Comparative Study of Four Jackfruit Genotypes: Morphology, Physiology and Physicochemical Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Morphological Analysis
2.3. Physiological Analysis
2.4. Physicochemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Morphological Analysis
3.2. Physiological Analysis
3.3. Physicochemical Analysis
3.4. Principal Component Analysis
4. Discussion
4.1. Morphological Analysis
4.2. Physiological Analysis
4.3. Physicochemical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, A.U.; Ema, I.J.; Faruk, M.R.; Tarapder, S.A.; Khan, A.U.; Noreen, S.; Adnan, M. Review on importance of Artocarpus heterophyllus L. (Jackfruit). J. Multidiscip. Appl. Nat. Sci. 2021, 1, 106–116. [Google Scholar] [CrossRef]
- Villalobos, M.C. Jaca (Artocarpus heterophyllus Lam.) Centenario de Nayarit. In Enciclopedia Centenario de Nayarit; Consejo Estatal para la Cultura y Las Artes de Nayarit: Tepic, Mexico, 2017; Volume 91, pp. 399–404. [Google Scholar]
- Luna-Esquivel, G.; Alejo-Santiago, G.; Ramírez-Guerrero, L.; Arévalo-Galarza, M. La yaca un fruto de exportación. Agro Product. 2013, 6, 65–70. [Google Scholar]
- SIAP. Anuario Estadístico de la Producción Agrícola. Servicio de Información Agroalimentaria Y Pesquera. 2021. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 22 March 2022).
- Haq, N. Jackfruit: Artocarpus heterophyllus; Southampton Centre for Underutilised Crops, University of Southampton: Southampton, UK, 2006; pp. 1–38. [Google Scholar]
- Elevitch, C.R.; Manner, H.I. Artocarpus heterophyllus (jackfruit). Species Profiles Pac. Isl. Agrofor. 2006, 1, 16. [Google Scholar]
- Saxena, A.; Bawa, A.S.; Raju, P.S. Jackfruit (Artocarpus heterophyllus Lam.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 275–298. [Google Scholar] [CrossRef]
- Dhakar, M.K.; Das, B.; Sarkar, P.K.; Nath, V.; Singh, A.K.; Bhatt, B.P. Diversity in jackfruit (Artocarpus heterophyllus Lam.): Insights into fruit characterization for the identification of superior genotypes. Plant Genet. Resour. Charact. Util. 2020, 18, 307–315. [Google Scholar] [CrossRef]
