Can Biostimulants Increase Resilience of Hydroponically-Grown Tomato to Combined Water and Nutrient Stress?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions, and Treatments
2.2. Root Zone and Drainage Solution Measurements
2.3. Biomass and Leaf Area Determination
2.4. Total Yield Determination
2.5. Leaves and Fruits Nutrient Analyses, Fruit Quality Characteristics
2.6. Statistical Analysis
3. Results
3.1. pH in Drainage Solution and Concentration of N, P, K in Tomato Root Zone
3.2. Fresh Biomass and Leaf Area of Plants
3.3. Tomato Yield Components
3.4. Leaf and Fruit Nutrient Concentrations, and Fruit Quality
4. Discussion
4.1. Effects of Combined Stress on Tomato Growth, Nutrition, and Yield
4.2. Biostimulants of Edypro
4.3. Strigolactone-Based Biostimulant
4.4. Maxicrop
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Katsoulas, N.; Savvas, D.; Kitta, E.; Bartzanas, T.; Kittas, C. Extension and evaluation of a model for automatic drainage solution management in tomato crops grown in semi-closed hydroponic systems. Comput. Electron. Agric. 2015, 113, 61–71. [Google Scholar] [CrossRef]
- Saadi, S.; Todorovic, M.; Tanasijevic, L.; Pereira, L.S.; Pizzigalli, C.; Lionello, P. Climate change and Mediterranean agriculture: Impacts on winter wheat and tomato crop evapotranspiration, irrigation requirements and yield. Agric. Water Manag. 2015, 147, 103–115. [Google Scholar] [CrossRef]
- Adams, P. Effects of increasing the salinity of the nutrient solution with major nutrients or sodium chloride on the yield, quality and composition of tomatoes grown in rockwool. J. Hortic. Sci. 2015, 66, 201–207. [Google Scholar] [CrossRef]
- Muñoz, P.; Paranjpe, A.; Montero, J.I.; Antón, A. Cascade crops: An alternative solution for increasing sustainability of greenhouse tomato crops in Mediterranean zone. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 927, Lisbon, Portugal, 22 August 2010; pp. 801–805. [Google Scholar]
- Wohanka, W. Nutrient solution disinfection. In Hydroponic Production of Vegetables and Ornamentals; Savvas, D., Passam, H.C., Eds.; Embryo Publishing: Athens, Greece, 2002; pp. 345–372. [Google Scholar]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Meric, M.K.; Tuzel, I.H.; Tuzel, Y.; Oztekin, G.B. Effects of nutrition systems and irrigation programs on tomato in soilless culture. Agric. Water Manag. 2011, 99, 19–25. [Google Scholar] [CrossRef]
- Sanjuan-Delmás, D.; Josa, A.; Muñoz, P.; Gassó, S.; Rieradevall, J.; Gabarrell, X. Applying nutrient dynamics to adjust the nutrient-water balance in hydroponic crops. A case study with open hydroponic tomato crops from Barcelona. Sci. Hortic. 2020, 261, 108908. [Google Scholar] [CrossRef]
