Twenty-Years of Hop Irrigation by Flooding the Inter-Row Did Not Cause a Gradient along the Row in Soil Properties, Plant Elemental Composition and Dry Matter Yield
Abstract
:1. Introduction
2. Materials and Methods
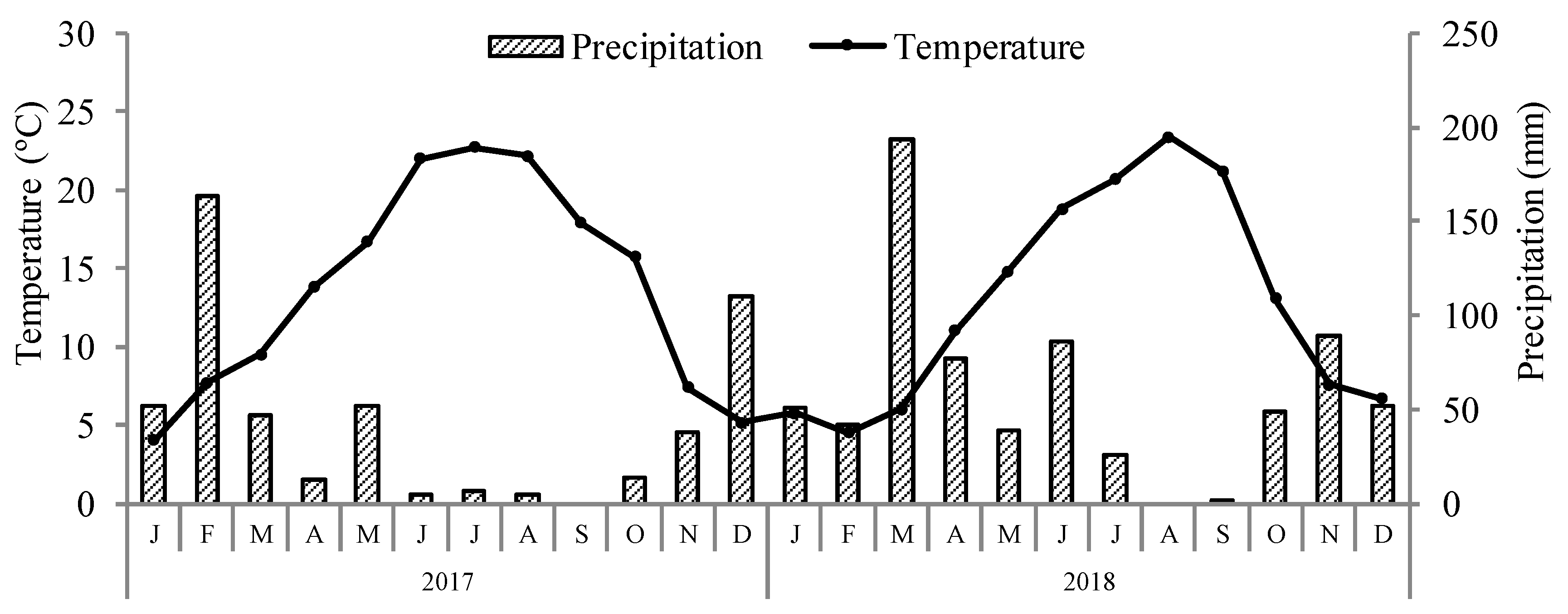
2.1. General Experimental Conditions
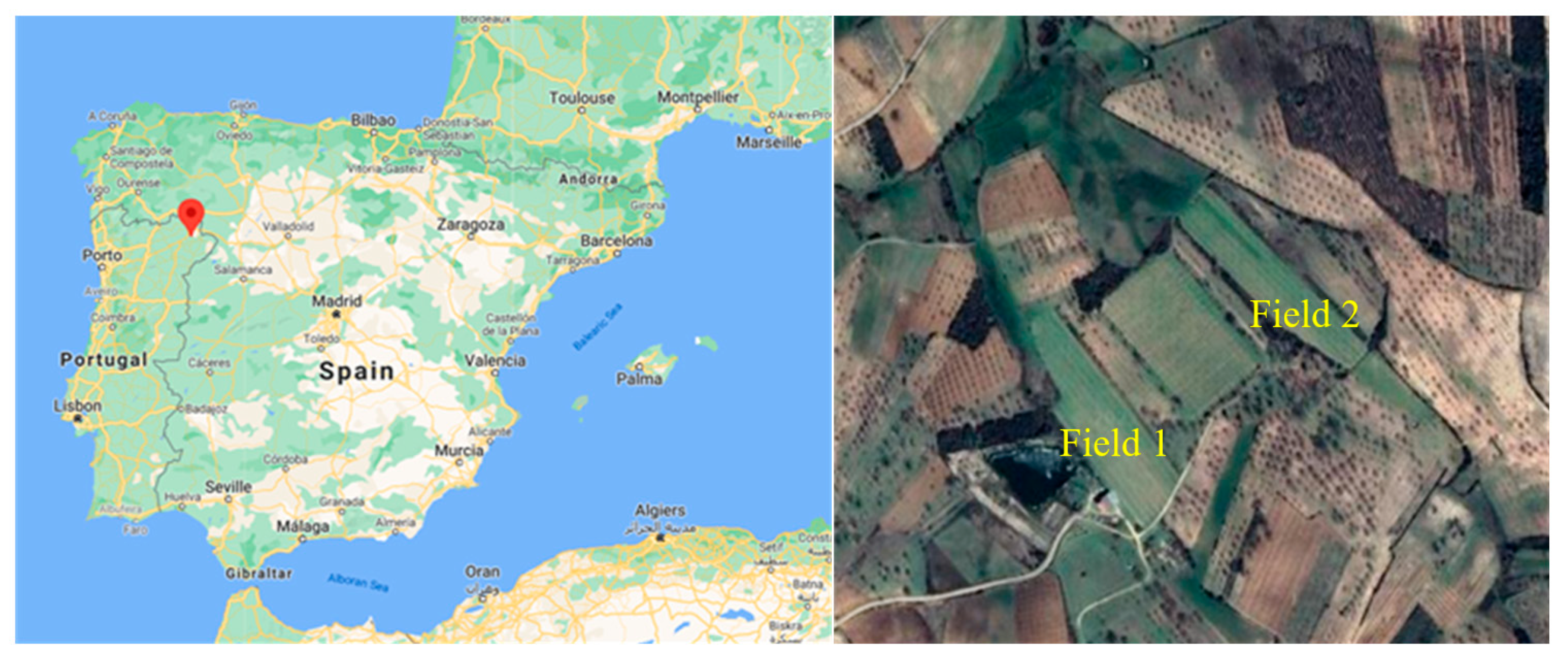
2.2. Field Experiments and Soil and Plant Sampling
2.3. Laboratory Analyses
2.4. Data Analysis
3. Results
3.1. Gradients in Soil and Plants along the Rows
3.1.1. Soil Properties
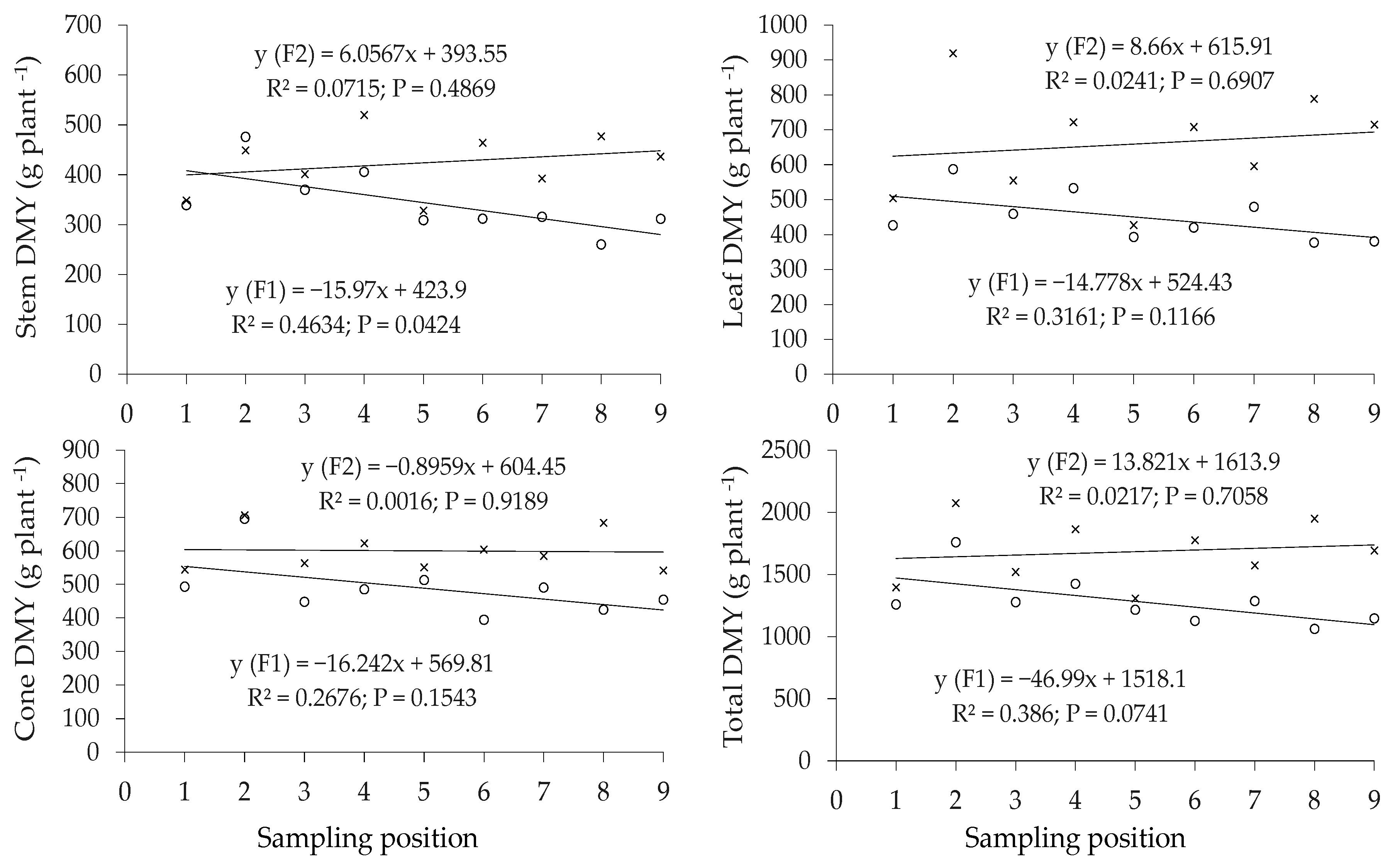
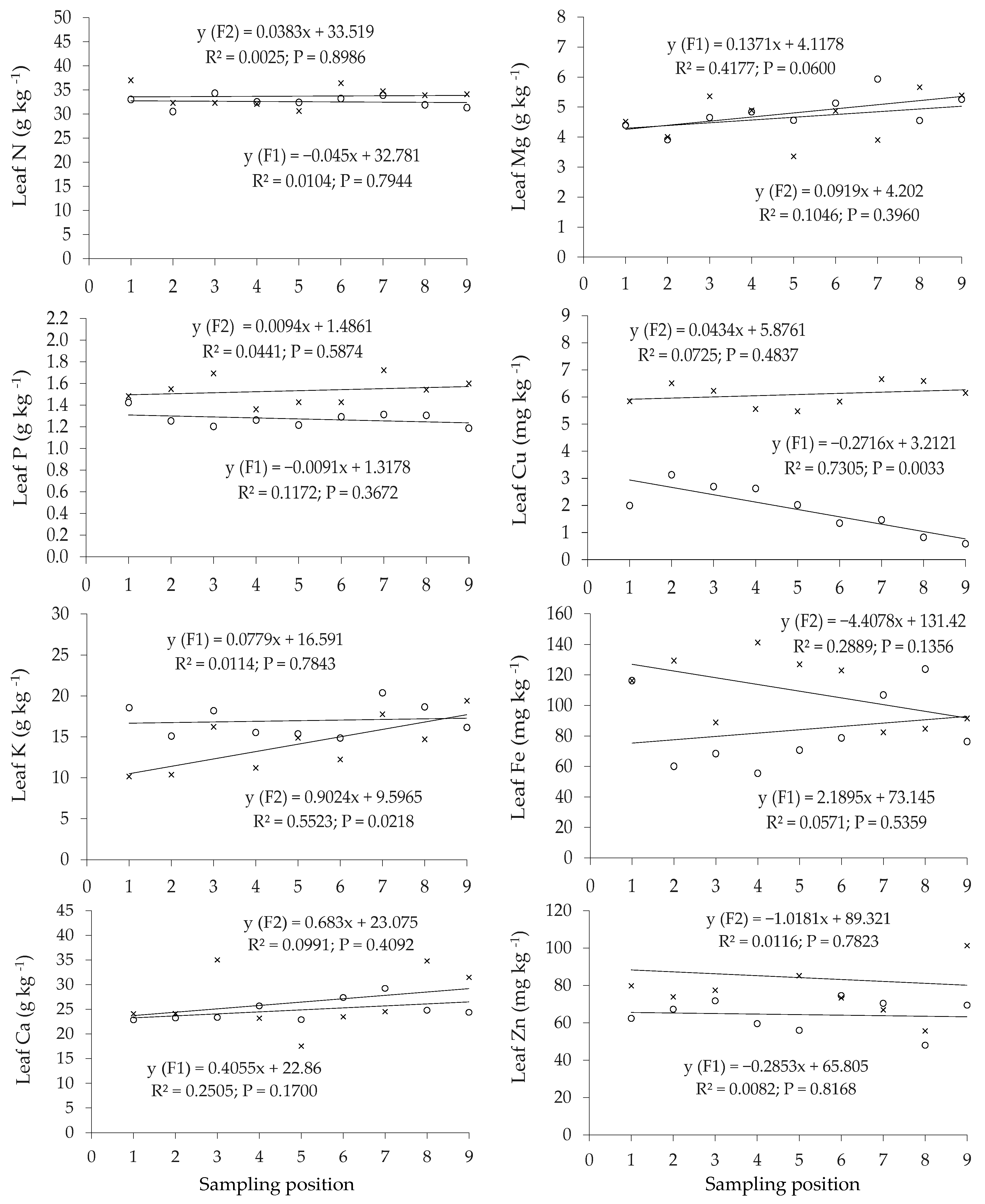
3.1.2. Hop Dry Mater Yield and Leaf Nutrient Concentration
3.1.3. Correlation Analysis between Soil Properties and Plant Dry Matter Yield
3.2. Liming Experiment
3.2.1. Soil Properties
3.2.2. Plant Response to Liming
3.2.3. Correlation Analysis between Soil pH and Plant Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turner, S.F.; Benedict, C.A.; Darby, H.; Hoagland, L.A.; Simonson, P.; Sirrine, J.R.; Murphy, K.M. Challenges and Opportunities for Organic Hop Production in the United States. Agron J. 2011, 103, 1645–1654. [Google Scholar] [CrossRef] [Green Version]
- Rossini, F.; Virga, G.; Loreti, P.; Iacuzzi, N.; Ruggeri, R.; Provenzano, M.E. Hops (Humulus lupulus L.) as a Novel Multipurpose Crop for the Mediterranean Region of Europe: Challenges and Opportunities of Their Cultivation. Agriculture 2021, 11, 484. [Google Scholar] [CrossRef]
- Hoque, M. The Way Ahead. In Biotechnology for Sustainable Agriculture; Singh, R.L., Mondal, S., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 375–397. [Google Scholar] [CrossRef]
- Afonso, S.; Arrobas, M.; Rodrigues, M.Â. Soil and Plant Analyses to Diagnose Hop Fields Irregular Growth. J. Soil Sci. Plant Nutr. 2020, 20, 1999–2013. [Google Scholar] [CrossRef]
- Hedley, C.B.; Knox, J.W.; Raine, S.R.; Smith, R. Water: Advanced Irrigation Technologies. In Encyclopedia of Agriculture and Food Systems; Van Alfen, N.K., Ed.; Academic Press: Oxford, UK, 2014; pp. 378–406. [Google Scholar] [CrossRef]
- Simmonds, M.B.; Plant, R.E.; Peña-Barragán, J.M.; van Kessel, C.; Hill, J.; Linquist, B.A. Underlying Causes of Yield Spatial Variability and Potential for Precision Management in Rice Systems. Precis. Agric. 2013, 14, 512–540. [Google Scholar] [CrossRef] [Green Version]
- Cox, C.; Jin, L.; Ganjegunte, G.; Borrok, D.; Lougheed, V.; Ma, L. Soil Quality Changes Due to Flood Irrigation in Agricultural Fields Along The Rio Grande in Western Texas. Appl. Geochem. 2018, 90, 87–100. [Google Scholar] [CrossRef]