- Ranasinghe, R.A.S.N.; Maduwanthi, S.D.T.; Marapana, R.A.U.J. Nutritional and health benefits of jackfruit (Artocarpus heterophyllus Lam.): A review. Int. J. Food Sci. 2019, 2019, 4327183. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Zawawi, N.; Razis, A.F.A.; Bakar, J.; Zarei, M. Antiviral activity of fermented foods and their probiotics bacteria towards respiratory and alimentary tracts viruses. Food Control. 2021, 127, 108140. [Google Scholar] [CrossRef]
- Vargas-Torres, A.; Becerra-Loza, A.S.; Sayago-Ayerdi, S.G.; Palma-Rodríguez, H.M.; del García-Magaña, M.; Montalvo-González, E. Combined effect of the application of 1-MCP and different edible coatings on the fruit quality of jackfruit bulbs (Artocarpus heterophyllus Lam.) during cold storage. Sci. Hortic. 2017, 214, 221–227. [Google Scholar] [CrossRef]
- Ningot, E.P.; Dahale, M.H.; Uikey, A.C.; Naitam, P.C. Variability studies on physico-chemical characteristics of jackfruit genotypes from Eastern Maharashtra, India. Int. J. Curr. Microbiol. Appl. Spec. Issue 2018, 6, 2294–2298. [Google Scholar]
- Biswajit, D.; Kartik, B. Morphological characterization of jackfruit (Artocarpus heterophyllus Lam.) of Assam, India. Int. J. Curr. Microbiol. Appl. 2019, 8, 1005–1016. [Google Scholar] [CrossRef]
- Chhetri, A.; Hazarika, B.N.; Wangchu, L.; Singh, S.; Alice, A.K.; Singh, M.C. Appraisement of variability and association among the jackfruit (Artocarpus heterophyllus Lam.) genotypes found in North-East India. Curr. J. Appl. Sci. 2019, 33, 1–13. [Google Scholar] [CrossRef]
- De Oca, M.M.-M.; Osuna-García, J.; Hernández-Estrada, A.; Ochoa-Villarreal, M.; Tovar-Gómez, B. Efecto del 1-metilciclopropeno (1-MCP) sobre la fisiología y calidad de frutos de jaca (Artocarpus heterophyllus Lam.). Rev. Chapingo Ser. Hortic. 2007, 13, 165–170. [Google Scholar] [CrossRef]
- Morelos-Flores, D.A.; Nolasco-González, Y.; Gutiérrez-Martínez, P.; Hernández-Fuentes, L.M.; Montalvo-González, E.; García-Magaña, M.L. Study of marketing simulation in jackfruit (Artocarpus heterophyllus Lam) treated with 1-methylcyclopropene. RIIT 2021, 9, 90–111. [Google Scholar]
- Bouzayen, M.; Latché, A.; Nath, P.; Pech, J.C. Mechanism of fruit ripening. In Plant Developmental Biology-Biotechnological Perspectives; Chong, E., Pua, M., Davey, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 1, pp. 319–339. [Google Scholar]