- Chen, J.G.; Crooks, R.M.; Seefeldt, L.C.; Bren, K.L.; Bullock, R.M.; Darensbourg, M.Y.; Holland, P.L.; Hoffman, B.; Janik, M.J.; Jones, A.K.; et al. Beyond fossil fuel–driven nitrogen transformations. Science 2018, 360, 6391. [Google Scholar] [CrossRef] [Green Version]
- Cordell, D.; White, S. Tracking phosphorus security: Indicators of phosphorus vulnerability in the global food system. Food Secur. 2015, 7, 337–350. [Google Scholar] [CrossRef]
- Yang, L.; Huang, B.; Mao, M.; Yao, L.; Niedermann, S.; Hu, W.; Chen, Y. Sustainability assessment of greenhouse vegetable farming practices from environmental, economic, and socio-institutional perspectives in China. Environ. Sci. Pollut. Res. 2016, 23, 17287–17297. [Google Scholar] [CrossRef]
- Craigie, J. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Sangha, J.S.; Kelloway, S.; Critchley, A.T.; Prithiviraj, B. Seaweeds (macroalgae) and their extracts as contributors of plant productivity and quality: The current status of our understanding. Sea Plants 2014, 71, 189–219. [Google Scholar]
- Koleška, I.; Hasanagić, D.; Todorović, V.; Murtić, S.; Klokić, I.; Parađiković, N.; Kukavica, B. Biostimulant prevents yield loss and reduces oxidative damage in tomato plants grown on reduced NPK nutrition. J. Plant Interact. 2017, 12, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Kunicki, E.; Grabowska, A.; Sękara, A.; Wojciechowska, R. The effect of cultivar type, time of cultivation, and biostimulant treatment on the yield of spinach (Spinacia oleracea L.). Folia Hortic. 2010, 22, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Zodape, S.T.; Gupta, A.; Bhandari, S.C.; Rawat, U.S.; Chaudhary, D.R.; Eswaran, K.; Chikara, J. Foliar application of seaweed sap as biostimulant for enhancement of yield and quality of tomato (Lycopersicon esculentum Mill). J. Sci. Ind. Res. 2011, 70, 215–219. [Google Scholar]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Francesca, S.; Cirillo, V.; Raimondi, G.; Maggio, A.; Barone, A.; Rigano, M.M. A Novel Protein Hydrolysate-Based Biostimulant Improves Tomato Performances under Drought Stress. Plants 2021, 10, 783. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. A meta-analysis and review of plant-growth response to humic substances: Practical implications for agriculture. Adv. Agron. 2014, 124, 37–89. [Google Scholar]
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, H.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar] [CrossRef]