- Cerdà, A.; Daliakopoulos, I.N.; Terol, E.; Novara, A.; Fatahi, Y.; Moradi, E.; Salvati, L.; Pulido, M. Long-Term Monitoring of Soil Bulk Density and Erosion Rates in Two Prunus persica (L) Plantations Under Flood Irrigation and Glyphosate Herbicide Treatment in La Ribera District, Spain. J. Environ. Manag. 2021, 282, 111965. [Google Scholar] [CrossRef] [PubMed]
- González-Méndez, B.; Webster, R.; Fiedler, S.; Siebe, C. Changes in Soil Redox Potential in Response to Flood Irrigation with Waste Water in Central Mexico. Eur. J. Soil Sci. 2017, 68, 886–896. [Google Scholar] [CrossRef]
- Batey, T. Soil Bompaction and Soil Management—A Review. Soil Use Manag. 2009, 25, 335–345. [Google Scholar] [CrossRef]
- Shapiro, C.A.; Elmore, R.W. Agricultural Crops. In Encyclopedia of Applied Plant Sciences, 2nd ed.; Thomas, B., Murray, B.G., Murphy, D.J., Eds.; Academic Press: Oxford, UK, 2017; pp. 1–8. [Google Scholar] [CrossRef]
- Arriaga, F.J.; Guzman, J.; Lowery, B. Conventional Agricultural Production Systems and Soil Functions. In Soil Health and Intensification of Agroecosytems; Al-Kaisi, M.M., Lowery, B., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 109–125. [Google Scholar] [CrossRef]
- Nawaz, M.F.; Bourrié, G.; Trolard, F. Soil Compaction Impact and Modelling. A Review. Agron. Sustain. Dev. 2013, 33, 291–309. [Google Scholar] [CrossRef] [Green Version]
- Indoria, A.K.; Sharma, K.L.; Reddy, K.S. Hydraulic properties of soil under warming climate. In Climate Change and Soil Interactions; Prasad, M.N.V., Pietrzykowski, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 473–508. [Google Scholar] [CrossRef]
- Sirrine, J.R.; Rothwell, N.; Lizotte, E.; Goldy, R.; Marquie, S.; Brown-Rytlewski, D. Sustainable Hop Production in the Great Lakes Region. Ext. Bull. E-3083 2010. Available online: https://www.uvm.edu/sites/default/files/media/Sirrine-Sustainable-Hop-Production-in-the-Great-Lakes-Region.pdf (accessed on 13 April 2019).
- Gent, D.H.; Sirrine, J.R.; Darby, H.M. Nutrient Management and Imbalances. In Field Guide for Integrated Pest Management in Hops; Washington Hop Commission: Moxee, WA, USA, 2015; pp. 98–100. [Google Scholar]
- Čeh, B.; Čremožnik, B. Soil pH and Hop (Humulus lupulus) Yield Related to Liming Material Rate. Hmelj. Bilt. 2015, 22, 49–57. [Google Scholar]
- Seng, V.; Bell, R.W.; Willett, I.R. Effect of Lime and Flooding on Phosphorus Availability and Rice Growth on Two Acidic Lowland Soils. Commun. Soil Sci Plant Anal. 2006, 37, 313–336. [Google Scholar] [CrossRef]
