Higher Temperatures Decrease Fruit Size in Strawberry Growing in the Subtropics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Growing Conditions
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Weather
3.2. Plant Growth
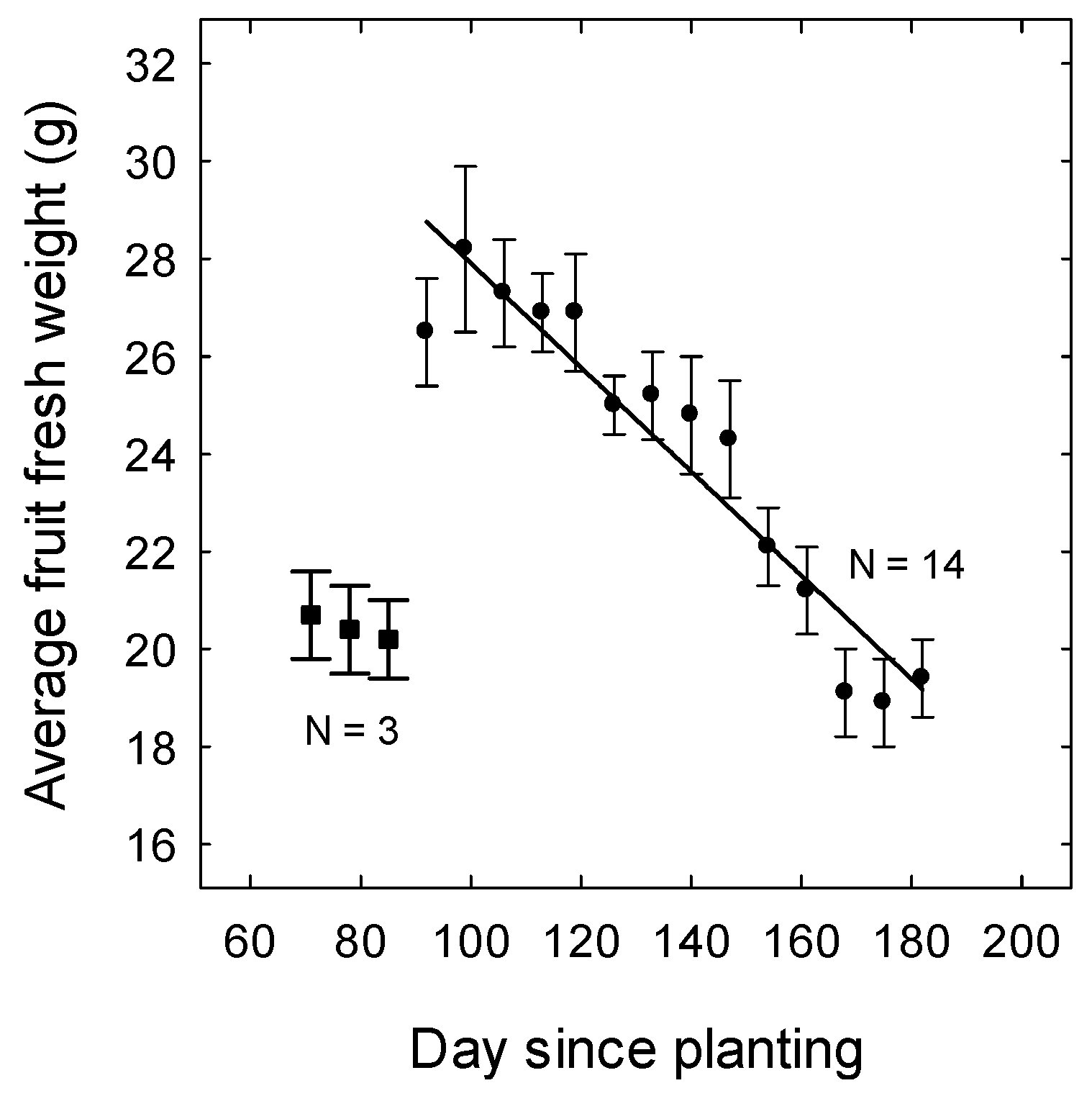
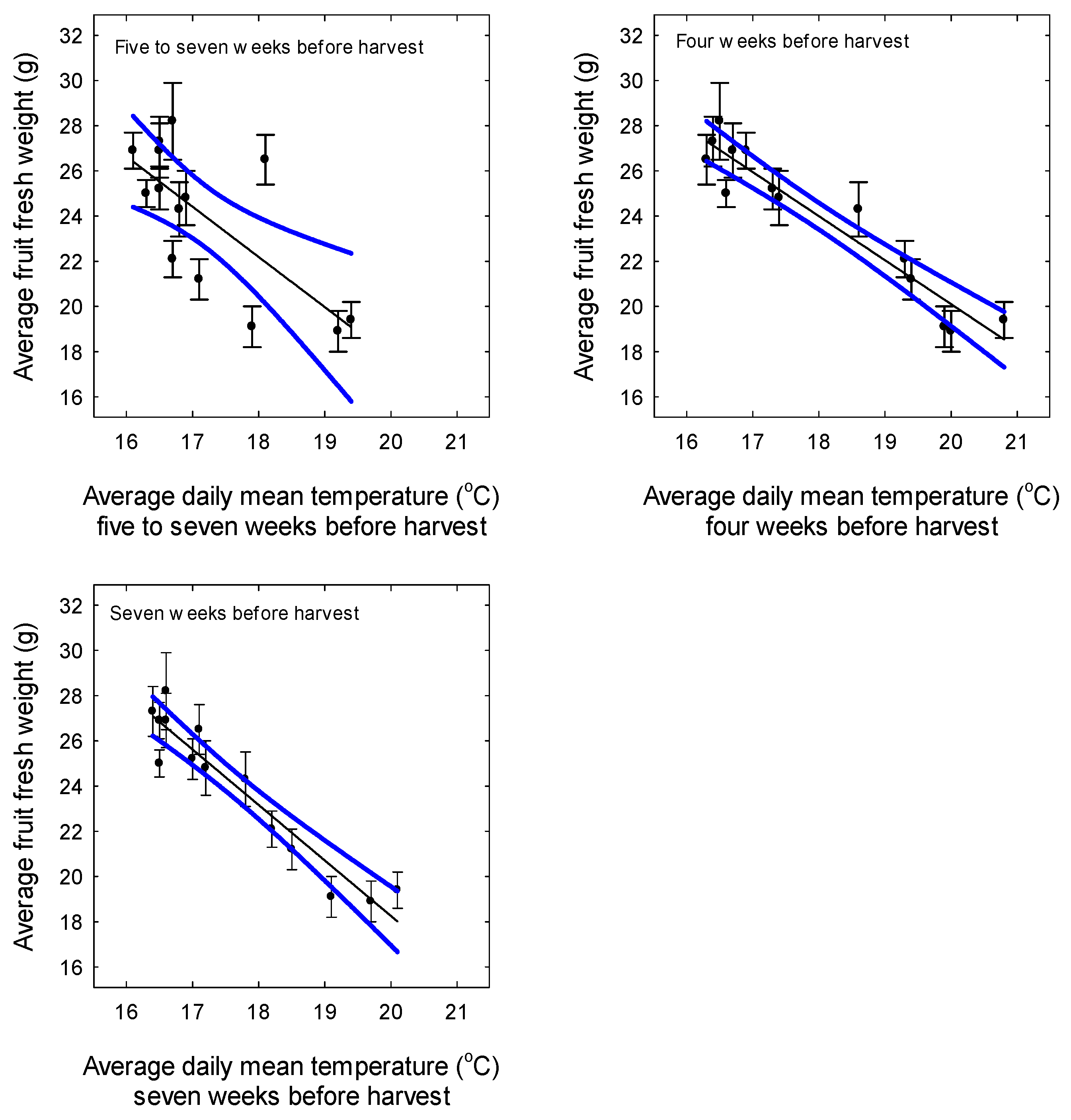
3.3. Yield and Fruit Quality
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, S.G.; Kim, S.K.; Lee, H.J.; Lee, H.S.; Lee, J.H. Impact of moderate and extreme climate change scenarios on growth, morphological features, photosynthesis, and fruit production of hot pepper. Ecol. Evol. 2017, 8, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, F.F.S.; Sánchez-Román, R.M.; González, A.M.G.O. Simulation model of the growth of sweet orange (Citrus sinensis L. Osbeck) cv. Natal in response to climate change. Clim. Chang. 2017, 143, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, V.; Kumar, P.; Long, S.P. Decreasing, not increasing, leaf area will raise crop yields under global atmospheric change. Glob. Chang. Biol. 2017, 23, 1626–1635. [Google Scholar] [CrossRef] [Green Version]
- Campoy, J.A.; Darbyshire, R.; Dirlewanger, E.; Quero-García, J. Yield potential definition of the chilling requirement reveals likely underestimation of the risk of climate change on winter chill accumulation. Int. J. Biomet. 2019, 63, 183–192. [Google Scholar] [CrossRef]
- Chavan, S.G.; Duursma, R.A.; Tausz, M.; Ghannoum, O. Elevated CO2 alleviates the negative impact of heat stress on wheat physiology but not on grain yield. J. Exp. Bot. 2019, 70, 6447–6459. [Google Scholar] [CrossRef]
- Mistry, M.N.; Wing, I.S.; de Cian, E. Simulated vs. empirical weather responsiveness of crop yields: US evidence and implications for the agricultural impacts of climate change. Environ. Res. Lett. 2017, 12, 075007. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Wang, C.; Chen, S. Using statistical model to simulate the impact of climate change on maize yield with climate and crop uncertainties. Theor. Appl. Climatol. 2017, 130, 1065–1071. [Google Scholar] [CrossRef]
- Benlloch-González, M.; Sánchez-Lucas, R.; Bejaoui, M.A.; Benlloch, M.; Fernández-Escobar, R. Global warming effects on yield and fruit maturation of olive trees growing under field conditions. Sci. Hortic. 2019, 249, 162–167. [Google Scholar] [CrossRef]