- Xu, C.; Leskovar, D.I. Effects of A. nodosum seaweed extracts on spinach growth, physiology and nutrition value under drought stress. Sci. Hortic. 2015, 183, 39–47. [Google Scholar] [CrossRef]
- Petrozza, A.; Santaniello, A.; Summerer, S.; Di Tommaso, G.; Di Tommaso, D.; Paparelli, E.; Piaggesi, A.; Perata, P.; Cellini, F. Physiological responses to Megafol® treatments in tomato plants under drought stress: A phenomic and molecular approach. Sci. Hortic. 2014, 174, 185–192. [Google Scholar] [CrossRef]
- Botta, A. Enhancing plant tolerance to temperature stress with amino acids: An approach to their mode of action. Acta Hortic. 2013, 1009, 29–35. [Google Scholar] [CrossRef]
- Cerdán, M.; Sánchez-Sánchez, A.; Jordá, D.J.; Juárez, M.; Andreu, J.S. Effect of commercial amino acids on iron nutrition of tomato plants grown underlime-induced iron deficiency. J. Plant Nutr. Soil Sci. 2013, 176, 1–8. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 2013, 364, 145–158. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and cropperformance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Visconti, F.; de Paz, J.M.; Bonet, L.; Jordà, M.; Quinones, A.; Intrigliolo, D.S. Effects of a commercial calcium protein hydrolysate on the salt tolerance of Diospyros kaki L. cv. Rojo Brillante grafted on Diospyros lotus L. Sci. Hortic. 2015, 185, 129–138. [Google Scholar] [CrossRef]
- Cardinale, F.; Korwin Krukowski, P.; Schubert, A.; Visentin, I. Strigolactones: Mediators of osmotic stress responses with a potential for agrochemical manipulation of crop resilience. J. Exp. Bot. 2018, 69, 2291–2303. [Google Scholar] [CrossRef]
- Marzec, M.; Muszynska, A.; Gruszka, D. The role of strigolactones in nutrient-stress responses in plants. Int. J. Mol. Sci. 2013, 14, 9286–9304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chesterfield, R.J.; Vickers, C.E.; Beveridge, C.A. Translation of strigolactones from plant hormone to agriculture: Achievements, future perspectives, and challenges. Trends Plant Sci. 2020, 25, 1087–1106. [Google Scholar] [CrossRef]
- Matusova, R.; Rani, K.; Verstappen, F.W.; Franssen, M.C.; Beale, M.H.; Bouwmeester, H.J. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol. 2005, 139, 920–934. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [Green Version]
- López-Ráez, J.A.; Bouwmeester, H. Fine-tuning regulation of strigolactone biosynthesis under phosphate starvation. Plant Signal. Behav. 2008, 3, 963–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Ráez, J.A.; Kohlen, W.; Charnikhova, T.; Mulder, P.; Undas, A.K.; Sergeant, M.J.; Verstappen, F.; Bugg, T.D.H.; Thompson, A.J.; Ruyter-Spira, C.; et al. Does abscisic acid affect strigolactone biosynthesis? New Phytol. 