- Sadiq, A.A.; Babagana, U. Influence of Lime Materials to Ameliorate Acidity on Irrigated Paddy Fields: A Review. Acad. Res. Int. 2012, 3, 413. [Google Scholar]
- Shi, L.; Guo, Z.; Liang, F.; Xiao, X.; Peng, C.; Zeng, P.; Feng, W.; Ran, H. Effect of Liming with Various Water Regimes on Both Immobilization of Cadmium and Improvement of Bacterial Communities in Contaminated Paddy: A Field Experiment. Int. J. Environ. Res. Public Health 2019, 16, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowell, D.L. Soil Science: Methods & Applications, 1st ed.; Longman Group UK Ltd.: Harlow, UK, 1994; p. 368. [Google Scholar] [CrossRef]
- Van Reeuwijk, L. Procedures for soil analysis (International Soil Reference and Information Centre). Tech. Pap. 2002, 9, 120. [Google Scholar]
- Lakanen, E.; Erviö, R. A Comparison of Eight Extractants for the Determination of Plant Available Micronutrients in Soils. Acta Agric. Fenn. 1971, 123, 223–232. [Google Scholar]
- Walinga, I.; Van Vark, W.; Houba, V.; Van der Lee, J. Soil and Plant Analysis, Part 7: Plant Analysis Procedures; Wageningen Agricultural University: Wageningen, The Netherlands, 1989. [Google Scholar]
- Delgado, A.; Gómez, J.A. The Soil. Physical, Chemical and Biological Properties. In Principles of Agronomy for Sustainable Agriculture; Villalobos, F.J., Fereres, E., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 15–26. [Google Scholar] [CrossRef]
- Hamza, M.A.; Al-Adawi, S.S.; Al-Hinai, K.A. Effect of Combined Soil Water and External Load on Soil Compaction. Soil Res. 2011, 49, 135–142. [Google Scholar] [CrossRef]
- Alaoui, A.; Rogger, M.; Peth, S.; Blöschl, G. Does Soil Compaction Increase Floods? A Review. J. Hydrol. 2018, 557, 631–642. [Google Scholar] [CrossRef]
- Chaudhari, P.R.; Ahire, D.V.; Ahire, V.D.; Chkravarty, M.; Maity, S. Soil Bulk Density as Related to Soil Texture, Organic Matter Content and Available Total Nutrients of Coimbatore Soil. Int. J. Sci. Res. Publ. 2013, 3, 1–8. [Google Scholar]
- Green, T.R.; Ahuja, L.R.; Benjamin, J.G. Advances and Challenges in Predicting Agricultural Management Effects on Soil Hydraulic Properties. Geoderma 2003, 116, 3–27. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Mineral Nutrition. In Plant Physiological Ecology; Lambers, H., Chapin, F.S., Pons, T.L., Eds.; Springer: New York, NY, USA, 2008; pp. 255–320. [Google Scholar] [CrossRef]
- Comerford, N.B. Soil Factors Affecting Nutrient Bioavailability. In Nutrient Acquisition by Plants: An Ecological Perspective; BassiriRad, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–14. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Global Edition: London, UK, 2017. [Google Scholar]
