0.2 AB | 3.90 ± 0.1 A | 3.61 ± 0.1 ABC | 3.47 ± 0.1 ABCD | 3.37 ± 0.1 BCD | 3.33 ± 0.1 CD | 3.2 ± 0.04 CD | 3.06 ± 0.04 D |
| P | 8.63 ± 0.1 BCD | 9.24 ± 0.2 ABC | 9.56 ± 0.1 AB | 9.95 ± 0.3 A | 7.78 ± 0.4 D | 7.64 ± 0.3 D | 8.2 ± 0.3 CD | 8.48 ± 0.3 BCD |
| K | 75.9 ± 1.1 A | 78.6 ± 2.4 A | 75.6 ± 0.6 A | 74.1 ± 1.2 AB | 66.0 ± 4.0 ABC | 62.1 ± 3.8 BC | 61.9 ± 2.3 BC | 58.1 ± 2.9 C |
| Ca | 7.09 ± 0.3 A | 7.02 ± 0.2 A | 6.57 ± 0.1 AB | 6.75 ± 0.3 AB | 5.95 ± 0.3 ABC | 5.76 ± 0.3 BC | 5.68 ± 0.2 BC | 5.08 ± 0.2 C |
| N | 43.4 ± 0.9 A | 43.1 ± 0.9 AB | 44.5 ± 0.5 A | 41.3 ± 0.5 ABC | 38.1 ± 1.3 CD | 37.5 ± 1.3 CD | 38.5 ± 0.8 BCD | 36.1 ± 1.2 D |
| S | 1.8 ± 0.1 A | 1.08 ± 0.04 BC | 1.0 ± 0.1 C | 1.88 ± 0.1 A | 1.93 ± 0.1 A | 1.34 ± 0.1 B | 2.05 ± 0.1 A | 2.07 ± 0.1 A |
| N/S | 24.4 ± 1.5 BC | 39.9 ± 1.3 A | 45.3 ± 3.1 A | 22.1 ± 0.9 CD | 19.9 ± 0.8 CD | 28.2 ± 1.7 B | 18.9 ± 1.3 CD | 17.5 ± 0.7 D |
| Micro mg/kg | ||||||||
| Mn | 32.9 ± 0.92 AB | 34.8 ± 0.91 A | 33.95 ± 1.03 A | 33.3 ± 0.89 A | 23.1 ± 1.30 C | 22.1 ± 2.02 C | 25.3 ± 2.26 BC | 23.4 ± 2.16 C |
| Fe | 100.9 ± 2.39 B | 120.6 ± 7.35 A | 93.57 ± 2.86 B | 99.6 ± 3.53 B | 98.9 ± 2.31 B | 104.2 ± 4.12 AB | 95.7 ± 3.93 B | 95.3 ± 2.16 B |
| Cu | 5.42 ± 0.30 A | 5.31 ± 0.65 A | 4.98 ± 0.62 A | 4.18 ± 0.34 A | 3.51 ± 0.16 A | 4.59 ± 0.49 A | 3.6 ± 0.40 A | 3.33 ± 0.44 A |
| Zn | 110.9 ±5.56 AB | 128.1 ± 5.50 A | 80.9 ± 4.26 C | 79.9 ± 3.57 C | 103.2 ± 2.62 B | 103.9 ± 2.16 B | 68.3 ± 1.89 C | 71.9 ± 4.88 C |
| Treatments | LC50 (mg/mL) |
|---|---|
| V1/Se0 | >1 |
| V1/Se1 | >1 |
| V1/Se2 | >1 |
| V1/Se3 | >1 |
| V2/Se0 | >1 |
| V2/Se1 | >1 |
| V2/Se2 | >1 |
| V2/Se3 | >1 |
| Doxorubicin | 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalla, M.A.; Wick, J.E.; Famuyide, I.M.; McGaw, L.J.; Mühling, K.H. Selenium Enrichment of Green and Red Lettuce and the Induction of Radical Scavenging Potential. Horticulturae 2021, 7, 488. https://doi.org/10.3390/horticulturae7110488
Abdalla MA, Wick JE, Famuyide IM, McGaw LJ, Mühling KH. Selenium Enrichment of Green and Red Lettuce and the Induction of Radical Scavenging Potential. Horticulturae. 2021; 7(11):488. https://doi.org/10.3390/horticulturae7110488
Chicago/Turabian StyleAbdalla, Muna Ali, Jürgen E. Wick, Ibukun M. Famuyide, Lyndy J. McGaw, and Karl H. Mühling. 2021. "Selenium Enrichment of Green and Red Lettuce and the Induction of Radical Scavenging Potential" Horticulturae 7, no. 11: 488. https://doi.org/10.3390/horticulturae7110488
APA StyleAbdalla, M. A., Wick, J. E., Famuyide, I. M., McGaw, L. J., & Mühling, K. H. (2021). Selenium Enrichment of Green and Red Lettuce and the Induction of Radical Scavenging Potential. Horticulturae, 7(11), 488. https://doi.org/10.3390/horticulturae7110488