2010, 187, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Toh, S.; Kamiya, Y.; Kawakami, N.; Nambara, E.; McCourt, P.; Tsuchiya, Y. Thermoinhibition uncovers a role for strigolactones in Arabidopsis seed germination. Plant Cell Physiol. 2012, 53, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Novero, M.; Charnikhova, T.; Ferrandino, A.; Schubert, A.; Ruyter-Spira, C.; Bonfante, P.; Lovisolo, C.; Bouwmeester, H.J.; Cardinale, F. Carotenoid cleavage dioxygenase 7 modulates plant growth, reproduction, senescence, and determinate nodulation in the model legume Lotus japonicus. J. Exp. Bot. 2013, 64, 1967–1981. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; He, H.; Vitali, M.; Visentin, I.; Charnikhova, T.; Haider, I.; Schubert, A.; Ruyter-Spira, C.; Bouwmeester, H.J.; Lovisolo, C.; et al. Osmotic stress represses strigolactone biosynthesis in Lotus japonicus roots: Exploring the interaction between strigolactones and ABA under abiotic stress. Planta 2015, 241, 1435–1451. [Google Scholar] [CrossRef]
- Koltai, H. Strigolactones activate different hormonal pathways for regulation of root development in response to phosphate growth conditions. Ann. Bot. 2013, 112, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Umehara, M. Strigolactone, a key regulator of nutrient allocation in plants. Plant Biotechnol. 2011, 28, 429–437. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, Á.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, G.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Li, W.; Nguyen, K.H.; Fujita, M.; Tran, L.S.P. Strigolactones in plant adaptation to abiotic stresses: An emerging avenue of plant research. Plant Cell Environ. 2018, 41, 2227–2243. [Google Scholar] [CrossRef] [PubMed]
- Vurro, M.; Prandi, C.; Baroccio, F. Strigolactones: How far is their commercial use for agricultural purposes? Pest Manag. Sci. 2016, 72, 2026–2034. [Google Scholar] [CrossRef]
- Ofner, I.; Lashbrooke, J.; Pleban, T.; Aharoni, A.; Zamir, D. Solanum pennellii backcross inbred lines (BIL s) link small genomic bins with tomato traits. Plant J. 2016, 87, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Savvas, D.; Gianquinto, G.P.; Tüzel, Y.; Gruda, N. Soilless culture. In Good Agricultural Practices for Greenhouse Vegetable Crops—Principles for Mediterranean Climate Areas; FAO: Rome, Italy, 2013; Volume 217, pp. 303–354. [Google Scholar]
- Stirk, W.A.; van Staden, J. Seaweed products as biostimulants in agriculture. In World Seaweed Resources; ETI Information Services Lts: Amesterdam, The Netherlands, 2006; pp. 80–84. [Google Scholar]