- Liu, X.; Herbert, S.; Hashemi, A.; Zhang, X.; Ding, G. Effects of Agricultural Management on Soil Organic Matter and Carbon Transformation—A Review. Plant. Soil Environ. 2006, 52, 531. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, S. Reactions of Boron with Soils. Plant Soil 1997, 193, 35–48. [Google Scholar] [CrossRef]
- Das, A.K.; Purkait, A. Boron Dynamics in Soil: Classification, Sources, Factors, Fractions, and Kinetics. Commun. Soil Sci. Plant Anal. 2020, 51, 2778–2790. [Google Scholar] [CrossRef]
- Husson, O. Redox Potential (Eh) and pH as Drivers of Soil/Plant/Microorganism Systems: A Transdisciplinary Overview Pointing to Integrative Opportunities for Agronomy. Plant Soil 2013, 362, 389–417. [Google Scholar] [CrossRef] [Green Version]
- Osman, K.T. Soils: Principles, Properties and Management, 1st ed.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- George, E.; Horst, W.J.; Neumann, E. Adaptation of Plants to Adverse Chemical Soil Conditions. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 409–472. [Google Scholar]
- Pezeshki, S.R.; DeLaune, R.D. Soil Oxidation-Reduction in Wetlands and its Impact on Plant Functioning. Biology 2012, 1, 196–221. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Dong, G.; Karthikeyan, R.; Li, L.; Harmel, R. Phosphorus Dynamics in Long-Term Flooded, Drained, and Reflooded Soils. Water 2017, 9, 531. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Xuan, J.; Du, C.; Xie, J. Effect of Potassium Nutrition of Rice on Rhizosphere Redox Status. Plant Soil 1997, 188, 131–137. [Google Scholar] [CrossRef]
- Kundu, D.; Neue, H.; Singh, R. Iron and Potassium Availability to Rice in Tropudalf and Sulfaquept as Influenced by Water Regime. J. Indian Soc. Soil Sci. 2001, 49, 130–134. [Google Scholar]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers: An Introduction to Nutrient Management, 8th ed.; Pearson: Boston, MA, USA, 2014. [Google Scholar]
- Gingrich, C.; Hart, J.; Christensen, N. Fertilizer Guide 79; Oregon State University, Extension Service: Corvallis, OR, USA, 1994. [Google Scholar]
- Bryson, G.; Mills, H.; Sasseville, D.; Jones, J.B., Jr.; Barker, A. Plant. Analysis Handbook III: A Guide to Sampling, Preparation, Analysis and Interpretation for Agronomic and Horticultural Crops; Micro-Macro Publishing: Athens, GA, USA, 2014. [Google Scholar]
- Marceddu, R.; Carrubba, A.; Sarno, M. Cultivation Trials of Hop (Humulus lupulus L.) in Semi-Arid Environments. Heliyon 2020, 6, e05114. [Google Scholar] [CrossRef]
