The Impact of Salt Stress on Plant Growth, Mineral Composition, and Antioxidant Activity in Tetragonia decumbens Mill.: An Underutilized Edible Halophyte in South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Location
2.2. Plant Preparation, Irrigation and Treatments
2.3. Determination of Plant Growth
2.3.1. Plant Weight
2.3.2. Shoot Length and Branch Number
2.4. Mineral Analysis
2.5. Chlorophyll Readings
2.6. The Antioxidant Analysis
2.6.1. Sample Preparation
2.6.2. Determination of Antioxidant Capacity and Content
2.6.3. Polyphenol Assay
2.6.4. ABTS Assay
2.6.5. Ferric Reducing Antioxidant Power (FRAP) Assay
2.7. Statistical Analysis
3. Results
3.1. Effects of Salt Stress on Plant Growth
3.1.1. Shoot Length and Lateral Branch Number
3.1.2. Fresh and Dry Weight of Shoots
3.1.3. Fresh and Dry Weight of Stem and Roots
3.1.4. Total Fresh and Dry Weight
3.2. Effect of Salt Stress on the Mineral Content of Dried Leaves of T. decumbens
3.2.1. Macronutrients
3.2.2. Micronutrients
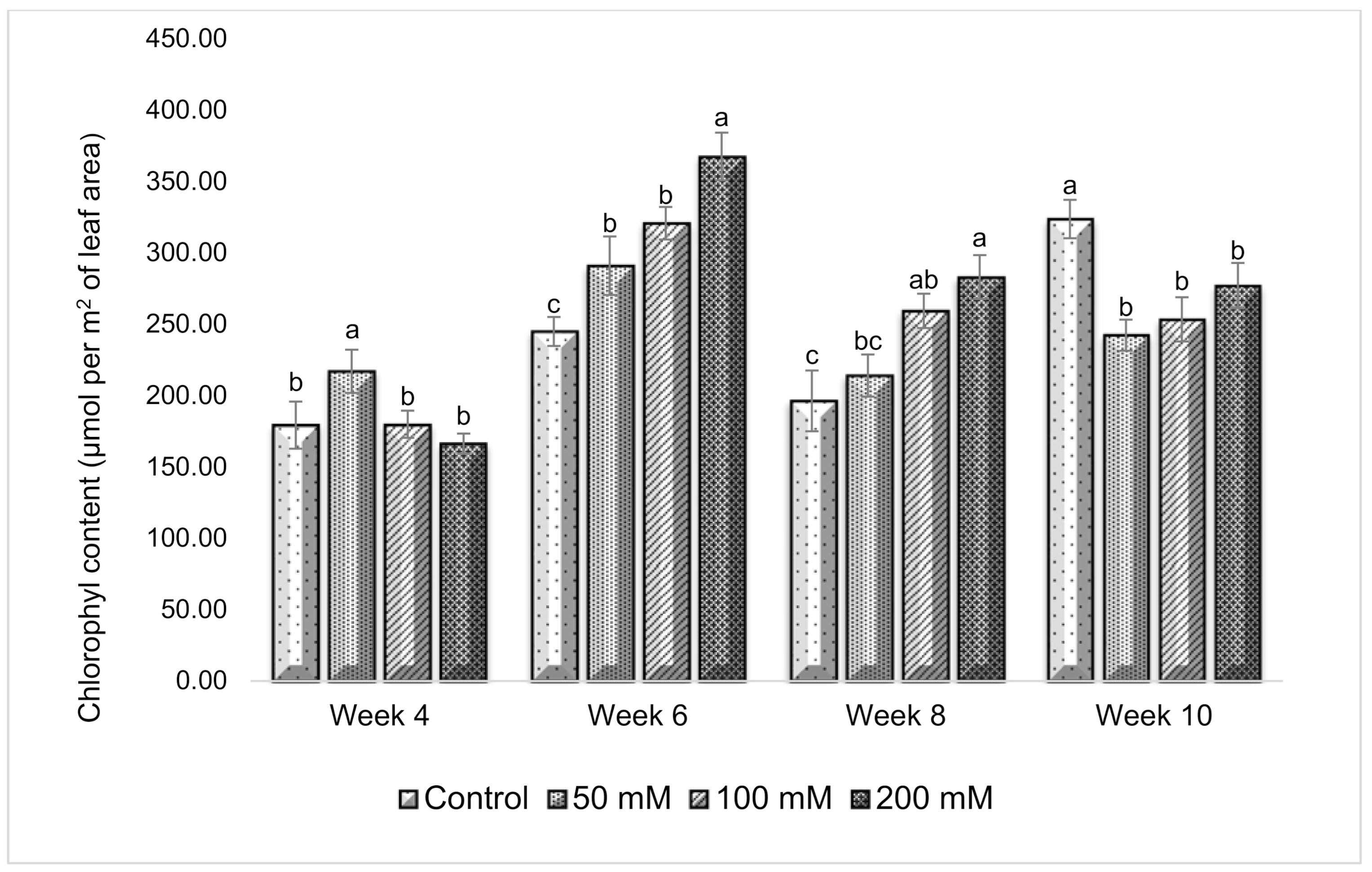
3.3. Effect of Salt Stress on Chlorophyll Content
3.4. Effects of Salt Stress on Phenolic Content and Antioxidant Capacity
3.4.1. Polyphenol Content
3.4.2. ABTS Capacity
3.4.3. FRAP Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molotoks, A.; Smith, P.; Dawson, T.P. Impacts of land use, population, and climate change on global food security. Food Energy Secur. 2021, 10, e261. [Google Scholar] [CrossRef]
- Gidamis, A.B.; Chove, B.E. Biotechnology and Biosafety: Exploring the Debate and Public Perception in Developing Countries. J. Knowl. Glob. 2009, 2, 45–62. [Google Scholar]
- Ogunniyi, A.I.; Mavrotas, G.; Olagunju, K.O.; Fadare, O.; Adedoyin, R. Governance quality, remittances and their implications for food and nutrition security in Sub-Saharan Africa. World Dev. 2020, 127, 104752. [Google Scholar] [CrossRef]
- World Bank. World Development Report 2008: Agriculture for Development; World Bank: Washington, DC, USA, 2007. [Google Scholar]
- Acquaah, G. Principles of Plant Genetics and Breeding; Blackwell Publishing: Oxford, UK, 2007. [Google Scholar]