- Steveni, C.M.; Norrington-Davies, J.; Hankins, S.D. Effect of seaweed concentrate on hydroponically grown spring barley. J. Appl. Phycol. 1992, 4, 173–180. [Google Scholar] [CrossRef]
- Passam, H.C.; Karapanos, I.C.; Bebeli, P.J.; Savvas, D. A review of recent research on tomato nutrition, breeding and post-harvest technology with reference to fruit quality. Eur. J. Plant Sci. Biotechnol. 2007, 1, 1–21. [Google Scholar]
- Bremner, J.M. Total nitrogen. In Methods of Soil Analysis; Black, C.A., Evans, D.D., White, I.L., Ensminger, L.E., Clark, F.E., Eds.; ACSESS: Madison, WI, USA, 1965; pp. 1149–11789. [Google Scholar]
- Kalozoumis, P.; Savvas, D.; Aliferis, K.; Ntatsi, G.; Marakis, G.; Simou, E.; Tampakaki, A.; Karapanos, I. Impact of Plant Growth-Promoting Rhizobacteria Inoculation and Grafting on Tolerance of Tomato to Combined Water and Nutrient Stress Assessed via Metabolomics Analysis. Front. Plant Sci. 2021, 12, 814. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, T.Q.; Tan, C.S.; Astatkie, T. Responses of fruit yield and quality of processing tomato to drip-irrigation and fertilizers phosphorus and potassium. Agronomy 2011, 103, 1339–1345. [Google Scholar] [CrossRef]
- Adams, P. Nutritional control in hydroponics. In Hydroponic Production of Vegetables and Ornamentals; Savvas, D., Passam, H.C., Eds.; Embryo Publishing: Athens, Greece, 2002; pp. 211–261. [Google Scholar]
- Sonneveld, C. Composition of nutrient solutions. In Hydroponic Production of Vegetables and Ornamentals; Savvas, D., Passam, H.C., Eds.; Embryo Publishing: Athens, Greece, 2002; pp. 179–210. [Google Scholar] [CrossRef]
- Sestili, F.; Rouphael, Y.; Cardarelli, M.; Pucci, A.; Bonini, P.; Canaguier, R.; Colla, G. Protein hydrolysate stimulates growth in tomato coupled with N-dependent gene expression involved in N assimilation. Front Plant Sci. 2018, 9, 1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaya, C.; Higgs, D. Inter-relationships between zinc nutrition, growth parameters, and nutrient physiology in a hydroponically grown tomato cultivar. J. Plant Nutr. 2001, 24, 1491–1503. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Nardi, S. Transcriptome-wide identification of differentially expressed genes in Solanum lycopersicon L. in response to an alfalfa-protein hydrolysate using microarrays. Front. Plant Sci. 2017, 8, 1159. [Google Scholar] [CrossRef] [Green Version]
- Lv, S.; Zhang, Y.; Li, C.; Liu, Z.; Yang, N.; Pan, L.; Wu, J.; Wang, J.; Yang, J.; Lv, Y.; et al. Strigolactone-triggered stomatal closure requires hydrogen peroxide synthesis and nitric oxide production in an abscisic acid-independent manner. New Phytol. 2018, 217, 290–304. [Google Scholar] [CrossRef] [Green Version]