- MacKinnon, D.; Viljem, P.; Čeh, B.; Naglič, B.; Pavlovic, M. The Impact of Weather Conditions on Alpha-Acid Content in Hop (Humulus lupulus L.) cv. Aurora. Plant. Soil Environ. 2020, 66, 519–525. [Google Scholar] [CrossRef]
- Rossini, F.; Virga, G.; Loreti, P.; Provenzano, M.E.; Danieli, P.P.; Ruggeri, R. Beyond Beer: Hop Shoot Production and Nutritional Composition under Mediterranean Climatic Conditions. Agronomy 2020, 10, 1547. [Google Scholar] [CrossRef]

| Clay | Silt | Sand | Bulk Density | Porosity | |
|---|---|---|---|---|---|
| (%) | (kg dm−3) | (%) | |||
| Sampling position (P) | |||||
| Lowest value | 15.6 a | 34.5 a | 59.7 a | 1.26 a | 52.1 a |
| Highest value | 11.8 a | 28.5 b | 49.9 b | 1.18 b | 48.0 b |
| Field (F) | |||||
| Field 1 | 16.0 a | 33.2 a | 50.8 b | 1.25 a | 49.1 b |
| Field 2 | 11.5 b | 32.1 a | 56.4 a | 1.21 b | 50.8 a |
| Prob (P) | 0.2770 | 0.0386 | 0.0307 | 0.0143 | 0.0020 |
| Prob (F) | 0.0005 | 0.3741 | 0.0072 | 0.0260 | 0.0259 |
| Prob (P × F) | 0.8998 | 0.0432 | 0.1221 | 0.0874 | 0.0256 |
| Extract. K | Extract. P | Conductivity | pHH2O | pHKCl | Organic C | Exchan. Ca | CEC | Base Saturation | Extract. Fe | Extract. Mn | Extract. Zn | Extract. Cu | Extract. B | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg K2O kg−1) 1 | (mg P2O5 kg−1) 1 | (µs/m) | (g kg−1) | (cmolc kg−1) 2 | (%) | (mg kg−1) 3 | (mg kg−1) 4 | |||||||
| Sampling site (S) | ||||||||||||||
| Ridge | 310.4 a | 349.7 a | 78.9 a | 5.42 b | 4.42 b | 20.9 a | 4.94 a | 7.34 a | 87.7 b | 213.4 a | 166.8 b | 5.03 a | 7.86 a | 1.16 a |
| Inter-row | 246.5 b | 292.7 b | 54.7 b | 5.53 a | 4.52 a | 18.3 b | 4.77 a | 6.63 b | 89.8 a | 222.1 a | 194.2 a | 4.28 b | 7.84 a | 0.79 b |
| Sampling position (P) | ||||||||||||||
| Lowest value | 228.3 a | 195.0 c | 60.2 a | 5.42 a | 4.35 a | 17.8 b | 3.80 c | 6.21 b | 86.0 b | 178.1 c | 136.0 d | 3.47 d | 6.64 d | 0.79 c |
| Highest value | 313.6 a | 399.7 a | 70.0 a | 5.60 a | 4.64 a | 21.3 a | 5.73 a | 7.68 a | 92.1 a | 262.2 a | 217.3 a | 5.64 a | 9.22 a | 1.17 a |
| Field (F) | ||||||||||||||
| Field 1 | 361.8 a | 286.3 b | 65.9 a | 5.75 a | 4.67 a | 18.4 b | 5.22 a | 7.31 a | 93.6 a | 207.2 b | 134.1 b | 4.96 a | 10.35 a | 0.91 b |
| Field 2 | 195.1 b | 356.2 a | 67.6 a | 5.21 b | 4.27 b | 20.8 a | 4.49 b | 6.66 b | 84.0 b | 228.4 a | 226.8 a | 4.35 b | 5.36 b | 1.04 a |
| Prob (S) | <0.0001 | 0.0022 | <0.0001 | 0.0028 | 0.0236 | <0.0001 | 0.4028 | 0.0069 | 0.0179 | 0.2836 | 0.0003 | 0.0009 | 0.9386 | <0.0001 |