- Hussain, M.; Park, H.W.; Farooq, M.; Jabran, K.; Lee, D.J. Morphological and physiological basis of salt resistance in different rice genotypes. Int. J. Agric. Biol. 2013, 15, 113–118. [Google Scholar]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H.M. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Atieno, J.; Li, Y.; Langridge, P.; Dowling, K.; Brien, C.; Berger, B.; Varshney, R.K.; Sutton, T. Exploring genetic variation for salinity tolerance in chickpea using image-based phenotyping. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Huang, Y.; Guan, C.; Liu, Y.; Chen, B.; Yuan, S.; Cui, X.; Zhang, Y.; Yang, F. Enhanced growth performance and salinity tolerance in transgenic switchgrass via overexpressing vacuolar Na+ (K+)/H+ antiporter gene (PvNHX1). Front. Plant Sci. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2020, 1–46. [Google Scholar] [CrossRef]
- Chao, Y.Y.; Hsu, Y.T.; Kao, C.H. Involvement of glutathione in heat shock- and hydrogen peroxide-induced cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant Soil 2009, 318, 37–45. [Google Scholar] [CrossRef]
- Hernández, J.A.; Ferrer, M.A.; Jiménez, A.; Barceló, A.R.; Sevilla, F. Antioxidant systems and O2-/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol. 2001, 127, 817–831. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Sharma, S. Antioxidant defence system, lipid peroxidation, proline-metabolizing enzymes, and biochemical activities in two Morus alba genotypes subjected to NaCl stress. Russ. J. Plant Physiol. 2010, 57, 509–517. [Google Scholar] [CrossRef]
- Rasheed, R.; Ashraf, M.A.; Iqbal, M.; Hussain, I.; Akbar, A.; Farooq, U.; Shad, M.I. Major Constraints for Global Rice Production: Changing Climate, Abiotic and Biotic Stresses. In Rice Research for Quality Improvement: Genomics and Genetic Engineering; Springer: Singapore, 2020; pp. 15–45. [Google Scholar]
- Chrysargyris, A.; Tzionis, A.; Xylia, P.; Tzortzakis, N. Effects of salinity on tagetes growth, physiology, and shelf life of edible flowers stored in passive modified atmosphere packaging or treated with ethanol. Front. Plant Sci. 2018, 871, 1–13. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Michailidi, E.; Tzortzakis, N. Physiological and biochemical responses of Lavandula angustifolia to salinity under mineral foliar application. Front. Plant Sci. 2018, 9, 489. [Google Scholar] [CrossRef]