- Visentin, I.; Vitali, M.; Ferrero, M.; Zhang, Y.; Ruyter-Spira, C.; Novák, O.; Strnad, M.; Lovisolo, C.; Schubert, A.; Cardinale, F. Low levels of strigolactones in roots as a component of the systemic signal of drought stress in tomato. New Phytol. 2016, 212, 954–963. [Google Scholar] [CrossRef]
- Heuvelink, E.; Bakker, M.J.; Elings, A.; Kaarsemaker, R.C.; Marcelis, L.F.M. Effect of leaf area on tomato yield. In Proceedings of the International Conference on Sustainable Greenhouse Systems-Greensys2004, Leuven, Belgium, 12–16 September 2004; Volume 691, pp. 43–50. [Google Scholar] [CrossRef]
- Ali, N.; Farrell, A.; Ramsubhag, A.; Jayaraman, J. The effect of Ascophyllum nodosum extract on the growth, yield and fruit quality of tomato grown under tropical conditions. J. Appl. Phycol. 2016, 28, 1353–1362. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Phycol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef]
- González-González, M.F.; Ocampo-Alvarez, H.; Santacruz-Ruvalcaba, F.; Sánchez-Hernández, C.V.; Casarrubias-Castillo, K.; Becerril-Espinosa, A.; Castañeda-Nava, J.J.; Hernández-Herrera, R.M. Physiological, ecological, and biochemical implications in tomato plants of two plant biostimulants: Arbuscular mycorrhizal fungi and seaweed extract. Front. Plant Sci. 2020, 11, 999. [Google Scholar] [CrossRef]
- Rolland, F.; Moore, B.; Sheen, J. Sugar sensing and signaling in plants. Plant Cell 2002, 14, S185–S205. [Google Scholar] [CrossRef] [Green Version]
- Singh, I.S.; Shono, M.A. Heat-stress effects on dry matter partitioning, pollen viability and fruit yield in tomato genotypes. Indian J. Plant Physiol. 2012, 17, 103–112. [Google Scholar]
- Vernieri, P.; Borghesi, E.; Tognoni, F.; Serra, G.; Ferrante, A.; Piagessi, A. Use of biostimulants for reducing nutrient solution concentration in floating system. Acta Hortic. 2006, 718, 477–484. [Google Scholar] [CrossRef]
- Parađiković, N.; Vinković, T.; Vinković Vrček, I.; Žuntar, I.; Bojić, M.; Medić-Šarić, M. Effect of natural biostimulants on yield and nutritional quality: An example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [CrossRef]
- Siddiqi, M.Y.; Kronzucker, H.J.; Britto, D.T.; Glass, A.D.M. Growth of a tomato crop at reduced nutrient concentrations as a strategy to limit eutrophication. J. Plant Nutr. 1998, 21, 1879–1895. [Google Scholar] [CrossRef]
- Vázquez-Hernández, M.C.; Parola-Contreras, I.; Montoya-Gómez, L.M.; Torres-Pacheco, I.; Schwarz, D.; Guevara-González, R.G. Eustressors: Chemical and physical stress factors used to enhance vegetables production. Sci. Hortic. 2019, 250, 223–229. [Google Scholar] [CrossRef]