| Prob (P) | 0.0758 | <0.0001 | 0.9345 | 0.3221 | 0.0710 | 0.0058 | <0.0001 | 0.0268 | 0.0012 | <0.0001 | <0.0001 | <0.0001 | 0.0003 | 0.0007 |
| Prob (F) | <0.0001 | 0.0002 | 0.5991 | <0.0001 | <0.0001 | <0.0001 | 0.0004 | 0.0130 | <0.0001 | 0.0100 | <0.0001 | 0.0070 | <0.0001 | 0.0075 |
| Prob (S × P) | 0.6500 | 0.9648 | 0.9341 | 0.9859 | 0.7781 | 0.8954 | 0.7956 | 0.7826 | 0.0938 | 0.9836 | 0.0644 | 0.8658 | 0.2274 | 0.5881 |
| Prob (S × F) | 0.8057 | 0.0001 | 0.0013 | 0.8860 | 0.7597 | 0.7527 | 0.0287 | 0.1220 | 0.0901 | 0.0053 | 0.6578 | 0.0738 | 0.1982 | 0.0810 |
| Prob (P × F) | 0.0904 | 0.0374 | 0.8663 | 0.1846 | 0.2467 | 0.0199 | 0.8269 | 0.6486 | 0.1179 | <0.0001 | <0.0001 | 0.0018 | <0.0001 | 0.0497 |
| Prob (S × P × F) | 0.4096 | 0.9991 | 0.9791 | 0.5470 | 0.9433 | 0.5569 | 0.9503 | 0.9365 | 0.2005 | 0.9005 | 0.5541 | 0.6590 | 0.6793 | 0.4975 |
| Soil † | Leaf Nutrient † | DMY | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pHH2O | pHKCl | N | P | K | Ca | Mg | Fe | Mn | Cu | Zn | B | Total ‡ | Cone † | |
| (g kg−1) | (mg kg−1) | (g Plant−1) | ||||||||||||
| Soil bulk density | ||||||||||||||
| D1 (0.0–0.10 m depth) | 0.422 * | 0.440 * | −0.442 | −0.190 | 0.043 | −0.209 | −0.130 | 0.128 | −0.067 | −0.322 | −0.515 | −0.333 | −0.243 | 0.139 |
| D2 (0.10–0.20 m depth) | 0.087 | 0.062 | −0.239 | −0.690 * | −0.046 | −0.512 | −0.249 | 0.626 | −0.220 | −0.525 | −0.312 | −0.459 | −0.706 * | −0.128 |
| Soil porosity | ||||||||||||||
| D1 (0.0–0.10 cm depth) | −0.396 * | −0.400 * | 0.418 | −0.038 | −0.110 | 0.055 | 0.075 | −0.055 | 0.139 | 0.097 | 0.370 | 0.285 | 0.168 | −0.261 |
| D2 (0.10–0.20 m depth) | −0.020 | 0.015 | 0.248 | 0.646 * | <0.0001 | 0.406 | 0.185 | −0.632 * | 0.273 | 0.535 | 0.309 | 0.418 | 0.714 * | 0.127 |
| Soil separates | ||||||||||||||
| Clay | 0.806 ** | 0.542 * | −0.241 | −0.563 * | 0.639 ** | 0.038 | 0.057 | −0.389 | −0.197 | −0.773 ** | −0.459 | −0.707 ** | −0.676 ** | −0.666 ** |
| Silt | 0.387 | 0.129 | −0.220 | 0.066 | 0.323 | −0.049 | −0.084 | 0.042 | −0.292 | −0.179 | −0.042 | −0.503 * | −0.117 | −0.005 |
| Sand | −0.703 ** | −0.391 | 0.276 | 0.339 | −0.562 * | 0.034 | 0.046 | 0.247 | 0.300 | 0.571 * | 0.307 | 0.639 ** | 0.427 | 0.410 |
| Extract. K | Extract. P | Conductivity | pHH2O | pHKCl | Organic C | Exchan. Ca | CEC | Base Saturation | Extract. Fe | Extract. Mn | Extract. Zn | Extract. Cu | Extract. B | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg K2O kg−1) 1 | (mg P2O5 kg−1) 1 | (µs/m) | (g kg−1) | (cmolc kg−1) 2 | (%) | (mg kg−1) 3 | (mg kg−1) 4 | |||||||