- International Plant Genetic Resources Institute (IPGRI). Descriptors for Jackfruit (Artocarpus heterophyllus); International Plant Genetic Resources Institute: Washington, DC, USA, 2000; p. 64. [Google Scholar]
- Tovar, B.; García, H.S.; Mata, M. Physiology of pre-cut mango. I. ACC and ACC oxidase activity of slices subjected to osmotic dehydration. Food Res. Int. 2000, 34, 207–215. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analysis Chemists International: Rockville, MD, USA, 2005. [Google Scholar]
- Rivera-Castro, V.; Bahena-Ortega, A.; Valenzuela-Lagarda, J.; Montaño-Lopez, A.; Mancillas-Paredes, J. Cambios fisicoquímicos durante maduración en condiciones locales de venta de frutos de mango Ataulfo de San Marcos Guerrero, Mex. Química Hoy Chem. Sci. 2022, 11, 22–25. [Google Scholar] [CrossRef]
- Castro-Benítez, M.; Restrepo-Sanchez, L.P.; Narváez-Cuenca, C.E. Actividad de clorofilasa durante la maduración del banano bocadillo (Musa accuminata) (Simons). Actual. Biol. 2005, 27, 151–158. [Google Scholar]
- Kader, A.A.; Yahia, E.M. Postharvest biology of tropical and subtropical fruits. In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 79–111. [Google Scholar] [CrossRef]
- Ibrahim, M.; Islam, M.S.; Helali, M.O.H.; Alam, A.K.M.S.; Shafique, M.Z. Morphological fruit characters and nutritional food value of different jackfruit (Artocarpus heterophyllus Lam.) cultivars in Rajshahi region of Bangladesh. BJSIB 2014, 48, 287–292. [Google Scholar] [CrossRef]
- Kavya, K.; Shyamalamma, S.; Gayatri, S. Morphological and molecular genetic diversity analysis using SSR markers in Jackfruit (Artocarpus heterophyllus Lam.) genotypes for pulp colour. Indian J. Agric. Res. 2019, 53, 8–16. [Google Scholar] [CrossRef]
- Akter, A.; Rahman, H. Evaluation of jackfruit (Artocarpus heterophyllus Lam.) germplasm. J. Bot. 2018, 7, 38–53. [Google Scholar] [CrossRef]
- Karunakaran, G.; Singh, P.; Ravishankar, H. New jackfruit for homesteads. Indian Hortic. 2017, 1, 46–48. [Google Scholar]
- Singh, A.K.; Gohain, I.; Shyamalamma, S. Morphological variability in jackfruit grown under agro-forestry system of Tripura. Indian J. Hortic. 2018, 75, 376–383. [Google Scholar] [CrossRef]