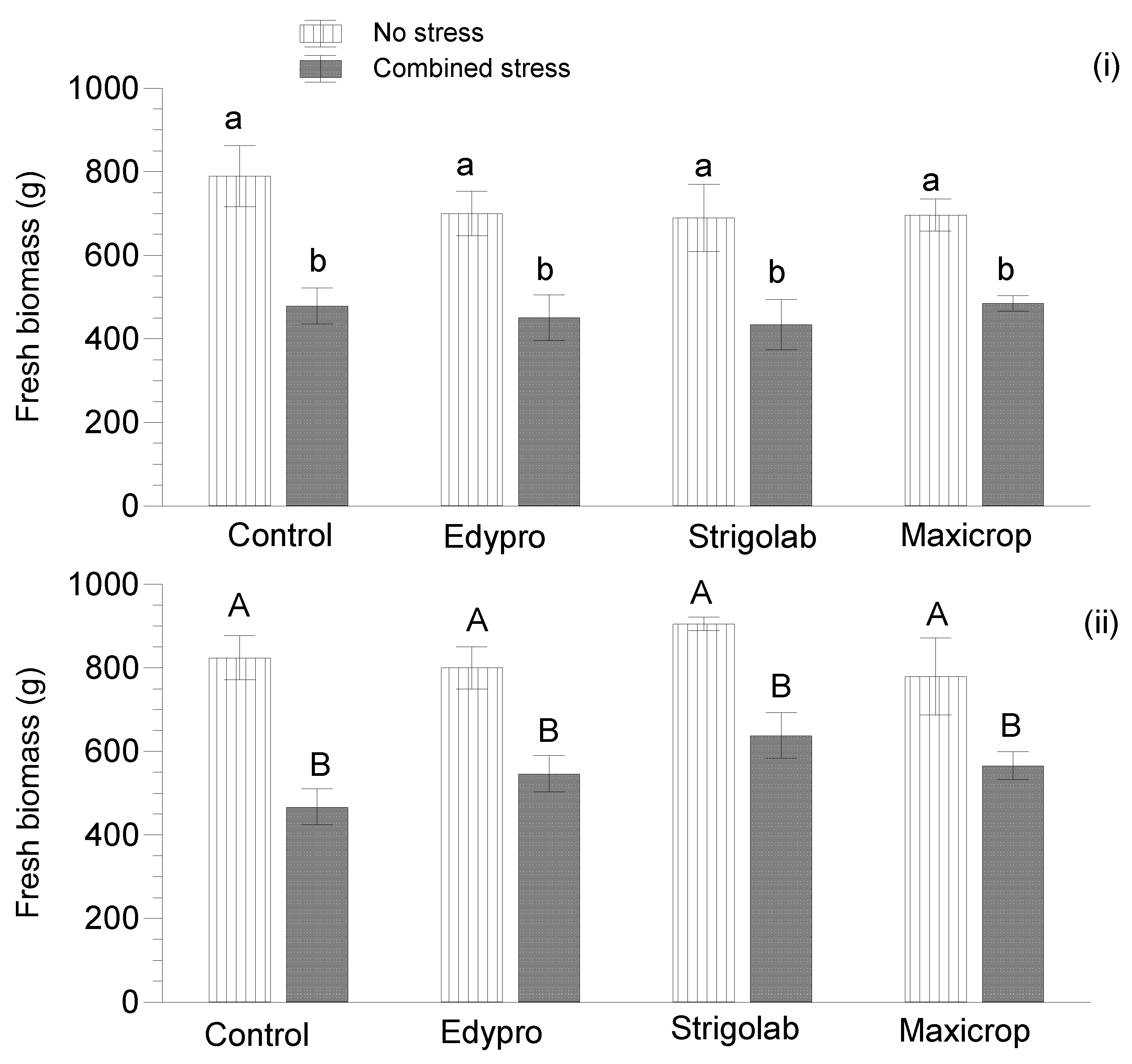
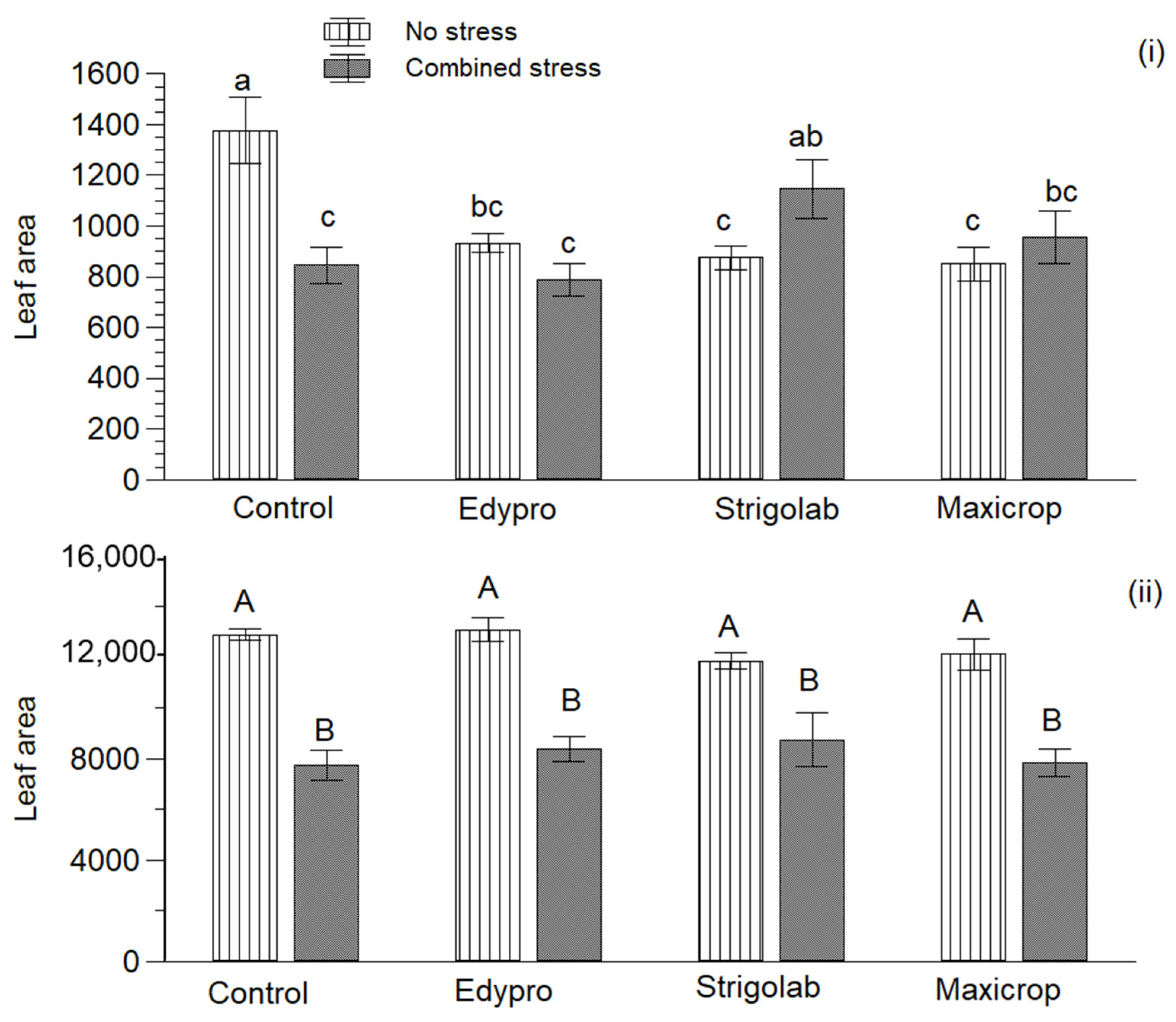

| Treatment | 30 DAT | 60 DAT | 90 DAT | 120 DAT |
|---|---|---|---|---|
| No stress | 11.5 b | 6.3 a | 6.9 a | 6.2 a |
| Combined Stress | 12.8 a | 3.9 b | 4.8 b | 4.6 b |
| Control | 12.3 | 4.7 | 6.0 | 5.2 |
| Edypro | 11.8 | 5.1 | 6.1 | 5.3 |
| Strigolactone-based BS | 11.9 | 4.9 | 5.1 | 5.4 |
| Maxicrop | 12.6 | 5.6 | 6.2 | 5.6 |
| Statistical interactions | ||||
| NS × Control | 13.3 ab | 6.6 | 7.6 | 6.4 |
| NS × Edypro | 10.7 cd | 6.7 | 7.4 | 6.1 |
| NS × Strigolactone-based BS | 9.7 d | 5.0 | 5.6 | 5.9 |
| NS × Maxicrop | 12.3 abc | 6.9 | 6.9 | 6.4 |
| CS × Control | 11.3 bcd | 2.8 | 4.6 | 4.1 |
| CS × Edypro | 12.9 abc | 3.5 | 4.6 | 4.6 |
| CS × Strigolactone-based BS | 14.2 a | 4.8 | 4.5 | 4.9 |
| CS × Maxicrop | 12.9 abc | 4.4 | 5.6 | 4.9 |
| Statistical interactions | ||||
| Stress | * | *** | *** | *** |
| BS | ns | ns | ns | ns |
| Stress × BS | ** | ns | ns | ns |
| Treatment | 30 DAT | 60 DAT | 90 DAT | 120 DAT |
|---|---|---|---|---|
| No stress | 10.3 | 10.7 a | 10.5 a | 10.2 a |
| Combined Stress | 10.3 | 8.0 b | 6.8 b | 5.3 b |
| Control | 9.9 | 9.4 | 7.9 | 7.8 |
| Edypro | 9.8 | 8.2 | 8.1 | 8.2 |
| Strigolactone-based BS | 10.4 | 10.1 | 9.7 | 7.9 |
| Maxicrop | 11.4 | 9.6 | 9.0 | 7.3 |
| Statistical interactions | ||||
| NS × Control | 9.8 bc | 11.1 | 11.9 a | 9.7 a |
| NS × Edypro | 10.2 abc | 10.6 | 10.4 ab | 9.6 a |
| NS × Strigolactone-based BS | 9.4 c | 9.8 | 9.4 ab | 10.9 a |
| NS × Maxicrop | 11.7 a | 11.5 | 10.5 ab | 10.7 a |
| CS × Control | 10.0 abc | 7.7 | 3.9 d | 5.8 bc |
| CS × Edypro | 9.4 c | 5.9 | 5.8 cd | 6.8 b |
| CS × Strigolactone-based BS | 11.4 ab | 10.5 | 9.9 ab | 4.8 cd |
| CS × Maxicrop | 10.5 abc | 7.7 | 7.5 bc | 3.9 d |
| Statistical interactions | ||||
| Stress | ns | * | *** | *** |
| BS | ns | ns | ns | ns |
| Stress × BS | * | ns | * | ** |
| Treatment | 30 DAT | 60 DAT | 90 DAT | 120 DAT |
|---|---|---|---|---|
| No stress | 1.7 | 1.9 a | 2.1 a | 1.4 a |
| Combined Stress | 1.7 | 0.9 b | 1.0 b | 0.9 b |
| Control | 1.5 | 1.6 | 1.5 b | 1.2 |
| Edypro | 1.7 | 1.4 | 2.0 a | 1.2 |
| Strigolactone-based biostimulant | 1.8 | 1.3 | 1.4 b | 1.2 |
| Maxicrop | 1.9 | 1.3 | 1.4 b | 1.0 |
| Statistical interactions | ||||
| NS × Control | 1.5 b | 2.0 | 2.2 b | 1.4 |
| NS × Edypro | 1.4 b | 2.2 | 2.8 a | 1.5 |
| NS × Strigolactone-based biostimulant | 1.9 ab | 1.6 | 1.8 b | 1.4 |
| NS × Maxicrop | 2.1 a | 1.6 | 1.7 b | 1.3 |
| CS × Control | 1.5 b | 1.1 | 0.9 c | 0.9 |