- Durazzo, A. Study approach of antioxidant properties in foods: Update and considerations. Foods 2017, 6, 17. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Nutrients and antinutrient constituents of Amaranthus caudatus L. Cultivated on different soils. Saudi J. Biol. Sci. 2020, 27, 3570–3580. [Google Scholar] [CrossRef]
- Salami, S.O.; Afolayan, A.J. Suitability of Roselle—Hibiscus sabdariffa L. as raw material for soft drink production. J. Food Qual. 2020, 2020, 8864142. [Google Scholar] [CrossRef]
- Debez, A.; Saadaoui, D.; Slama, I.; Huchzermeyer, B.; Abdelly, C. Responses of Batis maritima plants challenged with up to two-fold seawater NaCl salinity. J. Plant Nutr. Soil Sci. 2010, 173, 291–299. [Google Scholar] [CrossRef]
- Ventura, Y.; Sagi, M. Halophyte crop cultivation: The case for salicornia and sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Bueno, M.; Cordovilla, M.P. Ecophysiology and Uses of Halophytes in Diverse Habitats. In Handbook of Halophytes; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–25. [Google Scholar]
- Ruiz-Rodríguez, B.M.; Morales, P.; Fernández-Ruiz, V.; Sánchez-Mata, M.C.; Cámara, M.; Díez-Marqués, C.; Pardo-de-Santayana, M.; Molina, M.; Tardío, J. Valorization of wild strawberry-tree fruits (Arbutus unedo L.) through nutritional assessment and natural production data. Food Res. Int. 2011, 44, 1244–1253. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Vasilopoulou, E.; Hollman, P.; Chamalides, C.; Foufa, E.; Kaloudis, T.; Kromhout, D.; Miskaki, P.; Petrochilou, I.; Poulima, E.; et al. Nutritional composition and flavonoid content of edible wild greens and green pies: A potentially rich source of antioxidant nutrients in the Mediterranean diet. Food Chem. 2000, 70, 319–323. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef]
- Bittrich, V. AIZOACEAE. Bothalia 1990, 20, 217–219. [Google Scholar] [CrossRef]
- Klak, C.; Hanáček, P.; Bruyns, P.V. Out of southern Africa: Origin, biogeography and age of the Aizooideae (Aizoaceae). Mol. Phylogenet. Evol. 2017, 109, 203–216. [Google Scholar] [CrossRef]
- Forrester, J. Tetragonia decumbens. Plantz Africa. South African National Biodiversity Institute. 2004, pp. 1–3. Available online: http://opus.sanbi.org/bitstream/20.500.12143/3865/1/Tetragoniadecumbens_PlantzAfrica.pdf (accessed on 28 May 2021).