- Chandrashekar, K.G.; Vijayakumar, R.M.; Subramanian, S.; Kavino, M.; Joel, A.J. Fruit characterization of jackfruit (Artocarpus heterophyllus Lam.) local genotypes under coffee ecosystem of lower pulney hills in Tamil Nadu, India. J. Appl Hortic. 2018, 21, 47–52. [Google Scholar] [CrossRef]
- Anu, K.; Jayalekshmi, G.; Joseph, E.; Sabu, T. Assessment of physic- chemical properties of jackfruit collections from Kuttanand region of Kerala. Asian J. Hortic. 2015, 2, 262–266. [Google Scholar] [CrossRef]
- Mitra, S.K.; Maity, C.S. A summary of the genetic resources of jackfruit (Artocarpus heterophyllus Lam.) in West Bengal, India. Acta Hortic. 2002, 575, 269–271. [Google Scholar] [CrossRef]
- Rahman, M.; Mizanur, M.; Choedhury, F.; Khan, H. Preservation of jackfruit by osmotic dehydration. Bangladesh J. Agric. Res. 2016, 37, 67–75. [Google Scholar] [CrossRef]
- Rai, M.; Nath, V.; Das, B.; Rai, A.; Kumar, M. Evaluation of jackfruit genotypes for yield and quality attributes under eastern Indian condition. Orissa J. Hortic. 2002, 31, 1–5. [Google Scholar]
- Reddy, B.; Patil, P.; Kumar, S.; Govindraju, R. Studies on physico-chemical characteristics of jackfruit clones of south Karnataka. Karnataka J. Agric. Sci. 2004, 17, 279–820. [Google Scholar]
- Singh, S.; Narayanswami, P.; Banik, C.; Shyamalamy, S.; Simon, L. Evaluation of cracking and non-cracking genotypes of jackfruit. Crop. Res. 2011, 42, 157–162. [Google Scholar]
- Tiwari, V.K.; Verma, V.C.; Khushboo, A.; Kumar, K.; Tsewang, T.; Verma, A.; Norbu, T.; Acharya, S. Edible coating for postharvest management of fruits and vegetables. Pharm. Innov. J. 2022, 11, 970–978. [Google Scholar]
- Fishman, S.; Rodov, V.; Ben-Yehoshua, S. Mathematical model for perforation effect on oxygen and water vapour dynamics in modified atmosphere packages. J. Food Sci. 1996, 61, 956–961. [Google Scholar] [CrossRef]
- Kandasamy, A. Respiration rate of fruits and vegetables for modified atmosphere packaging: A mathematical approach. J. Postharv. Technol. 2022, 10, 88–102. [Google Scholar]
- Bolívar-Fernández, N.; Saucedo-Veloz, C.; Sauri-Duch, E. Respiración y parámetros relacionados durante la maduración del chicozapote cosechado en la Península de Yucatán. Rev. Bras. Frutic. 2011, 33, 261–266. [Google Scholar] [CrossRef][Green Version]