- Petersen, L.K. Impact of climate change on twenty-first century crop yields in the US. Climate 2019, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Ray, D.K.; West, P.C.; Clark, M.; Gerber, J.S.; Prishchepov, A.V.; Chatterjee, S. Climate change has likely already affected global food production. PLoS ONE 2019, 14, e0217148. [Google Scholar] [CrossRef]
- Vogel, E.; Donat, M.G.; Alexander, L.V.; Meinshausen, M.; Ray, D.K.; Karoly, D.; Meinshausen, N.; Frieler, K. The effects of climate extremes on global agricultural yields. Environ. Res. Lett. 2019, 14, 054010. [Google Scholar] [CrossRef]
- Hammer, G.L.; McLean, G.; van Oosterom, E.; Chapman, S.; Zheng, B.; Wu, A.; Doherty, A.; Jordan, D. Designing crops for adaptation to the drought and high temperature risks anticipated in future climates. Crop Sci. 2020, 60, 605–621. [Google Scholar] [CrossRef]
- Lollato, R.P.; Bavia, G.P.; Perin, V.; Knapp, M.; Santos, E.A.; Patrignani, A.; DeWolf, E.D. Climate-risk assessment for winter wheat using long-term weather data. Agron. J. 2020, 112, 2132–2151. [Google Scholar] [CrossRef]
- Ma, L.; Fang, Q.X.; Sima, M.W.; Burkey, K.O.; Harmel, R.D. Simulated climate change effects on soybean production using two crop modules in RZWQM2. Agron. J. 2021, 113, 112. [Google Scholar] [CrossRef]
- Leng, G. Keeping global warming within 1.5 °C reduces future risks of yield loss in the United States: A probabilistic modeling approach. Sci. Total Environ. 2018, 644, 52–59. [Google Scholar] [CrossRef]
- Qian, B.; Jing, Q.; Bélanger, G.; Shang, J.; Huffman, T.; Liu, J.; Hoogenboom, G. Simulated canola yield responses to climate change and adaptation in Canada. Agron. J. 2018, 110, 133–146. [Google Scholar] [CrossRef] [Green Version]
- Kinose, Y.; Masutomi, Y.; Shiotsu, F.; Hayashi, K.; Ogawada, D.; Gomez-Garcia, M.; Matsumura, A.; Takahashi, K.; Fukushi, K. Impact assessment of climate change on the major rice cultivar Ciherang in Indonesia. J. Agric. Meterol. 2020, 76, 19–28. [Google Scholar] [CrossRef]
- Cammarano, D.; Ceccarelli, S.; Grando, S.; Romagosa, I.; Benbelkacem, A.; Akar, T.; Al-Yassin, A.; Pecchioni, N.; Francia, E. The impact of climate change on barley yield in the Mediterranean basin. Eur. J. Agron. 2019, 106, 1–11. [Google Scholar] [CrossRef]