- Tembo-Phiri, C. Edible Fynbos Plants: A Soil Types and Irrigation Regime Investigation on Tetragonia decumbens and Mesembryanthemum Crystallinum; Stellenbosch University: Stellenbosch, South Africa, 2019. [Google Scholar]
- Bartels, D.; Sunkar, R. Drought and Salt Tolerance in Plants. CRC Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Glenn, E.P.; Brown, J.J.; Blumwald, E. Salt Tolerance and Crop Potential of Halophytes. CRC Crit. Rev. Plant Sci. 1999, 18, 227–255. [Google Scholar] [CrossRef]
- World Wide Fund. Scenarios for the Future of Water in South Africa; World Wide Fund For Nature: Cape Town, South Africa, 2017. [Google Scholar]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Otunola, G.A.; Afolayan, A.J. Nutritional evaluation of Kedrostis africana (L.) Cogn: An edible wild plant of South Africa. Asian Pac. J. Trop. Biomed. 2017, 7, 443–449. [Google Scholar] [CrossRef]
- Olatunji, T.L.; Afolayan, A.J. Comparison of nutritional, antioxidant vitamins and capsaicin contents in Capsicum annuum and C. frutescens. Int. J. Veg. Sci. 2019, 18, 1–18. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Heavy metal uptake and growth characteristics of Amaranthus caudatus L. under five different soils in a controlled environment. Not. Bot. Horti Agrobot. 2020, 48, 417–425. [Google Scholar] [CrossRef]
- Hoenig, M.; Baeten, H.; Vanhentenrijk, S.; Vassileva, E.; Quevauviller, P. Critical discussion on the need for an efficient mineralization procedure for the analysis of plant material by atomic spectrometric methods. Anal. Chim. Acta 1998, 358, 85–94. [Google Scholar] [CrossRef]
- Idris, O.A.; Wintola, O.A.; Afolayan, A.J. Comparison of the Proximate Composition, Vitamins Analysis of the Essential Oil of the Root and Leaf of Rumex crispus L. Plants 2019, 8, 51. [Google Scholar] [CrossRef]
- Nkcukankcuka, M.; Jimoh, M.O.; Griesel, G.; Laubscher, C.P. Growth characteristics, chlorophyll content and nutrients uptake in Tetragonia decumbens Mill. cultivated under different fertigation regimes in hydroponics. Crop Pasture Sci. 2021, 72, 1–12. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Antioxidant and phytochemical activities of Amaranthus caudatus L. harvested from different soils at various growth stages. Sci. Rep. 2019, 9, 12965. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Jimoh, M.A.; Idris, O.A.; Jimoh, M.O. Cytotoxicity, Phytochemical, Antiparasitic Screening, and Antioxidant Activities of Mucuna pruriens (Fabaceae). Plants 2020, 9, 1249. [Google Scholar] [CrossRef]
- Faber, R.J.; Laubscher, C.P.; Rautenbach, F.; Jimoh, M.O. Variabilities in alkaloid concentration of Sceletium tortuosum (L.) N.E. Br in response to different soilless growing media and fertigation regimes in hydroponics. Heliyon 2020, 6, e05479. [Google Scholar] [CrossRef]
- Hasegawa, P.M. Sodium (Na+) homeostasis and salt tolerance of plants. Environ. Exp. Bot. 2013, 92, 19–31. [Google Scholar] [CrossRef]