| CS × Edypro | 2.0 ab | 0.7 | 1.2 c | 0.8 |
| CS × Strigolactone-based biostimulant | 1.8 ab | 1.0 | 1.0 c | 1.0 |
| CS × Maxicrop | 1.6 ab | 1.0 | 1.1 c | 0.7 |
| Statistical interactions | ||||
| Stress | ns | * | *** | *** |
| BS | ns | ns | ** | ns |
| Stress × BS | * | ns | * | ns |
| Treatment | Early Fruit Yield (kg m−2) | Total Fruit Yield (kg m−2) | Number of Fruit m−2 | Mean Fruit Weight (g) | Extra Class (kg m−2) |
|---|---|---|---|---|---|
| No stress | 1.75 | 3.35 a | 13.4 a | 125.7 a | 0.85 a |
| Combined Stress | 1.68 | 2.69 b | 11.5 b | 115.9 b | 0.65 b |
| Control | 1.82 b | 3.29 a | 13.1 a | 125.6 | 0.92 a |
| Edypro | 1.27 c | 2.51 b | 10.8 b | 116.2 | 0.52 b |
| Strigolactone-based BS | 2.07 a | 3.17 a | 13.1 a | 121.2 | 0.83 a |
| Maxicrop | 1.71 b | 3.10 a | 12.9 a | 120.3 | 0.73 ab |
| Statistical interactions | |||||
| Stress | ns | *** | ** | *** | * |
| BS | *** | ** | * | ns | * |
| Stress × BS | ns | ns | ns | ns | ns |
| Leaves | Fruits | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | N (g kg−1 dw) | P (g kg−1 dw) | K (g kg−1 dw) | Zn (μg g−1) | P (g kg−1 dw) | K (g kg−1 dw) | TSS (oBrix) | Acidity (g Citric Acid per 100 g fw |
| No stress | 38.6 a | 8.6 a | 46.8 | 37.58 | 5.9 | 38.1 a | 3.45 | 0.34 |
| Combined Stress | 34.9 b | 7.0 b | 47.0 | 45.25 | 6.3 | 33.4 b | 3.74 | 0.35 |
| Control | 37.5 | 7.7 | 45.4 b | 31.75 b | 6.3 | 31.6 b | 3.68 | 0.34 |
| Edypro | 34.7 | 6.8 | 50.3 a | 58.50 a | 6.1 | 38.9 a | 3.66 | 0.35 |
| Strigolactone-based BS | 37.0 | 9.1 | 45.9 b | 44.50 ab | 5.9 | 34.9 ab | 3.59 | 0.35 |
| Maxicrop | 37.6 | 7.7 | 45.9 b | 30.92 b | 6.2 | 37.6 a | 3.46 | 0.33 |
| Statistical interactions | ||||||||
| Stress | ** | * | ns | ns | ns | * | ns | ns |
| BS | ns | ns | * | ** | ns | * | ns | ns |
| Stress × BS | ns | ns | ns | ns | ns | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalozoumis, P.; Vourdas, C.; Ntatsi, G.; Savvas, D. Can Biostimulants Increase Resilience of Hydroponically-Grown Tomato to Combined Water and Nutrient Stress? Horticulturae 2021, 7, 297. https://doi.org/10.3390/horticulturae7090297
Kalozoumis P, Vourdas C, Ntatsi G, Savvas D. Can Biostimulants Increase Resilience of Hydroponically-Grown Tomato to Combined Water and Nutrient Stress? Horticulturae. 2021; 7(9):297. https://doi.org/10.3390/horticulturae7090297
Chicago/Turabian StyleKalozoumis, Panagiotis, Christos Vourdas, Georgia Ntatsi, and Dimitrios Savvas. 2021. "Can Biostimulants Increase Resilience of Hydroponically-Grown Tomato to Combined Water and Nutrient Stress?" Horticulturae 7, no. 9: 297. https://doi.org/10.3390/horticulturae7090297