- Varma, V.; Bebber, D.P. Climate change impacts on banana yields around the world. Nat. Clim. Chang. 2019, 9, 752–757. [Google Scholar] [CrossRef] [Green Version]
- Pathak, T.B.; Maskey, M.L.; Dahlberg, J.A.; Kearns, F.; Bali, K.M.; Zaccaria, D. Climate change trends and impacts on California agriculture: A detailed review. Agronomy 2018, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Døving, A. Climate change and strawberry season in Norway. Acta Hortic. 2008, 842, 753–756. [Google Scholar] [CrossRef]
- Esitken, A.; Ercisli, S.; Yildiz, H.; Orhan, E. Does climate change have an effect on strawberry yield in colder growing areas? Acta Hortic. 2009, 838, 59–61. [Google Scholar] [CrossRef]
- Bethere, L.; Sīle, T.; Seņņikovs, J.; Bethers, U. Impact of climate change on the timing of strawberry phenological processes in the Baltic States. Est. J. Earth Sci. 2016, 65, 48–58. [Google Scholar] [CrossRef]
- Neri, D.; Baruzzi, G.; Massetani, F.; Faedi, W. Strawberry production in forced and protected culture in Europe as a response to climate change. Can. J. Plant Sci. 2012, 92, 1021–1036. [Google Scholar] [CrossRef]
- Husani, A.M.; Xu, Y.W. Challenges of Climate Change to Strawberry Cultivation: Uncertainty and Beyond. In Strawberry: Growth, Development and Diseases; Husani, A.M., Neri, D., Eds.; CABI: Wallingford, UK, 2016; pp. 262–287. [Google Scholar]
- Morton, L.W.; Peres, N.; Fraisse, C.; Gleason, M. Climate, weather and strawberries. Sociol. Tech. Rep. 2017, 1047, 16. [Google Scholar]
- Kerr, A.; Dialesandro, J.; Steenwerth, K.; Lopez-Brody, N.; Elias, E. Vulnerability of California specialty crops to projected mid-century temperature changes. Clim. Chang. 2018, 148, 419–436. [Google Scholar] [CrossRef]
- Lobell, D.; Field, C. California perennial crops in a changing climate. Clim. Chang. 2011, 109, 317–333. [Google Scholar] [CrossRef]
- Deschenes, O.; Kolstad, C. Economic impacts of climate change on California agriculture. Clim. Chang. 2011, 109, 365–386. [Google Scholar] [CrossRef] [Green Version]
- Lobell, D.; Cahill, K.N.; Field, C.B. Historical effects of temperature and precipitation on California crop yields. Clim. Chang. 2007, 81, 187–203. [Google Scholar] [CrossRef]
- Grez, J.; Contreras, E.; Sánchez, S.; Alcalde, J.A.; Gambardella, M. Floral induction and dormancy behaviour in ‘Chilean white strawberry’ (Fragaria chiloensis (L.) Mill. subsp. chiloensis f. chiloensis). Sci. Hortic. 2020, 274, 109648. [Google Scholar] [CrossRef]