- Rahneshan, Z.; Nasibi, F.; Moghadam, A.A. Effects of salinity stress on some growth, physiological, biochemical parameters and nutrients in two pistachio (Pistacia vera L.) rootstocks. J. Plant Interact. 2018, 13, 73–82. [Google Scholar] [CrossRef]
- Fageria, N.K.; Stone, L.F.; Santos, A.B. Dos Breeding for salinity tolerance. In Plant Breeding for Abiotic Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2012; pp. 103–122. ISBN 9783642305535. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Petretto, G.L.; Urgeghe, P.P.; Massa, D.; Melito, S. Effect of salinity (NaCl)on plant growth, nutrient content, and glucosinolate hydrolysis products trends in rocket genotypes. Plant Physiol. Biochem. 2019, 141, 30–39. [Google Scholar] [CrossRef] [PubMed]
- van Puijenbroek, M.E.B.; Teichmann, C.; Meijdam, N.; Oliveras, I.; Berendse, F.; Limpens, J. Does salt stress constrain spatial distribution of dune building grasses Ammophila arenaria and Elytrichia juncea on the beach? Ecol. Evol. 2017, 7, 7290–7303. [Google Scholar] [CrossRef] [PubMed]
- Grigore, M.N.; Villanueva, M.; Boscaiu, M.; Vicente, O. Do Halophytes Really Require Salts for Their Growth and Development? An Experimental Approach. Not. Sci. Biol. 2012, 4, 23–29. [Google Scholar] [CrossRef]
- Guo, J.; Li, Y.; Han, G.; Song, J.; Wang, B. NaCl markedly improved the reproductive capacity of the euhalophyte Suaeda salsa. Funct. Plant Biol. 2018, 45, 350–361. [Google Scholar] [CrossRef]
- Orlovsky, N.; Japakova, U.; Zhang, H.; Volis, S. Effect of salinity on seed germination, growth and ion content in dimorphic seeds of Salicornia europaea L. (Chenopodiaceae). Plant Divers. 2016, 38, 183–189. [Google Scholar] [CrossRef]
- Tian, F.; Hou, M.; Qiu, Y.; Zhang, T.; Yuan, Y. Salinity stress effects on transpiration and plant growth under different salinity soil levels based on thermal infrared remote (TIR) technique. Geoderma 2020, 357, 113961. [Google Scholar] [CrossRef]
- Benito, B.; Haro, R.; Amtmann, A.; Cuin, T.A.; Dreyer, I. The twins K+ and Na+ in plants. J. Plant Physiol. 2014, 171, 723–731. [Google Scholar] [CrossRef]
- Shabala, S.; Pottosin, I. Regulation of potassium transport in plants under hostile conditions: Implications for abiotic and biotic stress tolerance. Physiol. Plant. 2014, 151, 257–279. [Google Scholar] [CrossRef]
- Parvez, S.; Abbas, G.; Shahid, M.; Amjad, M.; Hussain, M.; Asad, S.A.; Imran, M.; Naeem, M.A. Effect of salinity on physiological, biochemical and photostabilizing attributes of two genotypes of quinoa (Chenopodium quinoa Willd.) exposed to arsenic stress. Ecotoxicol. Environ. Saf. 2020, 187, 109814. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Levizou, E.; Ntatsi, G.; Fernandes, Â.; Petrotos, K.; Akoumianakis, K.; Barros, L.; Ferreira, I.C.F.R. Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 2017, 214, 129–136. [Google Scholar] [CrossRef]