- Wahl, E.; Tan, S.; Koulikov, S.; Kharlamov, B.; Rella, C.; Crosson, E.; Biswell, D.; Paldus, B. Ultra-sensitive ethylene post-harvest monitor based on cavity ring-down spectroscopy. Opt. Express 2006, 14, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Carballo, S. Fisiología de la respiración y la transpiración de frutas y hortalizas. Programa Nac. Hortic. 2003, 1, 1–18. [Google Scholar]
- Ahmad, M.; Khalid, Z.M.; Farooqi, W.A. Effect of waxing and lining materials on a storage life of some citrus fruit. Proc. Fla. State Hortic. Soc. 1979, 92, 237–240. [Google Scholar]
- Becker, B.R.; Fricke, B.A. Transpiration and respiration of fruits and vegetables. In New Developments in Refrigeration for Food and Safety and Quality; International Institute of Refrigeration: Paris, France; American Society of Agricultural Engineers: St. Joseph, MI, USA, 1996; pp. 110–121. [Google Scholar]
- Sun, Y.; Singh, Z.; Tokala, V.; Heather, B. Harvest maturity stage and cold storage period influence lemon fruit quality. Sci. Hortic. 2019, 249, 322–328. [Google Scholar] [CrossRef]
- Montalvo-González, E.; González-Espinoza, N.G.; García-Galindo, H.S.; Tovar-Gómez, B.; de Oca, M.M.-M. Efecto del etileno exógeno sobre la desverdización del chile ‘Poblano’ en poscosecha. Rev. Chapingo Ser. Hortic. 2009, 15, 189–197. [Google Scholar] [CrossRef]
- Balamaze, J.; Muyonga, J.H.; Byaruhanga, Y.B. Physico-chemical characteristics of selected jackfruit (Artocarpus heterophyllus Lam.) varieties. J. Food Res. 2019, 8, 11–22. [Google Scholar] [CrossRef]
- Jordán, M.; Casaretto, J. Hormonas y reguladores del crecimiento: Etileno, ácido abscísico, brasinoesteroides, poliaminas, ácido salicílico y çacido jasmónico. In Fisiología Vegetal; Squeo, F.A., Cardemil, L., Eds.; Ediciones Universidad de La Serena: La Serena, Chile, 2006; Volume 16, pp. 1–28. [Google Scholar]
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001, 47, 311–339. [Google Scholar] [CrossRef]
- Payasi, A.; Sanwal, G.G. Ripening of climacteric fruits and their control. J. Food Biochem. 2010, 34, 679–710. [Google Scholar] [CrossRef]