- Yang, J.; Su, D.; Wei, S.; Chen, S.; Lou, Z.; Shen, X.; Zhang, Z.; Jamil, A.; Tong, J.; Cui, X. Current and future potential distribution of wild strawberry species in the biodiversity hotspot of Yunnan Province, China. Agronomy 2020, 10, 959. [Google Scholar] [CrossRef]
- Gamboa-Mendoza, A.P.; Delgadillo-Martínez, J.; Almaraz-Suárez, J.J.; Robledo-Paz, A.; Alarcón, A. Response of Fragaria mexicana and rhizosphere microbial communities to temperature increase. Rev. Biol. Trop. 2019, 67, 94–106. [Google Scholar]
- Sun, P.; Mantri, N.; Lou, H.; Hu, Y.; Sun, D.; Zhu, Y.; Dong, T.; Lu, H. Effects of elevated CO2 and temperature on yield and fruit quality of strawberry (Fragaria × ananassa Duch.) at two levels of nitrogen application. PLoS ONE 2012, 7, e41000. [Google Scholar] [CrossRef]
- Mackenzie, S.J.; Chandler, C.K.; Hasing, T.; Whitaker, V.M. The role of temperature in the late-season decline in soluble solids content of strawberry fruit in a subtropical production system. HortScience 2011, 46, 1562–1566. [Google Scholar] [CrossRef]
- Menzel, C.M. Temperature has a greater effect on fruit growth than defoliation or fruit thinning in strawberries in the subtropics. Agriculture 2019, 9, 127. [Google Scholar] [CrossRef] [Green Version]
- Fernandes Filho, C.C.; Andrade, M.H.M.L.; Souza Marçal, T.; Fernandes, M.O.; Bastos, A.J.R.; Guedes, M.L.; Ribeiro, S.R.R.d.P.; Pinto, C.A.B.P.; Nunes, J.A.R. Selection of potato (Solanum tuberosum L.) clones for heat tolerance and resistance to viruses X and Y for processing purposes. Crop Sci. 2021, 61, 552–565. [Google Scholar] [CrossRef]
- Chandler, C.K.; Legard, D.E.; Dunigan, D.D.; Crocker, T.E.; Sims, C.A. ‘Strawberry Festival’ strawberry. HortScience 2000, 35, 1366–1367. [Google Scholar] [CrossRef] [Green Version]
- Whitaker, V.M.; Peres, N.A.; Osorio, L.F.; Fan, Z.; Nunes, M.C.N.; Plotto, A.; Sims, C.A. ‘Florida Brilliance’ strawberry. HortScience 2019, 54, 2073–2077. [Google Scholar] [CrossRef] [Green Version]
- Herrington, M.; Neal, J.; Woolcock, L.; Paynter, M.; Gomez, A. ‘Red Rhapsody’ strawberry. HortScience 2019, 54, 1641–1643. [Google Scholar] [CrossRef] [Green Version]