- Lima, A.R.; Castañeda-Loaiza, V.; Salazar, M.; Nunes, C.; Quintas, C.; Gama, F.; Pestana, M.; Correia, P.J.; Santos, T.; Varela, J.; et al. Influence of cultivation salinity in the nutritional composition, antioxidant capacity and microbial quality of Salicornia ramosissima commercially produced in soilless systems. Food Chem. 2020, 333, 127525. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.H.; Wang, G.Z.; Si, W.T.; Zhou, Y.; Liu, Z.; Jia, J. Effects of Salt Stress on Photosynthetic Pigments and Activity of Ribulose-1,5-bisphosphate Carboxylase/Oxygenase in Kalidium foliatum. Russ. J. Plant Physiol. 2018, 65, 98–103. [Google Scholar] [CrossRef]
- Stepien, P.; Johnson, G.N. Contrasting responses of photosynthesis to salt stress in the glycophyte arabidopsis and the halophyte thellungiella: Role of the plastid terminal oxidase as an alternative electron sink. Plant Physiol. 2009, 149, 1154–1165. [Google Scholar] [CrossRef]
- Sui, N.; Yang, Z.; Liu, M.; Wang, B. Identification and transcriptomic profiling of genes involved in increasing sugar content during salt stress in sweet sorghum leaves. BMC Genom. 2015, 16, 1–18. [Google Scholar] [CrossRef]
- Ferreira, J.; Cornacchione, M.; Liu, X.; Suarez, D. Nutrient Composition, Forage Parameters, and Antioxidant Capacity of Alfalfa (Medicago sativa, L.) in Response to Saline Irrigation Water. Agriculture 2015, 5, 577–597. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Gil, A.M.; Duarte, I.F.; Godejohann, M.; Braumann, U.; Maraschin, M.; Spraul, M. Characterization of the aromatic composition of some liquid foods by nuclear magnetic resonance spectrometry and liquid chromatography with nuclear magnetic resonance and mass spectrometric detection. Anal. Chim. Acta 2003, 488, 35–51. [Google Scholar] [CrossRef]
- Daiponmak, W.; Theerakulpisut, P.; Thanonkao, P.; Vanavichit, A.; Prathepha, P. Changes of anthocyanin cyanidin-3-glucoside content and antioxidant activity in Thai rice varieties under salinity stress. ScienceAsia 2010, 36, 286–291. [Google Scholar] [CrossRef]
- Jafari, S.; Hashemi Garmdareh, S.E. Effects of salinity on morpho-physiological, and biochemical characteristics of stock plant (Matthiola incana L.). Sci. Hortic. 2019, 257, 108731. [Google Scholar] [CrossRef]
- Kim, H.J.; Fonseca, J.M.; Choi, J.H.; Kubota, C.; Dae, Y.K. Salt in irrigation water affects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, N.; Huang, L.; Zhao, Y.; Tang, X.; Wang, K. Effects of salt stress on plant growth, antioxidant capacity, glandular trichome density, and volatile exudates of schizonepeta tenuifolia briq. Int. J. Mol. Sci. 2018, 19, 252. [Google Scholar] [CrossRef] [PubMed]
| Treatments | SL (cm) | BN (n) | FWS (g) | DWS (g) | FWSR (g) | DWSR (g) | TFW (g) | TDW (g) |
|---|---|---|---|---|---|---|---|---|
| Control | 101.1 ± 3.8 a | 3.4 ± 0.2 c | 136.8 ± 6.7 c | 23.1 ± 1.2 c | 61.4 ± 5.4 b | 14.5 ± 1 b | 198.3 ± 8.3 c | 37.6 ± 1.1 c |
| 50 mM | 96.5 ± 3.1 ab | 5.4 ± 0.4 a | 210.8 ± 10.4 a | 32.1 ± 1.3 a | 89.3 ± 6.7 a | 18.6 ± 1 a | 300.2 ± 12.8 a | 50.6 ± 1.6 a |
| 100 mM | 90.7 ± 2.2 b | 4.7 ± 0.2 ab | 186.4 ± 6.8 b | 26.9 ± 0.7 b | 78.0 ± 3.8 a | 17.3 ± 0.8 ab | 264.4 ± 6.6 b | 44.1 ± 0.9 b |