| Treatment (T) | ||||||||||||||
| Control | 82.5 b | 162.2 b | 69.8 b | 5.20 b | 4.09 b | 14.3 a | 3.76 a | 7.38 a | 82.7 a | 160.4 a | 96.1 b | 2.54 b | 4.74 b | 0.82 b |
| Lime | 100.8 a | 216.7 a | 85.5 a | 5.40 a | 4.35 a | 14.6 a | 3.90 a | 7.64 a | 79.5 a | 165.8 a | 123.7 a | 3.49 a | 5.74 a | 1.11 a |
| Field (F) | ||||||||||||||
| Field 1 | 126.5 a | 244.6 a | 82.6 a | 5.54 a | 4.53 a | 14.7 a | 4.47 a | 8.21 a | 91.2 a | 153.2 b | 105.8 a | 3.32 a | 7.23 a | 1.11 a |
| Field 2 | 56.8 b | 134.3 b | 72.6 b | 5.06 b | 3.91 b | 14.2 a | 3.19 b | 6.81 b | 71.1 b | 173.0 a | 114.0 a | 2.71 b | 3.25 b | 0.82 b |
| Prob (T) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.6084 | 0.4582 | 0.1793 | 0.0638 | 0.5629 | 0.0009 | <0.0001 | 0.0001 | 0.0002 |
| Prob (F) | <0.0001 | <0.0001 | 0.0002 | <0.0001 | <0.0001 | 0.4112 | <0.0001 | <0.0001 | <0.0001 | 0.0357 | 0.3074 | 0.0052 | <0.0001 | 0.0003 |
| Prob (T × F) | 0.0009 | 0.1647 | <0.0001 | <0.0001 | <0.0001 | 0.8899 | 0.1608 | 0.4658 | 0.0098 | 0.8719 | 0.4696 | 0.4311 | 0.0661 | 0.0079 |
| N | P | K | Ca | Mg | Fe | Mn | Cu | Zn | B | Total DMY | Cone DMY | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g kg−1) | (mg kg−1) | (g Plant−1) | |||||||||||
| 2017 | Treatment (T) | ||||||||||||
| Control | 3.31 a | 0.14 b | 1.56 a | 2.57 a | 0.47 a | 96.7 a | 374.1 a | 3.97 a | 74.3 a | 71.7 a | 1483 a | 544.3 a | |
| Lime | 3.39 a | 0.15 a | 1.66 a | 2.72 a | 0.52 a | 95.3 a | 316.9 a | 4.59 a | 82.9 a | 69.3 a | 1379 a | 441.2 b | |
| Field (F) | |||||||||||||
| Field 1 | 3.39 a | 0.13 b | 1.76 a | 2.56 a | 0.49 a | 87.9 b | 355.9 a | 2.54 b | 64.35 b | 63.29 b | 1271 b | 446.2 b | |
| Field 2 | 3.31 a | 0.16 a | 1.46 b | 2.73 a | 0.50 a | 104.2 a | 335.1 a | 6.03 a | 92.88 a | 77.70 a | 1591 a | 539.3 a | |
| Prob. (T) | 0.2043 | 0.0440 | 0.3130 | 0.3597 | 0.1445 | 0.8597 | 0.0703 | 0.2111 | 0.2636 | 0.2542 | 0.2139 | 0.0033 | |
| Prob. (F) | 0.2180 | <0.0001 | 0.0049 | 0.2909 | 0.7214 | 0.0447 | 0.5024 | <0.0001 | 0.0007 | <0.0001 | 0.0005 | 0.0073 | |
| Prob. (T × F) | 0.0024 | 0.7290 | 0.8820 | 0.9293 | 0.4216 | 0.2588 | 0.0358 | 0.1327 | 0.2608 | 0.0918 | 0.3402 | 0.5728 | |
| 2018 | Treatment (T) | ||||||||||||
| Control | 3.48 a | 0.19 a | 3.26 a | 1.53 a | 0.56 a | 94.6 b | 513.0 a | 6.93 a | 21.0 a | 57.5 a | 1681 a | 475.2 a | |
| Lime | 3.56 a | 0.19 a | 3.19 a | 1.57 a | 0.61 a | 109.3 a | 495.2 a | 7.07 a | 19.5 a | 51.6 b | 1407 b | 380.2 b | |
| Field (F) | |||||||||||||
| Field 1 | 3.55 a | 0.20 a | 3.83 a | 1.43 b | 0.62 a | 91.0 b | 408.9 b | 6.26 b | 20.51 a | 52.37 b | 1421 b | 428.9 a | |