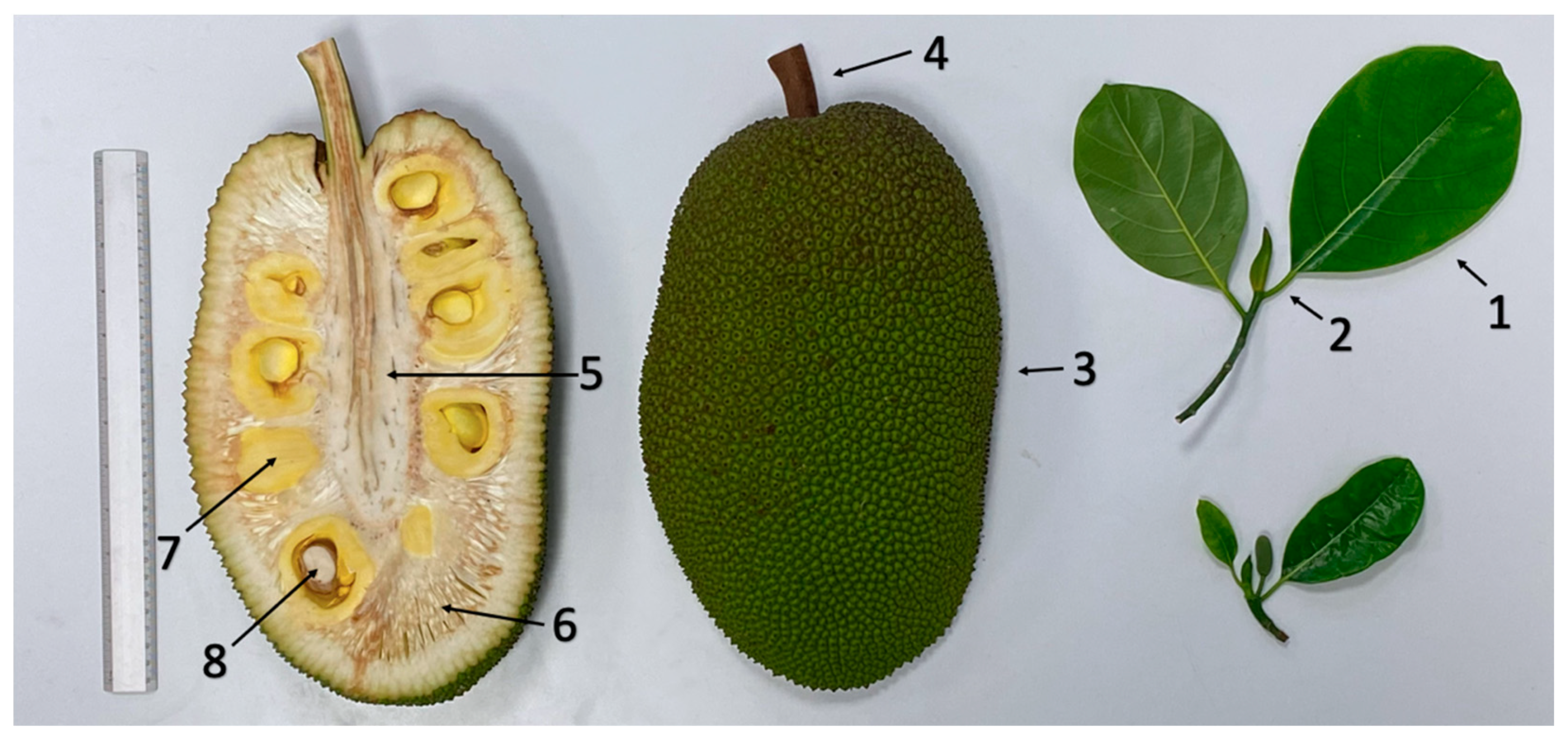
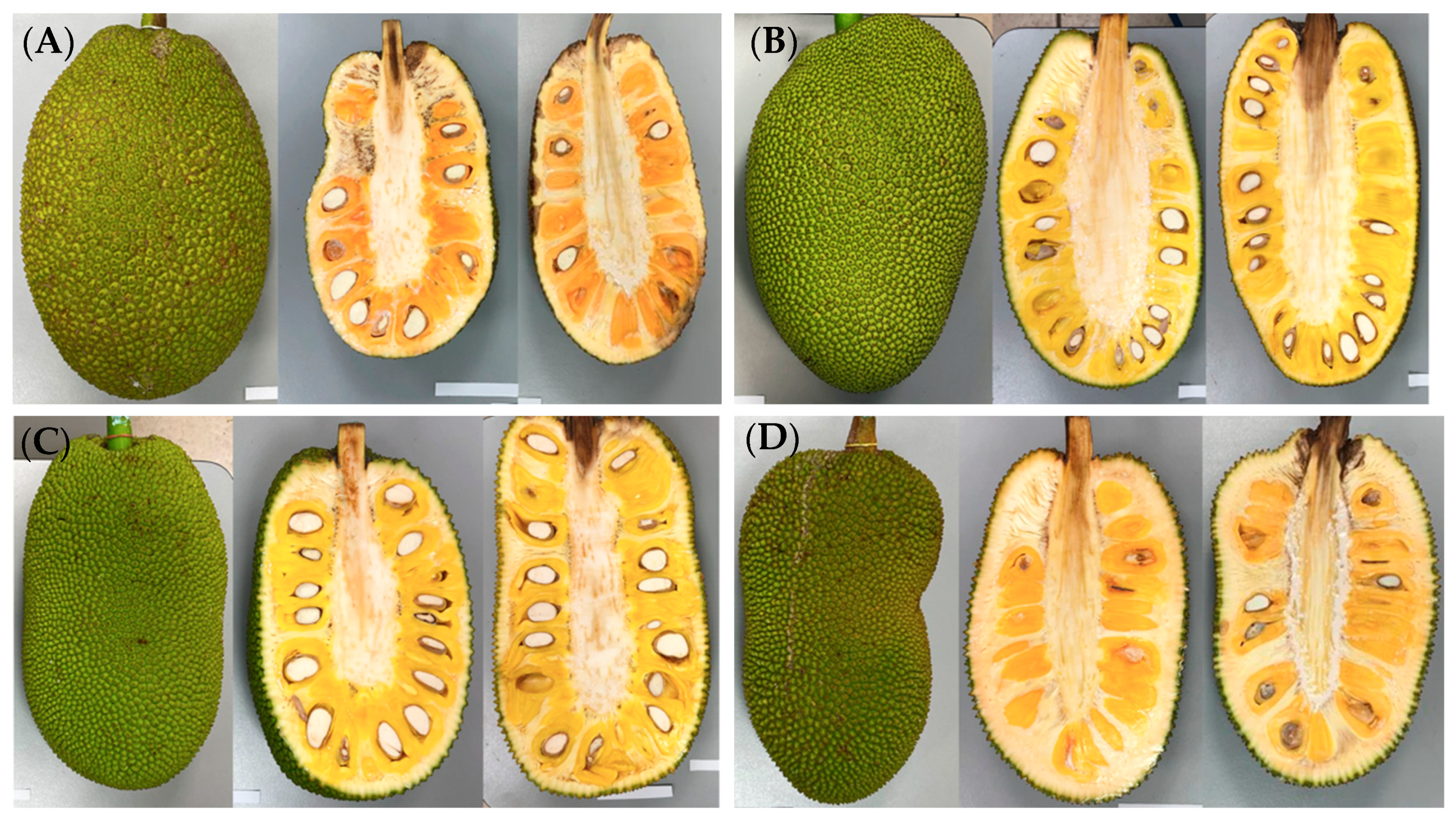


| Morphological Parameter | “Agüitada” | “Rumina” | “Licenciada” | “Virtud” |
|---|---|---|---|---|
| Length of the petiole (cm) | 1.93a | 2.31b | 1.74c | 2.16b |
| Length of the peduncle (cm) | 25.54a | 33.24b | 19.22c | 30.52ab |
| Equatorial diameter of the peduncle (cm) | 6.60a | 9.85a | 7.26b | 8.38c |
| Weight of the peel (kg) | 1.72a | 2.83b | 2.51bc | 2.12ac |
| Thickness of the peel (cm) | 1.21a | 1.27a | 1.27a | 2.08b |
| Length of the rachis (cm) | 20.60a | 27.38b | 24.35c | 19.91a |
| Rachis width (cm) | 6.50a | 8.74b | 6.65a | 4.11c |
| Bulb weight (kg) | 2.72a | 5.40b | 4.58b | 1.77a |
| Bulb length (cm) | 3.49a | 3.25a | 3.26a | 3.16a |
| Bulb width (cm) | 5.23a | 6.03b | 5.99b | 5.06a |
| Bulb thickness (cm) | 0.72a | 0.90a | 0.89a | 0.85a |
| Seed length (cm) | 3.02a | 3.10a | 3.60b | 3.34c |
| Seed width (cm) | 2.30a | 2.36ab | 2.51b | 2.30a |
| Number of seeds per fruit | 89.70a | 130.33b | 101.17a | 52.10c |
| Weight of seedless bulbs (kg) | 2.41a | 4.63b | 3.75c | 1.57a |
| Leaf length (cm) | 12.05a | 13.90b | 14.08b | 14.13b |
| Leaf width (cm) | 5.96a | 7.78b | 8.50c | 8.63c |
| Equatorial diameter of the fruit (cm) | 62.07a | 69.30b | 69.97b | 66.83b |
| Polar diameter of the fruit (cm) | 83.27a | 98.17bc | 101.27b | 93.63c |
| Storage Days (25 °C) | Agüitada | Rumina | Licenciada | Virtud |
|---|---|---|---|---|
| Respiration rate (mL of CO2 kg−1·h−1) | ||||
| 1 | 53.17aA | 71.40bA | 32.08cAD | 42.11cA |
| 2 | 125.53aB | 60.31bA | 52.35bBD | 56.48bAB |
| 3 | 118.37aB | 66.76bA | 66.92bC | 66.38bBC |
| 4 | 112.78aB | 77.81bcA | 63.59bBC | 80.02cC |
| 5 | 74.30aC | 69.71aA | 51.37bBCD | 63.81abBC |
| 6 | 75.80aC | 96.62bB | 69.20acBC | 60.55cB |
| 7 | 58.97aAC | 70.31aA | 24.63bA | 58.16aAB |
| 8 | 61.01aAC | 64.47aA | 42.37bD | 53.99abAB |
| 9 | 69.47aAC | 65.49abA | 44.82cD | 52.90bcAB |
| 10 | - | 65.75aA | 47.52bD | - |
| 11 | - | 93.43aB | 59.54bBC | - |
| Ethylene production (µL kg−1·h−1) | ||||
| 1 | 0.00aA | 0.00aA | 0.00aA | 0.00aAD |
| 2 | 10.16aBF | 5.04aAB | 7.43aBC | 23.52bA |
| 3 | 47.25aC | 6.81bBD | 6.06bBC | 84.56cB |
| 4 | 63.31aD | 54.92bC | 40.45cF | 27.15dA |