- Menzel, C.M.; Smith, L.A.; Moisander, J.A. The productivity of strawberry plants growing under plastic high tunnels in a wet subtropical environment. HortTechnology 2014, 24, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Menzel, C.M. Changes in the concentration of leaf nitrogen over the season affect the diagnosis of deficiency or sufficiency in strawberries in the subtropics. Agriculture 2018, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Menzel, C.M.; Gomez, A.; Smith, L.A. Control of grey mould and stem-end rot in strawberry plants growing in a subtropical environment. Australas. Plant Pathol. 2016, 45, 489–498. [Google Scholar] [CrossRef]
- Macías-Rodríguez, L.; Quero, E.; López, M.G. Carbohydrate differences in strawberry crowns and fruit (Fragaria × ananassa) during plant development. J. Agric. Food Chem. 2002, 50, 3317–3321. [Google Scholar] [CrossRef] [PubMed]
- Kallio, H.; Hakala, M.; Pelkkikangas, A.; Lapveteläinen, A. Sugars and acids of strawberry varieties. Eur. Food Res. Technol. 2000, 212, 81–85. [Google Scholar] [CrossRef]
- Levenberg, K. A method for the solution of certain non-linear problems in least squares. Quart. Appl. Math. 1944, 2, 164–168. [Google Scholar] [CrossRef] [Green Version]
- Marquardt, D. An algorithm for least-squares estimation of nonlinear parameters. SIAM J. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Tomczyk, K. Levenberg-Marquardt algorithm for optimization of mathematical models according to MINIMAX objective function of measurement systems. Metrol. Meas. Syst. 2009, 16, 599–606. [Google Scholar]
- Miura, H.; Imada, S.; Yabuuchi, S. Double sigmoid growth curve of strawberry fruit. J. Jpn. Soc. Hortic. Sci. 1990, 59, 527–531. [Google Scholar] [CrossRef] [Green Version]
- Chandler, C.K.; MacKenzie, S.J.; Herrington, M. Fruit development period in strawberry differs among cultivars, and is negatively correlated with average post bloom air temperature. Proc. Fla. State Hortic. Soc. 2004, 117, 83–85. [Google Scholar]
- Ariza, M.T.; Soria, C.; Martinez-Ferri, E. Developmental stages of cultivated strawberry flowers in relation to chilling sensitivity. AoB Plants 2015, 7, plv012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.Y.; Camp, M.J. Temperatures after bloom affect plant growth and fruit quality of strawberry. Sci. Hortic. 2000, 85, 183–199. [Google Scholar] [CrossRef]