| Field 2 | 3.49 a | 0.17 b | 2.63 b | 1.67 a | 0.55 b | 112.9 a | 599.4 a | 7.73 a | 19.96 a | 56.70 a | 1666 a | 426.4 a | |
| Prob. (T) | 0.2155 | 0.8374 | 0.6215 | 0.4655 | 0.0550 | 0.0035 | 0.7162 | 0.5532 | 0.1170 | 0.0024 | 0.0016 | 0.0021 | |
| Prob. (F) | 0.3461 | <0.0001 | <0.0001 | 0.0001 | 0.0279 | <0.0001 | 0.0004 | <0.0001 | 0.5648 | 0.0218 | 0.0043 | 0.9304 | |
| Prob. (T × F) | 0.6601 | 0.0185 | 0.2051 | 0.0983 | 0.2205 | 0.9394 | 0.8796 | 0.6927 | 0.1228 | 0.4463 | 0.0057 | 0.3068 | |
| Leaf Nutrient Concentration | DMY | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil | N | P | K | Ca | Mg | Fe | Mn | Cu | Zn | B | Total | Cone |
| (g kg−1) | (mg kg−1) | (g Plant−1) | ||||||||||
| 2017 | ||||||||||||
| pHH2O | 0.492 * | −0.648 ** | 0.323 | −0.131 | −0.088 | −0.157 | 0.324 | −0.531 ** | −0.503 * | −0.788 ** | −0.635 ** | −0.657 ** |
| pHKCl | 0.519 ** | −0.651 ** | 0.318 | −0.068 | −0.076 | −0.137 | 0.315 | −0.524 ** | −0.538 ** | −0.787 ** | −0.563 ** | −0.590 ** |
| 2018 | ||||||||||||
| pHH2O | 0.315 | 0.544 ** | 0.606 ** | −0.289 | 0.492 * | −0.290 | −0.714 ** | −0.611 ** | −0.144 | −0.477 * | 0.093 | −0.104 |
| pHKCl | 0.269 | 0.526 ** | 0.585 ** | −0.321 | 0.436 * | −0.317 | −0.717 ** | −0.670 ** | −0.127 | −0.477 * | 0.188 | −0.066 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonso, S.; Arrobas, M.; Rodrigues, M.Â. Twenty-Years of Hop Irrigation by Flooding the Inter-Row Did Not Cause a Gradient along the Row in Soil Properties, Plant Elemental Composition and Dry Matter Yield. Horticulturae 2021, 7, 194. https://doi.org/10.3390/horticulturae7070194
Afonso S, Arrobas M, Rodrigues MÂ. Twenty-Years of Hop Irrigation by Flooding the Inter-Row Did Not Cause a Gradient along the Row in Soil Properties, Plant Elemental Composition and Dry Matter Yield. Horticulturae. 2021; 7(7):194. https://doi.org/10.3390/horticulturae7070194
Chicago/Turabian StyleAfonso, Sandra, Margarida Arrobas, and Manuel Ângelo Rodrigues. 2021. "Twenty-Years of Hop Irrigation by Flooding the Inter-Row Did Not Cause a Gradient along the Row in Soil Properties, Plant Elemental Composition and Dry Matter Yield" Horticulturae 7, no. 7: 194. https://doi.org/10.3390/horticulturae7070194
APA StyleAfonso, S., Arrobas, M., & Rodrigues, M. Â. (2021). Twenty-Years of Hop Irrigation by Flooding the Inter-Row Did Not Cause a Gradient along the Row in Soil Properties, Plant Elemental Composition and Dry Matter Yield. Horticulturae, 7(7), 194. https://doi.org/10.3390/horticulturae7070194