| 5 | 49.52aC | 30.87bE | 17.75cD | 19.10cCF |
| 6 | 32.90aE | 18.80bF | 19.7bD | 11.10cDFE |
| 7 | 27.92aE | 26.03aE | 9.30bBE | 14.74cF |
| 8 | 14.23aF | 17.17aF | 16.24aD | 5.68bEG |
| 9 | 3.83aAB | 8.62aBG | 4.13aAC | 3.83aG |
| 10 | - | 7.72aBG | 2.23bA | - |
| 11 | - | 5.36aADG | 2.37aA | - |
| Physiological weigth loss (%) | ||||
| 1 | 0.00aA | 0.00aA | 0.00aA | 0.00aA |
| 2 | 1.12aB | 0.88aB | 1.08aB | 1.00aB |
| 3 | 2.62aC | 1.82bC | 2.05abC | 2.13abC |
| 4 | 3.97aD | 2.83bD | 3.25abD | 3.39abD |
| 5 | 5.78aE | 4.38bE | 4.86bE | 4.49bE |
| 6 | 5.90aE | 4.90bE | 5.22abE | 5.60abF |
| 7 | 6.90aF | 5.88bF | 6.16abF | 6.69aG |
| 8 | 8.06aG | 6.90bG | 7.12cG | 7.85acH |
| 9 | 8.92aG | 7.53bG | 7.72bG | 8.63aH |
| 10 | - | 8.57aH | 8.85aH | - |
| 11 | - | 9.39aH | 9.70aH | - |
| Storage Days (25 °C) | “Agüitada” | “Rumina” | “Licenciada” | “Virtud” |
|---|---|---|---|---|
| Peel color (°H) | ||||
| 1 | 90.58aA | 86.82aA | 092.69aA | 88.38aA |
| 3 | 86.93aAB | 88.86aA | 92.94aA | 86.45aA |
| 5 | 79.55aB | 84.25abAB | 91.42bAB | 82.34aA |
| 7 | 78.20aB | 82.72aAB | 83.49aBC | 81.82aA |
| 9 | 69.22aC | 71.23aC | 81.88bC | 72.25aB |
| 11 | − | 72.30abBC | 78.86aC | − |
| Bulbs color (°H) | ||||
| 1 | 67.55aA | 80.44 ± 2.23bA | 81.89bA | 72.84aA |
| 3 | 66.87aA | 79.61bA | 79.64bAB | 70.12aAB |
| 5 | 62.32aB | 77.70bA | 77.00bB | 69.57cAB |
| 7 | 60.29aB | 77.36bA | 75.16bB | 68.86cAB |
| 9 | 63.28aAB | 76.91bA | 76.17bB | 66.86aB |
| 11 | − | 76.04bA | 77.31bAB | − |
| Peel firmness (N) | ||||
| 1 | 308.38aA | 409.15bA | 348.79abA | 343.92aA |
| 3 | 305.30aA | 421.12bAB | 307.81aAC | 321.89aAB |
| 5 | 295.75aAB | 408.90bAB | 298.00aAC | 315.23aAB |
| 7 | 232.19acBC | 349.18bB | 230.18cB | 293.30abAB |
| 9 | 215.58aC | 250.03abC | 221.98aCB | 289.16bB |
| 11 | − | 293.45bC | 220.55aB | − |
| Bulb firmness (N) | ||||
| 1 | 34.27aA | 42.51bA | 26.52cA | 15.86dA |
| 3 | 19.10aB | 17.90aB | 21.58aB | 10.69aB |
| 5 | 3.85aC | 12.67aB | 7.76aC | 5.62 aC |
| 7 | 3.56aC | 6.91aC | 6.35aCD | 4.46aC |
| 9 | 3.48aC | 5.41aC | 4.67aCD | 3.87aC |
| 11 | − | 4.72aC | 2.96aD | − |
| Titratable acidity (%) | ||||
| 1 | 0.10aA | 0.06aA | 0.11aA | 0.29bA |
| 3 | 0.43acB | 0.48aB | 0.25bB | 0.41cB |