- Kadir, S.; Sidhu, G.; Al-Khatib, K. Strawberry (Fragaria × ananassa Duch.) growth and productivity as affected by temperature. HortScience 2006, 41, 1423–1430. [Google Scholar] [CrossRef] [Green Version]
- Menzel, C.M. Strawberry R&D Update; Department of Primary Industries: Sydney, Australia, 2007; pp. 11–13. [Google Scholar]
- Acuna-Maldonado, L.; Pritts, M. Seasonal patterns of carbohydrate and nitrogen accumulation and depletion in strawberry are affected by fruiting but not day neutrality. J. Am. Pomol. Soc. 2013, 67, 95–103. [Google Scholar]
- Kruger, E.; Josuttis, M.; Nestby, R.; Toldam-Andersen, T.B.; Carlen, C.; Mezzetti, B. Influence of growing conditions at different latitudes of Europe on strawberry growth performance, yield and quality. J. Berry Res. 2012, 2, 143–157. [Google Scholar] [CrossRef] [Green Version]
- Le Mière, P.; Hadley, P.; Darby, J.; Battey, N.H. The effect of thermal environment, planting date and crown size on growth, development and yield of Fragaria × ananassa Duch. cv. Elsanta. J. Hortic. Sci. Biotechnol. 1998, 73, 786–795. [Google Scholar] [CrossRef]
- Anjom, F.K.; Vougioukas, S.G.; Slaughter, D.C. Development of a linear mixed model to predict the picking time in strawberry harvesting processes. Biosyst. Eng. 2018, 166, 76–89. [Google Scholar] [CrossRef]
- Xiong, Y.; Ge, Y.; Grimstad, L.; From, P.J. An autonomous strawberry-harvesting robot: Design, development, integration, and field evaluation. J. Field Robot. 2020, 37, 202–224. [Google Scholar] [CrossRef] [Green Version]
- Whitehouse, A.B.; Simpson, D.W.; Johnson, A.W.; McLeary, K.J.; Passey, A.J.; Troop, S.W. ‘Malling Centenary’, a short-day strawberry cultivar from NIAB-EMR. Acta Hortic. 2017, 1156, 185–188. [Google Scholar] [CrossRef]
- Delphy. Production Guidelines—Malling Centenary; Delphy: Wageningen, The Netherlands, 2018; pp. 1–10. [Google Scholar]
- Faedi, W.; Baruzzi, G. Strawberry Breeding. In Strawberry: Growth, Development and Diseases; Husaini, A.M., Neri, D., Eds.; CABI: Wallingford, UK, 2016; pp. 26–40. [Google Scholar]
- Herrington, M.E.; Wegener, M.; Hardner, C.; Woolcock, L.L.; Dieters, M.J. Influence of plant traits on production costs and profitability of strawberry in southeast Queensland. Agric. Syst. 2012, 106, 23–32. [Google Scholar] [CrossRef]