| 200 mM | 73.7 ± 3.1 c | 3.8 ± 0.3 bc | 119.7 ± 6.3 c | 15.6 ± 0.8 d | 79.5 ± 6.2 a | 15.3 ± 1.1 b | 199.2 ± 7.8 c | 30.9 ± 1.2 d |
| F-statistic | 14.6 * | 7.6 * | 29.8 * | 45 * | 4.2 * | 3.2 * | 30 * | 45 * |
| Treatments | N (g/kg) | P (g/kg) | K (g/kg) | Ca (g/kg) | Mg (g/kg) | Na (g/kg) |
|---|---|---|---|---|---|---|
| Control | 23.5 ± 0.4 a | 4.7 ± 0.4 a | 44.1 ± 1.1 a | 6.9 ± 0.7 a | 6.9 ± 0.5 a | 6.9 ± 0.4 b |
| 50 mM | 20.8 ± 0.1 b | 3.3 ± 0.3 a | 26.8 ± 1.1 b | 3.6 ± 0.2 b | 3.3 ± 0.1 b | 35.8 ± 1.3 a |
| 100 mM | 20.9 ± 0.3 b | 3.4 ± 0.4 a | 22.6 ± 1 c | 3.7 ± 0.5 b | 3.2 ± 0.2 b | 54.7 ± 1.3 c |
| 200 mM | 24.4 ± 0.3 a | 4.8 ± 0.4 a | 21.9 ± 0.7 c | 2.5 ± 0.1 b | 2.8 ± 0.1 b | 59 ± 0.9 c |
| F-statistic | 14.9 * | 1.9 ns | 81.3 * | 18.6 * | 18.6 * | 58.8 * |
| Treatments | Mn (mg/kg) | Fe (mg/kg) | Cu (mg/kg) | Zn (mg/kg) | B (mg/kg) |
|---|---|---|---|---|---|
| Control | 74.3 ± 1.3 a | 66.4 ± 2.2 b | 1.7 ± 0.3 a | 56.7 ± 1.8 a | 35.1 ± 1.5 a |
| 50 mM | 67.5 ± 1.9 a | 61.3 ± 0.83 bc | 1.7 ±0.2 a | 35.4 ± 0.5 b | 20.7 ± 0.7 b |
| 100 mM | 81.3 ± 1.9 a | 89.2 ± 2.7 a | 4.2 ±1.9 a | 51.6 ± 1.4 a | 24.7 ± 2.4 b |
| 200 mM | 64.4 ± 1.2 a | 57.9 ± 2.2 c | 2.3 ± 0.5 a | 36 ± 0.3 b | 22.1 ± 0.9 b |
| F-statistic | 0.4 ns | 44.9 * | 1.2 ns | 17.9 * | 5.2 * |
| Treatments | Total Polyphenols (mg GAE/g DW−1) | ABTS (µM TE/g DW−1) | FRAP (µM AAE/g DW−1) |
|---|---|---|---|
| Control | 1.3 ± 0.2 b | 82.5 ± 8 a | 10.9 ± 0.1 b |
| 50 mM | 1.6 ± 0.3 b | 78.4 ± 6 a | 14.3 ± 0.4 a |
| 100 mM | 1.7 ± 0.4 ab | 70.4 ± 4 a | 12.1 ± 1.1 ab |
| 200 mM | 2.6 ± 0.2 a | 77.8 ± 4.4 a | 11.8 ± 0.7 b |
| F-statistic | 3.3 * | 0.41 ns | 3.6 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sogoni, A.; Jimoh, M.O.; Kambizi, L.; Laubscher, C.P. The Impact of Salt Stress on Plant Growth, Mineral Composition, and Antioxidant Activity in Tetragonia decumbens Mill.: An Underutilized Edible Halophyte in South Africa. Horticulturae 2021, 7, 140. https://doi.org/10.3390/horticulturae7060140
Sogoni A, Jimoh MO, Kambizi L, Laubscher CP. The Impact of Salt Stress on Plant Growth, Mineral Composition, and Antioxidant Activity in Tetragonia decumbens Mill.: An Underutilized Edible Halophyte in South Africa. Horticulturae. 2021; 7(6):140. https://doi.org/10.3390/horticulturae7060140
Chicago/Turabian StyleSogoni, Avela, Muhali Olaide Jimoh, Learnmore Kambizi, and Charles Petrus Laubscher. 2021. "The Impact of Salt Stress on Plant Growth, Mineral Composition, and Antioxidant Activity in Tetragonia decumbens Mill.: An Underutilized Edible Halophyte in South Africa" Horticulturae 7, no. 6: 140. https://doi.org/10.3390/horticulturae7060140
APA StyleSogoni, A., Jimoh, M. O., Kambizi, L., & Laubscher, C. P. (2021). The Impact of Salt Stress on Plant Growth, Mineral Composition, and Antioxidant Activity in Tetragonia decumbens Mill.: An Underutilized Edible Halophyte in South Africa. Horticulturae, 7(6), 140. https://doi.org/10.3390/horticulturae7060140