| 5 | 0.33aC | 0.41bB | 0.26aB | 0.29aA |
| 7 | 0.25aD | 0.30aC | 0.17bA | 0.28aA |
| 9 | 0.25aD | 0.27aC | 0.21aB | 0.22aA |
| 11 | − | 0.23aC | 0.21aB | − |
| pH | ||||
| 1 | 5.82aA | 6.25aA | 5.52aA | 4.49bA |
| 3 | 4.35aB | 4.35aB | 4.18aA | 4.39aA |
| 5 | 4.55aB | 4.17aB | 4.67aA | 4.49aA |
| 7 | 5.41aAC | 5.20aC | 5.59aB | 5.31aB |
| 9 | 5.30aC | 5.27aC | 5.51aB | 5.29aB |
| 11 | − | 5.11aC | 4.90aA | − |
| Total soluble solids (TSS) | ||||
| 1 | 4.85aA | 3.70aA | 8.6bA | 13.13cA |
| 3 | 20.28aB | 19.38aB | 16.85bB | 20.60aB |
| 5 | 25.38aC | 23.55aC | 22.30aC | 21.06bB |
| 7 | 26.45aC | 26.15aC | 21.57bC | 23.73aBC |
| 9 | 27.46aC | 26.20aC | 23.01bC | 25.57aC |
| 11 | − | 26.83aC | 23.05bC | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morelos-Flores, D.A.; Montalvo-González, E.; Chacón-López, M.A.; Santacruz-Varela, A.; Zamora-Gasga, V.M.; Torres-García, G.; de Lourdes García-Magaña, M. Comparative Study of Four Jackfruit Genotypes: Morphology, Physiology and Physicochemical Characterization. Horticulturae 2022, 8, 1010. https://doi.org/10.3390/horticulturae8111010
Morelos-Flores DA, Montalvo-González E, Chacón-López MA, Santacruz-Varela A, Zamora-Gasga VM, Torres-García G, de Lourdes García-Magaña M. Comparative Study of Four Jackfruit Genotypes: Morphology, Physiology and Physicochemical Characterization. Horticulturae. 2022; 8(11):1010. https://doi.org/10.3390/horticulturae8111010
Chicago/Turabian StyleMorelos-Flores, David Antonio, Efigenia Montalvo-González, Martina Alejandra Chacón-López, Amalio Santacruz-Varela, Víctor Manuel Zamora-Gasga, Gerardo Torres-García, and María de Lourdes García-Magaña. 2022. "Comparative Study of Four Jackfruit Genotypes: Morphology, Physiology and Physicochemical Characterization" Horticulturae 8, no. 11: 1010. https://doi.org/10.3390/horticulturae8111010
APA StyleMorelos-Flores, D. A., Montalvo-González, E., Chacón-López, M. A., Santacruz-Varela, A., Zamora-Gasga, V. M., Torres-García, G., & de Lourdes García-Magaña, M. (2022). Comparative Study of Four Jackfruit Genotypes: Morphology, Physiology and Physicochemical Characterization. Horticulturae, 8(11), 1010. https://doi.org/10.3390/horticulturae8111010