- Heide, O.M.; Stavang, J.A.; Sønsteby, A. Physiology and genetics of flowering in cultivated and wild strawberries—A review. J. Hortic. Sci. Biotechnol. 2013, 88, 1–18. [Google Scholar] [CrossRef]
- Nakano, Y.; Higuchi, Y.; Yishida, Y.; Hisamatsu, T. Environmental responses of the FT/TFL1 gene family and their involvement in flower induction in Fragaria × ananassa. J. Plant Physiol. 2015, 177, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Sønsteby, A.; Heide, O.M. Flowering performance and yield of established and recent strawberry cultivars (Fragaria × ananassa) as affected by raising temperature and photoperiod. J. Hortic. Sci. Biotechnol. 2017, 92, 367–375. [Google Scholar] [CrossRef]
- Heide, O.M.; Sønsteby, A. Climate-photothermographs, a tool for ecophysiological assessment of effects of climate warming in crop plants: Examples with three berry crops. J. Berry Res. 2020, 10, 411–418. [Google Scholar] [CrossRef]

| Period | May | June | July | August | September | October |
|---|---|---|---|---|---|---|
| 2020 | ||||||
| Mean daily max. temperature (°C) | 23.2 | 22.2 | 21.3 | 23.3 | 24.6 | 26.6 |
| Mean daily min. temperature (°C) | 13.5 | 13.0 | 11.2 | 10.1 | 13.7 | 15.7 |
| Mean daily solar radiation (MJ/m2) | 13.3 | 12.4 | 13.5 | 16.3 | 17.4 | 20.6 |
| Total monthly rainfall (mm) | 51 | 59 | 113 | 17 | 76 | 112 |
| Long-term average | ||||||
| Mean daily max. temperature (°C) | 23.5 | 21.3 | 20.8 | 22.3 | 24.6 | 26.5 |
| Mean daily min. temperature (°C) | 11.7 | 8.5 | 7.0 | 7.4 | 9.8 | 13.2 |
| Mean daily solar radiation (MJ/m2) | 13.7 | 11.7 | 13.1 | 16.1 | 18.9 | 20.9 |
| Total monthly rainfall (mm) | 108 | 115 | 50 | 58 | 90 | 80 |
| Cultivar & Time of Sampling | No. of Leaves/Plant | Leaf Area (cm2/plant) | Leaf Dry Weight (g/plant) | Crown Dry Weight (g/plant) | Root Dry Weight (g/plant) |
|---|---|---|---|---|---|
| 26 August | |||||
| Festival | 15.3 b | 1450 c | 12.4 c | 2.5 bc | 1.2 bc |
| Brilliance | 13.8 ab | 843 a | 7.3 a | 1.9 a | 0.7 a |
| Red Rhapsody | 13.4 ab | 1138 b | 11.1 c | 2.6 c | 1.5 c |
| Scarlet Rose | 11.4 a | 990 a | 9.3 b | 2.0 ab | 0.9 ab |
| Sundrench | 14.9 b | 1008 ab | 9.0 ab | 1.8 a | 1.0 ab |
| Mean ± SE | 13.8 ± 0.6 | 1086 ± 92 | 9.8 ± 0.8 | 2.2 ± 0.1 | 1.1 ± 0.1 |
| 28 October | |||||
| Festival | 25.1 b | 1249 c | 17.2 c | 4.8 b | 1.7 b |
| Brilliance | 24.9 b | 1280 a | 10.4 a | 3.4 a | 1.1 a |
| Red Rhapsody | 22.5 ab | 1710 b | 16.8 c | 4.4 b | 2.1 c |
| Scarlet Rose | 19.5 a | 1642 b | 14.8 b | 3.5 a | 1.2 a |
| Sundrench | 21.2 a | 1142 a | 9.8 a | 2.9 a | 1.5 b |
| Mean ± SE | 22.6 ± 1.0 | 1585 ± 159 | 13.8 ± 1.4 | 3.8 ± 0.3 | 1.5 ± 0.2 |
| Cultivar | Concentration of Non-Structural Carbohydrates (% DW) | |||
|---|---|---|---|---|
| Leaves on 26 August | Crowns and Roots on 26 August | Leaves on 28 October | Crowns and Roots on 28 October | |
| Festival | 8.9 a | 4.8 a | 7.1 b | 3.8 a |
| Brilliance | 7.7 a | 4.9 a | 5.6 a | 3.5 a |
| Red Rhapsody | 8.2 a | 5.3 a | 5.9 a | 3.6 a |
| Scarlet Rose | 7.7 a | 4.5 a | 6.3 ab | 3.5 a |
| Sundrench | 7.9 a | 5.4 a | 7.1 b | 4.3 a |
| Mean ± SE | 8.1 ± 0.3 | 5.0 ± 0.1 | 6.4 ± 0.2 | 3.7 ± 0.1 |
| Cultivar | Marketable Yield (g/plant) | Av. Fruit Fresh Weight (g) | Percentage of Small Fruit | Soluble Solids Content (%) | Titratable Acidity (%) |
|---|---|---|---|---|---|
| Festival | 616 b | 20.3 a | 29.0 c | 8.3 c | 0.64 b |
| Brilliance | 457 a | 22.7 b | 28.2 c | 7.3 b | 0.57 a |
| Red Rhapsody | 617 b | 23.9 c | 16.2 a | 7.3 b | 0.64 b |
| Scarlet Rose | 592 b | 24.4 cd | 21.7 b | 8.9 d | 0.83 c |
| Sundrench | 656 b | 25.5 d | 16.0 a | 6.8 a | 0.57 a |
| Cultivar | Sm ± SE | k ± SE | m ± SE | R2 Value |
|---|---|---|---|---|
| Festival | 670 ± 12 | 0.050 ± 0.002 | 129 ± 1 | 0.99 |
| Brilliance | 481 ± 11 | 0.047 ± 0.002 | 125 ± 1 | 0.99 |
| Red Rhapsody | 698 ± 13 | 0.046 ± 0.001 | 139 ± 1 | 0.99 |
| Scarlet Rose | 643 ± 14 | 0.049 ± 0.002 | 133 ± 1 | 0.99 |
| Sundrench | 679 ± 12 | 0.052 ± 0.002 | 127 ± 1 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menzel, C. Higher Temperatures Decrease Fruit Size in Strawberry Growing in the Subtropics. Horticulturae 2021, 7, 34. https://doi.org/10.3390/horticulturae7020034
Menzel C. Higher Temperatures Decrease Fruit Size in Strawberry Growing in the Subtropics. Horticulturae. 2021; 7(2):34. https://doi.org/10.3390/horticulturae7020034
Chicago/Turabian StyleMenzel, Christopher. 2021. "Higher Temperatures Decrease Fruit Size in Strawberry Growing in the Subtropics" Horticulturae 7, no. 2: 34. https://doi.org/10.3390/horticulturae7020034
APA StyleMenzel, C. (2021). Higher Temperatures Decrease Fruit Size in Strawberry Growing in the Subtropics. Horticulturae, 7(2), 34. https://doi.org/10.3390/horticulturae7020034





